Abstract
Although the rat has been the predominant rodent used to investigate the pathophysiology and treatment of experimental spinal cord injury (SCI), the increasing availability of transgenic animals has led to greater use of mouse models. However, behavioral assessment after SCI in mice has been less extensively investigated than in rats and few studies have critically examined the correlation between behavioral tests and injury severity or tissue damage. The present study characterized hind-limb functional performance in C57Bl/6 mice after contusion SCI at T9 using the weight drop method. A number of behavioral tests were examined with regard to variability, inter-rater reliability, and correlation to injury severity and white matter sparing. Mice were subjected to sham, mild-moderate or moderate-severe SCI and evaluated at day 1 and weekly up to 42 days using the Basso mouse scale (BMS), ladder climb, grid walk, inclined plane, plantar test and tail flick tests. The ladder climb and grid walk tests proved sub-optimal for use in mice, but modifications enhanced their predictive value with regard to injury severity. The inclined plane, plantar test and tail flick test showed far too much variability to have meaningful predictive value. The BMS score proved reliable, as previously reported, but a combined score (BLG) using BMS, Ladder climb (modified), and Grip walk (modified grid walk) provided better separation across injury levels and less variability than the individual tests. These data provide support for use of a combined scoring method to follow motor recovery in mice after SCI contusion injury.
Keywords: Spinal cord injury, Behavioral testing, BMS, Grid walk, Ladder climb
Introduction
Spinal cord injury (SCI) often results in a permanent loss of sensory and motor function (Lu et al., 2000). Following the initial damage after SCI, delayed biochemical changes lead to secondary injury resulting in additional tissue damage and cell death (Panter & Faden, 1992), glial scar formation , and impaired regeneration. Contusion models in rodents recapitulate certain features of human injury, exhibiting a central core lesion with spared peripheral white matter with the latter decreases in volume associated with an increase in the severity of the injury (Basso et al., 1996).
Most behavioral tests for rodent SCI have been developed to assess functional recovery in rats. These include the Basso, Beattie, Bresnahan (BBB) scale (Basso et al., 1995), ladder climb (Apostolova et al., 2006), grid walk (Metz et al., 2000; Schucht et al., 2002; Dijkstra et al., 2006), inclined plane (Rivlin & Tator, 1977; Panjabi & Wrathall, 1988; Fehlings & Tator, 1995), plantar test (Hargreaves et al., 1988), and tail flick (Merkler et al., 2001), among others. However, as transgenic mice are increasingly used to examine mechanisms of secondary injury, well characterized behavioral measures for mouse SCI are needed.
The Basso Mouse Scale (BMS) was developed to examine open-field locomotion in spinal cord injured mice (Basso et al., 2006) and has become widely used in mouse functional testing research as an indicator of recovery. This test evaluates different locomotor categories such as paralysis, weight support, and stepping. However, in order to increase the sensitivity of the BMS, the need for more quantitative assessments is suggested in the literature (Engesser-Cesar et al., 2005; Cummings et al., 2007). Other tests currently utilized in assessing mouse function include grid walk and ladder climb (Metz et al., 2000; Apostolova et al., 2006; Cummings et al., 2007; Gulino et al., 2007), in which mice walk on a ladder angled on a horizontal or vertical plane, respectively. In these tests, the number of footfalls - the number of times that the animals’ feet slide through the rungs- is counted. Unfortunately, in moderate-severely injured mice, their hind-paws often slide over the bars and the footfalls are not clearly observed; this results in significant underestimation of recorded footfalls.
In the current study, we assessed the utility of a number of functional performance tests in mice, including BMS, grid walk, ladder climb, inclined plane, tail flick and plantar test latencies. In order to improve the sensitivity of several tests, we included assessment of hind-paw ‘gripping’, instead of ‘footfalls’, to assess function of the hind-paws. We demonstrated that a combined score (BLG) utilizing BMS, and modified ladder climb, and grip walk provides better discrimination of injury severity and tissue preservation than any of the individual tests, with low inter-rater variability.
Materials and methods
Spinal Cord Injury
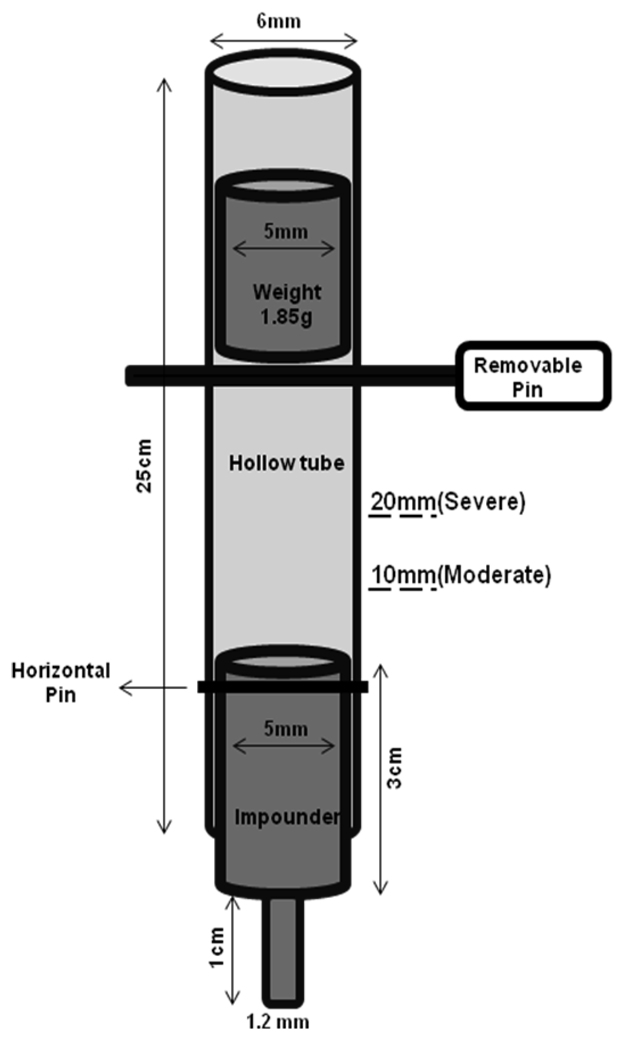
Contusion SCI was performed in adult male C57BL/6 mice (20–25g, Taconic). Mice were anesthetized using isoflurane (Baxter, Deerfield, IL) and a laminectomy was performed at the T-9 level. The spinal cord was stabilized using mouse transverse clamps (Stoelting, Wood Dale, IL). The weight drop apparatus was a modified version of device used in rat studies (Yakovlev & Faden, 1994; Byrnes et al., 2007). Briefly, a steel impounder inserted in a Teflon base with a 1.2mm tip diameter was lowered onto the exposed spinal cord. The impounder was attached by a horizontal pin to the end of a hollow tube, which guided the weight and prevented it from bouncing after impact. To produce a contusive injury, a1.85 g weight was dropped from 10 mm (mild-moderate injury, n=11) or 20 mm (moderate-severe injury, n=9) onto the impounder. The weight was a Teflon-coated stainless steel supported by a removable pin, that was taken away to release the weight (Figure 1). A rod magnet was used to retrieve the weight after injury by lowering the magnet in the hollow tube. To obtain a symmetrical injury, it was imperative for the impounder to be perpendicularly placed in the center of the spinal cord at the time of impact. Sham animals (n=9) received a laminectomy without weight drop. Bladder expression on injured animals was performed twice daily until autonomic bladder expression returned, typically by day 10. From each group, 3 mice were euthanized at 28 days for histological analyses and the rest were euthanized at either 35 or 42 days after injury. All experiments complied fully with the principles set forth in the ‘Guide for the Care and Use of Laboratory Animals’ and were approved by the Georgetown University Animal Care and Use Committee.
Figure 1.
Schematic of the spinal cord injury device. A horizontal pin attached the impounder to the end of a hollow tube, which guided the weight and prevented it from bouncing after impact. The weight was supported by a removable pin, which would be located at 10 (mild-moderate) or 20mm (moderate-severe) above the impounder to produce mild-moderate or moderate-severe injuries, respectively.
Behavioral Testing
BMS
Mice were tested for hindlimb functional deficits at 1, 7, 14, 21, 28 (n=9 per group), 35 (n=6 per group), and 42 days (n=3 per group) after SCI. Hindlimb locomotor recovery was assessed in an open field using the BMS previously described in detail (Basso et al., 2006)or other tests (see below). This scale ranges from 0, indicating complete paralysis, to 9, indicating normal movement of the hindlimbs. Performance of the left and right hindlimbs was averaged in order to obtain the BMS score.
Grip Walk (GW)
Based on prior publications (Z'Graggen et al., 1998), a grid walk was constructed for mice using two parallel pieces of wood (1m long) to hold 100 rungs (round wooden steps, 2mm in diameter, 10cm in length) 1cm apart. Prior to injury, mice were trained for 3 days on the apparatus. Each mouse was allowed to cross the grid walk 3 consecutive times, resting 25 seconds in a dark box between each trial. The grid walk was video-taped on the third day and a baseline was obtained from the number of grips for each mouse.
The grid walk was renamed to grip walk following modification of the method to count the number of grips instead of the footfalls. A grip was defined as placing the toes on the rung as well as pushing the hindlimb off to go to the next step. The mice were evaluated on 50cm of the grid using three patterns: easy (50 steps, 1cm apart), medium (every third step was removed), and hard (every other step was removed). The sum of the number of the grips for all three patterns was used for the analysis of grip walk. Similar to BMS, the grip walk was performed weekly starting on day 7 until euthanasia.
Ladder Climb (LC)
The grip walk apparatus with the ‘easy pattern’ (steps 1 cm apart) was inclined to a 55° angle. The mice were trained for 3 days and were video-taped on the third day to obtain a baseline. Missing more than two rungs during the baseline recording disqualified mice from the experiment; no mice used in this study were disqualified. The ladder climb was performed on the same days as the grip walk and the number of grips was counted.
Inclined Plane
Mice were placed head down on an inclined plane platform (50cm × 75cm) with a grooved 1mm thick rubber surface, while the angle was gradually increased until the mouse was unable to hold its position for 5 seconds without sliding. The last angle that the mouse was able to hold for 5 seconds was recorded on days 1, 7, 14, 21, 28 and days.
Plantar Test and Tail Flick
Mice were acclimated individually in heated glass floor in a plexiglass chambers (IITC Life sciences, Plantar Analgesia Meter for thermal paw) for 15 min. Mice were tested under the same conditions, i.e. 7:00–9:00AM after 15min of acclimating. The testing environment was washed thoroughly between animals to eliminate any odor related stress cues. A radiant heat source with a locator light was positioned under the plantar surface of the hind-paw and tail and the latency to withdrawal was determined. The settings for the heat were optimized to get an average withdrawal latency of 6–8 seconds in uninjured animals. A cutoff time of 20 seconds was used to prevent tissue damage. The light beam was directed on the plantar surface of the hind-paw or tail until the animal responded, or 20 seconds, whichever occurred first. The latency to a flick or flinch of the hind-paw or tail, placement of the hind-paw into the mouth, or jumping away from the heat source was recorded with a built-in timer, which displayed reaction time in 0.01 second increments. Tests were performed three times in sequence on the right hind-paw, left hind-paw, and the tail, with a 5 minute rest between sequences. The average latency for each hind-paw and the tail was recorded. All functional testing or tape-viewing were done by two investigators blinded to the injury severity.
Histology
Animals were anesthetized (100 mg/Kg sodium pentobarbital, I.P.) and intracardially perfused with 50 ml of 0.9% heparinized saline followed by 50 ml of 10% buffered formalin. A 1 cm section of the spinal cord centered at the lesion epicenter was dissected, post-fixed in 10% buffered formalin overnight and cryoprotected in 30% sucrose for 48 hours. Sections were embedded in OCT compound (Andwin Scientific Tissue-Tek) and were cut at 20µm onto SuperFrost slides (Fisher, Cat#12-550-15). Every 50th section of the 1 cm spinal cord block, with a random starting section, was processed with a standard eriochrome cyanine R staining protocol for histological analysis. NIH Image J software was used to determine the area in mm2 of blue pixels representing the eriochrome cyanine R stained myelin (Kuhn & Wrathall, 1998)
Statistical analyses
Quantitative data are presented as mean ± SEM. BMS scores and were analyzed with one-way ANOVA and repeated measures. Inter-rater reliability was assessed using Pearson correlation to compare scores obtained by two independent investigators blinded to injury severity. All statistical tests were performed using the GraphPad Prism Program, Version 4 for Windows (GraphPad Software, Inc. San Diego, CA). A p value < 0.05 was considered statistically significant.
Results
BMS reached a plateau between 21and 42 days after SCI
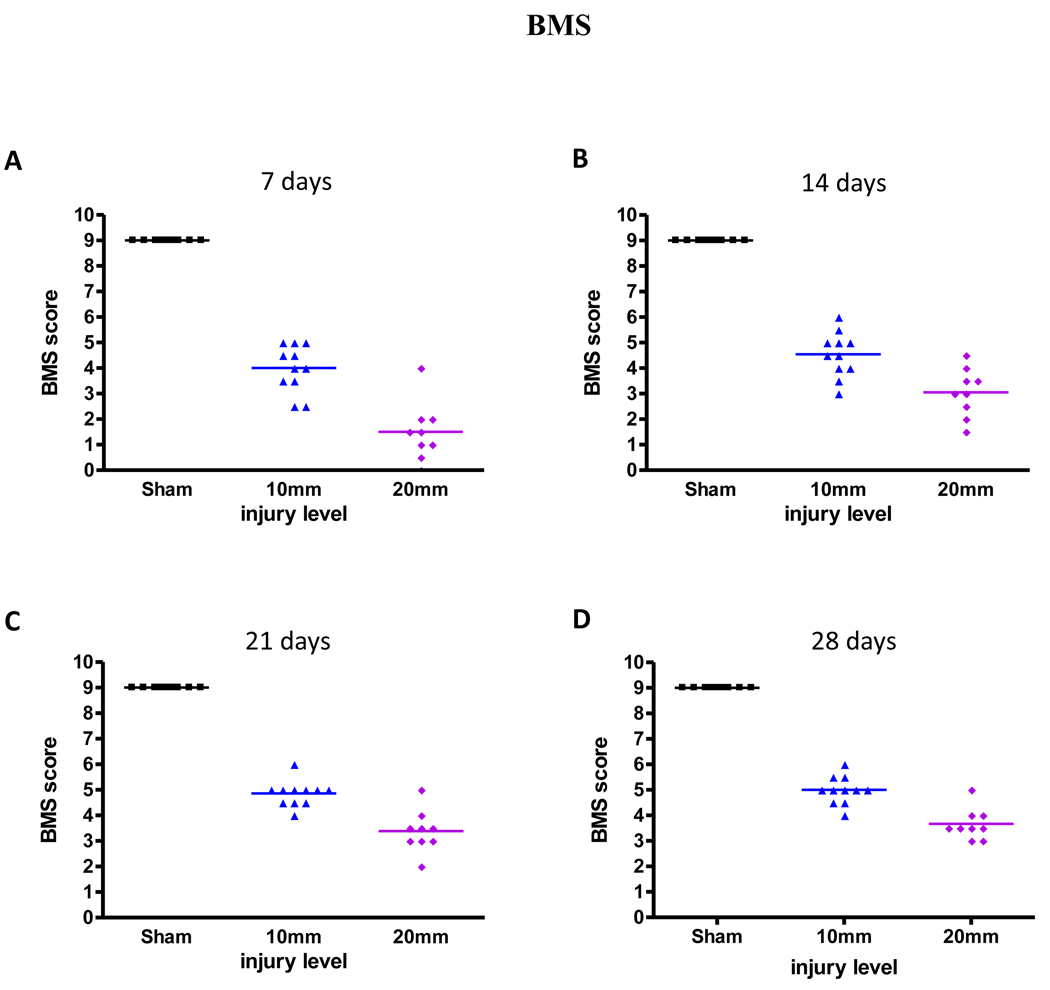
Mice were observed in an open field and scored for BMS on days 1, 7, 14, 28, 35, and 42 days post-injury. Sham mice showed a score of 9 for all time points, which indicates no functional impairments. Mild-moderate and moderate-severe injury groups had a BMS score of 0–0.5 one day post-injury (data not shown). BMS scores increased on days 7 through 21 (Figures 2A–C, Table 1) and stabilized between 21 and 42 days (to prevent redundancy, data for 35 and 42 days are not shown) post-injury (Figure 2D, Table 1). The BMS score reached about 5 in mild-moderate and 3 in moderate-severely injured mice at 21 days after injury. These data indicate that the injury was performed consistently within groups and with the published literature for contusion injury, with acute functional loss one day after injury followed by gradual limited recovery.
Figure 2.
BMS scores 7, 14, 21, and 28 days after injury (n=9/sham, n=11/mild-moderate, and n=9/moderate-severe). BMS scores were between 0–0.5 on day one (data not shown), increased on days 7 to 21 (A–C) and plateaued between 21 to 28 (C–D) days after injury.
Table 1.
Statistical data for BMS, grip walk, ladder climb, inclined plane, plantar test, and BLG score are reported at 7 and 28 days after injury.
| Test | 7days | 28days | ||||
|---|---|---|---|---|---|---|
| Sham | 10mm | 20mm | Sham | 10mm | 20mm | |
| BMS | 9.0±0 | 4.0±0.3 | 1.5±0.4 | 9.0±0 | 5.0±0.2 | 3.7±0.2 |
| Grip Walk | 65.6±2.3 | 12.7±2.8 | 0 | 67.8±2.4 | 34.8±4.0 | 9.5±3.2 |
| Ladder Climb | 20.4±0.4 | 11.1±1.4 | 0.9±0.8 | 20.6±0.7 | 18.4±0.8 | 8.4±1.9 |
| Inclined Plane | 52.0±1.4 | 41.2±1.5 | 35.9±0.6 | 46.1±1.5 | 43.0±1.7 | 35.5±1.8 |
| Planter Test | 3.4±0.4 | 4.3±0.4 | N/O | 4.1±0.2 | 5.9±0.8 | 4.7±0.8 |
| BLG | 26.9±0.1 | 10.2±1.2 | 1.9±0.6 | 26.8±0.1 | 18.1±0.8 | 8.4±1.5 |
Modified grip walk and ladder climb are reliable tests to evaluate recovery after SCI in mice
The grid walk and ladder climb tests have been used previously to assess function after SCI in rats and mice (Metz et al., 2000; Apostolova et al., 2006). Using the published method of assessment, a high degree of variability was observed in individual animals in both tests. Review of the videotapes revealed that mice could slide the hind-paws over the rungs in a way that prevented the paws from falling below the plane of the rung, thereby reducing the number of observed ‘footfalls’.
To correct for this behavior and more accurately assess hind-paw function, the test was modified to count the number of hind-paw ‘grips’ instead of ‘footfalls’ in the grid walk and the ladder climb tests. We therefore renamed the task to ‘grip walk’. A grip was defined as placing the foot on the rung as well as pushing the hind-limb off the rung to go to the next step. In order to prevent adaptation to the stepping pattern, the grip walk was performed using three different patterns: easy, medium, and hard, as described in the methods section. The score for the 3 patterns were then added to give a sum of grips. For the ladder climb, the grip walk apparatus with the rungs spaced 1 cm apart (easy pattern) was inclined to a 55° angle and the number of grips was counted.
The ladder climb score using grip versus footfalls was compared in a subset of animals (n=5) at days 14 and 21. The grip counts (14 days/mild-moderate: 14.4±1.9; 14 days/moderate-severe: 2.8±1.5; 21 days/mild-moderate: 16.6±3.1; 21 days/moderate-severe: 4.8±4.1) gave less variability as well as a better separation between the groups than the “footfalls” (14 days/mild-moderate: 8.8±2.6; 14 days/moderate-severe: 21.2±1.2; 21 days/mild-moderate: 9.4±3.2; 21 days/moderate-severe: 23.6±2.8).
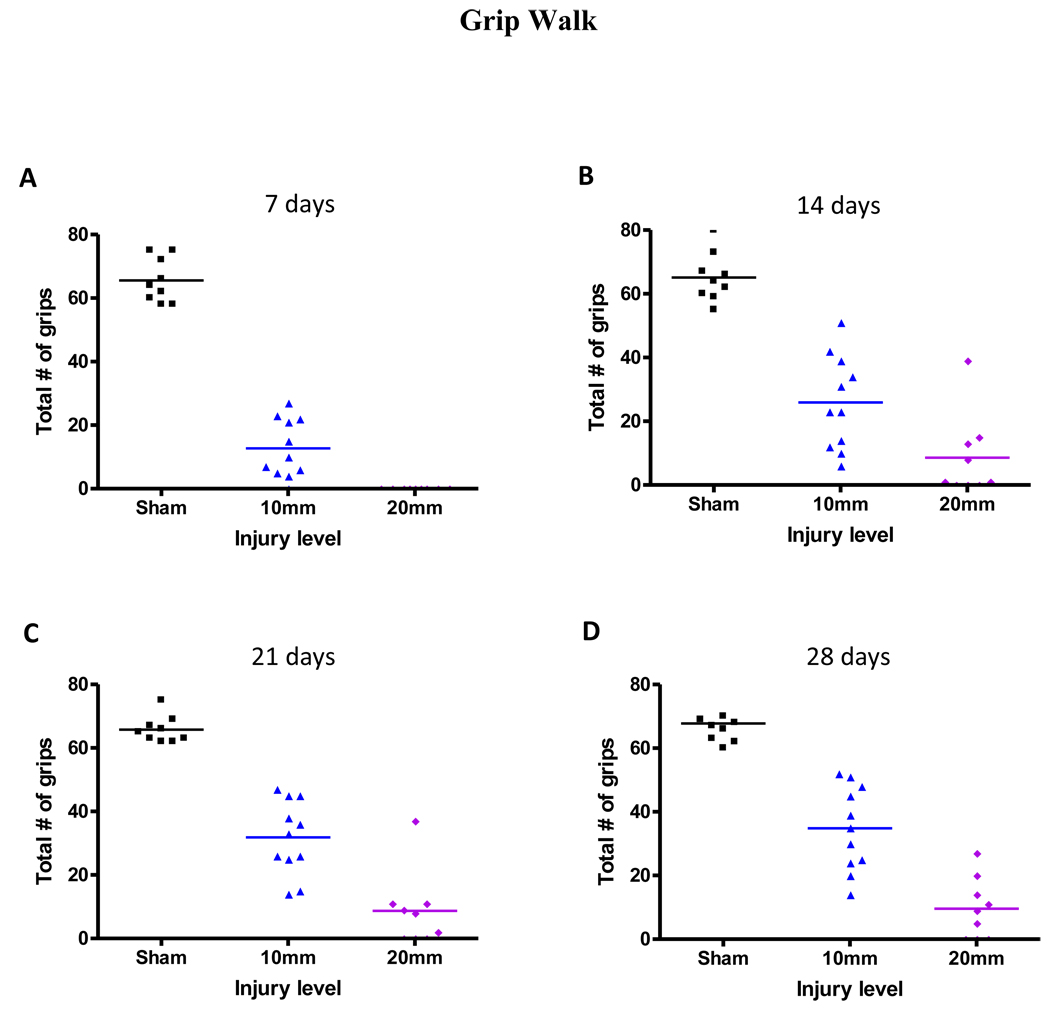
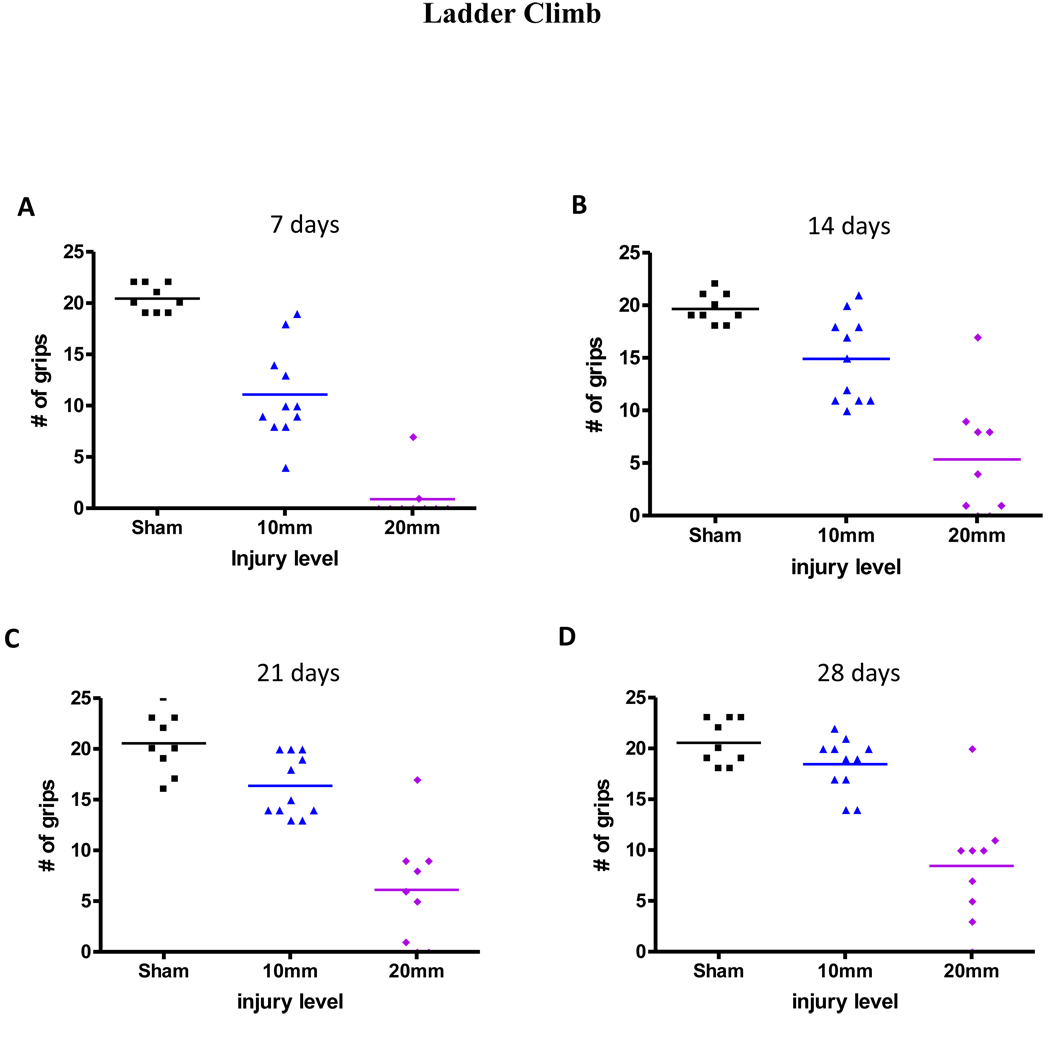
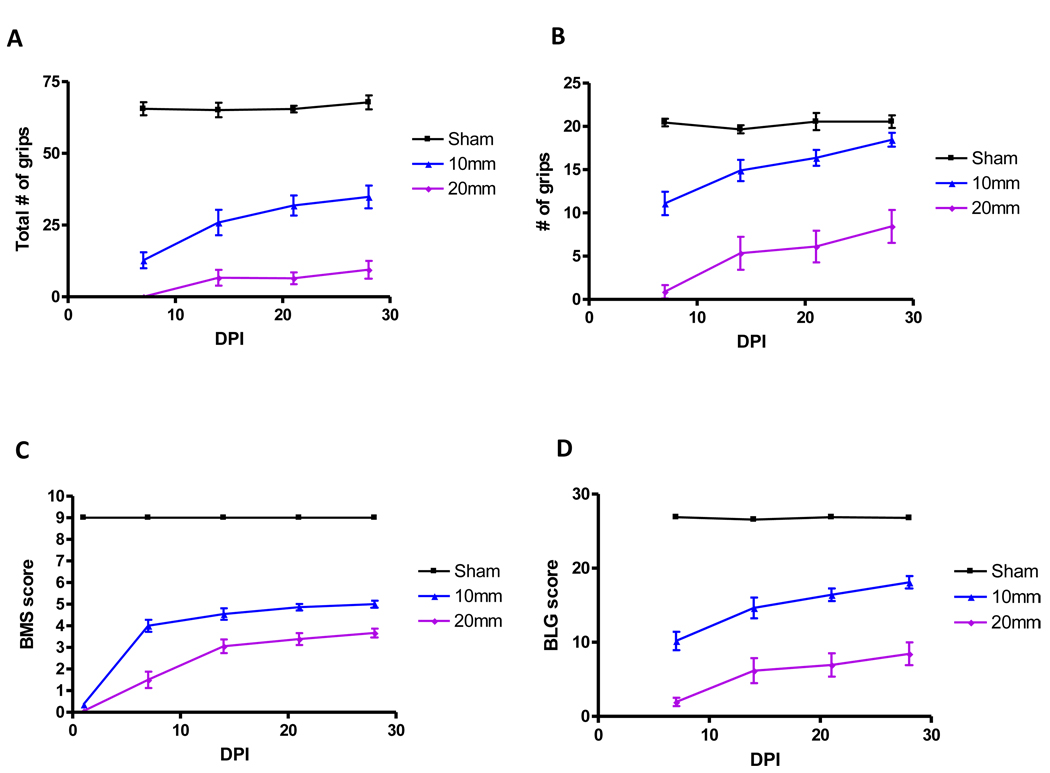
Mice were unable to perform either the grip walk or the ladder climb first day after the surgery. In the grip walk test, mice could grip the rungs at 7 days after the mild-moderate injury but not after the moderate-severe injury (Figure 3A, Table 1). Grips were observed in the moderate-severe group when tested at 14 days after injury. At 28 days (Figure 3D) after injury, the number of grips was increased compared to day 7, even in the –moderate-severely injured mice (Table 1). The scores were ranged from 0, indicating no grips, to a maximum of 85 grips in sham mice. Mice performed similarly in the ladder climb test, in which mice could grip the rungs at 7 days after the mild-moderate injury but not after the moderate-severe injury (Figure 4A, Table 1). At 28 days after injury, the number of correct grips was significantly increased, even in the moderate-severely injured mice (Figure 4D, Table 1). The scores ranged from 0, indicating no grips, to a maximum of 25 grips in case of sham mice.
Figure 3.
Number of grips in the grip walk (GW) test at 7, 14, 21, and 28 days post injury. The number of grips were added for easy, medium, and hard test (n=9/sham, n=11/mild-moderate, and n=9/moderate-severe). The test began at day 7 after injury.
Figure 4.
Number of grips in the ladder climb (LC) test at 7, 14, 21, and 28 days post injury (n=9/sham, n=11/mild-moderate, and n=9/moderate-severe). The test begins at day 7 after injury.
Inclined plane, plantar test, and tail flick show high variability after SCI in mice
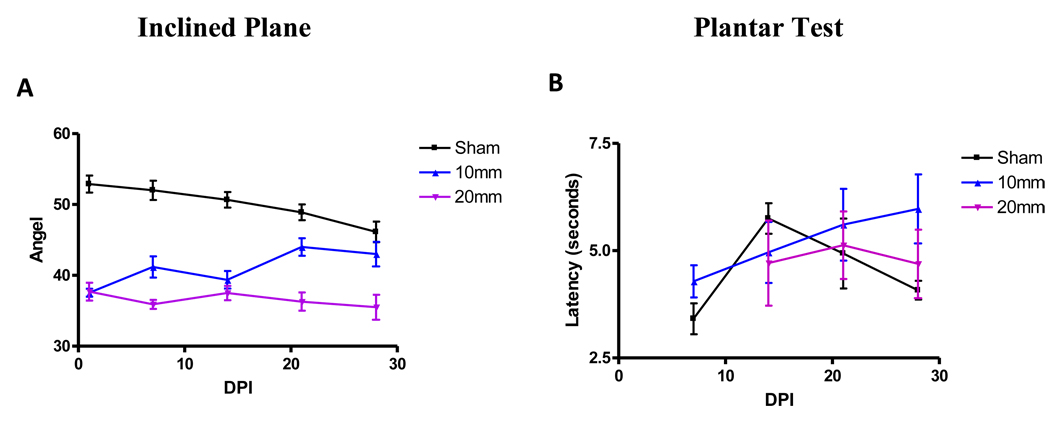
Mice were tested in their performance on an inclined plane and in response to heat (plantar test and tail flick test) (Figure 5, Table 1). Over time, sham and moderate-severely-injured mice showed a trend of reduction in the maximum angle of the inclined plane (Figure 5A, Table 1), whereas mild-moderate injured mice showed a slight increase (Table 1). However, the high variability in the data prevented the detection of any significant differences between groups or over time, which led to the termination of the test at 28 days. The angles measured ranged from 28° for injured animals to 60° for sham mice.
Figure 5.
The highest angle in the inclined plane (A) and the latency in the plantar (B) test at 1, 7, 14, 21, and 28 days post injury (DPI). Tests were not carried to day 35 due to no significant differences between injury groups or high variability observed among sham animals (n=9/sham, n=11/mild-moderate, and n=9/moderate-severe).
Similarly, there was a high level of variability in the results obtained from the plantar (Figure 5B, Table 1) and tail flick (data not shown) tests. In addition, although all the animals were tested under the same conditions, there was high variability even in the sham group. Moderate-severely injured mice were unable to perform the plantar test 7 days after injury. Further, neither the plantar (Figure 5B) nor the tail flick (data not shown) revealed any understandable pattern in response in any of the groups (sham, mild-moderate or moderate-severe).
BLG combined score gives a better separation between injury groups
In order to assess whether combined scoring would increase the correlation of injury severity and tissue preservation with hindlimb function, a novel scale was introduced utilizing performance on the three tests showing the best separation among groups: the BMS score, the modified Ladder climb, and the Grip walk (BLG). The number of grips for ladder climb and grip walk were converted to an ordinal scale ranging from 0–9 (Table 2). The scale is based on 10% divisions of the mean grip walk and ladder climb scores calculated for the sham animals (66 and 20, respectively; Table 2). The summation of these individual scores and the unaltered BMS score resulted in the BLG score, which ranged from 0 to 27. The modified grip walk (Figure 6A), ladder climb (Figure 6B), BMS (Figure 6C), and BLG score (Figure 6D) are demonstrated at 28 days. The BLG score allowed for clear distinction of injury severity (Table 1). The BLG combined score provided high inter-rater reliability (Pearson r = 0.98), low inter-group variability, and high correlation (r2 = 0.9055) to injury severity (Figure 7C), which is essential for a functional test to discriminate differences due to pharmacological or genetic manipulations.
Table 2.
Conversion table for BLG score.
| BLG | LC | GW | % of sham |
|---|---|---|---|
| 0 | 0–2 | 0–7 | 10% |
| 1 | 3–4 | 8–13 | 20% |
| 2 | 5–6 | 14–20 | 30% |
| 3 | 7–8 | 21–26 | 40% |
| 4 | 9–10 | 27–33 | 50% |
| 5 | 11–12 | 34–40 | 60% |
| 6 | 13–14 | 41–46 | 70% |
| 7 | 15–16 | 47–53 | 80% |
| 8 | 17–18 | 54–59 | 90% |
| 9 | ≥19 | ≥60 | 100% |
Figure 6.
The modified grip walk (A), ladder climb (B), the BMS alone (C), and the BLG score (D) are demonstrated at 28 days. The BMS score, and converted GW and LC were added to obtain the BLG score at indicated days post injury (DPI) and (n=9/sham, n=11/mild-moderate, and n=9/moderate-severe).
Figure 7.
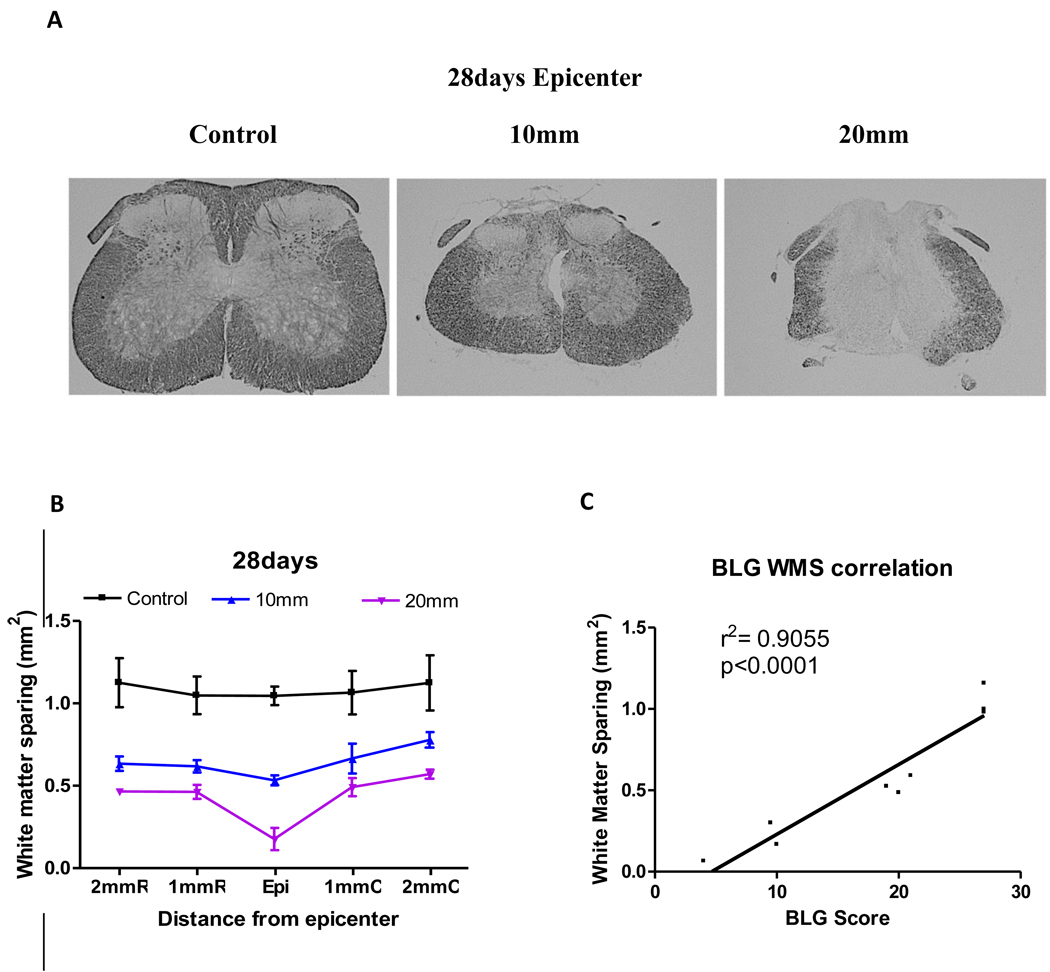
Sections from sham, mild-moderate, and moderate-severely injured mice were stained with eriochrome cyanine R at 28 (A) days after injury and white matter sparing was assessed. The spinal cord shrinks at the epicenter as shown in mild-moderate and moderate-severe injury. White matter sparing correlation with the injury severity (B) and a positive correlation with the BLG score (C). White matter sparing was significantly different in both groups from the control group (p<0.01). C shows a direct correlation between the BLG score and the white matter sparing (r2= 0.9055, p<0.0001) Scalebar = 500µm.
Spared white matter correlates with BLG score
To evaluate the correlation between spared white matter and functional recovery as assessed by the BLG, spinal cord samples from 28 days post-injury were stained with eriochrome cyanine R, to identify spared white matter (Figures 7A). Staining revealed a central core lesion with spared peripheral white matter at 28 days after injury. The spared peripheral white matter area decreased as the severity of the injury increased (Figure 7B). In accordance with the literature (Kuhn & Wrathall, 1998), the spared white matter was also increased as the distance from the epicenter increased. Correlation analysis demonstrated a significant positive correlation between spared white matter and the BLG score at 28 days (Figure 7C, r2=0.9055, p<0.0001).
Discussion
Assessment of behavioral recovery after SCI is important for evaluating injury severity and response to therapeutic interventions. To date, the majority of behavioral tests have been designed and characterized for studies in rats (Rivlin & Tator, 1977; Hargreaves et al., 1988; Panjabi & Wrathall, 1988; Basso et al., 1995; Fehlings & Tator, 1995; Metz et al., 2000; Merkler et al., 2001; Schucht et al., 2002; Apostolova et al., 2006; Dijkstra et al., 2006) and few tests are available that have been rigorously characterized for mouse SCI models (Apostolova et al., 2006; Basso et al., 2006; Li et al., 2006; Cummings et al., 2007; Gulino et al., 2007). In this study, we evaluated a number of tests previously used primarily for assessment in rat SCI models – grid walk, ladder climb, inclined plane, Plantar test, and tail flick – in combination with a test designed for mouse assessment, the BMS scoring method (Basso et al., 2006). We examined the intra-animal, intra-group and inter-group variability of each test and evaluated whether a combined scoring method could improve correlations to injury severity, white matter sparing, reduce variability, and/or increase separation between injury groups. Analysis of the outcomes of these tests led to the development of a new scoring method, the BLG score, which combined the BMS, Ladder climb and Grip walk tests, using ordinal scaling. The BLG combined score provides essential factors –high inter-rater reliability, low inter-group variability, significant correlation to injury severity, and excellent separation between injury groups – for a functional test, which should readily permit distinguishing differences caused by genetic or pharmacological manipulations.
The grid walk or ‘foot fault’ test evaluates the sensory-motor coordination between hindlimbs and forelimbs and examines the deficits in descending motor control (Metz et al., 2000; Sedy et al., 2008). In this test, animals are allowed to cross a grid and the number of missed rungs, known as ‘footfalls’ or ‘foot faults’ is counted for different patterns. The number of errors is then rated in a non- parametric grid walk score in a manner that the higher the error, the lower the score would be. In the ladder climb, which is a modified ladder walking test, the grid walk apparatus is inclined to a 55° angle for analysis of hindlimb function with the benefit of additional forelimb support (Apostolova et al., 2006). In both the ladder climb and grid walk tests, it is customary to count the number of ‘footfalls’, or the number of times that the animals’ hindpaws fall through the rungs as the animal crosses a long grid (McEwen & Springer, 2006; Sedy et al., 2008). However, it has been shown that the number of footfalls does not correlate well with BBB scores in rats (McEwen & Springer, 2006). It is possible that this low correlation is due to the loss of ‘footfalls’ caused by the sliding of hindpaws across ladder rungs rather than dropping below the plane of the ladder, resulting in falsely low numbers of ‘footfalls’. To compensate for this behavior in mice, we modified both the grid walk and ladder climb tests by counting the number of hind-paw ‘grips’ instead of ‘footfalls’. A grip was defined as placing the foot on the rung, grabbing it, and pushing the hindlimb off the rung to go to the next step. After video-taping the mice on grid walk and ladder climb, direct comparison of ‘footfalls’ and ‘grip’ counts indicated that ‘grip’ counting resulted in lower intra-animal and intra-group variability at 14 and 21 days post-injury and better discrimination of injury severity. We have thus renamed the task to ‘grip walk’ and present all data as the summation of ‘grips’ over trials in both the grip walk and ladder climb tasks.
The BBB score, which is performed in an open-field, is the most commonly and accepted test for recovery of locomotor function. It was originally developed for assessment of function after contusion SCI in rats (Basso et al., 1995) and it grades the hindlimb locomotion from 0 (no movement) to 21 (normal movement). Since the BBB test was not able to effectively discriminate the recovery pattern differences in mice after SCI, the BMS test was derived from the BBB (Basso et al., 2006). The BMS ranges from 0 for complete paralysis to 9 for a normal mouse. Mice are measured according to 7 main categories: ankle movement, plantar placement, stepping, coordination, paw position, trunk instability, and tail. Using our weight drop apparatus, we obtained a BMS score of about 5 for the mild-moderate (10mm) and 3 for the moderate-severe (20mm) injury. These scores are similar to scores obtained from other devices, namely Infinite Horizon (Nishi et al., 2007). Although the BMS gives a reliable measure of recovery of function after SCI in mice, it is difficult to distinguish between different degrees of coordination. An expanded quantitative assessment may provide more sensitivity especially once some degree of hindlimb-forelimb coordination has been achieved (Cummings et al., 2007). weight drop injury device
Three functional tests, the inclined plane, plantar test, and tail flick, were found to be poor indicators of injury severity. These tests had high intra-animal, intra-group and inter-group variability. The inclined plane evaluates the ability of the animal to maintain its body position on a surface which is gradually raised to increasing angles. In rat SCI models of spinal cord injury, this test has been shown to be reliable, consistent, sensitive and has been used to assess therapeutic modalities (Rivlin & Tator, 1977; Panjabi & Wrathall, 1988; Fehlings & Tator, 1995). However, according to the inclined plane data, the sham animals held a slightly lower angle at 28 days after the laminectomy than the first day. This result is counter-intuitive, as the angle was expected to remain constant or increase as the animal became used to the testing situation. The cause for the reduction in value is unclear, but its occurrence in sham animals calls the reliability of the test into question in our mouse SCI model. Also, the lack of significant differences between the mild-moderate and the moderate-severely injured animals at several time points after injury indicates a low ability to discriminate between injury severities.
The plantar and tail flick tests have been used to detect sensory system disorders after SCI in rats. The plantar test has been successful in discerning differences in does-related hyperalgesia (Hargreaves et al., 1988). The tail flick on the other hand, has not shown any significant differences (Gale et al., 1985; Merkler et al., 2001) up to 35 days after SCI. In this study, each of these tests had very high intra-animal and intra-group variability, including within the sham group. Both tests proved unpredictable and failed to provide any meaningful data. Therefore, although these tests have been used in rat SCI models by some, our data do not support the effectiveness of their use in mouse SCI. Due to this high variability, the tests were terminated on day 28 post-injury.
Functional assessments have often found that combinations of multiple tests result in the finest discrimination in outcome measures. For example, a combined behavioral score (CBS) (Gale et al., 1985) was developed to discern functional performance acutely after SCI in rats. The CBS included tasks such as toe spread reflex, placing reflex, withdrawal in response to stimulation and hot plate test. Using a battery of tests increased the sensitivity of the combined scores compared to the individual tests assessed. The BLG score was developed in the current work in order to provide a scale that incorporated the most predictive tests found in the study (the BMS, ladder climb and grip walk) but also operated to help discriminate between different injury severities. In the BLG, the number of grips for ladder climb and grip walk scores were converted to an ordinal scale that were then added to the BMS score. Transformation of the data into limited ordinal scales — that were then summed without weighting — worked to effectively incorporate the inter-group discrimination provided by all 3 tests. Furthermore, the summation of the three tests for a final range of 0–27 will give the data parametric characteristics allowing for the use of parametric statistical analyses. In addition, at any given time point, there is a greater degree of difference between the two injury level. For instance, at 28 days (Figures 6C–D) the difference between 10mm and 20mm injured animals vary from score of 5 to score of 15 in the BLG score. However, the same animals vary from 3 to 5 in the BMS score. This increased distance between scores will allow for more discrimination between groups in genetic or pharmological studies. The BLG score demonstrated significant correlation with injury severity as defined by spared white matter, low intra-animal variability, low intra-group variability and high inter-group discrimination. It also demonstrated high inter-rater reliability, with a Pearson reliability of > 0.98, demonstrating high viability as a functional assessment method.
Further, spared peripheral white matter surrounding a central core lesion is one of the characteristics of contusion models in rodents and human (Steward et al., 1999). Our data and others have clearly demonstrated that white matter decreases as the severity of the injury increases (Figure 7) (McEwen & Springer, 2006). The area of the residual white matter at the epicenter and 1mm caudal and rostral to the injury site with our model is in accordance with the classic study done by Kuhn and Wrathall (1998). Several studies have demonstrated that improved functional performance is highly correlated with an increase in spared white matter in rats (Ballermann & Fouad, 2006; Ballermann et al., 2006; McEwen & Springer, 2006; Smith et al., 2006). Analysis of spared white matter showed significant positive correlation (r2 =0.9055, p<0.0001) between white matter sparing and BLG score.
In summary, the combined BLG score shows high inter-rater reliability, low variability, and significant correlations to injury severity and tissue preservation. To date, there are few functional tests to clearly discriminate injury severity or recovery in mouse, as most of the functional/behavioral work has been performed in rats (Collazos-Castro et al., 2006; Hendriks et al., 2006). Many researchers attempt to use the rat tests in mice; however, studies have shown that there is a significant difference in the progression of the injury in mice in comparison to rats (Steward et al., 1999). Our current findings demonstrate that modifications of these tests may be required for effective use in mice.
Acknowledgments
The authors would like to thank Drs. Jean Wrathall, Linda MacArthur, and David Loane for helpful advice and careful reading of the manuscript. We would also like to thank Mr. David Zapple and Ms. Kyni Jones for their technical support. This work was supported by NIH Grant 5R01NS054221-03(AIF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apostolova I, Irintchev A, Schachner M. Tenascin-R Restricts Posttraumatic Remodeling of Motoneuron Innervation and Functional Recovery after Spinal Cord Injury in Adult Mice. J Neurosci. 2006;26:7849–7859. doi: 10.1523/JNEUROSCI.1526-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballermann M, Fouad K. Spontaneous Locomotor Recovery in Spinal Cord Injured Rats Is Accompanied by Anatomical Plasticity of Reticulospinal Fibers. Eur J Neurosci. 2006;23:1988–1996. doi: 10.1111/j.1460-9568.2006.04726.x. [DOI] [PubMed] [Google Scholar]
- Ballermann M, Tse AD, Misiaszek JE, Fouad K. Adaptations in the Walking Pattern of Spinal Cord Injured Rats. J Neurotrauma. 2006;23:897–907. doi: 10.1089/neu.2006.23.897. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded Histological and Locomotor Outcomes after Spinal Cord Contusion Using the Nyu Weight-Drop Device Versus Transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso Mouse Scale for Locomotion Detects Differences in Recovery after Spinal Cord Injury in Five Common Mouse Strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Byrnes KR, Stoica BA, Fricke S, Di Giovanni S, Faden AI. Cell Cycle Activation Contributes to Post-Mitotic Cell Death and Secondary Damage after Spinal Cord Injury. Brain. 2007;130:2977–2992. doi: 10.1093/brain/awm179. [DOI] [PubMed] [Google Scholar]
- Collazos-Castro JE, Lopez-Dolado E, Nieto-Sampedro M. Locomotor Deficits and Adaptive Mechanisms after Thoracic Spinal Cord Contusion in the Adult Rat. J Neurotrauma. 2006;23:1–17. doi: 10.1089/neu.2006.23.1. [DOI] [PubMed] [Google Scholar]
- Cummings BJ, Engesser-Cesar C, Cadena G, Anderson AJ. Adaptation of a Ladder Beam Walking Task to Assess Locomotor Recovery in Mice Following Spinal Cord Injury. Behav Brain Res. 2007;177:232–241. doi: 10.1016/j.bbr.2006.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra S, Duis S, Pans IM, Lankhorst AJ, Hamers FP, Veldman H, Bar PR, Gispen WH, Joosten EA, Geisert EE., Jr Intraspinal Administration of an Antibody against Cd81 Enhances Functional Recovery and Tissue Sparing after Experimental Spinal Cord Injury. Exp Neurol. 2006;202:57–66. doi: 10.1016/j.expneurol.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Anderson AJ, Basso DM, Edgerton VR, Cotman CW. Voluntary Wheel Running Improves Recovery from a Moderate Spinal Cord Injury. J Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Tator CH. The Relationships among the Severity of Spinal Cord Injury, Residual Neurological Function, Axon Counts, and Counts of Retrogradely Labeled Neurons after Experimental Spinal Cord Injury. Exp Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Gale K, Kerasidis H, Wrathall JR. Spinal Cord Contusion in the Rat: Behavioral Analysis of Functional Neurologic Impairment. Exp Neurol. 1985;88:123–134. doi: 10.1016/0014-4886(85)90118-9. [DOI] [PubMed] [Google Scholar]
- Gulino R, Dimartino M, Casabona A, Lombardo SA, Perciavalle V. Synaptic Plasticity Modulates the Spontaneous Recovery of Locomotion after Spinal Cord Hemisection. Neurosci Res. 2007;57:148–156. doi: 10.1016/j.neures.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A New and Sensitive Method for Measuring Thermal Nociception in Cutaneous Hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Hendriks WT, Eggers R, Ruitenberg MJ, Blits B, Hamers FP, Verhaagen J, Boe GJ. Profound Differences in Spontaneous Long-Term Functional Recovery after Defined Spinal Tract Lesions in the Rat. J Neurotrauma. 2006;23:18–35. doi: 10.1089/neu.2006.23.18. [DOI] [PubMed] [Google Scholar]
- Kuhn PL, Wrathall JR. A Mouse Model of Graded Contusive Spinal Cord Injury. J Neurotrauma. 1998;15:125–140. doi: 10.1089/neu.1998.15.125. [DOI] [PubMed] [Google Scholar]
- Li Y, Oskouian RJ, Day YJ, Kern JA, Linden J. Optimization of a Mouse Locomotor Rating System to Evaluate Compression-Induced Spinal Cord Injury: Correlation of Locomotor and Morphological Injury Indices. J Neurosurg Spine. 2006;4:165–173. doi: 10.3171/spi.2006.4.2.165. [DOI] [PubMed] [Google Scholar]
- Lu J, Ashwell KW, Waite P. Advances in Secondary Spinal Cord Injury: Role of Apoptosis. Spine. 2000;25:1859–1866. doi: 10.1097/00007632-200007150-00022. [DOI] [PubMed] [Google Scholar]
- McEwen ML, Springer JE. Quantification of Locomotor Recovery Following Spinal Cord Contusion in Adult Rats. J Neurotrauma. 2006;23:1632–1653. doi: 10.1089/neu.2006.23.1632. [DOI] [PubMed] [Google Scholar]
- Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor Recovery in Spinal Cord-Injured Rats Treated with an Antibody Neutralizing the Myelin-Associated Neurite Growth Inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K. Efficient Testing of Motor Function in Spinal Cord Injured Rats. Brain Res. 2000;883:165–177. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- Nishi RA, Liu H, Chu Y, Hamamura M, Su MY, Nalcioglu O, Anderson AJ. Behavioral, Histological, and Ex Vivo Magnetic Resonance Imaging Assessment of Graded Contusion Spinal Cord Injury in Mice. J Neurotrauma. 2007;24:674–689. doi: 10.1089/neu.2006.0204. [DOI] [PubMed] [Google Scholar]
- Panjabi MM, Wrathall JR. Biomechanical Analysis of Experimental Spinal Cord Injury and Functional Loss. Spine. 1988;13:1365–1370. doi: 10.1097/00007632-198812000-00007. [DOI] [PubMed] [Google Scholar]
- Panter SS, Faden AI. Pretreatment with Nmda Antagonists Limits Release of Excitatory Amino Acids Following Traumatic Brain Injury. Neurosci Lett. 1992;136:165–168. doi: 10.1016/0304-3940(92)90040-e. [DOI] [PubMed] [Google Scholar]
- Rivlin AS, Tator CH. Objective Clinical Assessment of Motor Function after Experimental Spinal Cord Injury in the Rat. J Neurosurg. 1977;47:577–581. doi: 10.3171/jns.1977.47.4.0577. [DOI] [PubMed] [Google Scholar]
- Schucht P, Raineteau O, Schwab ME, Fouad K. Anatomical Correlates of Locomotor Recovery Following Dorsal and Ventral Lesions of the Rat Spinal Cord. Exp Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- Sedy J, Urdzikova L, Jendelova P, Sykova E. Methods for Behavioral Testing of Spinal Cord Injured Rats. Neurosci Biobehav Rev. 2008;32:550–580. doi: 10.1016/j.neubiorev.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Smith RR, Burke DA, Baldini AD, Shum-Siu A, Baltzley R, Bunger M, Magnuson DS. The Louisville Swim Scale: A Novel Assessment of Hindlimb Function Following Spinal Cord Injury in Adult Rats. J Neurotrauma. 2006;23:1654–1670. doi: 10.1089/neu.2006.23.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Schauwecker PE, Guth L, Zhang Z, Fujiki M, Inman D, Wrathall J, Kempermann G, Gage FH, Saatman KE, Raghupathi R, McIntosh T. Genetic Approaches to Neurotrauma Research: Opportunities and Potential Pitfalls of Murine Models. Exp Neurol. 1999;157:19–42. doi: 10.1006/exnr.1999.7040. [DOI] [PubMed] [Google Scholar]
- Yakovlev AG, Faden AI. Sequential Expression of C-Fos Protooncogene, Tnf-Alpha, and Dynorphin Genes in Spinal Cord Following Experimental Traumatic Injury. Mol Chem Neuropathol. 1994;23:179–190. doi: 10.1007/BF02815410. [DOI] [PubMed] [Google Scholar]
- Z'Graggen WJ, Metz GA, Kartje GL, Thallmair M, Schwab ME. Functional Recovery and Enhanced Corticofugal Plasticity after Unilateral Pyramidal Tract Lesion and Blockade of Myelin-Associated Neurite Growth Inhibitors in Adult Rats. J Neurosci. 1998;18:4744–4757. doi: 10.1523/JNEUROSCI.18-12-04744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]