Abstract
Aims
This study sought to evaluate the feasibility and early outcomes of a percutaneous edge-to-edge repair approach for mitral valve regurgitation with the MitraClip® system (Evalve, Inc., Menlo Park, CA, USA).
Methods and results
Patients were selected for the procedure based on the consensus of a multidisciplinary team. The primary efficacy endpoint was acute device success defined as clip placement with reduction of mitral regurgitation to ≤2+. The primary acute safety endpoint was 30-day freedom from major adverse events, defined as the composite of death, myocardial infarction, non-elective cardiac surgery for adverse events, renal failure, transfusion of >2 units of blood, ventilation for >48 h, deep wound infection, septicaemia, and new onset of atrial fibrillation. Thirty-one patients (median age 71, male 81%) were treated between August 2008 and July 2009. Eighteen patients (58%) presented with functional disease and 13 patients (42%) presented with organic degenerative disease. A clip was successfully implanted in 19 patients (61%) and two clips in 12 patients (39%). The median device implantation time was 80 min. At 30 days, there was an intra-procedural cardiac tamponade and a non-cardiac death, resulting in a primary safety endpoint of 93.6% [95% confidence interval (CI) 77.2–98.9]. Acute device success was observed in 96.8% of patients (95% CI 81.5–99.8). Compared with baseline, left ventricular diameters, diastolic left ventricular volume, diastolic annular septal–lateral dimension, and mitral valve area significantly diminished at 30 days.
Conclusion
Our initial results with the MitraClip device in a very small number of patients indicate that percutaneous edge-to-edge mitral valve repair is feasible and may be accomplished with favourable short-term safety and efficacy results.
Keywords: Edge-to-edge, Percutaneous mitral valve repair, MitraClip
See page 1301 for the editorial comment on this article (doi:10.1093/eurheartj/ehq088)
Introduction
Moderate or severe mitral regurgitation (MR) is the second most common valve disease requiring surgical treatment in Europe.1 Although some controversies about the optimal timing of intervention in asymptomatic patients are currently present, a consensus exists that valvular surgery should be advised in symptomatic patients with severe MR, as shown by the corresponding class I recommendation in both the American and European clinical guidelines.2,3
Valve repair, when feasible, is the preferred surgical treatment in patients with severe MR because of lower perioperative mortality, improved survival, better preservation of post-operative left ventricular function, and lower long-term morbidity compared with valve replacement.2–4 Edge-to-edge repair has been used as a surgical technique for the treatment of MR since the early 1990s.5–7 With this technique, the edge of the middle scallop of the posterior leaflet is sutured to the corresponding portion of the anterior leaflet, creating a point of permanent coaptation of the two leaflets and resulting in a double-orifice.
The MitraClip® System (Evalve, Inc., Menlo Park, CA, USA) is a percutaneous edge-to-edge attachment system that mimics the surgical procedure. This technique creates a tissue bridge between the anterior and posterior leaflets by means of one clip deployed through transseptal catheterization. Early trials suggested that percutaneous valve repair with the MitraClip system is feasible and safe, with ∼60% of patients being discharged with a clip and mild or little MR.8 This report details the preliminary results of the Italian experience with the MitraClip system.
Methods
Study design
This prospective registry includes 31 patients treated in the two centres presently performing percutaneous mitral valve repair with the MitraClip system in Italy, namely Ferrarotto Hospital (FH) of Catania and San Raffaele Hospital (SRH) of Milan. The study was approved by the local medical ethic committees. After receiving a complete oral and written explanation of the issues surrounding the procedure, all patients signed written consent for inclusion in the study.
MitraClip system and procedural technique
The MitraClip system includes a MitraClip device, a 24-F Steerable Guide Catheter (SGC), and a Clip Delivery System (CDS). The Clip is pre-assembled to the tip of the disposable delivery catheter. Opening, closing, locking, and detaching the clip are all controlled by the delivery catheter handle mechanism, which is firmly lodged on a metal, sterilized external support placed outside the patient, on the bottom of a small table above the upper leg.
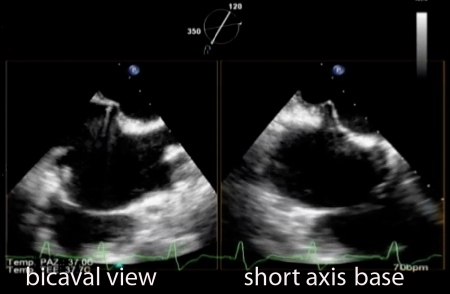
The procedure is performed under general anaesthesia to avoid any discomfort due to transoesophageal echocardiography (TOE) monitoring. Invasive arterial pressure is monitored through the radial or femoral artery, and a central venous catheter is placed in the right internal jugular or subclavian vein. The right femoral vein is cannulated with a 7-F introducer sheath, and a baseline right heart catheterization is performed. Then the 7-F introducer is exchanged with an 8-F Mullins sheath (S. Jude Medical, Minnesota, USA) over a 0.32 guidewire, and a transseptal puncture is performed using a Brockenbrough needle under TOE guidance. This is a critical point of the procedure, because the puncture has to be located in the postero-superior part of the interatrial septum in order to obtain enough room in the left atrium for a safe and optimal orientation of the steerable distal part of the CDS (Figure 1). Once the left atrium is entered with the 8-F sheath, the left pulmonary vein is cannulated using a 6-F multipurpose catheter (MPA2, Cordis, Johnson & Johnson, Miami, FL, USA) and a 260 cm Amplatz Super stiff guidewire is left in place. After administration of 100 IU/kg of unfractioned heparin, the 24-F SGC is introduced in the left atrium and the dilator is carefully and slowly retrieved for avoiding vacuum air bubbles. The CDS is then advanced in the left atrium, and the distal steerable part is manipulated in the atrium for obtaining a perpendicular and central position with respect to the mitral valve leaflets coaptation line. The correct trajectory of the clip and the perpendicularity of the two arms with respect to the mitral leaflet coaptation line are checked using three echocardiographic views: (i) the three-chamber in which the left atrium, left ventricle, and aortic root are visualized; (ii) the dual-chamber, in which both left atrium and ventricle are obtained, and (iii) the trans-gastric short axis view to visualize the coaptation line of the mitral leaflets.
Figure 1.
Bicaval and modified short-axis transoesophageal echocardiographic views. Transseptal puncture performed by means of the Brockenbrough needle.
Once the system has been aligned, the clip with opened arms is advanced into the left ventricle and under TOE guidance the arms grasp the leaflets. When a double-orifice has been created and the echocardiography confirms the regurgitation reduction and the optimal and stable grasp of both leaflets, there are two options: if the position is suboptimal, the clip can be reopened and repositioned; if the result is good and the grasp is stable, the clip arms are closed, locked, and detached and the SGC and CDS are withdrawn (Figure 2). When necessary, for example, in case of degenerative MR or ruptured chordae tendineae with wide prolapse, a second clip can be implanted (Figure 3). Although the implantation of a second clip is predictable, when a flail is present, the need for a second clip in other scenarios is evaluated on a case-by-case basis. Right cardiac catheterization is finally performed to record the post-procedural pressure and the final results (Figure 4). The guiding catheter is removed, and venous femoral access is closed using a ‘figure-of-eight’ superficial stitch.9
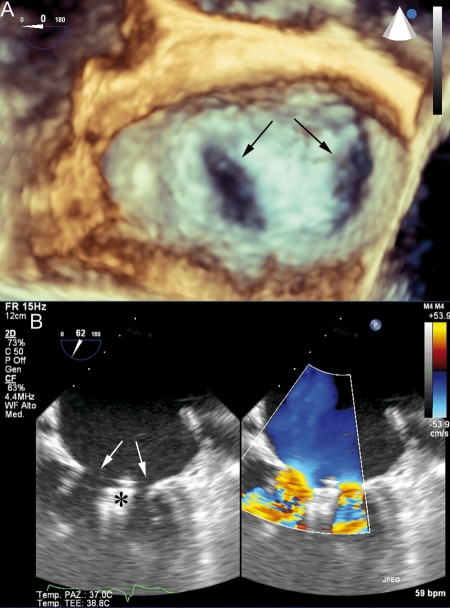
Figure 2.
Post-procedural imaging of the mitral valve after clip deployment. The newly created double-orifice is indicated by arrows in the three-dimensional (A) and two-dimensional echocardiographic views (B). Echoes generated by the clip grasping the leaflets (*) are evident below the mitral annulus plane in the two-dimensional view.
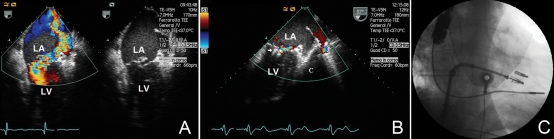
Figure 3.
(A) Two-chamber transesophageal echocardiographic view. Colour-Doppler examination shows severe mitral regurgitation from the left ventricle (LV) to the left atrium (LA). The two-dimensional image shows a free-floating chord and the major degree of prolapse of the posterior leaflet in the LA. (B) Two-chamber transoesophageal echocardiographic view. The colour-Doppler image demonstrates a significant reduction of the mitral regurgitation after the successful implantation and release of two clips (arrow). (C) The fluoroscopy right anterior oblique projection shows the two clips attached to the mitral valve leaflets. The second clip is going to be released from the delivery catheter system.
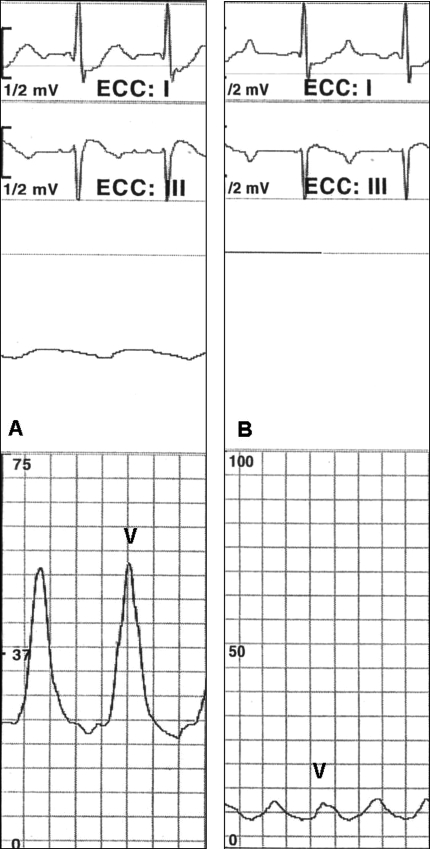
Figure 4.
Pulmonary wedge pressure recorded in basal condition shows a high V wave (A) secondary to severe mitral regurgitation. After clip implantation, the V wave is reduced (B). The parallel A-wave reduction indicates diminished end-diastolic pressure. These variations in aggregate result in diminished mean wedge pressure after clip implantation. In this case example, the systemic pressure varied from 100/60 mmHg before clip implantation to 130/70 mmHg after clip implantation, following fluid challenge and norepinephrine administration.
Post-procedural pharmacologic management included aspirin 100 mg lifelong and clopidogrel 75 mg for 3 months in patients without atrial fibrillation. Patients with atrial fibrillation were prescribed aspirin 100 mg and vitamin K antagonists.
Patient selection
Key inclusion and exclusion criteria are listed in Table 1. Patients were selected for the procedure if they met basic criteria for intervention from the European Society of Cardiology Task Force recommendation on the management of valvular heart disease.2 Hence, candidates included patients with moderate to severe (3+) or severe (4+) functional or degenerative MR with symptoms or without symptoms but left ventricular ejection fraction (LVEF) <60% or left ventricle end systolic diameter >45 mm. In addition to meeting guidelines criteria, patients were high-risk candidates for mitral valve surgery including cardiopulmonary bypass. High risk was established based on the consensus between a local independent cardiologist and a cardiac surgeon that conventional surgery would be associated with excessive morbidity and mortality. Criteria of high risk included European System for Cardiac Operative Risk Evaluation (EuroSCORE) >20%, hepatic cirrhosis, autoimmune disease, severe renal failure requiring haemodialysis or any contraindication to extracorporeal circulation. Given that certain leaflet anatomy is deemed to hamper a proper clip positioning on the leaflets with sufficient reduction in MR, echocardiographic criteria played a crucial role among clinical considerations for patient selection. These anatomic considerations included: (i) flail segment width ≥15 mm or a flail gap ≥10 mm; (ii) severe bileaflet flail or severe bileaflet prolapse; (iii) evidence of calcification or cleft of the grasping area; and (iv) lack of both primary and secondary chordae support (Table 1). As part of pre-interventional screening, patients underwent trans-thoracic echocardiography, TOE, chest X-ray, periodontal radiography, and invasive cardiac evaluation with coronary angiogram, left ventriculography, and right catheterization.
Table 1.
Major inclusion and exclusion criteria
| Inclusion criteria | |
| Age 18 years or older | |
| Moderate to severe (3+) or severe (4+) chronic mitral valve regurgitation with symptoms or without symptoms but LVEF < 60% or left ventricle end systolic diameter > 45 mm | |
| High-risk candidates for mitral valve surgery including cardiopulmonary bypass | |
| Primary regurgitant jet originating from malcoaptation of the A2 and P2 scallops of the mitral valve; if a secondary jet exists, it must be considered clinically insignificant | |
| Presence of sufficient leaflet tissue for a mechanical coaptation | |
| Non-rheumatic/endocarditic valve morphology | |
| Transseptal catheterization determined to be feasible by the treating physician | |
| Exclusion criteria | |
| Evidence of an acute myocardial infarction in the 12 weeks prior to the intended treatment | |
| Need for any other cardiac surgery including surgery for coronary artery disease, atrial fibrillation, pulmonic, aortic, or tricuspid valve disease | |
| Mitral valve orifice area <4.0 cm2 | |
| If leaflet flail is presenta: | |
| flail width ≥15 mmb | |
| flail gap ≥10 mmc | |
| If leaflet tethering is present: | |
| coaptation depth ≥11 mmd | |
| coaptation length <2 mme | |
| Severe mitral annular calcification | |
| Any leaflet anatomy which may preclude clip implantation, proper clip positioning on the leaflets, or sufficient reduction in MR | |
| Haemodynamic instability defined as systolic pressure <90 mmHg without afterload reduction or cardiogenic shock or the need for inotropic support or intra-aortic balloon pump | |
| Need for emergency surgery for any reason | |
| Systolic anterior motion of the mitral valve leaflet | |
| Hypertrophic cardiomyopathy | |
| Echocardiographic evidence of intra-cardiac mass, thrombus, or vegetation | |
| History of, or active, endocarditis | |
| History of, or active, rheumatic heart disease | |
| History of atrial septal defect, whether repaired or not | |
| History of patent foramen ovale associated with clinical symptoms (e.g. cerebral ischaemia) or previously repaired or when, in the judgement of the investigator, an atrial septal aneurysm is present that may interfere with transseptal crossing | |
| History of a stroke or documented TIA within the prior 6 months | |
| Patients in whom TOE is contraindicated | |
LVEF, left ventricular ejection fraction; TIA, transient ischaemic attack; TOE, transoesophageal echocardiography.
aFlail is defined as when a leaflet has both ruptured chordae and a free edge that extends above the opposing leaflet or above the plane of the annulus during systole.
bFlail width is defined as the width of flail leaflet segment as measured along the line of coaptation in the short-axis view.
cFlail gap is defined as the greatest distance between the ventricular side of the flail leaflet segment to the atrial side of the opposing leaflet edge.
dCoaptation depth is defined as the shortest distance between the coaptation of the leaflets and the annular plane.
eCoaptation length is defined as the vertical length of leaflets that is in contact, or is available for contact, during mid-systole in the atrial-to-ventricular direction in the four-chamber view.
Study endpoints
The primary acute safety endpoint was freedom from major adverse events (MAEs) at 30 days, defined as the composite of death, myocardial infarction, non-elective cardiac surgery for adverse events, renal failure, transfusion of >2 units of blood, ventilation for >48 h, deep wound infection, septicaemia, and new onset of atrial fibrillation.
Echocardiograms were performed according to a pre-specified protocol at baseline, pre-discharge, and at 30 days. The primary efficacy endpoint was acute device success defined as clip implant with reduction of MR to ≤2+, based on current guidelines.3 MR grade was assigned as recommended by the American Society of Echocardiography based on a validated integrative method10,11 and the consensus of two expert observers. In case of disagreement, the opinion of a third observer was obtained and the final decision was made by consensus. As vena contracta width and regurgitant orifice area have not been validated for a double-orifice valve, these parameters were not included among methods to appraise the severity of MR.
Statistical analysis
Continuous variables were analysed for a normal distribution with the Shapiro–Wilk test. Continuous variables following a normal distribution are presented as mean ± standard deviations and were compared using Student's unpaired t-test. Variables not following a normal distribution are expressed as median [interquartile range (IQR)] and were compared with Mann–Whitney rank sum test. One-way analysis of variance or Jonckheere–Terpstra test were used as appropriate for comparisons across multiple groups and to generate P-values for trend tests. Categorical variables are presented as counts and percentages. All probability values reported are two-sided, and a value of P < 0.05 was considered to be significant. All data were processed using the Statistical Package for Social Sciences, version 15 (SPSS, Chicago, IL, USA).
Results
Patient characteristics
Thirty-one patients [age 71 (IQR 62–79) years, male 81%] were treated between August 2008 and July 2009. Demographic and clinical characteristics are shown in Table 2. Eighteen patients (58%) presented with functional disease and 13 patients (42%) with organic degenerative disease (Table 3). Among patients with functional MR, 67% had a previous history of coronary artery disease. Logistic EuroSCORE and Society of Thoracic Surgeons (STS) score were 14.3 ± 11.9 and 10.3 ± 8.8%, respectively. Mean pulmonary and wedge pressures assessed by right catheterization were 25.2 ± 10.8 and 18.4 ± 9.1 mmHg, respectively.
Table 2.
Baseline demographic and clinical characteristics
| n = 31 | |
|---|---|
| Age, median (IQR) | 71 (62–79) |
| Age > 65 years | 22 (71) |
| Male sex | 25 (81) |
| Diabetes mellitus | 10 (32) |
| Hypertension | 18 (58) |
| Chronic kidney diseasea | 6 (19) |
| Chronic obstructive pulmonary disease | 0 |
| History of congestive heart failureb | 11 (36) |
| History of coronary artery disease | 14 (45) |
| Previous coronary artery bypass graft | 2 (6) |
| Atrial fibrillation | 8 (26) |
| High-risk criteria for mitral valve surgery with cardiopulmonary bypassc | |
| Contraindications to extracorporeal circulation | 12 (39) |
| Logistic EuroSCORE > 20% | 7 (23) |
| Autoimmune disease | 5 (16) |
| Severe renal failure requiring haemodialysis | 4 (13) |
| Hepatic cirrhosis | 3 (10) |
Data are expressed as counts and percentages if not otherwise specified. IQR, interquartile range.
aDefined as estimated glomerular filtration rate less than 60 mL/min/1.73 m2.
bDefined as prior hospitalization with documentation of exertional dyspnoea, fatigue, bilateral pedal oedema, orthopnoea, paroxysmal nocturnal dyspnoea, acute pulmonary oedema, or rales.
cHigh-risk criteria in patients with EuroSCORE < 20% included contraindications to cardiopulmonary bypass (e.g. pre-existing cancer, pulmonary, or cerebral disease) and co-morbidities not included in the EuroSCORE but deemed at high risk, based on the consensus between a local independent cardiologist and a cardiac surgeon that conventional surgery would be associated with excessive morbidity and mortality.
Table 3.
Mitral regurgitation aetiology
| Functional/ischaemic | 18 (58%) |
| Degenerative | 13 (42%) |
| P2 prolapse/flail | 8 (26%) |
| Bileaflets prolapse/flail | 3 (10%) |
| A2 prolapse/flail | 2 (6%) |
Procedure results
Twenty-one procedures were performed at one site (FH) and 10 at the other site (SRH). General anaesthesia was employed in all patients except one, who was treated under a deep conscious sedation because of contraindications to anaesthetic drugs. Mean general anaesthesia time was 166 ± 54 min. A clip was successfully implanted in 19 patients (61%) and two clips in 12 patients (39%). In no case, was clip implantation unsuccessful. The clip was implanted in the central portion of the valve in 97% of patients. In one case, was the clip implanted in the lateral commissure. The aetiology of MR was degenerative in 10% of patients who required one clip and 58% of patients who required two clips.
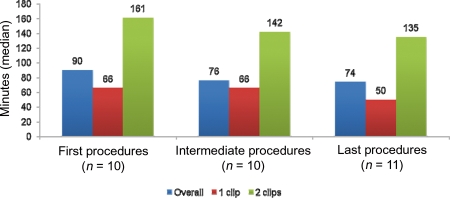
The device implantation time, defined as the time from guide insertion until CDS removal, did not significantly diminish with experience (Figure 5). Overall, the median device implantation time was 80 (IQR 61–137) min, ranging from 35 to 188 min. The first 10 procedures had a median device implantation time of 90 (IQR 65–158) min, the next 10 procedures had a median device implantation time of 76 (IQR 55–140) min, and the final 11 procedures had a mean device implantation time of 74 (IQR 50–113) min (P for trend = 0.468). The median device implantation time varied significantly between one-clip and two-clip cases [65 (IQR 50–74) vs. 151 (IQR 115–169) min, respectively, P < 0.001] and non-significantly between functional and degenerative aetiologies [73 (IQR 58–117) vs. 113 (IQR 59–161) min, respectively, P = 0.357]. There was no procedural mortality.
Figure 5.
The learning curve of the study series displayed a non-significant reduction of the device time throughout the study, which was also consistently seen in one- and two-clip cases.
Primary safety and efficacy endpoint
MAEs occurred in two patients at 30 days, resulting in a primary safety endpoint of 93.6% [95% confidence interval (CI) 77.2–98.9]. One patient, a 76-year-old man with thrombocytopenia and renal failure on haemodialysis, died 2 weeks after the procedure from gastrointestinal bleeding. Another patient experienced intra-procedural cardiac tamponade, after transseptal puncture, requiring surgical subxiphoid drainage and blood transfusion. Despite this complication, the clip was implanted successfully. No patient underwent emergency cardiac surgery for a failed clip implantation. No cases of clip detachment or embolization were observed. There were no other complications, including access site bleedings and transient ischaemic attacks.
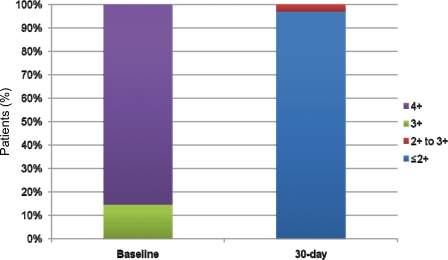
Acute device success was observed in 30 of 31 patients (96.8%, 95% CI 81.5–99.8) (Figure 6). At 30 days, of the 28 patients with MR = 4+ before the procedure, 18 (58%) had an MR graded as trivial to mild (0 to 1+), 9 (29%) had an MR graded as mild to moderate (1 to 2+), and 1 patient had an MR graded as moderate to severe (2 to 3+). This latter patient was treated with two clips for a degenerative MR. All three patients with MR = 3+ before the procedure had an MR graded as trivial to mild (0 to 1+) at 30 days.
Figure 6.
Baseline and 30-day mitral regurgitation grading.
At baseline, New York Heart Association (NYHA) functional class was I/II in 13% of patients and III/IV in 87% of patients. Thirty days after the procedure, all patients (100%) were in NYHA functional class I/II. Clinical symptoms were improved in all patients. In none of the patients the dosage of diuretics was increased after the procedure. Compared with baseline, diastolic left ventricular diameter, left ventricular volume, and annular septal–lateral dimension significantly diminished at 30 days (Table 4). The mitral valve area by planimetry was 4.4±1.1 cm2 at baseline and 2.8 ± 0.5 cm2 at 30 days (P < 0.001). Systolic left atrial dimension and LVEF did not vary significantly.
Table 4.
Dimensional echocardiographic parameters
| Baseline | 30-day follow-up | P-value | |
|---|---|---|---|
| Systolic left atrial dimension (mm) | 49 ± 8 | 47 ± 7 | 0.204 |
| Diastolic left ventricular diameter (mm) | 59 ± 10 | 56 ± 10 | <0.001 |
| Systolic left ventricular diameter (mm) | 38 ± 12 | 34 ± 8 | <0.001 |
| Diastolic left ventricular volume (mL) | 158 ± 59 | 134 ± 59 | <0.001 |
| LVEF (%) | 42 ± 17 | 43 ± 14 | 0.541 |
| Diastolic annular septal–lateral dimension (mm) | 37 ± 5 | 32 ± 3 | 0.013 |
| Mitral valve area (cm2)a | 4.4 ± 1.1 | 2.8 ± 0.5 | <0.001 |
| Systolic pulmonary pressure (mmHg) | 47 ± 15 | 36 ± 7 | <0.001 |
Data are expressed as mean ± standard deviation.
aAssessed by planimetry.
Discussion
The results of the initial Italian experience with the MitraClip device in a cohort of 31 patients indicate that the percutaneous edge-to-edge mitral valve repair is feasible and may be accomplished with favourable acute results. For the first time, these findings apply to a different population from that enrolled in the pivotal Endovascular Valve Edge-to-Edge Repair Study (EVEREST) trial.8,12
Surgery is the current gold standard of treatment for patients with symptomatic severe MR or asymptomatic severe MR with evidence of left ventricular dysfunction or dilatation, as it represents the only approach with defined clinical success, providing sustained relief of symptoms or heart failure.2,3 On the downside, despite its proven effectiveness, cardiac surgery is associated with significant trauma, risk of complications, and extended post-operative recovery period. Importantly, many patients with co-morbidities do not undergo surgery because of the high perceived risk of perioperative morbidity and mortality.13
A broad consensus exists that surgical mitral valve repair should be the first choice of treatment for MR, when feasible, because of improved clinical outcomes and avoidance of chronic anti-coagulation compared with valve replacement surgery. Among surgical repair techniques, the edge-to-edge or ‘double-orifice repair’ has gained wider use over time because of durable results shown in selected surgically treated patients.7,14 The suture guarantees that the two leaflets move together properly during systole and results in a 40–50% smaller effective diastolic orifice area than prior the procedure.15
The MitraClip system has been designed and developed to enable physicians to perform edge-to-edge valve repair while the heart is beating as an alternative to the open chest, arrested heart approach. Benefits of using this device include avoidance of chest incisions, cardiopulmonary bypass, and cardiac arrest, as well as the ability to identify the optimum point for creating an edge-to-edge repair and to assess the result of the mitral valve repair while the heart is fully functional prior to completion of the procedure. In addition, the procedure allows fully reversing all steps prior to complete deployment of clip device. This enables to test multiple sites along the line of coaptation if the physician is not satisfied with initial MitraClip device placement after the mitral valve leaflets have been grasped and approximated. Of note, differently from surgery, the percutaneous edge-to-edge procedure does not allow the concomitant execution of mitral annuloplasty. Most surgeons perform annuloplasty at the time of the initial surgical repair procedure to reduce the likelihood of re-operation in the future. However, it has been suggested that edge-to-edge repair, when used alone, preserves the anatomic sphincter mechanism of the mitral valve and the systolic performance of the base of the heart.16
Thirty-day safety and efficacy outcomes of a series of 107 patients enrolled in the EVEREST and in the pre-randomization phase of the EVEREST II (clinicaltrials.gov id NCT00209274) pivotal trials have been recently reported.12 In that cohort, 62% of patients were older than 65 years, mean ejection fraction was 62%, and functional aetiology accounted for 21% of patients. Acute procedural success was obtained in 74% of patients, and 64% were discharged with MR ≤ 1+.
Different from the inclusion criteria of the pivotal trial, patients deemed at high risk of periprocedural complications with surgery were included in the present study. As a result, our patients reflected a higher risk profile than those enrolled in the EVEREST trial: 71% of patients were older than 65 years, mean ejection fraction was 42%, and a functional aetiology, with all the inherent cardiac and extra-cardiac characteristics, was more frequently the cause of MR, being observed in 58% of patients. Despite this higher risk at baseline, we observed encouraging results in our study population. Reductions on MR were achieved in all patients, with 68% of patients who met the procedural goal of reducing MR to mild (1+) or less and 97% of patients with MR ≤2+.
Although the first 10 procedures had a device implantation time of 104 min and the final 11 procedures were on average 15 min shorter, we did not appreciate a statistically significant learning curve throughout the study period. However, the overall device implantation time was more than 1 h shorter than that reported in the EVEREST trial, despite a higher rate of procedures requiring the placement of two clips. This difference may be partly explained by lessons learnt by the initial experience with the device and a higher rate of functional MR. Not surprisingly, in fact, device implantation time was longer in procedures on degenerative valve disease which more often required two-clip deployment.
Importantly, despite the relatively large MitraClip system being manipulated inside the left chambers across the mitral orifice for more than 1 h in the majority of the cases, all the procedures were characterized by haemodynamic stability of the patients, including those with severe left ventricular dysfunction. The ability of the device to grasp and remove the clip before deployment was critical to address some initial unsatisfactory degrees of improvement in MR and then reposition the clip to achieve the optimal result.
The safety of the device was also remarkable, with no cases of clip detachment or embolization. One case of intra-procedural cardiac tamponade was successfully managed. Haemostasis of the 24-F femoral venous access was obtained without any bleeding issue. The early mortality rate of the study population was lower than that predicted by its average baseline EuroSCORE, an index which has also been shown to overestimate the risk of cardiac surgery in high-risk patients.17 Death occurred in one patient, who suffered from multiple co-morbidities, including end-stage chronic kidney disease and thrombocytopaenia. Percutaneous mitral valve repair was offered as the only palliative alternative of treatment to this patient, who presented with worsening symptoms of dyspnoea and died due to gastrointestinal bleeding 2 weeks after a successful procedure followed by symptoms relief.
Study limitations
Although positive, this study reports results obtained in a non-randomized fashion on a very small sample of patients with functional or degenerative MR selected on the basis of numerous exclusion criteria. In addition, due to the novelty of the technique, only a short follow-up was documented. Therefore, these study results must be intended as preliminary. Larger series and longer follow-up are warranted to determine the safety, efficacy, and durability of the MitraClip system enabling further investigation on different patient populations, including patients with functional and degenerative MR aetiology.
Another caveat is that the echocardiograms were not reviewed by an independent Core-Lab, because the study was performed in a clinical setting reproducing real world practice. In this study, however, the grading of MR was accomplished by means of a validated integrative method and based on the consensus among at least two expert observers.
Conclusions
The preliminary Italian experience with the MitraClip device indicates that percutaneous edge-to-edge mitral valve repair is a feasible approach that may be accomplished with favourable early outcomes. Until more experience can be gained and indications can be defined, this new technology could apply for a high-risk population in whom no viable option for mitral repair is available. Whether mitral valve repair by percutaneous clip implantation may represent a preferable alternative to surgery or medical therapy alone is currently undefined and needs to be proven by specifically designed randomized trials.
Funding
Funding to pay the Open Access publication charges for this article was provided by Abbott Vascular.
Conflict of interest: none declared.
References
- 1.Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. 2009;373:1382–1394. doi: 10.1016/S0140-6736(09)60692-9. [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology, ESC Committee for Practice Guidelines. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Carabello BA, Kanu C, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons. ACC/AHA 2006 Guidelines for the Management of Patients with Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 4.Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve repair improves the outcome of surgery for mitral regurgitation: a multivariate analysis. Circulation. 1995;91:1022–1028. doi: 10.1161/01.cir.91.4.1022. [DOI] [PubMed] [Google Scholar]
- 5.Alfieri O, Maisano F, De Bonis M, Stefano PL, Torracca L, Oppizzi M, La Canna G. The double-orifice technique in mitral valve repair: a simple solution for complex problems. J Thorac Cardiovasc Surg. 2001;122:674–681. doi: 10.1067/mtc.2001.117277. [DOI] [PubMed] [Google Scholar]
- 6.Maisano F, Torracca L, Oppizzi M, Stefano PL, D'Addario G, La Canna G, Zogno M, Alfieri O. The edge-to-edge technique: a simplified method to correct mitral insufficiency. Eur J Cardiothorac Surg. 1998;13:240–245. doi: 10.1016/s1010-7940(98)00014-1. [DOI] [PubMed] [Google Scholar]
- 7.Maisano F, Schreuder JJ, Oppizzi M, Fiorani B, Fino C, Alfieri O. The double-orifice technique as a standardized approach to treat mitral regurgitation due to severe myxomatous disease: surgical technique. Eur J Cardiothorac Surg. 2000;17:201–205. doi: 10.1016/s1010-7940(00)00351-1. [DOI] [PubMed] [Google Scholar]
- 8.Feldman T, Wasserman HS, Herrmann HC, Gray W, Block PC, Whitlow P, StGoar F, Rodriguez L, Silvestry F, Schwartz A, Sanborn TA, Condado JA, Foster E. Percutaneous mitral valve repair using the edge-to-edge technique: six-month results of the EVEREST Phase I Clinical Trial. J Am Coll Cardiol. 2005;46:2134–2140. doi: 10.1016/j.jacc.2005.07.065. [DOI] [PubMed] [Google Scholar]
- 9.Bagai J, Zhao D. Subcutaneous ‘figure-of-eight’ stitch to achieve hemostasis after removal of large-caliber femoral venous sheaths. Cardiac Interventions Today. 2008;5:22–23. doi: 10.1002/ccd.22946. [DOI] [PubMed] [Google Scholar]
- 10.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ American Society of Echocardiography. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 11.Foster E, Wasserman HS, Gray W, Homma S, Di Tullio MR, Rodriguez L, Stewart WJ, Whitlow P, Block P, Martin R, Merlino J, Herrmann HC, Wiegers SE, Silvestry FE, Hamilton A, Zunamon A, Kraybill K, Gerber IL, Weeks SG, Zhang Y, Feldman T. Quantitative assessment of severity of mitral regurgitation by serial echocardiography in a multicenter clinical trial of percutaneous mitral valve repair. Am J Cardiol. 2007;100:1577–1583. doi: 10.1016/j.amjcard.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 12.Feldman T, Kar S, Rinaldi M, Fail P, Hermiller J, Smalling R, Whitlow PL, Gray W, Low R, Herrmann HC, Lim S, Foster E, Glower D EVEREST Investigators. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. J Am Coll Cardiol. 2009;54:686–694. doi: 10.1016/j.jacc.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 13.Mirabel M, Iung B, Baron G, Messika-Zeitoun D, Détaint D, Vanoverschelde JL, Butchart EG, Ravaud P, Vahanian A. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28:1358–1365. doi: 10.1093/eurheartj/ehm001. [DOI] [PubMed] [Google Scholar]
- 14.Maisano F, Viganò G, Blasio A, Colombo A, Calabrese C, Alfieri O. Surgical isolated edge-to-edge mitral valve repair without annuloplasty: clinical proof of the principle for an endovascular approach. EuroIntervention. 2006;2:181–186. [PubMed] [Google Scholar]
- 15.Akins CW, Hilgenberg AD, Buckley MJ, Vlahakes GJ, Torchiana DF, Daggett WM, Austen WG. Mitral valve reconstruction versus replacement for degenerative or ischemic mitral regurgitation. Ann Thorac Surg. 1994;58:668–675. doi: 10.1016/0003-4975(94)90725-0. [DOI] [PubMed] [Google Scholar]
- 16.Umaña JP, Salehizadeh B, DeRose JJ, Jr, Nahar T, Lotvin A, Homma S, Oz MC. ‘Bow-tie’ mitral valve repair: an adjuvant technique for ischemic mitral regurgitation. Ann Thorac Surg. 1998;66:1640–1646. doi: 10.1016/s0003-4975(98)00828-5. [DOI] [PubMed] [Google Scholar]
- 17.Shanmugam G, West M, Berg G. Additive and logistic EuroSCORE performance in high-risk patients. Interact Cardiovasc Thorac Surg. 2005;4:299–303. doi: 10.1510/icvts.2004.104042. [DOI] [PubMed] [Google Scholar]