Summary
Human glioma incidence, malignancy and treatment resistance are directly proportional to patient age. Cell intrinsic factors are reported to contribute to human age-dependent glioma malignancy but suitable animal models to examine the role of aging are lacking. Here we developed an orthotopic syngeneic glioma model to test the hypothesis that the age of neural progenitor cells (NPCs), presumed cells of glioma origin, influences glioma malignancy. Gliomas generated from transformed donor 3-, 12-, and 18-month-old NPCs in same-aged adult hosts all formed highly invasive glial tumors that phenocopied the human disease. Survival analysis indicated increased malignancy of gliomas generated from older 12- and 18-month-old transformed NPCs compared with their 3-month counterparts (median survival of 38.5 and 42.5 vs. 77 days, respectively). This study showed for the first time that age of target cells at the time of transformation can affect malignancy and demonstrated the feasibility of a syngeneic model using transformed NPCs for future examination of the relative impacts of age-related cell intrinsic and cell-extrinsic factors in glioma malignancy.
Keywords: Gliomagenesis, aging, progenitor, malignancy, tumor suppressor, animal model
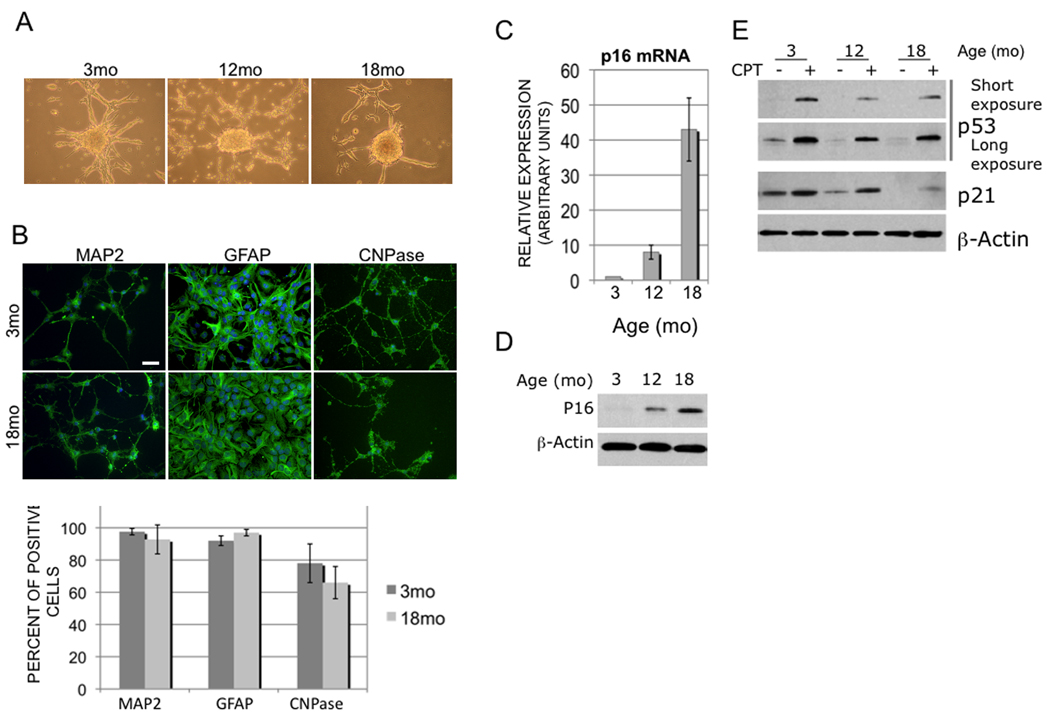
Increased age has a negative impact on human malignant glioma survival and directly correlates with increased incidence, malignancy and treatment resistance (Curran et al. 1993; Barker et al. 2001; CBTRUS 2005). However, little is known about age-related mechanisms that impact glioma malignancy. Previous animal studies reported conflicting impacts of host age related immune function and neural progenitor cell (NPC) recruitment on survival (Wheeler et al. 2003; Glass et al. 2005) while human data suggests that age-related cell-intrinsic factors may affect glioma malignancy (Rosenblum et al. 1982). To examine the role of host age, we implanted GL261 glioma cells into syngeneic 3- and 18-month-old host C57Bl/6 mice and found no difference in survival (see supplemental data). Since NPCs are presumed glioma cells of origin (Stiles & Rowitch 2008), we tested how NPC age influences glioma malignancy. NPCs isolated from 3-, 12-, and 18-month-old C57Bl/6 mice formed neurospheres and demonstrated multi-potent differentiation (Fig 1A, B). To validate molecular changes reported in aging NPCs (Molofsky et al. 2006), we confirmed that p16 tumor suppressor expression increased with NPC age (Fig 1C,D). Like p16, loss of p53 tumor suppressor (TS) activity contributes significantly to human glioma pathogenesis, but in a non-overlapping set of gliomas (Ohgaki & Kleihues 2005). We found a striking age-related inverse relationship between p16 and p53 expression levels, with marked reductions in p53 expression and activity with increasing NPC age (Fig 1E). These results indicated that NPCs of all ages demonstrated similarities but were distinguished by unique profiles of TS expression/activity highly relevant to human glioma pathogenesis.
Figure 1. Isolation and characterization of NPCs from different-aged donor brain.
(A) Representative neurospheres generated from NPCs isolated from 3-, 12- and 18- mo mouse forebrain cultures. (B) Multipotency in 3- and 18-mo NPC cultures under differentiation conditions assayed by neuronal (MAP2), astrocytic (GFAP) and oligodendroglia (CNPase) marker expression (quantified in histogram below), scale bar = 20 ⎧m. Expression levels of p16 message (C) and protein (D) increase proportionally with NPC age. E) Basal and CPT-induced expression of p53 and its target gene, p21WAF1, decrease with increased NPC age. Long exposure panel demonstrates steady state p53 expression.
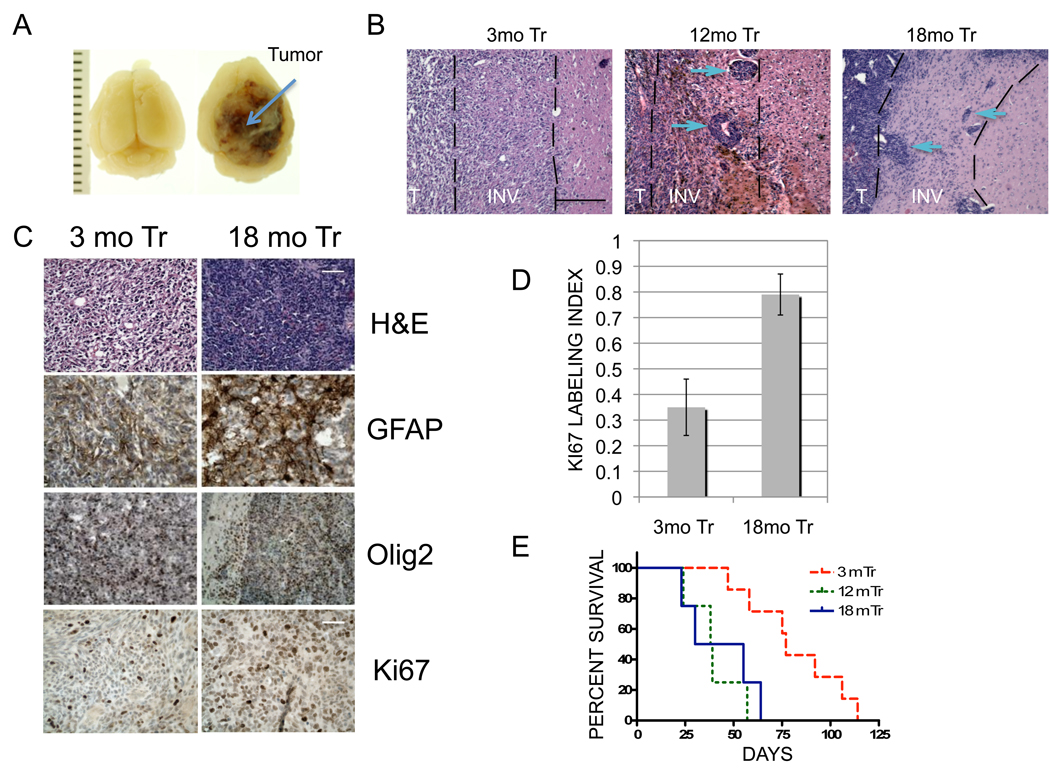
To determine the impact of NPC age on glioma malignancy, NPCs of different ages were transformed by over-expression of activated Ha-Ras (Ha-RasV12) and HPV18 E6E7 (to inhibit both p53 and the p16/Rb axis). All cultures demonstrated equivalent levels of exogenous Ras expression (Fig S2), and expected alterations in p53 and Rb expression in vitro (data not shown). Intracranial implantation of equal numbers of transformed 3-, 12-, and 18- month-old NPCs into 4- to 6-month-old adult C57Bl/6 mice produced highly invasive GFAP and Olig2-positive glial tumors in all cases (Fig 2A, B, C). Gliomas derived from 12- and 18-mo transformed NPCs were distinguished by increased cellularity, less differentiated morphology, increased proliferation (Ki-67 LIs) and a distinct pattern of invasion with large cell aggregates as well as diffuse cellular invasion (Fig 2B, C, D). Most importantly, animals with gliomas derived from 3-mo NPCs survived nearly twice as long as those with gliomas from 12- and 18-mo NPCs (77 vs. 38.5 and 42.5 days, respectively; log rank p=0.01132 and 0.002, respectively; Fig 2D).
Figure 2. Age-dependent malignancy of transformed NPCs in vivo.
(A) Macroscopic appearance of representative 18-mo tumor (arrow) compared to normal brain (left- ruler units=1mm) (B) Distinct age-related invasive growth patterns indicated by aggregate (arrows) and diffuse cell invasion in 12- and 18 mo gliomas and lack of aggregate invasion in 3 mo tumors. T-solid tumor, INV-invasive margin (dashed lines). Scale bar 170⎧m. (C) Top panel- H&E histology demonstrates increased cellularity and less differentiated morphology of 18-versus 3-mo gliomas. Middle panels- GFAP and Olig2 expression confirm glial differentiation in both 3- and 18-mo tumors. Lower panel- Representative images of Ki-67 positive cells (brown nuclei of various intensity) indicates increased proliferation in 18 mo versus 3 mo glioma. Scale bars 90 ⎧m/50 ⎧m (top 3/lower panel respectively). (D) Histogram demonstrates significant difference in mean Ki-67 LIs between 3 and 18 mo gliomas (35% vs 78%, respectively, p<0.0026). (E) Kaplan-Meier survival analysis. Animals injected with 3-mo transformed cells survived significantly longer than animals injected with 12- or 18-mo transformed NPCs (77 vs 38.5 and 42.5 days, respectively; log rank p=0.01132 and 0.002, respectively).
This study of gliomas provides the first experimental evidence that increased age of transformation impacts the malignant potential of cancer target cells. The current findings are concordant with clinical observations that age-related cell intrinsic factors may play a leading role in the impact of age on glioma malignancy. The highly invasive glial growth patterns further support the clinical relevance of this syngeneic model and its usefulness for future studies to i) determine how specific oncogenic mechanisms affect age-related malignancy, ii) examine the role of p53 activity in NPC malignant potential, iii) define the relative importance of target cell versus host age on glioma malignancy, and iv) determine how age-related cell-extrinsic host mechanisms such as immune function and recruitment of endogenous progenitor cells impact glioma malignancy.
Supplementary Material
Acknowledgments
We thank Rosemary Kimmel for her expert editorial assistance in the manuscript preparation. E.S. is supported by the University of Washington training grant in developmental biology. This work is supported by NIH grants: Clinical Neuroscience Training Grant NS007144, AG229406, NS046724 and NS35533.
References
- Barker FG, 2nd, Chang SM, Larson DA, Sneed PK, Wara WM, Wilson CB, Prados MD. Age and radiation response in glioblastoma multiforme. Neurosurgery. 2001;49:1288–1297. doi: 10.1097/00006123-200112000-00002. discussion 1297-1288. [DOI] [PubMed] [Google Scholar]
- CBTRUS. Statistical Report: Primary brain tumors in the United States, 1998–2002. 2005. [Google Scholar]
- Curran WJ, Jr, Scott CB, Horton J, Nelson JS, Weinstein AS, Fischbach AJ, Chang CH, Rotman M, Asbell SO, Krisch RE, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- Glass R, Synowitz M, Kronenberg G, Walzlein J-H, Markovic DS, Wang L-P, Gast D, Kiwit J, Kempermann G, Kettenmann H. Glioblastoma-Induced Attraction of Endogenous Neural Precursor Cells Is Associated with Improved Survival. J. Neurosci. 2005;25:2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- Rosenblum ML, Gerosa M, Dougherty DV, Reese C, Barger GR, Davis RL, Levin VA, Wilson CB. Age-related chemosensitivity of stem cells from human malignant brain tumours. Lancet. 1982;1:885–887. doi: 10.1016/s0140-6736(82)92154-7. [DOI] [PubMed] [Google Scholar]
- Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58:832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Wheeler CJ, Black KL, Liu G, Ying H, Yu JS, Zhang W, Lee PK. Thymic CD8+T cell production strongly influences tumor antigen recognition and age-dependent glioma mortality. J Immunol. 2003;171:4927–4933. doi: 10.4049/jimmunol.171.9.4927. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.