Abstract
Aims
To examine the extent of delay from initial hospital presentation to fibrinolytic therapy or primary percutaneous coronary intervention (PCI), characteristics associated with prolonged delay, and changes in delay patterns over time in patients with ST-segment elevation myocardial infarction (STEMI).
Methods and results
We analysed data from 5170 patients with STEMI enrolled in the Global Registry of Acute Coronary Events from 2003 to 2007. The median elapsed time from first hospital presentation to initiation of fibrinolysis was 30 min (interquartile range 18–60) and to primary PCI was 86 min (interquartile range 53–135). Over the years under study, there were no significant changes in delay times to treatment with either strategy. Geographic region was the strongest predictor of delay to initiation of fibrinolysis >30 min. Patient's transfer status and geographic location were strongly associated with delay to primary PCI. Patients treated in Europe were least likely to experience delay to fibrinolysis or primary PCI.
Conclusion
These data suggest no improvements in delay times from hospital presentation to initiation of fibrinolysis or primary PCI during our study period. Geographic location and patient transfer were the strongest predictors of prolonged delay time, suggesting that improvements in modifiable healthcare system factors can shorten delay to reperfusion therapy even further.
Keywords: Percutaneous coronary intervention, ST-segment elevation myocardial infarction, Reperfusion, Fibrinolysis
Introduction
Percutaneous coronary intervention (PCI) is superior to fibrinolysis for the treatment of ST-segment elevation myocardial infarction (STEMI) when performed with minimal delay in high-volume angioplasty centres by experienced operators.1–4 Nevertheless, given the importance of timely reperfusion to successful patient outcomes, guidelines strongly recommend proceeding with immediate fibrinolysis for patients with STEMI who present to hospitals without interventional capability and who will experience delays of >90 min if transferred to another hospital for primary PCI.5 The recently published European Society of Cardiology (ESC) guidelines suggest that fibrinolysis should be performed if delay to PCI is >90 min for patients presenting within 120 min of symptom onset or if delay to PCI is ≥120 min for patients presenting within >120 min of symptoms.6
Unfortunately, there is still considerable debate about whether patients may incur longer delays in treatment (up to 3 h) and still benefit more from PCI than earlier fibrinolysis. It has been suggested that in the midst of this controversy, a more important goal—that of trying to shorten total ischaemic time in patients with STEMI—is being ignored.7 As noted in a separate review, ‘what is needed now is an integrated system of care to minimize delays between symptom onset and reperfusion, rather than a continuing debate about primary PCI and fibrinolysis’.8 Using data from a multinational registry of patients with acute coronary syndromes (ACS), we examined changes over time in delay to treatment (time of initial hospital presentation to fibrinolysis or primary angioplasty) in the community. The impact of patient demographic and clinical characteristics, delay from symptom onset to hospital presentation, transfer status, and reperfusion modality on delay times were also examined.
Methods
Full details of the Global Registry of Acute Coronary Events (GRACE) rationale and methods have been published and are outlined below.9–11
Site selection
A total of 123 hospitals located in 14 countries (Argentina, Australia, Austria, Belgium, Brazil, Canada, France, Germany, Italy, New Zealand, Poland, Spain, UK, and USA) across four continents have contributed data to this observational study. To enhance the generalizability of the study findings, these hospitals were selected from 18 geographic clusters—in the majority, patients within each cluster were drawn from a defined geographic area. A more detailed description of cluster characteristics is given elsewhere.9 In each cluster, a sample of hospitals representative of those from that region were selected. Accordingly, enrolling hospitals within each cluster were of varying size, characteristics, and treatment capabilities.
Patient enrolment at each study hospital was intended to reflect an unbiased sample of admissions for ACS, independent of the annual volume of ACS patients seen at each of the participating hospitals. Individual hospital enrolment targets were uniformly established as the first 10 qualifying cases of ACS discharged each month. Regular audits were performed at all participating hospitals.
Patient population
Patients entered in the registry had to be at least 18 years old, be admitted for ACS as a presumptive diagnosis, and have at least one of the following: electrocardiographic changes consistent with ACS, serial increases in biochemical markers of cardiac necrosis, and/or documentation of coronary artery disease.9–11 The qualifying ACS must not have been precipitated by trauma or surgery. For purposes of the present study, we included only those patients experiencing an STEMI during calendar year 2003 through 2007 who presented to the index hospital of admission within 12 h of symptom onset, had no contraindications to fibrinolytic therapy, and were treated with either primary PCI or fibrinolysis within 12 h of presentation to the initial hospital. As complete data regarding administration of fibrinolytics were not available for patients treated with pre-hospital fibrinolytics (n = 315), these subjects were excluded. Where required, study investigators received approval from their hospital ethics or institutional review board, and a signed consent form for follow-up contact was obtained.
Data collection
Data were collected at each site by a trained coordinator using a standardized case report form. Demographic and clinical characteristics, myocardial infarction characteristics, and data on delay times [symptom onset to presentation, transfer time, first hospital presentation to treatment (door to fibrinolysis, D2L, or door to primary PCI, D2B)] were collected. Data abstractors were instructed to use time of first balloon inflation as time of primary PCI. Standardized definitions of all patient-related variables, clinical diagnoses, and treatments were used.
Data analysis
Subjects were grouped according to type of reperfusion therapy received (fibrinolysis vs. primary PCI). Within each treatment group, subjects were further dichotomized by delay time from the initial hospital presentation to initiation of reperfusion treatment (≤30 vs. >30 min for fibrinolysis; ≤90 vs. >90 min for primary PCI). These cutpoints were selected on the basis of current guidelines from the American College of Cardiology (ACC), the American Heart Association (AHA), and the ESC.5,10 Differences in the demographic and clinical characteristics of patients in these strata were assessed. The Wilcoxon rank sum test was used to analyse differences between respective comparison groups for continuous variables while the χ2 test was used to assess between-group differences in categorical variables. The Mantel–Haenszel test was used to assess time trends in dichotomous outcomes.
Multivariable logistic regression analyses were used to examine the association between patient demographic characteristics and clinical characteristics (including symptom onset to presentation and hospital transfer status) and prolonged time to reperfusion (>30 min for fibrinolysis; >90 min for primary PCI). Candidate variables for inclusion in our regression models included the demographic, clinical, and treatment characteristics included in Table 1. Candidate variables possibly associated with prolonged delay to treatment (P ≤ 0.20 after univariate analysis) were included in the multivariable models. Variables with P > 0.05 were eliminated in a backward fashion so that only variables with a statistically significant association with the outcome of interest were included in the final regression models.
Table 1.
Characteristics of subjects stratified by perfusion type and delay to reperfusion
| Delay in minutes | Fibrinolysis |
Primary PCI |
||||
|---|---|---|---|---|---|---|
| ≤30 n = 1071 | >30 n = 1042 | P-value | ≤90 n = 1626 | >90 n = 1431 | P-value | |
| Sociodemographic | ||||||
| Age in years, median | 60 | 61 | 0.11 | 61 | 61 | 0.47 |
| Women, % | 22 | 28 | 0.001 | 24 | 28 | 0.02 |
| Region, % | ||||||
| Australia/New Zealand/Canada | 26 | 29 | <0.0001 | 4.4 | 6.7 | <0.0001 |
| Europe, % | 54 | 37 | 67 | 53 | ||
| Argentina/Brazil, % | 11 | 22 | 16 | 20 | ||
| USA, % | 8.0 | 12 | 13 | 20 | ||
| Year admitted, % | ||||||
| 2003 | 33 | 32 | 0.86 | 20 | 20 | 0.65 |
| 2004 | 25 | 25 | 23 | 23 | ||
| 2005 | 20 | 19 | 21 | 23 | ||
| 2006 | 12 | 12 | 20 | 20 | ||
| 2007 | 10 | 11 | 15 | 14 | ||
| Medical history, % | ||||||
| Myocardial infarction | 12 | 16 | 0.01 | 11 | 16 | 0.0001 |
| Angiogram (+) CAD | 9.7 | 13 | 0.03 | 12.0 | 16 | 0.003 |
| Prior PCI | 6.9 | 7.5 | 0.61 | 9.6 | 11 | 0.37 |
| Prior CABG | 2.3 | 3.9 | 0.03 | 2.8 | 3.8 | 0.15 |
| Congestive heart failure | 2.5 | 3.3 | 0.36 | 2.5 | 2.2 | 0.63 |
| Peripheral arterial disease | 3.1 | 4.8 | 0.04 | 4.1 | 5.3 | 0.10 |
| Hypertension | 46 | 53 | 0.001 | 46 | 53 | <0.0001 |
| Hyperlipidaemia | 37 | 37 | 0.96 | 39 | 40 | 0.88 |
| Prior stroke/TIA | 3.0 | 4.2 | 0.13 | 2.9 | 4.8 | 0.01 |
| Former/current smoking | 64 | 64 | 0.99 | 63 | 62 | 0.29 |
| Diabetes | 16 | 17 | 0.64 | 16 | 19 | 0.02 |
| Renal insufficiency | 1.8 | 2.7 | 0.18 | 1.9 | 3.6 | 0.005 |
| Presentation characteristics | ||||||
| Admission weight in kg, median | 80 | 78 | 0.42 | 78 | 78 | 0.17 |
| Admission height in cm, median | 170 | 170 | 0.33 | 171 | 170 | 0.04 |
| Body mass index in kg/m2, median | 27 | 27 | 0.56 | 26 | 27 | 0.001 |
| Initial creatinine in mg/dL, median | 1.01 | 1.02 | 0.13 | 1.00 | 1.00 | 0.06 |
| GRACE risk score, median | 134 | 136 | 0.49 | 137 | 138 | 0.45 |
| Killip class (%) | ||||||
| I | 87 | 87 | 0.82 | 86 | 87 | 0.62 |
| II | 9.8 | 9.4 | 9.6 | 9.6 | ||
| III | 2.5 | 2.1 | 1.7 | 2.0 | ||
| IV | 1.0 | 1.2 | 2.3 | 1.8 | ||
| Pulse ≥100 b.p.m., % | 11 | 13 | 0.17 | 12 | 14 | 0.22 |
| Systolic BP <90 mmHg, % | 4.0 | 3.3 | 0.48 | 4.6 | 3.6 | 0.14 |
| >2 h of symptoms before hospital presentation | 40 | 45 | 0.04 | 53 | 53 | 0.86 |
| Patient transferred to hospital no. 2 for reperfusion, % | 23 | 27 | 0.06 | 6.3 | 31 | <0.0001 |
| Prior medicationsa % | ||||||
| Aspirin | 17 | 20 | 0.10 | 16 | 20 | 0.002 |
| ACE-inhibitor | 14 | 17 | 0.05 | 12 | 16 | 0.01 |
| Angiotensin II receptor blocker | 4.1 | 5.1 | 0.30 | 5.5 | 5.3 | 0.87 |
| Beta-blocker | 12 | 17 | 0.001 | 15 | 17 | 0.04 |
| Calcium antagonist | 8.2 | 9.9 | 0.20 | 8.5 | 9.6 | 0.31 |
| Clopidogrel | 2.1 | 1.6 | 0.52 | 3.3 | 3.0 | 0.76 |
| Diuretic | 5.8 | 8.3 | 0.03 | 8.6 | 11 | 0.02 |
| Insulin | 2.8 | 2.6 | 0.79 | 2.7 | 3.9 | 0.05 |
| Nitrate | 3.7 | 6.4 | 0.005 | 3.7 | 4.3 | 0.40 |
| Statin | 14 | 17 | 0.15 | 14 | 17 | 0.01 |
Variables with missing data >1% of total study sample include thrombolyis cohort/PCI cohort: admission weight (n = 239/306), admission height (n = 488/414), admission BMI (n = 524/458), initial creatinine (n = 114/128), GRACE risk score (n = 238/333), Killip class (n = 43/42), pulse (n = 44/106), systolic BP (n = 44/83), prior angiotensin II blocker (n = -/46), prior calcium channel blocker (n = -/41), insulin (n = -/36), nitrate (n = -/34).
ACE, angiotensin-converting enzyme; CABG, coronary artery bypass graft; CAD, coronary artery disease; PCI, percutaneous coronary intervention; TIA, transient ischaemic attack.
aMedications with <2% utilization not listed.
Given that we have clustered binary data (random hospitals nested in geographic regions), we fit our data using a logistic regression model with a generalized estimating equation approach and an exchangeable correlation structure (SAS GENMOD procedure for binary outcomes). This produces population average model estimates, estimates averaged over the distribution of the random hospitals. Model assumptions (linearity of continuous covariates, lack of multicollinearity, model goodness of fit) were adequately met. Linear trends in delay time over the study period (2003 through 2007) were evaluated using regression models of the form delay time in minutes (ranked from shortest to longest) = study year (an ordinal variable). SAS Version 9.1 (SAS Institute Inc., Cary, NC, USA) was used for all statistical analyses.
Results
The study population consisted of 5170 men and women with STEMI and no contraindications to fibrinolytics presenting within 12 h of the onset of acute coronary symptoms and treated within 12 h of hospital presentation. Of these, 2113 patients (41%) were treated with fibrinolytics and 3057 patients (59%) were treated with primary PCI.
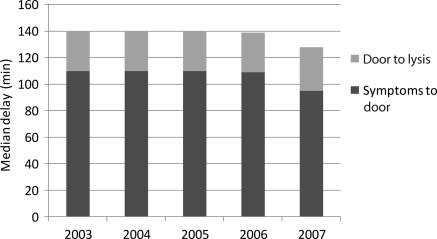
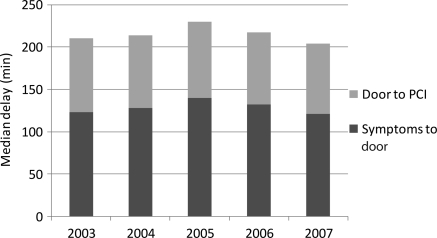
Median delay from symptom onset to fibrinolysis during our study period was 150 min [interquartile range (IQR) 100–243]. There was a slight but not statistically significant decrease in this delay period from 2003 (151 min) to 2007 (140 min) (Figure 1). Median delay from symptom onset to PCI during our study period was 235 min (IQR 165–370). No change in this delay was seen between 2003 and 2007 (Figure 2).
Figure 1.
Temporal trends in delay times in patients with acute myocardial infarction undergoing fibrinolysis.
Figure 2.
Temporal trends in delay times in patients with acute myocardial infarction undergoing percutaneous coronary intervention.
Fibrinolysis-treated patients
Among patients treated with fibrinolysis, the median elapsed D2L time was 30 min (IQR 18–60). Over the 5 years of study, there was no statistically significant change in median D2L time or in median time from symptom onset to hospital presentation (Figure 1). Approximately 53% of patients with symptom onset to hospital presentation time of <2 h experienced a D2L time of ≤30 min. Over time, this proportion increased slightly (50% in 2003; 51% in 2004; 55% in 2005; 54% in 2006; 56% in 2007).
Patients treated with fibrinolysis >30 min after hospital presentation were more often female or have a history of coronary artery disease by angiogram, myocardial infarction, coronary artery bypass graft surgery, peripheral arterial disease, or hypertension (Table 1). They were more likely to present already on angiotensin-converting enzyme inhibitors or beta-blockers, or nitrates. They were more likely to present to the index hospital >120 min after acute symptom onset. They were less likely to be treated in Europe (compared with other regions). Patients at the extremes of age (<40 or >80 years) were more likely to have a delay to treatment >30 min compared with other patients (<40 years, 59%; 40–50 years, 48%; 51–60 years, 47%; 61–70 years, 52%; 71–80 years, 52%; >80 years, 59%).
After logistic regression modelling, five variables were associated with D2L time >30 min: advancing age, female sex, previous use of beta-blockers or nitrates, and geographic region (Table 2). Patients treated in any region other than Europe were significantly more likely to experience a delay in fibrinolytic reperfusion.
Table 2.
Predictors of delay from hospital presentation to fibrinolysis >30 min (n = 2073 patients with complete covariate data) and percutaneous coronary intervention >90 min (n = 2838 patients with complete covariate data)
| Odds ratio (95% confidence interval) | P-value | |
|---|---|---|
| Fibrinolysis >30 mina | ||
| Age per 10 year increase | 1.1 (1.0–1.2) | 0.02 |
| Female sex | 1.4 (1.1–1.7) | 0.003 |
| Chronic beta-blocker therapy | 1.3 (1.0–1.6) | 0.06 |
| Chronic nitrate therapy | 1.5 (1.1–2.0) | 0.01 |
| Region (reference category = Europe) | ||
| USA | 2.1 (1.3–3.5) | <0.001 |
| Argentina/Brazil | 2.5 (1.5–4.1) | |
| Australia/New Zealand/Canada | 1.8 (1.1–2.8) | |
| PCI >90 min | ||
| Systolic blood pressure per 20 mmHg increase | 1.1 (1.0–1.2) | 0.02 |
| Creatinine per additional mg/dL | 1.3 (1.1–1.5) | 0.01 |
| Female sex | 1.3 (1.1–1.5) | <0.001 |
| History of myocardial infarction | 1.5 (1.2–1.8) | <0.001 |
| Transferred for percutaneous coronary intervention | 9.8 (6.2–15.6) | <0.001 |
| Region (reference category = Europe) | ||
| USA | 2.7 (1.6–4.7) | <0.001 |
| Argentina/Brazil | 2.4 (1.4–4.0) | |
| Australia/New Zealand/Canada | 2.8 (1.8–4.5) | |
ac-statistic 0.63; Hosmer–Lemeshow goodness-of-fit P-value 0.67.
Primary percutaneous coronary intervention
Among patients treated with primary PCI, the median D2B time was 86 min (53–135). Over the 5 years of study, there was no statistically significant change in median D2B time or in median time from symptom onset to hospital presentation (Figure 2). The median D2B time was 150 min (105–205) in patients who required transfer for the procedure. Approximately 52% of patients with symptom onset to hospital presentation time of <2 h experienced a D2B time of ≤90 min. This improved slightly over time (52% in 2003; 53% in 2004; 48% in 2005; 54% in 2006; 56% in 2007). The number of patients transferred from a non-PCI hospital to a PCI-capable hospital for primary PCI did not change over time (from 2003: 19%, 2004: 21%, 2005: 22%, 2006:18%, 2007:20%; P=0.24 for linear trend).
Patients with a delay from hospital presentation to primary PCI of >90 min had a slightly greater body mass index, were more often female, or were more likely to have a history of coronary artery disease diagnosed by angiogram, previous myocardial infarction, hypertension, transient ischaemic attack/stroke, diabetes, or renal insufficiency than patients who were treated in a more timely manner (Table 1). They were more likely to present already on angiotensin-converting enzyme inhibitors, beta-blockers, diuretics, insulin, or a statin. They were much more likely to have been transferred for PCI. They were less likely to be treated in Europe (compared with other regions). Patients <40 years of age were more likely to have a delay to PCI of >90 min (<40 years, 58%; 40–50 years, 45%; 51–60 years, 44%; 61–70 years, 49%; 71–80 years, 49%; >80 years, 49%).
After logistic regression modelling, six variables were associated with D2B >90 min: increasing systolic blood pressure, increasing serum creatinine levels, female sex, previous myocardial infarction, geographic region, and transfer to a PCI-capable facility (Table 2). Transfer for PCI was the strongest predictor of delay from hospital presentation to treatment.
Discussion
Data from this large multinational registry suggest that the overall median delay time from initial hospital presentation to receipt of fibrinolysis is 30 min and to primary PCI is 86 min for patients with STEMI. These delay times are within the limits of those recommended by current guidelines.5,6 Taken from a global perspective (of developed nations), this is encouraging. However, there remain a large proportion of patients who are not treated as promptly as possible. Furthermore, there has been no improvement in these delay patterns during the years under study (2003–7). Increased delay times to restoration of coronary flow are associated with increased infarction size, increased risk of subsequent congestive heart failure, and higher mortality. In a review of 2635 patients with STEMI enrolled in 10 randomized trials of primary PCI vs. thrombolysis, patients with symptom onset to presentation time of >4 h had significantly increased rates of a combined endpoint of death, non-fatal re-infarction, and stroke compared with those with symptom onset to presentation times <2 h (thrombolysis: 19.4 vs. 12.5%; PCI 7.7 vs. 5.8%).12 In another review of 22 trials comparing primary PCI with thrombolysis, patients with D2B times of >79–120 min had increased mortality compared with those with delay of only 0–35 min (6.6 vs. 2.8%).13 Finally, in an analysis of data from over 27 000 patients with STEMI enrolled in the 2nd National Registry of Myocardial Infarction, the adjusted odds of in-hospital mortality were increased ∼1.5-fold in patients with D2B times >120 min compared with those with D2B times <120 min.14
Fibrinolysis
Clinical trials of fibrinolysis have demonstrated clearly the benefit of initiating fibrinolytic therapy as early as possible after the symptoms of acute myocardial infarction have been noted. Early studies suggest that fibrinolytic therapy administered within the first hour of symptom onset decreases mortality by ∼27%, and between the first and second hour by ∼23%.15 Thereafter, the benefit associated with fibrinolytic therapy declines with increasing duration of delay.
Given the importance of timely reperfusion, current recommendations suggest that fibrinolytic therapy be administered within 30 min of hospital presentation. In our study, the median time from hospital presentation to fibrinolysis was ∼30 min. This represents a significant improvement in treatment efficiencies compared with previous reports. In an analysis of patients with STEMI enrolled in Global Utilization of Streptokinase and tPA for Occluded Coronary Arteries-1 (GUSTO-1; completed in 1993) and GUSTO 3 (completed in 1997), median delay times from hospital presentation to treatment were 66 and 54 min, respectively.16 Similarly, in a substudy of the National Registry of Myocardial Infarction (NRMI), which enrolled patients with STEMI between 1993 and 1994, the median delay time was 54 min.17 Data from the Myocardial Infarction National Audit Project, which collected data from >100 000 patients enrolled at 222 of 235 hospitals in England and Wales, also showed improvements in delay to fibrinolysis. From the first quarter of 2001 to the third quarter of 2002, 67% of patients received fibrinolytic therapy within 30 min of hospital presentation.18 Nevertheless, approximately half of the patients in our study still were not treated within the currently recommended time period. Furthermore, no improvement in delay from hospital presentation to fibrinolysis was seen from 2003 to 2007.
Not surprisingly, older individuals and women were less likely to be treated within the recommended time frame than were younger patients and men. Concerns about the risk for major bleeding associated with fibrinolytic therapy in elderly patients, particularly intracranial haemorrhage, creates a treatment dilemma for physicians caring for these patients. Appropriately, primary PCI is preferred to fibrinolysis in elderly patients, given a superior safety profile. Nevertheless, as with younger patients, if fibrinolysis is to be used, it should be initiated without delay. Ours and a number of other studies suggest delayed utilization of fibrinolytics in elderly patients with STEMI.19–21 We hypothesize that in at least some of these elderly patients, indecision about type of reperfusion and/or failed attempts to arrange transfer for PCI may result in increased delays to fibrinolysis. This healthcare system issue requires closer attention.
Previous studies have also shown increased delay to fibrinolysis in women.17,22 Differences in symptom presentation and the speed at which STEMI is diagnosed in women vs. men may account for some of this delay. In the NRMI substudy, door to electrocardiogram time was longer in women.17 Interestingly, while there was no difference in the presence of ST elevation on the initial electrocardiogram (86%), there was still a greater delay in making the decision to use fibrinolysis in women compared with men.
Geographic region of enrolment was the strongest predictor of delays in the administration of thrombolytic therapy: patients in Europe had a median delay of 26 min compared with 35 min in the USA, 49 min in Argentina/Brazil, and 32 min in Australia/New Zealand/Canada. The reasons for geographic disparity in time to fibrinolysis after hospital presentation cannot be determined by our study; a number of health system variables (e.g. utilization of pre-hospital electrocardiograms, treatment pathways, staffing models) may account for this difference.
Primary percutaneous coronary intervention
Similar to the effective utilization of fibrinolytic therapy, the overarching goal of primary PCI is timely restoration of flow in the infarct-related artery. The overall median D2B time in our study was at the upper limit of current recommendations. The strongest predictors of delay >90 min were geographic region and patient transfer for PCI. Patients transferred for PCI had an eight-fold increased risk of delay and a median delay time of 150 min. Patients treated in regions other than Europe had a significantly increased likelihood of prolonged D2B time. Interestingly, this occurred despite Europe having the highest transfer rate for PCI (22 vs. 19% vs. 7 vs. 8%, respectively). Decreased distances between hospitals in Europe and increased use of central myocardial infarction triage networks among area hospitals may allow for increased use of a transfer status without concomitant prolonged delay.
These findings are not surprising since these two variables (transfer for PCI and geography) are at the core of ongoing debate regarding the optimal treatment of STEMI. In the Danish Multicenter Randomized Study on Fibrinolytic Therapy vs. Acute Coronary Angioplasty in Acute Myocardial Infarction (DANAMI-2) patients enrolled at referral hospitals who were randomized to transfer for primary angioplasty had a lower incidence of the composite endpoint of death, myocardial infarction, or stroke at 30 days (8.5%) than those who remained at the referral hospital and received immediate fibrinolysis (14.2%).4 Most of this benefit was driven by a reduction in recurrent myocardial infarction. There was no significant difference seen in mortality or stroke. It should also be noted that the median transfer time for PCI was only 67 min. Subsequently, concerns about whether such efficient transfer strategies could be reproduced in the ‘real world’ were raised.
More recently, a meta-analysis of 22 trials comparing fibrinolysis with PCI suggested that primary PCI was more effective even if delayed up to 120 min after hospital presentation compared with when fibrinolysis could be administered.13 Others have argued that even delays of up to 180 min in PCI may still be preferable to fibrinolysis.23 But as reviewed by Antman7 in a recent editorial, under-representation of patients with short symptom-to-presentation delay as well as a biologically implausible relationship between PCI-related delay and mortality in patients treated with fibrinolytics render these findings suspect.
Unfortunately, median delay from symptom onset to hospital presentation remained relatively fixed between 120 and 140 min over our study period. This component of overall delay has proved relatively refractory to patient-education initiatives.24 Duration of symptom onset before hospital presentation should also be considered when deciding on whether to administer fibrinolysis or to transfer the patient for primary PCI.25 In patients who present within 60–120 min of symptom onset, early reperfusion without delay is critical to maximize myocardial salvage. In such patients, primary PCI is still preferable if it can be performed within 90 min of initial presentation. However, if there is anticipated excessive delay (>90 min) incurred by transferring the patient for PCI, fibrinolysis followed by immediate transfer may be more appropriate. Conversely, in patients who present >3 h after symptom onset, delays in reperfusion would not be expected to have as much of a negative impact on myocardial salvage—the relative safety and efficacy of PCI compared with fibrinolysis, even if necessitating some delay, may make this a better option. Unfortunately, we cannot examine variables directly associated with decision to transfer for PCI in our database. However, symptom duration of 120 min before hospital presentation was not associated with delay in treatment for either fibrinolysis or primary PCI in our study.
As seen in our study, most healthcare systems (and particularly those outside of Europe) have not been able to replicate the short D2B times for transfer patients observed in DANAMI-2 and similar studies. Nevertheless, it appears many healthcare providers remain enamoured with this approach (transfer for primary PCI regardless of delay time). Even among patients who do not require transfer for reperfusion, a substantial proportion suffers needless delays from door to fibrinolysis or PCI. Moreover, delay from symptom onset to hospital presentation remains the largest component of the overall delay time to treatment and did not appreciably change over our 5-year study period. As such, there is still great potential for substantial improvements in STEMI-related care and outcomes.
Although not examined directly in our study, use of pre-hospital thrombolytics and acute myocardial infarction triage networks to decrease transfer time to hospitals with cardiac catheterization facilities may be one way in which these improvements can be realized. In order to improve the use of STEMI therapy in Vienna, Austria, the Viennese Ambulance System was developed in conjunction with recommendations to initiate thrombolysis (in-hospital or pre-hospital) if PCI could not be offered in a timely fashion (particularly in patients with duration of symptoms of <2 h).26 This resulted in an increase in the use of reperfusion therapy (66–87%). Among patients receiving pre-hospital fibrinolytics, symptom onset to fibrinolysis time was 76 min, 91% went on to coronary angiography, and ∼50% had rescue or facilitated PCI. Among patients receiving primary PCI, first medical contact-to-balloon time was 81 min. In-hospital mortality decreased from 16% before establishment of the network to 9.5% after establishment of the network. Similarly, data from 1714 patients enrolled during a 1-month period in 2005 in the French Registry on Acute ST-Elevation Myocardial Infarction (FAST-MI) found that utilization of timely thrombolysis (∼60% pre-hospital) followed by liberal use of early PCI resulted in similar 1-year survival (94%) as primary PCI (92%).27 Whether such strategies can be successfully and broadly implemented in other regions of the world remains to be seen.
Study limitations
As with the interpretation of findings from any observational study, we cannot claim to have adequately controlled for all variables that may have impacted delay in reperfusion. It must also be recognized that numerous sociodemographic, clinical, and healthcare system variables must be considered when selecting reperfusion strategy and deciding on patient transfer. We purposely grouped our cohorts using recommended treatment times given clinical interest in these recommendations and based on the results of prior studies, which have utilized similar cutpoints. It should be noted that this dichotomization of the delay time variable in our regression analyses may lead to some loss of information with respect to factors that impact duration of delay.
Although GRACE was designed to include a broad representation of hospital types, it must be acknowledged that some of the participating centres may not be fully representative of their country with respect to acute myocardial infarction management and time delays. It is likely that systems of care designed to improve delay times or decrease patients initially referred to non-PCI centres were initiated or ongoing at some of the >100 hospitals participating in GRACE. Such centres were not excluded from this analysis. Unfortunately, data on the development of such systems in specific centres during our study years and impact of these systems were not collected in GRACE. Nevertheless, as this is an observational study rather than a randomized trial, patients were not excluded for comorbidity and hence the results are more likely to reflect clinical practice. We cannot comment directly on the appropriateness of such decisions—we can only identify an association between transfer and prolonged delay. Finally, it is possible that a small number of patients receiving primary PCI at a GRACE hospital were transferred to another hospital for subsequent care. Obviously, this would not be anticipated to impact D2B times—as such, inclusion of these patients would tend to minimize the observed association between transfer and delay to PCI.
Conclusions
Data from this large multinational registry suggest that the overall median delay time from initial hospital presentation to fibrinolysis is 30 min and for primary PCI is 84 min for patients with STEMI. However, this means that at least half of patients with an evolving STEMI have an unacceptably long delay. Furthermore, there has been no improvement in these delay patterns from 2003 to 2007. Geographic location of enrolment was the strongest predictor of delay in patients receiving fibrinolysis; transfer status and geographic location were the strongest predictors of delay time in patients undergoing PCI. These data suggest that improvements in modifiable healthcare system factors (nationally and locally) are still needed to shorten delay and improve patient outcomes.28 It remains to be seen whether recently initiated national efforts such as the ACC's D2B Alliance and the AHA's Mission Lifeline programmes can achieve significant gains in time to reperfusion at a systems level.29,30
Funding
This work was supported by an unrestricted educational grant from sanofi-aventis (Paris, France) to the Center for Outcomes Research, University of Massachusetts Medical School. Sanofi-aventis had no involvement in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. F.A.S. is supported by a Career Investigator Award from the Ontario Heart and Stroke Foundation.
Conflict of interest: Sanofi-aventis had no involvement in the collection, analysis, and interpretation of data, in the writing of the manuscript, or in the decision to submit the paper for publication. The design, conduct, and interpretation of the GRACE data are undertaken by an independent steering committee.
Potential conflicts of interest are as follows: F.A.S.: consultant for sanofi-aventis; honoraria from Bristol-Myers Squibb, Pfizer, and EISAI. G.M.: research grants from sanofi-aventis, Schering, Lilly, MSD, and Pfizer. K.A.A.F.: research grants from the British Heart Foundation, Medical Research Council, and the Wellcome Trust; research grants and lecture fees from sanofi-aventis, GlaxoSmithKline, and Bristol-Myers Squibb. S.G.G.: research grant support and/or speaker/consulting honoraria from Astra Zeneca, Bayer, Biovail, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Guidant, Hoffman La-Roche, Johnson & Johnson, Key Schering/Schering Plough, Merck Frosst, Pfizer, sanofi-aventis, and The Medicines Company. C.B.G.: research grants from Alexion, Astra Zeneca, Boehringer Ingelheim, Bristol-Myers Squibb, decode Genetics, Genentech, GlaxoSmithKline, Novartis, Proctor and Gamble, sanofi-aventis, The Medicines Company, INO Therapeutics, Medicure, and Proctor and Gamble; consultant/advisory board member for Alexion, Astra Zeneca, GlaxoSmithKline, INO Therapeutics, Medicure, Novartis, Proctor and Gamble, sanofi-aventis, and The Medicines Company. R.J.G.: research grants from sanofi-aventis. G.B.F.O.: none. F.A.A.: research grants from sanofi-aventis, Scios, and The Medicines Company; consultant/advisory board member for sanofi-aventis, GlaxoSmithKline, Scios, and The Medicines Company. K.A.E.: research grants from Biosite, Bristol-Myers Squibb, Blue Cross Blue Shield of Michigan, Hewlett Foundation, Mardigian Fund, Pfizer, sanofi-aventis, and the Varbedian Fund; consultant/advisory board member for NIH NHLBI, Pfizer, sanofi-aventis, and the Robert Wood Johnson Foundation. G.F.: none. J.M.G.: research grants from sanofi-aventis and The Medicines Company.
Acknowledgements
We thank the physicians and nurses participating in GRACE. Further information about the project, along with the complete list of participants, can be found at www.outcomes.org/grace. Sophie Rushton-Smith, PhD, provided editorial support on the final version of this manuscript and was funded by sanofi-aventis through the GRACE registry.
References
- 1.Grines CL, Browne KF, Marco J, Rothbaum D, Stone GW, O'Keefe J, Overlie P, Donohue B, Chelliah N, Timmis GC. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1993;328:673–679. doi: 10.1056/NEJM199303113281001. doi:10.1056/NEJM199303113281001. [DOI] [PubMed] [Google Scholar]
- 2.Zijlstra F, de Boer MJ, Hoorntje JC, Reiffers S, Reiber JH, Suryapranata H. A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med. 1993;328:680–684. doi: 10.1056/NEJM199303113281002. doi:10.1056/NEJM199303113281002. [DOI] [PubMed] [Google Scholar]
- 3.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. doi:10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 4.Andersen HR, Nielsen TT, Rasmussen K, Thuesen L, Kelbaek H, Thayssen P, Abildgaard U, Pedersen F, Madsen JK, Grande P, Villadsen AB, Krusell LR, Haghfelt T, Lomholt P, Husted SE, Vigholt E, Kjaergard HK, Mortensen LS. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733–742. doi: 10.1056/NEJMoa025142. doi:10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- 5.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC, Jr, Anbe DT, Kushner FG, Ornato JP, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW. 2007 Focused Update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: developed in collaboration With the Canadian Cardiovascular Society endorsed by the American Academy of Family Physicians: 2007 Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction, Writing on Behalf of the 2004 Writing Committee. Circulation. 2008;117:296–329. doi: 10.1161/CIRCULATIONAHA.107.188209. doi:10.1161/CIRCULATIONAHA.107.188209. [DOI] [PubMed] [Google Scholar]
- 6.Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Aguirre FV, Al-Attar N, Alegria E, Andreotti F, Benzer W, Breithardt O, Danchin N, Mario CD, Dudek D, Gulba D, Halvorsen S, Kaufmann P, Kornowski R, Lip GY, Rutten F. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: The Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. doi:10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 7.Antman EM. Time is muscle: translation into practice. J Am Coll Cardiol. 2008;52:1216–1221. doi: 10.1016/j.jacc.2008.07.011. doi:10.1016/j.jacc.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Fox KA, Huber K. A European perspective on improving acute systems of care in STEMI: we know what to do, but how can we do it? Nat Clin Pract Cardiovasc Med. 2008;5:708–714. doi: 10.1038/ncpcardio1343. doi:10.1038/ncpcardio1343. [DOI] [PubMed] [Google Scholar]
- 9.The GRACE Investigators. Rationale and design of the GRACE (Global Registry of Acute Coronary Events) Project: a multinational registry of patients hospitalized with acute coronary syndromes. Am Heart J. 2001;141:190–199. doi: 10.1067/mhj.2001.112404. doi:10.1067/mhj.2001.112404. [DOI] [PubMed] [Google Scholar]
- 10.Steg PG, Goldberg RJ, Gore JM, Fox KA, Eagle KA, Flather MD, Sadiq I, Kasper R, Rushton-Mellor SK, Anderson FA. Baseline characteristics, management practices, and in-hospital outcomes of patients hospitalized with acute coronary syndromes in the Global Registry of Acute Coronary Events (GRACE) Am J Cardiol. 2002;90:358–363. doi: 10.1016/s0002-9149(02)02489-x. doi:10.1016/S0002-9149(02)02489-X. [DOI] [PubMed] [Google Scholar]
- 11.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. doi:10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 12.Zijlstra F, Patel A, Jones M, Grines CL, Ellis S, Garcia E, Grinfeld L, Gibbons RJ, Ribeiro EE, Ribichini F, Granger C, Akhras F, Weaver WD, Simes RJ for the PCAT collaboration. Clinical characteristics and outcome of patients with early (<2 h), intermediate (2–4 h) and late (>4 h) presentation treated by primary coronary angioplasty or thrombolytic therapy for acute myocardial infarction. Eur Heart J. 2002;23:550–557. doi: 10.1053/euhj.2001.2901. doi:10.1053/euhj.2001.2901. [DOI] [PubMed] [Google Scholar]
- 13.Boersma E. Does time matter? A pooled analysis of randomized clinical trials comparing primary percutaneous coronary intervention and in-hospital fibrinolysis in acute myocardial infarction patients. Eur Heart J. 2006;27:779–788. doi: 10.1093/eurheartj/ehi810. doi:10.1093/eurheartj/ehi810. [DOI] [PubMed] [Google Scholar]
- 14.Cannon CP, Gibson CM, Lambrew CT, Shoultz DA, Levy D, French WJ, Gore JM, Weaver WD, Rogers WJ, Tiefenbrunn AJ. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality for patients undergoing angioplasty for acute myocardial infarction. JAMA. 2000;283:2941–2947. doi: 10.1001/jama.283.22.2941. doi:10.1001/jama.283.22.2941. [DOI] [PubMed] [Google Scholar]
- 15.Fibrinolytic Therapy Trialists' (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Lancet. 1994;343:311–322. [PubMed] [Google Scholar]
- 16.Gibler WB, Armstrong PW, Ohman EM, Weaver WD, Stebbins AL, Gore JM, Newby LK, Califf RM, Topol EJ. Persistence of delays in presentation and treatment for patients with acute myocardial infarction: The GUSTO-I and GUSTO-III experience. Ann Emerg Med. 2002;39:123–130. doi: 10.1067/mem.2002.121402. doi:10.1067/mem.2002.121402. [DOI] [PubMed] [Google Scholar]
- 17.Lambrew CT, Bowlby LJ, Rogers WJ, Chandra NC, Weaver WD. Factors influencing the time to thrombolysis in acute myocardial infarction. Time to Thrombolysis Substudy of the National Registry of Myocardial Infarction-1. Arch Intern Med. 1997;157:2577–2582. doi:10.1001/archinte.157.22.2577. [PubMed] [Google Scholar]
- 18.Birkhead J. Where are we today? Early results from MINAP, the National Audit of Myocardial Infarction Project. Heart. 2003;89(Suppl. 2):ii13–ii15. doi: 10.1136/heart.89.suppl_2.ii13. discussion ii35–17 doi:10.1136/heart.89.suppl_2.ii13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandra H, Yarzebski J, Goldberg RJ, Savageau J, Singleton C, Gurwitz JH, Gore JM. Age-related trends (1986–1993) in the use of thrombolytic agents in patients with acute myocardial infarction. The Worcester Heart Attack Study. Arch Intern Med. 1997;157:741–746. doi:10.1001/archinte.157.7.741. [PubMed] [Google Scholar]
- 20.Krumholz HM, Murillo JE, Chen J, Vaccarino V, Radford MJ, Ellerbeck EF, Wang Y. Thrombolytic therapy for eligible elderly patients with acute myocardial infarction. JAMA. 1997;277:1683–1688. doi:10.1001/jama.277.21.1683. [PubMed] [Google Scholar]
- 21.McLaughlin TJ, Gurwitz JH, Willison DJ, Gao X, Soumerai SB. Delayed thrombolytic treatment of older patients with acute myocardial infarction. J Am Geriatr Soc. 1999;47:1222–1228. doi: 10.1111/j.1532-5415.1999.tb05203.x. [DOI] [PubMed] [Google Scholar]
- 22.Weaver WD, White HD, Wilcox RG, Aylward PE, Morris D, Guerci A, Ohman EM, Barbash GI, Betriu A, Sadowski Z, Topol EJ, Califf RM. Comparisons of characteristics and outcomes among women and men with acute myocardial infarction treated with thrombolytic therapy. GUSTO-I investigators. JAMA. 1996;275:777–782. doi:10.1001/jama.275.10.777. [PubMed] [Google Scholar]
- 23.Stone GW. Primary angioplasty versus ‘earlier’ thrombolysis—time for a wake-up call. Lancet. 2002;360:814–816. doi: 10.1016/S0140-6736(02)11010-5. doi:10.1016/S0140-6736(02)11010-5. [DOI] [PubMed] [Google Scholar]
- 24.Luepker RV, Raczynski JM, Osganian S, Goldberg RJ, Finnegan JR, Jr, Hedges JR, Goff DC, Jr, Eisenberg MS, Zapka JG, Feldman HA, Labarthe DR, McGovern PG, Cornell CE, Proschan MA, Simons-Morton DG. Effect of a community intervention on patient delay and emergency medical service use in acute coronary heart disease: The Rapid Early Action for Coronary Treatment (REACT) Trial. JAMA. 2000;284:60–67. doi: 10.1001/jama.284.1.60. doi:10.1001/jama.284.1.60. [DOI] [PubMed] [Google Scholar]
- 25.Gersh BJ, Stone GW, White HD, Holmes DR., Jr Pharmacological facilitation of primary percutaneous coronary intervention for acute myocardial infarction: is the slope of the curve the shape of the future? JAMA. 2005;293:979–986. doi: 10.1001/jama.293.8.979. doi:10.1001/jama.293.8.979. [DOI] [PubMed] [Google Scholar]
- 26.Kalla K, Christ G, Karnik R, Malzer R, Norman G, Prachar H, Schreiber W, Unger G, Glogar HD, Kaff A, Laggner AN, Maurer G, Mlczoch J, Slany J, Weber HS, Huber K. Implementation of guidelines improves the standard of care: the Viennese registry on reperfusion strategies in ST-elevation myocardial infarction (Vienna STEMI registry) Circulation. 2006;113:2398–2405. doi: 10.1161/CIRCULATIONAHA.105.586198. doi:10.1161/CIRCULATIONAHA.105.586198. [DOI] [PubMed] [Google Scholar]
- 27.Danchin N, Coste P, Ferrieres J, Steg PG, Cottin Y, Blanchard D, Belle L, Ritz B, Kirkorian G, Angioi M, Sans P, Charbonnier B, Eltchaninoff H, Gueret P, Khalife K, Asseman P, Puel J, Goldstein P, Cambou JP, Simon T. Comparison of thrombolysis followed by broad use of percutaneous coronary intervention with primary percutaneous coronary intervention for ST-segment-elevation acute myocardial infarction: data from the French Registry on Acute ST-Elevation Myocardial Infarction (FAST-MI) Circulation. 2008;118:268–276. doi: 10.1161/CIRCULATIONAHA.107.762765. doi:10.1161/CIRCULATIONAHA.107.762765. [DOI] [PubMed] [Google Scholar]
- 28.Jollis JG, Roettig ML, Aluko AO, Anstrom KJ, Applegate RJ, Babb JD, Berger PB, Bohle DJ, Fletcher SM, Garvey JL, Hathaway WR, Hoekstra JW, Kelly RV, Maddox WT, Jr, Shiber JR, Valeri FS, Watling BA, Wilson BH, Granger CB. Implementation of a statewide system for coronary reperfusion for ST-segment elevation myocardial infarction. JAMA. 2007;298:2371–2380. doi: 10.1001/jama.298.20.joc70124. doi:10.1001/jama.298.20.joc70124. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs AK, Antman EM, Faxon DP, Gregory T, Solis P. Development of systems of care for ST-elevation myocardial infarction patients: executive summary. Circulation. 2007;116:217–230. doi: 10.1161/CIRCULATIONAHA.107.184043. doi:10.1161/CIRCULATIONAHA.107.184043. [DOI] [PubMed] [Google Scholar]
- 30.Bradley EH, Nallamothu BK, Stern AF, Byrd JR, Cherlin EJ, Wang Y, Yuan C, Nembhard I, Brush JE, Jr, Krumholz HM. Contemporary evidence: baseline data from the D2B Alliance. BMC Res Notes. 2008;1:23. doi: 10.1186/1756-0500-1-23. doi:10.1186/1756-0500-1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]