Abstract
Aims
Abnormal exercise test defined as the occurrence of exercise limiting symptoms, fall in blood pressure below baseline, or complex ventricular arrhythmias is useful to predict clinical events in asymptomatic patients with aortic stenosis (AS). The purpose of this study was to determine whether exercise-stress echocardiography (ESE) adds any incremental prognostic value to resting echocardiography in patients with AS having a normal exercise response.
Methods and results
One hundred and eighty-six asymptomatic patients with at least moderate AS and preserved LV ejection fraction (≥50%) were assessed by Doppler-echocardiography at rest and during a maximum ramp semi-supine bicycle exercise test. Fifty-one (27%) patients had an abnormal exercise test and were excluded from the present analysis. Among the 135 patients with normal exercise test, 67 had an event (aortic valve replacement motivated by symptoms or cardiovascular death) at a mean follow-up of 20 ± 14 months. The variables independently associated with events were: age ≥65 years [hazard ratio (HR) = 1.96; 95% confidence interval (CI): 1.15–3.47; P = 0.01], diabetes, (HR = 3.20; 95% CI: 1.33–6.87; P = 0.01), LV hypertrophy (HR = 1.96; 95% CI: 1.17–3.27; P = 0.01), resting mean gradient >35 mmHg (HR = 3.60; 95% CI: 2.11–6.37; P < 0.0001), and exercise-induced increase in mean gradient >20 mmHg (HR = 3.83; 95% CI: 2.16–6.67; P < 0.0001).
Conclusion
The exercise-induced increase in transvalvular gradient may be helpful to improve risk stratification in asymptomatic AS patients with normal exercise response. These results thus suggest that ESE may provide additional prognostic information over that obtained from standard exercise testing and resting echocardiography.
Keywords: Aortic valve, Aortic stenosis, Exercise, Doppler-echocardiography, Surgery
See page 1295 for the editorial comment on this article (doi:10.1093/eurheartj/ehq078)
The management of asymptomatic patients with severe aortic stenosis (AS) remains controversial. According to ESC and AHA/ACC guidelines, only patients having severe AS associated with either symptoms and/or LV ejection fraction <50% have a class I indication for aortic valve replacement (AVR).1,2 However, the slow progressive nature of AS combined to the relatively old age of the population affected by this disease predispose to under-reporting and/or underestimation of symptoms. Furthermore, sudden deaths have been reported in patients with severe AS in the absence of previous report of symptoms.3,4
In addition, Rosenhek et al.5 showed that a substantial proportion of patients with moderate AS progresses rapidly to the severe stage and these patients display excess mortality. Hence, moderate AS is not necessarily a benign disease and may require close follow-up and additional tests for prognostication.
In light of these findings, proper risk stratification of AS patients is crucial to determine: (i) the most appropriate time interval (i.e. 6, 12, or 24 months) between follow-up visits in the individual patient; and (ii) the optimal timing for AVR. In this regard, several factors have been proposed to identify the patients who are at higher risk for rapid disease progression and occurrence of adverse events including: higher peak jet velocity or gradient and smaller aortic valve area measured by Doppler-echocardiography,5–7 higher degree of valve calcification measured by echocardiography or computed tomography,5,7,8 occurrence of exercise-limiting symptoms on exercise stress test,9–13 and elevated plasma levels of natriuretic peptides.14,15
An important proportion (>20%) of the AS patients who claim to be asymptomatic exhibit exercise-limiting symptoms during exercise testing and these patients have a worse outcome.9–13 Hence, abnormal exercise test defined as the occurrence of exercise-limiting symptoms or fall in blood pressure below baseline is useful to predict clinical events in asymptomatic patients with AS and is now included in the ESC1 and AHA/ACC2 guidelines as an indication for AVR. However, whether or not exercise-stress echocardiography (ESE) provides any incremental predictive value beyond that obtained from standard exercise testing (i.e. without echocardiography) remains relatively unexplored and controversial.13,16 Lancellotti et al.12 demonstrated that an absolute increase in mean gradient >18 mmHg during exercise is an independent predictor of rapid progression to symptoms onset in patients with asymptomatic severe AS. On the other hand, Weisenberg et al.17 reported that ESE has limited added value to exercise testing alone in this population.
Given that the predictive value of abnormal exercise test is now well established and incorporated in the guidelines, the objective of this study was to determine if ESE adds any incremental value to resting echocardiography in the subset of patients who have a normal exercise response.
Methods
Study population
Doppler-echocardiographic and clinical data were prospectively collected in 186 asymptomatic patients with at least moderate AS (aortic valve area <1.5 cm2 and indexed aortic valve area <0.9 cm2/m2) who underwent ESE in four hospitals. Ninety-three patients were recruited in three European hospitals and 42 in one Canadian Center. The data of these four centres were pooled together and retrospectively analysed. Exclusion criteria were: (i) symptoms including dyspnoea, angina, syncope, or heart failure; (ii) LV ejection fraction <50%; (iii) moderate/severe aortic or mitral regurgitation or mitral stenosis; (iv) coronary artery disease (history of myocardial infarction or coronary artery stenosis on coronary angiography); (v) known pulmonary disease; (vi) atrial fibrillation or flutter; and (vii) inability to perform physical exercise.
Clinical data
Clinical data included age, gender, history of smoking, documented diagnosis of hypertension [patients receiving antihypertensive medications or having known, but untreated, hypertension (blood pressure ≥140/90 mmHg)], hypercholesterolaemia (patients on cholesterol lowering medication or in the absence of such medication low-density lipoprotein cholesterol level > 160 mg dL−1), and diabetes (patients currently receiving oral hypoglycaemic medication or insulin).
Exercise-stress echocardiography
Exercise protocol
A symptom-limited graded maximum bicycle exercise test was performed in the semi-supine position on an ergometer table tilted to 20°. After an initial workload of 20–25 W maintained for 3 min, the workload was increased every 3 min by 20–25 W. A 12-lead ECG was monitored continuously and blood pressure was measured at rest and every 2 min during exercise. If patients were on beta-blocker, they were asked to stop their medication 24 h before the test. The other medications, if any, were left unchanged. Abnormal exercise test was defined as: (i) occurrence of limiting breathlessness or fatigue at low workload, or of angina, dizziness, or syncope; (ii) fall in systolic blood pressure below baseline; (iii) complex ventricular arrhythmia. Patients with abnormal exercise test were excluded from the present study. The maximum workload (Watts) was recorded and the percent of maximum age and gender predicted workload was calculated.18
Doppler-echocardiographic measurements
Doppler-echocardiographic data were obtained at rest and at peak exercise with the use of a Vivid 7 (General Electrics, Chalfont St Giles, UK) or Sonos 7500 (Philips, Andover, MA, USA) ultrasound system and were stored in digital format for subsequent analysis. The Doppler-echocardiographic measurements included the LV end-diastolic diameter and thickness, the LV ejection fraction determined by the modified biplane Simpson's method, the transvalvular gradients by the simplified Bernoulli equation, and the aortic valve area by the continuity equation. For each measurement, at least three cardiac cycles were averaged. The LV mass was calculated with the corrected formula of the American Society of Echocardiography and indexed for body surface area and LV hypertrophy was defined as LV mass index >115 g m−2 in men and >95 g m−2 in women.
Follow-up and study endpoint
The vast majority of the patients had an annual follow-up in the centre where they had their baseline ESE. However, some patients were followed by cardiologists in centres not participating to this study. In these patients, the follow-up was performed by phone interview with the patient and treating cardiologist. If an event occurred, it was documented by review of patient's chart.
The primary outcome variable was the time to occurrence of the first composite endpoint of cardiovascular death or need for AVR motivated by the development of symptoms or LV systolic dysfunction.
Statistical analysis
Categorical data were given as a percentage and compared with a χ2 test. Continuous variables were tested for distribution normality using the Shapiro–Wilk test. Continuous data were expressed as mean ± SD and compared using the two-sided Student's t-test. Rest and exercise echocardiographic data were compared using the paired Student's t-test. If normality test failed, data were expressed as median and interquartile range and compared using the two-sided Wilcoxon's rank-sum test.
Cumulative probability of event-free survival was estimated using the Kaplan–Meier method and compared between groups using a log-rank test. The effect of the clinical, Doppler-echocardiographic, and exercise variables on event-free survival was assessed with the use of Cox proportional hazard model. Proportionality of the hazard was visually inspected from log-minus-log survival curves, stratified by the variable of interest. Hazard proportionality was also assessed by testing the interaction between variables of interest and time. All the variables presented in Table 1 were tested in univariate analysis and those with a P-value <0.10 on univariate analysis were incorporated into the multivariate models. To avoid colinearity among a subset of several variables measuring the same phenomenon (e.g. aortic valve area and mean gradient), we entered in the multivariate models the variable that had the strongest association with cardiac events on univariate analysis. We constructed a first series of multivariate models with independent variables entered in continuous format and then a second series with these variables entered in dichotomous formats.
Table 1.
Baseline clinical, exercise-stress, and Doppler-echocardiographic data at rest and at peak exercise in the whole cohort (n = 135)
| Variables | Rest | Peak exercise |
|---|---|---|
| Follow-up duration (months) | 20 ± 14 | – |
| Clinical data | ||
| Age (years) | 64 ± 15 | – |
| Female gender, n (%) | 48 (36) | – |
| Body surface area (m2) | 1.8 ± 0.2 | – |
| Body mass index (kg/m2) | 26 ± 4 | – |
| Hypertension, n (%) | 63 (47) | – |
| Diabetes, n (%) | 13 (10) | – |
| Hypercholesterolaemia, n (%) | 50 (37) | – |
| Exercise-stress data | ||
| Heart rate (b.p.m.) | 71 ± 12 | 126 ± 24a |
| Systolic blood pressure (mmHg) | 138 ± 21 | 178 ± 27a |
| Exercise duration (min) | – | 13 ± 5 |
| Peak workload (watt) | – | 90 (65–120)b |
| Percent workload (%) | – | 73 (54–89)b |
| ST segment depression ≥2 mm (%) | – | 14 (10%) |
| Doppler-echocardiographic data | ||
| Bicuspid valve | 23 (17%) | – |
| LV mass index (g/m2) | 105 ± 34 | – |
| LV hypertrophy, n (%) | 55 (41) | – |
| LV ejection fraction (%) | 65 ± 7 | 71 ± 10a |
| LV stroke volume (mL) | 83 ± 17 | 85 ± 22a |
| Mean transvalvular flow rate (mL/s) | 269 ± 55 | 345 ± 87a |
| Aortic valve area (cm2) | 0.97 ± 0.22 | 1.07 ± 0.27a |
| Aortic valve area index (cm2/m2) | 0.53 ± 0.12 | 0.59 ± 0.14a |
| Peak aortic jet velocity (m/s) | 3.8 ± 0.8 | 4.5 ± 0.8a |
| Peak pressure gradient (mmHg) | 61 ± 24 | 82 ± 27a |
| Mean pressure gradient (mmHg) | 36 ± 15 | 49 ± 19a |
aSignificant (P < 0.05) difference peak exercise vs. rest.
bValues are median (interquartile range).
A multivariate linear regression analysis was used to identify the independent determinants of the exercise-induced changes in echocardiographic variables. A P-value < 0.05 was considered statistically significant. Statistical analyses were performed with the use of JMP 7.0.2 software.
Results
Among the 186 patients who underwent ESE, 51 (27%) had an abnormal exercise test and were excluded from the present analysis. Table 1 shows the baseline clinical, resting echocardiographic, and ESE data of the study population composed of the 135 patients who had a normal exercise test. This study population consisted of 87 (64%) men and 48 (36%) women with a mean age 64 ± 15 years. The range of aortic valve area and indexed aortic valve area was 0.42–1.50 cm2 and 0.28–0.89 cm2/m2, respectively. Fifty-three percent had a severe AS defined as aortic valve area <1.0 cm2, 41% had LV hypertrophy, 47% had a history of hypertension, 37% had a history of hypercholesterolaemia, and 10% had diabetes. Left ventricular ejection fraction, stroke volume, mean transvalvular flow rate, aortic valve area, peak aortic jet velocity, and transvalvular pressure gradients increased significantly during exercise (Table 1).
Analysis of outcome
Description of clinical events
Follow-up was complete in all patients. Mean follow-up time was 20 ± 14 months (median 19 months). Sixty-seven (50%) patients reached an endpoint during follow-up: 58 underwent AVR motivated by development of symptoms; 1 had a sudden cardiac arrest 12 months after ESE, was resuscitated, and underwent AVR 3 weeks later; 4 developed severe symptoms but did not undergo AVR because they had severe comorbidities and were considered at prohibitive surgical risk; 1 developed severe symptoms and was waiting for surgery at the time of last follow-up; and 3 died from cardiovascular causes at 20, 31, and 50 months after ESE. All these deaths were preceded by the development of symptoms: one patient developed severe dyspnoea (NYHA class III) and died 2 months later from acute pulmonary oedema; this patient was in poor general condition and was not referred to surgery by the treating physician. Two patients developed congestive heart failure: one died while waiting for surgery and the other one was declined surgery because of severe comorbidities and died 2 months later. The median time between ESE and occurrence of endpoint was 13 months (range: 0.6–50 months).
In addition, eight patients had censoring events which were not considered as endpoints for this study. Five patients underwent elective AVR not motivated by development of symptoms or LV dysfunction; the reason for AVR was: rapid progression of stenosis severity in two patients; aneurysm of ascending aorta in one; and elective AVR prior to urologic surgery in one. Three patients died of non-cardiovascular cause.
Analyses with variables expressed in continuous format
On univariate analysis, older age, increased LV wall thickness and mass, smaller aortic valve area and higher transvalvular gradients at rest, and larger increase in gradients during exercise were significantly (P < 0.05) associated with increased risk of event (Table 2). Hazard proportionality was confirmed for all variables. The exercise duration, maximum exercise workload, percent of age and gender predicted workload, peak exercise heart rate, change in blood pressure, and occurrence of ST depression ≥2 mm during exercise were not significantly associated with clinical outcome.
Table 2.
Univariate analysis of association between baseline variables and event risk in the whole cohort (n = 135) with variables entered in continuous format
| Variables | Increment category | Univariate analysis |
|
|---|---|---|---|
| HR (95% CI) | P-value | ||
| Age (years) | 10 years increase | 1.33 (1.12–1.61)a | 0.001 |
| Diabetes | Yes | 2.1 (0.90–4.10)a | 0.08 |
| Rest systolic blood pressure | 10 mmHg increase | 1.10 (1.00–1.23)a | 0.06 |
| LV mass index (g/m2) | 10 g/m2 increase | 1.12 (1.04–1.20)a | 0.004 |
| Exercise LV ejection fraction (%) | 10% decrease | 1.22 (0.97–1.50)a | 0.09 |
| Rest peak gradient (mmHg) | 10 mmHg increase | 1.22 (1.12–1.34) | <0.0001 |
| Exercise peak gradient (mmHg) | 10 mmHg increase | 1.26 (1.15–1.38) | <0.001 |
| Exercise Δ peak gradient (mmHg) | 10 mmHg increase | 1.24 (1.03–1.45) | 0.04 |
| Rest mean gradient (mmHg) | 10 mmHg increase | 1.44 (1.25–1.66)a | <0.0001 |
| Exercise mean gradient (mmHg) | 10 mmHg increase | 1.50 (1.30–1.72) | <0.0001 |
| Exercise Δ mean gradient (mmHg) | 10 mmHg increase | 1.35 (1.05–1.72)a | 0.02 |
| Rest aortic valve area (cm2) | 0.1 cm2 decrease | 1.27 (1.14–1.41) | <0.0001 |
| Exercise aortic valve area (cm2) | 0.1 cm2 decrease | 1.14 (1.04–1.25) | 0.004 |
This table shows the variables having a P-value <0.10 on univariate analysis. The hazard ratio (HR) reflects the increase in risk of event per increment category: e.g. the risk of event is increased by 1.44-fold per 10 mmHg increase in rest gradient.
aIndicates the variables that were entered in multivariate analysis (Table 3). Exercise Δ indicates absolute difference between peak exercise and rest data.
On multivariate analysis including variables in continuous format (Table 3), those independently associated with events were: older age (P = 0.01), diabetes (P = 0.006), higher resting mean gradient (P < 0.0001), and larger exercise-induced increase in mean gradient (P < 0.0001). Indexed LV mass and peak exercise LV ejection fraction also had P-values of borderline significance (P = 0.06 and 0.15, respectively).
Table 3.
Multivariate analysis of association between baseline variables entered in continuous format and event risk in the whole cohort (n = 135), in patients with severe aortic stenosis (n = 72), and in those with moderate aortic stenosis (n = 63)
| Variables) | Increment category | Whole cohort (n = 135) |
Severe aortic stenosis (n = 72) |
Moderate aortic stenosis (n = 63) |
|||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age (years) | 10 years increase | 1.27 (1.06–1.53) | 0.01 | 1.17 (0.94–1.47) | 0.16 | 1.82 (1.26–2.78) | 0.001 |
| Diabetes | Yes | 3.61 (1.49–7.83) | 0.006 | 3.75 (1.39–9.12) | 0.01 | – | – |
| Rest systolic blood pressure | 10 mmHg increase | 1.07 (0.92–1.22) | 0.36 | – | – | 1.17 (0.90–1.49) | 0.23 |
| LV mass index (g/m2) | 10 g/m2 increase | 1.08 (1.00–1.15) | 0.06 | 1.12 (1.01–1.22) | 0.02 | – | – |
| Rest mean gradient (mmHg) | 10 mmHg increase | 1.50 (1.27–1.77) | <0.0001 | 1.32 (1.05–1.86) | 0.02 | 1.72 (1.03–2.86) | 0.04 |
| Exercise Δ mean gradient (mmHg) | 10 mmHg increase | 1.67 (1.32–2.13) | <0.0001 | 1.49 (1.12–2.00) | 0.008 | 2.08 (1.26–3.56) | 0.004 |
| Exercise LV ejection fraction (%) | 10% decrease | 1.20 (0.94–1.54) | 0.15 | 1.22 (0.88–1.67) | 0.23 | – | – |
The variables marked by superscript ‘a’ in Table 2 were entered in the multivariate model for the whole cohort. We selected the same variables to construct the models in the subsets of patients with severe and moderate aortic stenosis. However, the variables were entered in these models only if the P-value was <0.1 on univariate analysis in the given subset.
The hazard ratios reflect the increase in risk of event per increment category.
Importantly, the increase in mean gradient during exercise did not correlate with the rest gradient (r = 0.16; P = 0.17) or with any other rest echocardiographic data. The independent determinants of larger exercise-induced increase in gradient were: younger age (P = 0.04), smaller increase in aortic valve area (P < 0.0001), and larger increases in stroke volume (P < 0.0001) and heart rate (P = 0.004) during exercise. However, after further adjustment for these variables in the multivariate model for the prediction of event risk (Table 3), the significance and hazard ratio (HR) of exercise increase in gradient remained similar [P = 0.001; HR = 1.64, 95% confidence interval: 1.22–2.23].
Separate multivariate analyses in patients with moderate vs. severe AS revealed that the exercise-induced increase in gradient was strongly associated with event risk in both categories (Table 3).
Patients from the Canadian Center were significantly younger (60 ± 13 vs. 65 ± 15 years; P = 0.03) and had lower prevalence of severe AS (45 vs. 60%) compared with European Centers. Adjustment for location of the participating centre (Canada vs. Europe) in the multivariate model had no or minimal impact on the significance and HRs of the independent variables.
Analyses with variables expressed in dichotomous format
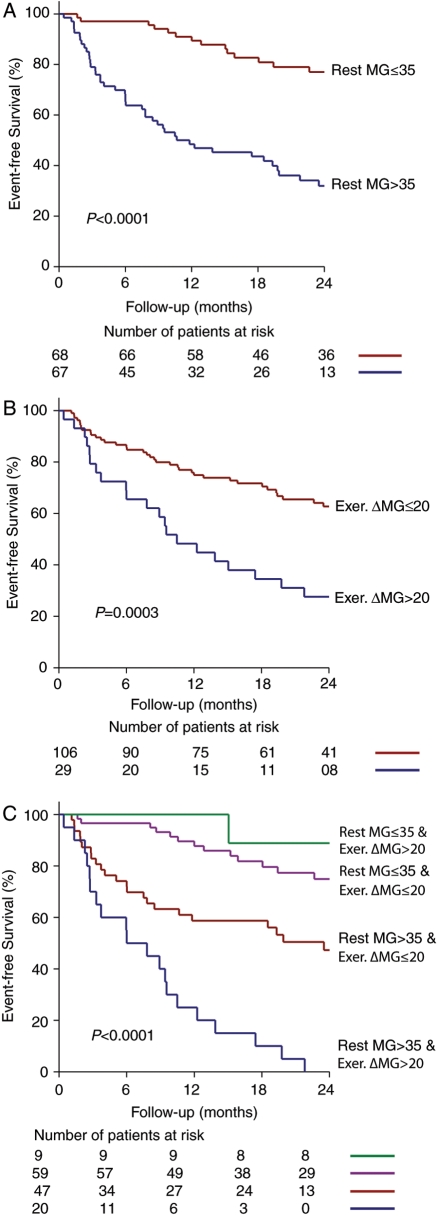
Univariate analyses with variables expressed in dichotomous format revealed that age ≥65 years, rest systolic blood pressure >135 mmHg, LV hypertrophy, resting mean gradient >35 mmHg (Figure 1A), exercise-induced increase in mean gradient >20 mmHg (Figure 1B), and peak exercise LV ejection fraction <70% were associated with increased risk of events. On multivariate analysis including variable in dichotomous format (Table 4), those independently associated with events were: age ≥65 years (HR = 1.96; P = 0.01); diabetes (HR = 3.20; P = 0.01), LV hypertrophy (HR = 1.96; P = 0.01), resting mean gradient >35 mmHg (HR = 3.60; P < 0.0001), and exercise-induced increase in mean gradient >20 mmHg (HR = 3.83; P < 0.0001). There also was a strong trend for an association with peak exercise LV ejection fraction <70%.
Figure 1.
Event-free survival as a function of the level of rest mean gradient (A), increase in gradient during exercise (B), and combination of rest gradient and exercise-induced increase in gradient (C). MG, mean gradient; Exer. ΔMG, exercise-induced increase in mean gradient.
Table 4.
Univariate and multivariate analysis of association between baseline variables and event risk in the whole cohort (n = 135) with variables entered in dichotomous format
| Variables | (%) of patients with variable | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age ≥65 years | 58 | 2.16 (1.30–3.72) | 0.003 | 1.96 (1.15–3.47) | 0.01 |
| Diabetes | 10 | 2.10 (0.90–4.10) | 0.08 | 3.20 (1.33–6.87) | 0.01 |
| Rest systolic blood pressure >135 mmHg | 55 | 1.71 (0.78–2.85) | 0.03 | 1.30 (0.78–2.23) | 0.32 |
| LV hypertrophy | 41 | 1.90 (1.17–3.08) | 0.009 | 1.96 (1.17–3.27) | 0.01 |
| Rest mean gradient >35 mmHg | 50 | 3.70 (2.21–6.41) | <0.0001 | 3.60 (2.11–6.37) | <0.0001 |
| Exercise Δ mean gradient >20 mmHg | 21 | 2.10 (1.22–2.52) | 0.008 | 3.83 (2.16–6.67) | <0.0001 |
| Exercise LV ejection fraction <70% | 38 | 1.61 (1.00–2.62) | 0.05 | 1.61 (0.95–2.71) | 0.07 |
When varying the cut-off value of rest gradient between 25 and 50 mmHg in this dichotomous model, the value of >35 mmHg yielded to the highest P-value, χ2 of likelihood ratio, and HR. For the exercise-induced increase in gradient, we used a cut-off value of >20 mmHg that was close to the cut-off value (>18 mmHg) proposed by Lancellotti et al.12 With a cut-off value >18 mmHg, the significance and HR (P = 0.002; HR = 3.05) were slightly inferior to those obtained with cut-off value >20 mmHg.
The combination of a rest gradient >35 mmHg and an exercise-induced increase in gradient >20 mmHg was associated with markedly increased risk of event (HR = 9.6; P < 0.0001) compared to the absence of these two factors (HR = 1.0, referent group) (Figure 1C). Patients with a rest gradient >35 mmHg and an increase in gradient ≤20 mmHg during exercise had a 2.5-fold increase in the risk of event compared with the referent group, whereas those with a rest gradient ≤35 mmHg and an increase in gradient >20 mmHg did not display a significant increase in event risk (HR = 0.8; P = NS). There were however very few patients (n = 9) in this latter group.
Discussion
The main finding of this study is that ESE provides incremental prognostic information beyond that obtained by resting echocardiography or exercise testing alone. An increase in mean gradient >20 mmHg during exercise was independently associated with a 3.8-fold increase in the risk of event after adjusting for other risk factors including age, diabetes, LV hypertrophy, rest gradient, and peak exercise LV ejection fraction. Moreover, patients having both a rest gradient >35 mmHg and an exercise-induced increase in gradient >20 mmHg display a 9.6-fold increase in the event risk compared to 2.5-fold in patients with a rest gradient >35 mmHg and a gradient augmentation ≤20 mmHg during exercise (Figure 1C). Importantly, the exercise-induced increase in gradient could not be predicted from the clinical or resting echocardiographic data.
Exercise stress vs. other predictors of outcomes in aortic stenosis
Several of the risk factors identified in the present study including older age, diabetes, LV hypertrophy, and higher transvalvular gradient have been reported in previous studies.3,5–7 Previous studies also demonstrated that abnormal exercise test is a powerful predictor of outcome.9–11,13,16,19 Accordingly, current guidelines recommend surgery in asymptomatic patients with severe AS exhibiting exercise-limiting symptoms (ESC: Class I; ACC/AHA: IIb), fall in blood pressure (ESC: IIa, ACC/AHA: IIb), or occurrence of complex ventricular arrhythmias (ESC: IIb) during exercise.1,2 In the present study that included only patients with a normal exercise test, we found no significant association between indices of maximum exercise capacity (e.g. percent of age- and gender-predicted workload) and outcome. Hence, as was found in 27% of patients initially enrolled in this study, standard exercise testing is useful to unmask symptoms in 20–30% of AS patients who claim to be asymptomatic and this information is useful to enhance prognostication. However, in the remaining group of patients (70–80%) with normal response to exercise, the measures of maximum exercise capacity fail to identify patients at higher risk for rapid disease progression.
Incremental value of exercise-stress echocardiography
Lancellotti et al.12 previously demonstrated that an exercise-induced increase in gradient >18 mmHg is an independent predictor of outcome. Their study included a series of 69 patients of whom 25 had abnormal exercise test. The present study extends this previous observation by demonstrating that a large increase in gradient during exercise is associated with a marked increase in event risk even in the subset of patients with normal exercise test, i.e. the patients with ‘true’ asymptomatic AS.
In a series of 50 patients, Marechaux et al.20 also reported that lower increase in LV ejection fraction is associated with abnormal exercise test and higher incidence of cardiac events. This factor was however not found to be associated with outcome in the present series of patients with normal exercise response. Nonetheless, there was a strong trend for an association between reduced peak exercise LV ejection fraction and risk of events. This index in fact incorporates the resting LV ejection fraction and the exercise-induced ‘ejection’ reserve. In light of the results of the present study, patients with a LV ejection fraction remaining below 70% at peak exercise may be at higher risk of event. Interestingly, reduced peak stress LV ejection fraction measured during dobutamine echocardiography was found to be one of the main independent risk factors for poor outcome in the series of patients with low flow, low gradient AS included in the TOPAS study.21 Further studies are needed to confirm the potential usefulness of peak exercise LV ejection fraction for risk stratification in patients with asymptomatic AS.
Potential mechanisms underlying the large exercise increase in gradient
A larger increase in gradient during exercise may reflect a more severe stenosis. Indeed, the more severe is the stenosis at rest, the higher is the increase in gradient for a given flow rate during exercise.22,23 However, the fact that the exercise-induced increase in gradient remained strongly associated with outcome even after adjusting for the resting gradient may also suggest the implication of other mechanisms such as differences in intrinsic valve compliance. Hence, of two patients having the same valve orifice area at rest, the one with a non-compliant and rigid valve will exhibit no or minimal enlargement in valve orifice area (i.e. fixed orifice) and a large increase in gradient during exercise, whereas the one with residual valve compliance, i.e. with valve orifice opening reserve during exercise will have less pronounced increase in gradient for a similar increase in flow. And logically, the former patient would be at higher risk for the rapid progression to symptoms and events. In the present study, lower exercise-induced increase in aortic valve area did not correlate with events. This finding may be due to the larger variability inherent to the measurement of aortic valve area, especially during exercise.
The higher increase in gradient during exercise may also be related to a larger increase in stroke volume and transvalvular flow rate. The stroke volume often has a biphasic behaviour during the course of exercise test: i.e. it may indeed increase markedly at low workload levels and then plateau or decrease at higher workloads due to tachycardia and associated reduction in LV diastolic filling and pump function. Hence, a substantial increase in stroke volume combined to a large increase in gradient may reflect exercise termination at relatively low workload with early and rapid rise in gradient due to a non-compliant stenotic valve. In future studies, it would be interesting to determine if the kinetics of the gradient increase during the exercise test (i.e. rapid rise in the early exercise stages vs. progressive increase culminating in the later stages) provides any incremental predictive value over that obtained from the magnitude of the gradient augmentation per se.
Clinical implications
This study is the first to demonstrate the incremental prognostic value of ESE in patients with normal exercise test. These findings support the utilization of ESE rather than exercise testing alone for prognostication in AS patients who claim to be asymptomatic. In the subset of patients exhibiting abnormal exercise test (i.e. ‘false asymptomatic’ patients), AVR should be considered. In the subset with normal exercise response (‘true asymptomatic’ patients), a large increase in gradient during exercise may help identify patients who are at higher risk for rapid progression to symptoms and events. In particular, this study reveals that patients presenting with a rest gradient >35 mmHg and an exercise-induced increase in gradient >20 mmHg have a very high risk for event in the short-term (Figure 1C). Additional tests such as measurement of plasma BNP may also be useful to refine risk stratification in these patients15 and future prospective studies will be necessary to determine if prognosis of these patients would be best served by closer watchful waiting (e.g. q. 3–6 months) or earlier referral to surgery.
The results of this study suggest that ESE may help to improve clinical management not only in patients with severe AS but also in those with moderate AS. Several prospective studies revealed that moderate AS is not a benign disease and that the outcome is worse than commonly assumed.5,24,25 Patients with moderate AS, however, constitute a highly heterogeneous group of patients with regard to the risk of adverse events: some patients indeed remain event-free for several years, whereas others have a rapid progression to symptoms and/or LV systolic dysfunction. Notwithstanding this important inter-individual variability in disease progression rate, the most recent guidelines recommend an echocardiographic follow-up every 2 years for patients with moderate AS. In light of the data published in the literature,5,24,25 it would appear that this recommendation may not be appropriate for all patients with moderate AS and that some of these patients require more frequent and closer clinical and echocardiographic follow-up. The results of the present study suggest that patients with moderate AS presenting with a rest mean gradient >35 mmHg and an exercise-induced increase in gradient ≤20 mmHg should have a follow-up at least every year, whereas those with a rest gradient >35 mmHg and an increase in gradient >20 mmHg should potentially have a follow-up every 6 months. Hence, ESE may provide important incremental information that may help the clinician to adjust and optimize the interval of follow-up according to the predicted risk of event.
Limitations
Plasma BNP and valve calcification score were not systematically measured in this study. These parameters have been shown to be useful to predict stenosis progression and occurrence of adverse events in asymptomatic AS.5,7,8,15,26,27 The calcification score measured by echocardiography has some limitations including its semi-quantitative nature and the difficulty to standardize this measurement among different centres and operators. Computed tomography is probably superior to echocardiography for the quantification of valvular calcification, but the exposure to ionizing radiation may limit its utilization for serial follow-up. Further studies are needed to compare the predictive performance and cost-benefit ratio of these tests to those of ESE.
To perform several Doppler-echocardiographic measurements at peak exercise is challenging and it is possible that the resulting higher measurement variability may have limited our ability to detect significant association between some ESE variables, such as peak exercise LV ejection fraction or exercise change in aortic valve area, and the risk of events.
Conclusion
Indices of valve haemodynamic behaviour measured by ESE but not indices of maximum exercise capacity are associated with outcome in patients with true asymptomatic AS (i.e. with normal exercise response). The exercise-induced increase in transvalvular gradient may be helpful to improve risk stratification in this population. These results show that ESE provides important incremental prognostic information that is unrevealed by standard exercise testing or resting echocardiography. These findings support the utilization of ESE for risk stratification in asymptomatic AS patients.
Funding
This study was funded, in part, by a grant (MOP-79342) from Canadian Institutes of Health Research, Ottawa, Canada. Funding to pay the Open Access publication charges for this article was provided by the Canada Research Chair in Valvular Heart Disease.
Conflict of interest: none declared.
Acknowledgement
P.P. holds the Canada Research Chair in Valvular Heart Diseases, Canadian Institutes of Health Research.
References
- 1.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A, Priori SG, Blanc JJ, Budaj A, Camm J, Dean V, Deckers J, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo J, Zamorano JL, Zamorano JL, Angelini A, Antunes M, Fernandez MA, Gohlke-Baerwolf C, Habib G, McMurray J, Otto C, Pierard L, Pomar JL, Prendergast B, Rosenhek R, Uva MS, Tamargo J. Guidelines on the management of valvular heart disease: The Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology. Eur Heart J. 2007;28:230–268. doi: 10.1093/eurheartj/ehl428. [DOI] [PubMed] [Google Scholar]
- 2.Bonow RO, Carabello BA, Kanu C, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84–e231. doi: 10.1161/CIRCULATIONAHA.106.176857. doi:10.1161/CIRCULATIONAHA.106.176857. [DOI] [PubMed] [Google Scholar]
- 3.Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, Barnes ME, Tajik AJ. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation. 2005;111:3290–3295. doi: 10.1161/CIRCULATIONAHA.104.495903. doi:10.1161/CIRCULATIONAHA.104.495903. [DOI] [PubMed] [Google Scholar]
- 4.Pai RG, Kapoor N, Bansal RC, Varadarajan P. Malignant natural history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg. 2006;82:2116–2122. doi: 10.1016/j.athoracsur.2006.07.043. doi:10.1016/j.athoracsur.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 5.Rosenhek R, Klaar U, Schemper M, Scholten C, Heger M, Gabriel H, Binder T, Maurer G, Baumgartner H. Mild and moderate aortic stenosis: natural history and risk stratification by echocardiography. Eur Heart J. 2004;25:199–205. doi: 10.1016/j.ehj.2003.12.002. doi:10.1016/j.ehj.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft C, Miyake-Hull CY, Schwaegler RG. Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation. 1997;95:2262–2270. doi: 10.1161/01.cir.95.9.2262. [DOI] [PubMed] [Google Scholar]
- 7.Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med. 2000;343:611–617. doi: 10.1056/NEJM200008313430903. doi:10.1056/NEJM200008313430903. [DOI] [PubMed] [Google Scholar]
- 8.Messika-Zeitoun D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Nkomo VT, Breen JF, Maalouf J, Scott C, Tajik AJ, Enriquez-Sarano M. Aortic valve calcification: determinants and progression in the population. Arterioscler Thromb Vasc Biol. 2007;27:642–648. doi: 10.1161/01.ATV.0000255952.47980.c2. doi:10.1161/01.ATV.0000255952.47980.c2. [DOI] [PubMed] [Google Scholar]
- 9.Amato MC, Moffa PJ, Werner KE, Ramires JA. Treatment decision in asymptomatic aortic valve stenosis: role of exercise testing. Heart. 2001;86:381–386. doi: 10.1136/heart.86.4.381. doi:10.1136/heart.86.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alborino D, Hoffmann JL, Fournet PC, Bloch A. Value of exercise testing to evaluate the indication for surgery in asymptomatic patients with valvular aortic stenosis. J Heart Valve Dis. 2002;11:204–209. [PubMed] [Google Scholar]
- 11.Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J. 2005;26:1309–1313. doi: 10.1093/eurheartj/ehi250. doi:10.1093/eurheartj/ehi250. [DOI] [PubMed] [Google Scholar]
- 12.Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation. 2005;112(Suppl.):I377–I382. doi: 10.1161/CIRCULATIONAHA.104.523274. [DOI] [PubMed] [Google Scholar]
- 13.Ennezat PV, Marechaux S, Iung B, Chauvel C, LeJemtel TH, Pibarot P. Exercise testing and exercise stress echocardiography in asymptomatic aortic valve stenosis. Heart. 2009;95:877–884. doi: 10.1136/hrt.2008.150011. doi:10.1136/hrt.2008.150011. [DOI] [PubMed] [Google Scholar]
- 14.Gerber IL, Legget ME, West TM, Richards AM, Stewart RA. Usefulness of serial measurement of N-terminal pro-brain natriuretic peptide plasma levels in asymptomatic patients with aortic stenosis to predict symptomatic deterioration. Am J Cardiol. 2005;95:898–901. doi: 10.1016/j.amjcard.2004.11.053. doi:10.1016/j.amjcard.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 15.Bergler-Klein J, Klaar U, Heger M, Rosenhek R, Mundigler G, Gabriel H, Binder T, Pacher R, Maurer G, Baumgartner H. Natriuretic peptides predict symptom-free survival and postoperative outcome in severe aortic stenosis. Circulation. 2004;109:2302–2308. doi: 10.1161/01.CIR.0000126825.50903.18. doi:10.1161/01.CIR.0000126825.50903.18. [DOI] [PubMed] [Google Scholar]
- 16.Picano E, Pibarot P, Lancellotti P, Monin JL, Bonow RO. The emerging role of exercise testing and stress echocardiography in valvular heart disease. J Am Coll Cardiol. 2009;54:2251–2260. doi: 10.1016/j.jacc.2009.07.046. doi:10.1016/j.jacc.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 17.Weisenberg D, Shapira Y, Vaturi M, Monakier D, Iakobishvili Z, Battler A, Sagie A. Does exercise echocardiography have an added value over exercise testing alone in asymptomatic patients with severe aortic stenosis? J Heart Valve Dis. 2008;17:376–380. [PubMed] [Google Scholar]
- 18.Gibbons RJ, Balady GJ, Beasley JW, Bricker JT, Duvernoy WF, Froelicher VF, Mark DB, Marwick TH, McCallister BD, Thompson PD, Jr, Winters WL, Yanowitz FG, Ritchie JL, Gibbons RJ, Cheitlin MD, Eagle KA, Gardner TJ, Garson A, Jr, Lewis RP, O'Rourke RA, Ryan TJ. ACC/AHA Guidelines for Exercise Testing. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) J Am Coll Cardiol. 1997;30:260–311. doi: 10.1016/s0735-1097(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 19.Lancellotti P, Karsera D, Tumminello G, Lebois F, Pierard LA. Determinants of an abnormal response to exercise in patients with asymptomatic valvular aortic stenosis. Eur J Echocardiogr. 2008;3:338–343. doi: 10.1016/j.euje.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Marechaux S, Ennezat PV, LeJemtel TH, Polge AS, de Groote P, Asseman P, Neviere R, Le Tourneau T, Deklunder G. Left ventricular response to exercise in aortic stenosis: an exercise echocardiographic study. Echocardiography. 2007;24:955–959. doi: 10.1111/j.1540-8175.2007.00501.x. doi:10.1111/j.1540-8175.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- 21.Clavel MA, Fuchs C, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, Bergler-Klein J, Beanlands RS, Mathieu P, Magne J, Pibarot P. Predictors of outcomes in low-flow, low-gradient aortic stenosis: results of the multicenter TOPAS Study. Circulation. 2008;118(Suppl.):S234–S242. doi: 10.1161/CIRCULATIONAHA.107.757427. doi:10.1161/CIRCULATIONAHA.107.757427. [DOI] [PubMed] [Google Scholar]
- 22.Otto CM, Pearlman AS, Kraft CD, Miyake-Hull CY, Burwash IG, Gardner CJ. Physiologic changes with maximal exercise in asymptomatic valvular aortic stenosis assessed by Doppler echocardiography. J Am Coll Cardiol. 1992;20:1160–1167. doi: 10.1016/0735-1097(92)90373-u. [DOI] [PubMed] [Google Scholar]
- 23.Burwash IG, Pearlman AS, Kraft CD, Miyake-Hull C, Healy NL, Otto CM. Flow dependence of measures of aortic stenosis severity during exercise. J Am Coll Cardiol. 1994;24:1342–1350. doi: 10.1016/0735-1097(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 24.Rossebo AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, Gerdts E, Gohlke-Barwolf C, Holme I, Kesaniemi YA, Malbecq W, Nienaber CA, Ray S, Skjaerpe T, Wachtell K, Willenheimer R. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. doi:10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 25.Kume T, Kawamoto T, Okura H, Watanabe N, Toyota E, Neishi Y, Okahashi N, Yamada R, Yoshida K. Rapid progression of mild to moderate aortic stenosis in patients older than 80 years. J Am Soc Echocardiogr. 2007;20:1243–1246. doi: 10.1016/j.echo.2007.03.022. doi:10.1016/j.echo.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Messika-Zeitoun D, Aubry MC, Detaint D, Bielak LF, Peyser PA, Sheedy PF, Turner ST, Breen JF, Scott C, Tajik AJ, Enriquez-Sarano M. Evaluation and clinical implications of aortic valve calcification measured by electron-beam computed tomography. Circulation. 2004;110:356–362. doi: 10.1161/01.CIR.0000135469.82545.D0. doi:10.1161/01.CIR.0000135469.82545.D0. [DOI] [PubMed] [Google Scholar]
- 27.Monin JL, Lancellotti P, Monchi M, Lim P, Weiss E, Pierard L, Gueret P. Risk score for predicting outcome in patients with asymptomatic aortic stenosis. Circulation. 2009;120:69–75. doi: 10.1161/CIRCULATIONAHA.108.808857. doi:10.1161/CIRCULATIONAHA.108.808857. [DOI] [PubMed] [Google Scholar]