Abstract
DNA vaccines delivered subcutaneously by gene-gun have generated strong protective and therapeutic immunity in rabbits. Recent studies have shown that peptides delivered by the mucosal routes also stimulate local and systemic immune responses. Since mucosal delivery is easier to administer and more cost-effective when compared to gene-gun delivery, we were interested to learn whether mucosally-delivered peptides would prime protective immunity comparable to that of gene-gun delivered DNA in rabbits. Our newly developed HLA-A2.1 transgenic rabbit model was used to test the hypothesis. We chose an HLA-A2.1 restricted cottontail rabbit papillomavirus (CRPV) E1 epitope (E1/303–311, MLQEKPFQL) for the peptide immunization studies because it provided complete protection when used as a DNA vaccine. Adjuvant has been widely used to boost immunity for vaccines. In this study, three adjuvants reported to be effective for rabbits (TT helper motif, PADRE and CpG2007) were tested with the peptide vaccine. Peptide alone or fused to TT helper or PADRE to create chimeric peptides was delivered by two mucosal routes (ocular and intranasal) together. Partial protection was found in HLA-A2.1 transgenic rabbits when peptide was delivered mucosally in the presence of adjuvant. When a subsequent booster of a half dose of the corresponding DNA vaccine was delivered, complete protections were achieved. We conclude that mucosal peptide immunization can be combined with a single DNA vaccination to provide strong protective immunity in rabbits.
Keywords: peptide delivery, immunization, CRPV, HHD mice, HLA-A2.1 transgenic rabbits, DNA vaccine, mucosal delivery, adjuvant, TT helper, CpG motif, PADRE, immunogenicity, CTL
1. Introduction
Peptide immunization has been shown to be effective in stimulating T cell mediated immune responses in the mouse model [1, 2]. Using an HLA-A2.1 transgenic mouse model, investigators have identified numerous HLA-A2.1 restricted epitopes from different pathogens and proteins that have shown strong immunogenicity using this immunization strategy[3–10]. Some of these epitopes were later confirmed in their natural infection hosts and have become promising prophylactic and therapeutic vaccine candidates[9, 11, 12]. We and others have studied papillomavirus immunology in a rabbit model and successfully stimulated both strong protective and therapeutic immunity using DNA vaccination strategies[13–17]. A helium-driven gene gun delivery system has proven to be the most effective delivery vehicle for DNA vaccines when compared with intramuscular injection or biojector [18]. However, the cost associated with the gene gun system has limited its application in the clinic. We hypothesized that peptide immunization would work in our rabbit model with some modifications.
We have established an HLA-A2.1 transgenic rabbit model to facilitate studies on human pathogens to which the rabbit is also susceptible [19, 20]. We have demonstrated that a multivalent DNA vaccine containing five HLA-A2.1 restricted epitopes from cottontail rabbit papillomavirus (CRPV) E1 was immunogenic and protective for these transgenic rabbits [19]. We further demonstrated that one of the epitopes (E1/303–311, MLQEKPFQL) provided complete protection when applied as a DNA vaccine [21]. In the study described here, we chose CRPVE1/303–311 for the peptide immunization test. Adjuvants have been used to augment the immune responses [22–26]. Several adjuvants have been found to be effective for rabbits. Among them,1) a TT helper motif (Tetanus Toxin 830–844, QYIKANSKFIGIT ELMLQEKPFQL); 2) a pan-HLA-DR-binding T-helper epitope [PADRE965.10, AK(X) VAAWTLKAAKMLQEKPFQL] and 3) CpG2007 ODNs (TCGTCGTTGTCGTTT TGTCGTT) have been tested in combination with DNA or peptide immunization and have shown promise in boosting immunity in rabbits[23–25]. In addition, peptide fused to adjuvant to form a chimeric peptide has been shown to be as effective as mixing peptide and adjuvant [24–26]. We therefore tested two chimeric CRPVE1/303–311 peptides by fusing them at the C-terminus of either TT helper motif or PADRE.
A series of experiments were conducted to adapt the peptide immunization method to our rabbit model. Different delivery routes such as intradermal and mucosal (including intranasal and ocular) [27–30], and different adjuvants such as TT helper, PADRE and CpG2007 as well as combined immunization strategies such as peptide and DNA vaccine were investigated [27]. Our data suggested that both an appropriate adjuvant and a well-chosen delivery route were critical for effective peptide immunization. We found that peptide delivered by mucosal routes such as intranasal and ocular provided substantial protective immunity. TT helper appeared to be a stronger adjuvant than PADRE when immunizations were done with peptide; however, no significant difference was found between these two adjuvants when the peptide immunization was combined with DNA vaccination in rabbits. Peptide priming with single half dose DNA booster immunization achieved protection comparable to DNA vaccination alone.
2. Materials and Methods
2.1 Animals
Four to six week old HLA-A2.1 transgenic and non-transgenic sibling rabbits were maintained in the animal facilities of the Pennsylvania State University College of Medicine. All animal care and handling procedures were approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University College of Medicine. The expression of HLA-A2.1 on the rabbit cell surface was confirmed with immunohistochemistry or fluorescent flow cytometry analysis. The HLA-A2.1 gene has been stably passed on to EIII/JC inbred offspring for ten generations without diminishing expression level [31].
2.2 Peptides, adjuvants and DNA vaccines
All the peptides were synthesized in the core facility of Pennsylvania State University College of Medicine. Peptides include the test peptide CRPVE1/303–311(MLQEKPFQL) and three HLA-A2.1 restricted control peptides 1) CRPVE6/ 93–101 (DLVDLGPGV); 2) HIVGagP17/77–85(SLYNTVATL) and 3)HPV16E7/82–90 (LLMGTLGIV); three adjuvant peptides: 1)HBV core T helper peptide (TPPAYRP PNAPIL); 2)TT helper motif (QYIKANSKFIGITELMLQEKPFQL) and 3) PADRE [AK(X)VAAWTLKAAKMLQEKPFQL]; and four chimeric peptides 1) CRPVE1/303–311 fused at the C-terminus of TT helper motif and 2) CRPV E1/303–311 fused at the C-terminus with PADRE and two control chimeric peptides containing 1) HPV16E7/82–90 fused at the C-terminus of TT helper motif and 2) HPV16E7/82–90 fused at the C-terminus with PADRE (Table 1).
Table 1.
Chimeric peptide composition and amino acid sequences
| Peptide ID | Chimeric peptide composition | AA sequences |
|---|---|---|
| NDCPe15 | TT helper +CRPVE1/303–311 | QYIKANSKFIGITELMLQEKPFQL |
| NDCPe16 | PADRE +CRPVE1/303–311 | AK(X)VAAWTLKAAAMLQEKPFQL |
| NDCPe17 | TT helper +HPV16E7/82–90 | QYIKANSKFIGITELLLMGTLGIV |
| NDCPe18 | PADRE +HPV16E7/82–90 | AK(X)VAAWTLKAAALLMGTLGIV |
X indicates cyclohexylalanine
Adjuvant CpG2007 motif ODNs (TCGTCGTTGTCGTTTTGTCGTT) was purchased from Coley pharmaceutical group.
DNA vaccines: Two DNA vaccines containing CRPVE1/303–311 and HPV16E7/82–90 epitopes were generated as described previously [19, 21]. A TT helper motif and an ubiquitin motif were added at the N-terminus and C-terminus of these epitopes respectively. These epitope DNA vaccines have been tested in HLA-A2.1 transgenic rabbits [20].
2.3. T2 binding assay
Four chimeric peptides (HLA-A2.1 restricted peptides CRPVE1/303–311 and HPV16E7/82–90 fused with TT helper or PADRE) were tested for their binding affinity to T2 cells with the protocol described previously [7, 10].
2.4 Peptide and DNA immunization
For peptide immunization, the rabbits did not require anesthesia. For the intradermal delivery, the tested peptides were dissolved at a concentration of 4mg/ml in 1×PBS buffer containing 5% DMSO. HBV core T helper peptide was dissolved at a concentration of 5.6mg/ml in 1×PBS buffer containing 5% DMSO. Each peptide was mixed with HBV core T helper peptide and incomplete Freund’s adjuvant (IFA) at a 1:1:2 (V: V: V) ratio with a homogenizer until the mixture was emulsified [2]. Each mouse was injected with 50 µl of emulsion on both sides of the base of the tail. Each rabbit was injected with 200 µl of emulsion at the back site. HHD mice or HLA-A2.1 transgenic rabbits were immunized for two to four times with a 2-week interval between injections respectively.
For peptide immunization delivered mucosally, 100µg peptide alone or mixed with 100µg adjuvant (TT helper or PADRE) was introduced into the nose and eye by dropper for three times with a two-week interval. One week after the final immunization, the HLA-A2.1 transgenic rabbits were challenged with CRPV DNA.
For peptide and DNA combination vaccinations, 100 µg chimeric peptides mixed with 25µg CpG2007 were delivered by ocular and intranasal routes ; the peptide immunization was followed by a half dose (10µg /rabbit) DNA vaccination. For DNA vaccination, the animals were anesthetized with 40mg/kg Ketamine and 5mg/kg Xylazine. The CRPVE1/303–311 or reference HPV16E7/82–90 epitope DNA plasmid was coated on gold particles and delivered by a helium-driven gene-gun as booster as described previously [10]. The rabbits were challenged with CRPV DNA one week after the DNA vaccination.
2.5 Tetramer binding assay
Rabbit and mouse spleens were harvested and cultured in vitro for T cell assays as reported previously[19, 21]. After two rounds of in vitro stimulations, the bulk CTLs from HLA-A2.1 transgenic mice or rabbits were labeled with corresponding CD8-FITC antibodies and then with specific PE conjugated tetramers (generously provided by the tetramer core facility of the National Institute of Health). A two-color flow cytometry analysis for detecting specific tetramer binding CD8 T cells was performed at the Core Facility of Pennsylvania State University College of Medicine [32].
2.6 Intracellular cytokine assay
Because no commercial anti-rabbit IFN-gamma antibody is available, the intracellular cytokine assay was conducted only for mouse CTLs. Bulk mouse CTLs were cultured in triplicate wells of a 96-well plate with 1µM peptide (either E1 peptides or a reference peptide HIVGagP17/77–85) and 1µM Brefeldin A at 37 °C for 3–4 hours. The cells were then labeled with FITC conjugated anti-mouse CD8 and PE conjugated anti-mouse interferon gamma and analyzed by two-color flow cytometry at the Core Facility of Pennsylvania State University College of Medicine as described previously [19].
2.7 Viral DNA challenge on rabbits
For DNA challenge, the rabbits were anesthetized with 40mg/kg Ketamine and 5mg/kg Xylazine. One week after peptide or DNA vaccination, the rabbits will be challenged with two different CRPV constructs at four left and right back sites respectively according to the method established recently[33]. Several CRPV genomes which displayed distinct phenotypes in our previous studies were used for the studies combining peptide and DNA immunizations. These included 1) wild type CRPV (wtCRPV) which produces progressive papillomas; 2) codon optimized CRPV (identified as coC CRPV which produces more aggressive tumors than does wt CRPV; 3) CRPV with E8 ATG knockout (identified as CRPVE8 ATGko) which produces persistent but slow-growing, small papillomas, and 4) CRPV containing an HLA-A2.1 restricted epitope from HPV16E7 (identified as CRPV/HPV16 E7/82–90). The plasmids were grown up and purified by cesium chloride ultracentrifugation. The rabbit back skin was scarified as described previously [33]. Three days later, each site was challenged with 5µg viral plasmid DNA. Beginning three weeks after DNA challenge, the rabbits were monitored weekly for papilloma development.
2.8 Statistical analysis
Papilloma size was determined by calculating the cubic root of the product of length × width × height of individual papillomas in millimeters to obtain a geometric mean diameter (GMD). Data were represented as the means (± SEMs) of the GMDs for each test group. Statistical significance of papilloma size from different groups was determined by unpaired student t-test comparison between pooled challenge sites on test and control animals (P<0.05 was considered significant). The protection rate was identified by the frequency of tumor free sites and was calculated as the number of sites without papillomas/total challenged sites in a given group of animals. Statistical significance was determined by Fisher’s Exact Test (P<0.05 was considered significant).
3. Results
3.1 Intradermally delivered peptide failed to provide protection against CRPV infection in HLA-A2.1 transgenic rabbits
We demonstrated previously that CRPVE1/303–311 DNA vaccination delivered by gene-gun stimulated complete protection in outbred HLA-A2.1 transgenic rabbits [20]. This epitope was also proven to stimulate strong specific T cell responses in HHD mice following intradermally delivered peptide immunization. We therefore first tested whether this same peptide immunization method could generate strong protective and specific immunity in our HLA-A2.1 transgenic rabbits. Six HLA-A2.1 transgenic and seven normal outbred rabbits were injected intradermally with the emulsion prepared as for previous HHD mouse studies. Each rabbit was immunized four times with 200µl of emulsion (CRPVE/303–311+HBV core in incomplete Freund adjuvant). One week after the final booster immunization, the rabbits were challenged with the two CRPV DNAs that had been used in our previous DNA vaccination study: 1) wild type CRPV DNA (wtCRPV) and CoCRPV DNA [7].
No protection was found in HLA-A2.1 transgenic rabbits against either wtCRPV DNA or coCRPV DNA challenge following peptide immunization. The papilloma size was comparable between HLA-A2.1 transgenic rabbits and normal rabbits (data not shown).
Two factors might contribute to the failure: first, the adjuvant HBV core is an optimal adjuvant for HHD mice but may not be effective for rabbits; second, peptide delivered intradermally might not be the best route for the rabbits. Therefore, the peptide immunization protocol used here, although effective for HHD mice, is not a useful method for stimulating protective cell-mediated immune responses in rabbits.
3.2 Mucosally delivered peptide stimulated strong protective immunity in HLA-A2.1 transgenic rabbits
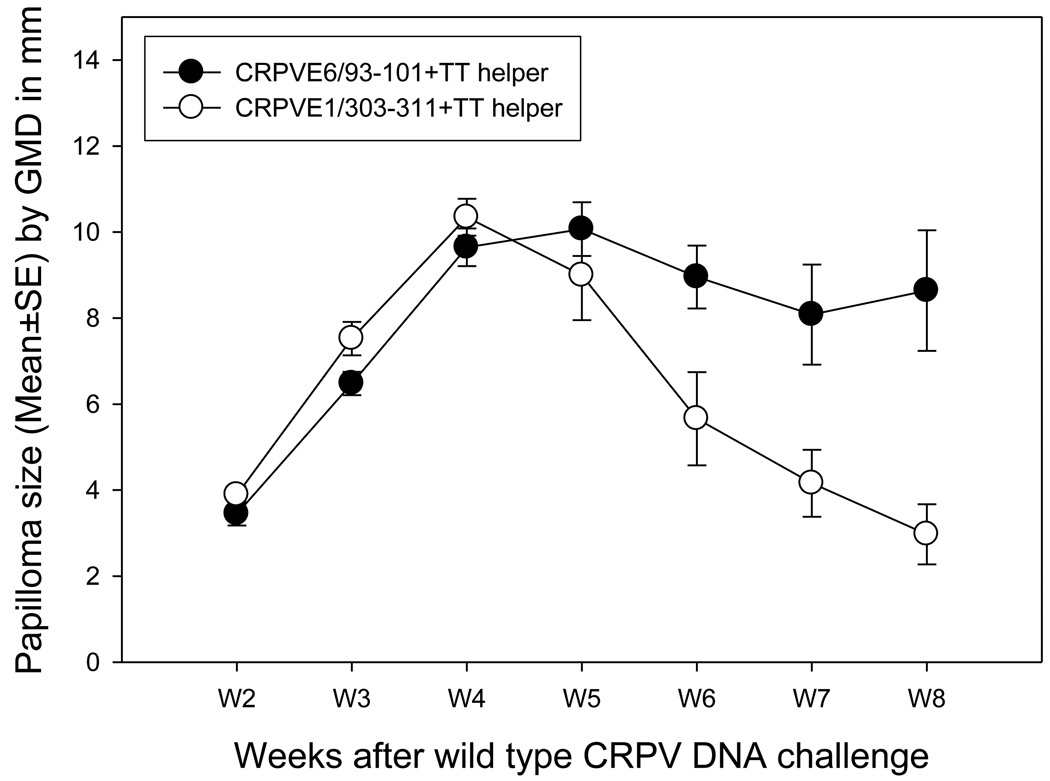
Because peptide mixed with HBV core motif failed to provide protection when delivered intradermally to HLA-A2.1 transgenic rabbits, we wondered whether an alternative strategy might work. We had previously demonstrated that the TT helper motif was an effective adjuvant in our DNA vaccine. We wondered if this adjuvant, when used as a peptide in combination with mucosally delivered peptide might be effective in rabbits. CRPVE1/303–311 and another HLA-A2.1 restricted epitope CRPVE6/93–101, demonstrated to lack immunogenicity in HHD mice when delivered as a peptide (unpublished observation), were used for this experiment. 100µg peptide plus 100µg TT helper peptide were mixed in 1×PBS buffer. Four and five HLA-A2.1 transgenic rabbits were immunized with 50µl of these test or control peptide mixtures to mucosal sites (both nasal and ocular routes) for a total of three times with a two week interval respectively (Table 2). In this experiment, besides wtCRPV, we also challenged rabbits with a CRPVE8ATGko mutant. The rationale for including the CRPVE8ATGko mutant papillomavirus DNA in this study was that this genome produces slow-growing, small papillomas. We hypothesized that the potentially weaker immunity generated by the peptide vaccine would be able to more effectively deal with these smaller tumors. Significantly smaller papillomas were found in CRPVE1/303–311 peptide immunized rabbits when compared to those found in CRPVE6/93–101 peptide group (Figure 1, P<0.05, unpaired student t test) when papillomas were induced by wild type CRPV DNA. Significantly fewer papillomas induced by CRPVE8 ATGko mutant DNA were also found in CRPVE1/303–311 peptide immunized rabbits when compared with the control group (Table 2, P<0.05, Fisher’s exact test).
Table 2.
Protective immunity generated by peptides mixed with TT helper and delivered mucosally(intranasal and ocular) at week 11 following viral CRPV DNA challenge in HLA-A2.1 transgenic rabbits.
| Vaccine | Protection Rate | |
|---|---|---|
| Wild type CRPV DNA | CRPVE8ATGko mutant DNA | |
| TT helper with CRPVE1/303–311 (N=4) |
6/16 (37.5%) a | 15/16 (93.75%) b |
| TT helper with CRPVE6/93–101 (N=5) |
3/20 (15%) | 12/20 (60%) |
P>0.05,
P<0.05 vs. CRPVE6/93–101 peptide immunization group respectively, Fisher’s exact test
Figure 1.
Papilloma outgrowth following peptide mucosal immunization. TT helper motif together with CRPVE1/303–311 or CRPVE6/93–101 was delivered by intranasal and ocular routes to HLA-A2.1 transgenic rabbits (N=5/per group) for three times with a two-week interval between immunizations. A week after the final booster immunization, the rabbits were challenged with wild type CRPV and CRPVE8ATGko mutant DNA at four left and right back sites respectively. The rabbits were monitored for papilloma outgrowth at week 2 after DNA challenge. Comparative growth was found in both immunization groups before week 6. Papillomas on CRPVE1/303–311 immunized rabbits began to regress after week 6 and the papilloma size was significantly smaller when compared with that on CRPVE6/93–101 peptide immunized rabbits (P<0.05, unpaired student t test).
3.3 Chimeric peptides could bind to HLA-A2.1 molecules but failed to stimulate immune responses in HHD mice
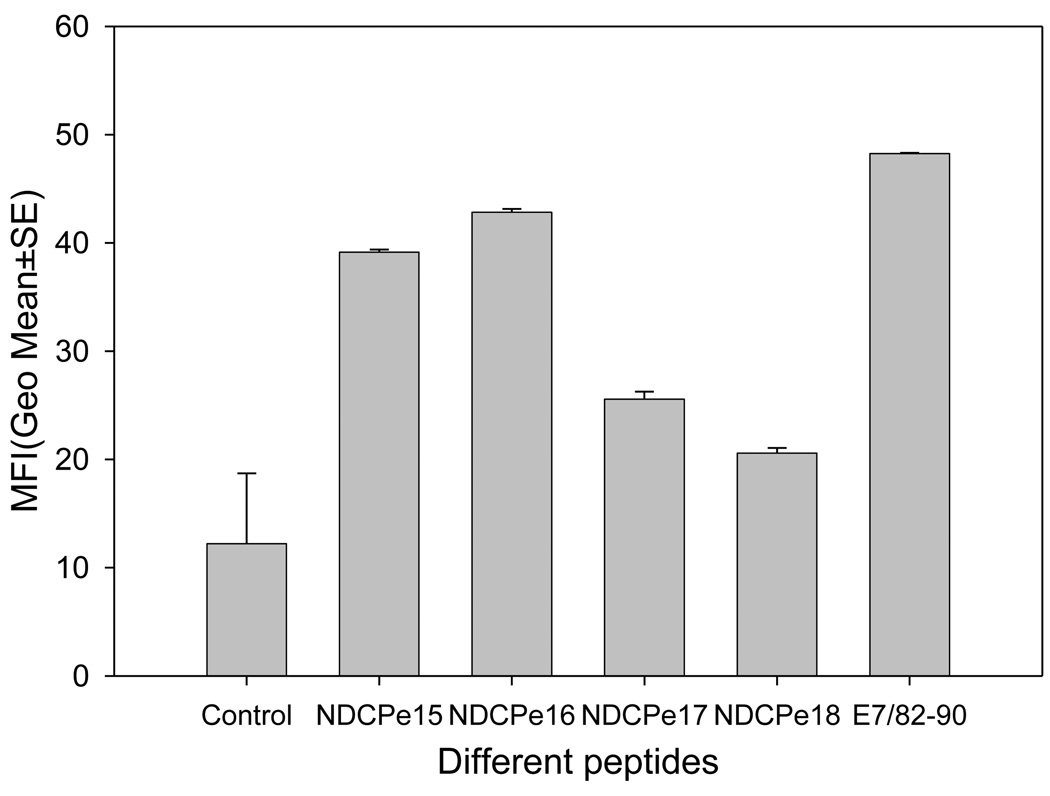
Recent studies demonstrated that fusing adjuvant Th1 helper motif with a peptide could achieve as strong immunity as that induced by mixing them together [24]. To investigate whether this strategy would work well in our rabbit model, the N termini of both CRPVE1/303–311 and HPV16 E7/82–90 were fused to the C-terminus of either TT helper motif or PADRE (a pan-HLA-DR-binding T-helper epitope) (Table 1). These chimeric peptides were tested for their affinity to HLA-A2.1 molecules by T2 binding. Although the binding ability of these chimeric peptides was reduced when compared to that of the original peptides, they were capable of binding (Figure 2, P<0.05 unpaired student t test).
Figure 2.
T2 binding assay for the chimeric peptides. NDCP15–18 represented TT helper fused CRPVE1/303–311, PADRE fused CPRVE1/303–311, TT helper fused HPV16E7/82–90 and PADRE fused HPV16E7/82–90 respectively. HPV16E7/82–90 and medium were positive and negative controls. T2 cells were incubated with each peptide (10µM) and MFI of A2 expression was detected with flow cytometry. All chimeric peptides showed binding although HPV16E7/82–90 chimeric peptides showed decreased affinity to A2 molecules when compared with positive control.
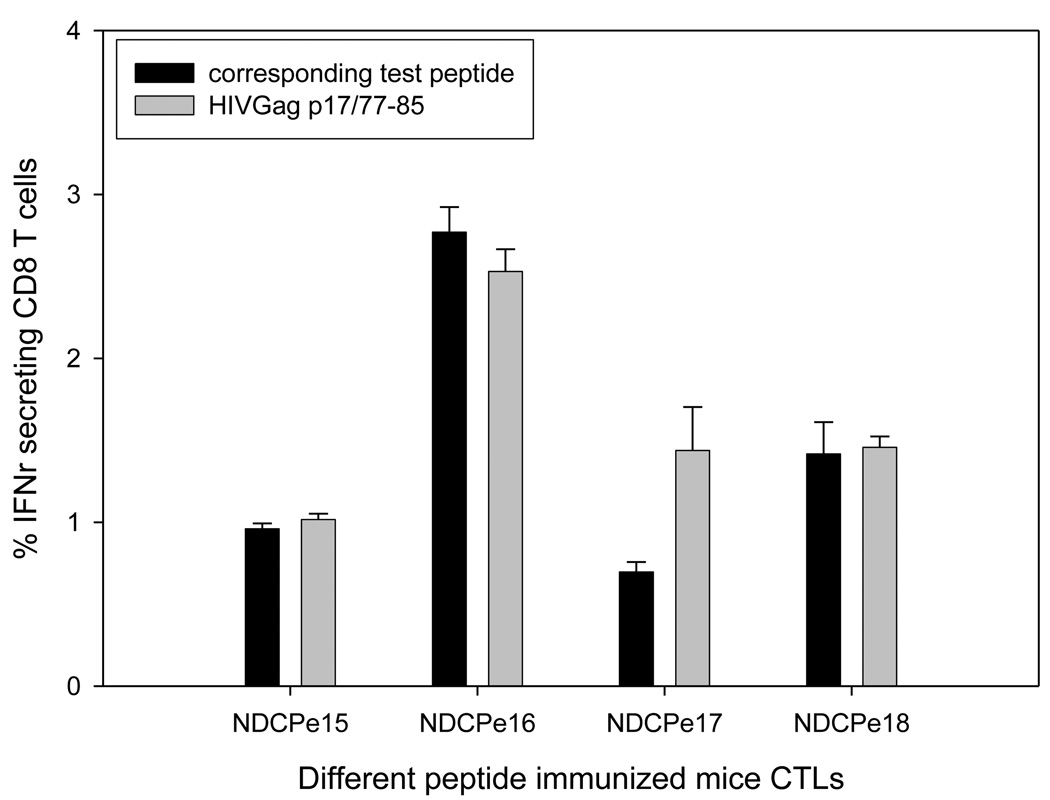
Next, we tested to see if these chimeric peptides could stimulate strong immune responses in HHD mice. Using peptide immunization methods for HHD mice as described above, we failed to detect specific CD8 T cells; no IFN-gamma secreting CD8 T cells were found in mice splenocytes after two rounds of in vitro stimulation (Figure 3, P>0.05 unpaired student t test).
Figure 3.
T cell immune response promoted by chimeric peptide immunization in HLA-A2.1 transgenic mice. NDCP15–18 represented TT helper fused CRPVE1/303–11, PADRE fused CPRVE1/303–311, TT helper fused HPV16E7/82–90 and PADRE fused HPV16E7/82–90 respectively. Two HLA-A2.1 transgenic mice were immunized with each chimeric peptide subcutaneously twice with a two-week interval. One week after booster immunization, spleens were harvested and cultured in vitro with HLA-A2.1 mouse dendritic cells pulsed twice with corresponding peptides. The bulk CTLs were then tested for intracellular interferon gamma secretion. No significant increase in IFNr secreting CD8 T cells was in any of the mice (P>0.05, unpaired student t test) when compared with a reference peptide HIVGagp17/77–85. Therefore, chimeric peptides failed to stimulate specific immune responses in HLA-A2.1 transgenic mice in this study.
We know from our immunization studies that delivery routes play an important role in stimulating strong immunity. For example, as noted above, our peptide immunization in rabbits showed promising results by intranasal and ocular routes. We hypothesized that our failure to achieve immunity via chimeric peptide immunization in HHD mice could have resulted from a suboptimal delivery method. We therefore examined whether nasal delivery of chimeric peptides in HHD mice would provoke a strong immune response.
Two HHD mice were used for each chimeric peptide. After one booster immunization, the spleen cells were harvested and cultured in vitro by stimulation with mouse dendritic cells pulsed with corresponding peptides. No T cell-mediated immune response was detected in these mice (data not shown).
3.4 Mucosally delivered chimeric peptides together with CpG motif stimulated strong and specific immunity in HLA-A2.1 transgenic rabbits
Although the chimeric peptides failed to generate a strong immune response in HHD mice, peptide mixed with TT helper did provoke strong protective immunity in HLA-A2.1 transgenic rabbits. Another promising adjuvant, CpG motif, has been tested in different hosts and shown to augment immune responses [22, 34, 35]. CpG2007 has been shown to be most effective in rabbits [29]. In an attempt to maximize the outcome of our immunization in rabbits, we applied CpG2007 together with our chimeric peptide in the present study.
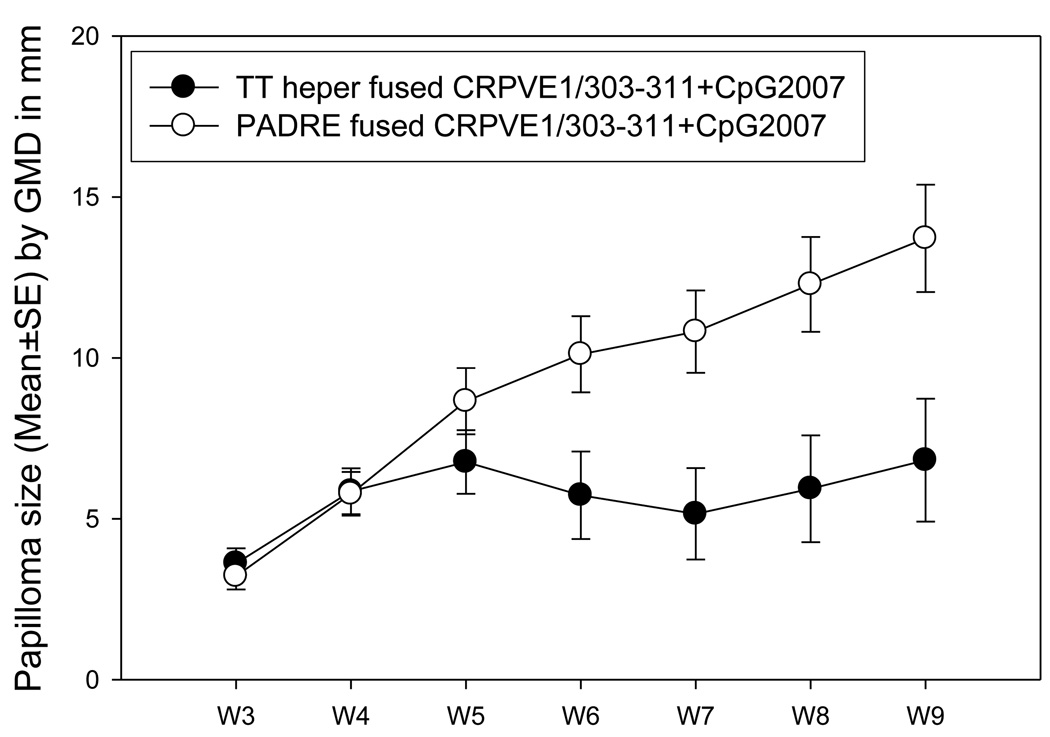
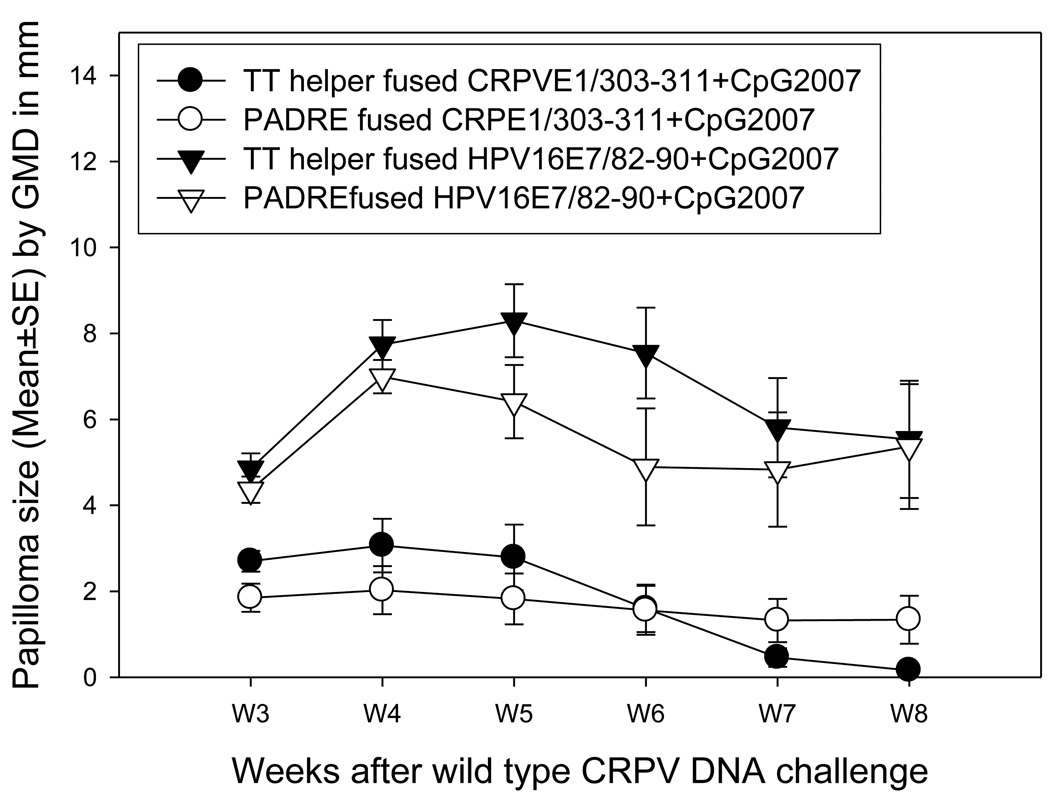
We compared the protective immunity provoked by two chimeric peptides (TThelper vs. PADRE) CRPVE1/303-11 when delivered to HLA-A2.1 transgenic rabbits by mucosal routes (the intranasal and ocular routes) together with CpG2007 at two week intervals for three times. The rabbits were then challenged with wt CRPV DNA. Significantly smaller papillomas were found in rabbits immunized with TT helper fused CRPVE1/303–311 when compared with PADRE fused peptide at week 5 after infection; this trend held until the termination of the experiment (Figure 4, P<0.05, unpaired student t test). Significantly more challenge sites were free of tumors in TT helper fused CRPVE1/303–311 when compared with PADRE fused CRPVE1/303–311 immunized animals (Table 3, P<0.05, unpaired student t test). As discussed, all animals also received the CpG2007 adjuvant. Therefore, TThelper motif plus CpG2007 adjuvant combination provided better protection than that of PADRE motif plus CpG2007 adjuvant combination in our animals.
Figure 4.
Comparison of immunity between two chimeric peptides delivered mucosally in HLA-A2.1 transgenic rabbits. 100µl of TT helper or PADRE fused peptides together with 25µg of CpG2007 were delivered to HLA-A2.1 transgenic rabbits (N=5/per group) by intranasal and ocular route for three times with a two-week interval. A week after the final booster immunization, the rabbits were challenged with wild type CRPV DNA at four back sites. The rabbits were monitored for papilloma outgrowth at week 2 after the infection. Comparable growth was found in both immunization groups before week 6. Papillomas on rabbits immunized with TT helper fused CRPVE1/303–311 peptide then began to grow more slowly and the mean papilloma size was significantly smaller when compared with that on PADRE fused CRPVE1/303–311 peptide immunized rabbits (P<0.05, unpaired student t test).
Table 3.
Protective immunity generated by mucosally delivered TT helper and PADRE chimeric peptide together with CpG2007 at week 9 following wild type CRPV DNA challenge in HLA-A2.1 transgenic rabbits
| Vaccine | Site free of papillomas |
Sites with papillomas |
Protection rate |
|---|---|---|---|
| TT helper fused CRPVE1/303–311 (N=5) |
11 | 9 | 11/20 (55%)a |
| PADRE fused CRPVE1/303–311 (N=5) |
4 | 16 | 4/20 (20%) |
P<0.05 vs. PADRE fused peptide immunization group, Fisher’s exact test
3.5 Complete protective immunity was induced in HLA-A2.1 transgenic rabbits when mucosally delivered chimeric peptide immunization was followed by a single DNA booster vaccination
The above study with chimeric peptides demonstrated that these peptide immunizations delivered mucosally to rabbits provided strong but not complete protection; DNA vaccination by gene gun, on the other hand, provides complete protection when the vaccine is administered a minimum of two times. Our previous data demonstrated that a single time of DNA vaccination was not sufficient to provide protective immunity in rabbits [10]. We wanted to test whether a combined immunization strategy could yield optimal protection in our rabbit model. 19 HLA-A2.1 transgenic rabbits were divided into four groups as shown in tables 4 and 5. The rabbits were immunized with chimeric peptides (both TT-helper and PADRE) plus CpG2007 three times with a two-week interval mucosally by intranasal and ocular routes. One week after final peptide immunization, the rabbits were challenged at left and right back sites respectively with wild type CRPV and a CRPV hybrid DNA containing HPV16E7/82–90 inserted at the end of E7 gene. Three days after DNA challenge, the rabbits were given a corresponding DNA vaccination using half of the normal dose (6µg). Papillomas were monitored and recorded starting three weeks after viral DNA challenge.
Table 4.
Specific protection after combined chimeric peptide and DNA vaccination at week 9 following wild type CRPV DNA challenge in HLA-A2.1 transgenic rabbits
| Vaccine | Site free of papillomas |
Sites with papillomas |
Protection rate |
|---|---|---|---|
| TT helper fused CRPVE1/303–311 (N=5) |
19 | 1 | 19/20 (95%)a |
| PADRE fused CRPVE1/303–311 (N=5) |
15 | 5 | 15/20 (75%)b |
| TT helper fused HPV16E7/82–90 (N=5) |
8 | 12 | 8/20 (40%) |
| PADRE fused HPV16E7/82–90 (N=4) |
6 | 10 | 6/16 (37.5%) |
P<0.01 and
P<0.05 vs. corresponding control group respectively, Fisher’s exact test
Table 5.
Protective immunity generated by both HLA-A2.1 restricted epitopes after combined peptide and DNA vaccination at week 9 following CRPV-HPV16E7/82–90 hybrid DNA challenge
| Vaccine | Site free of papillomas |
Sites with papillomas |
Protection rate |
|---|---|---|---|
| TT helper fused CRPVE1/303–311 (N=5) |
20 | 0 | 20/20 (100%)a |
| PADRE fused CRPVE1/303–311 (N=5) |
16 | 4 | 16/20 (80%)b |
| TT helper fused HPV16E7/82–90 (N=5) |
15 | 5 | 15/20 (75%) |
| PADRE fused HPV16E7/82–90 (N=4) |
9 | 7 | 9/16 (56.25%) |
P<0.05 and
P>0.05 vs. corresponding control group respectively, Fisher’s exact test
Although we failed to detect any tetramer positive CD8 T cells from CRPVE1/303-11 peptide immunized HLA-A2.1 transgenic rabbits (data not shown), significantly smaller papillomas were induced by wild type CRPV DNA challenge in rabbits immunized with CRPVE1/303–311 epitope fused with either TT helper motif or PADRE when compared with those immunized with corresponding fusion HPV16E7/82–90 epitope at all time points (Figure 5, P<0.05, unpaired student t test). Interestingly, most of the papillomas in CRPVE1/303–311 fusion epitope -immunized rabbits regressed by the termination of the experiment (table 4, P<0.05, Fischer’s exact test). No significant difference was found between these two adjuvants in this experiment (Table 4, P<0.05, Fischer’s exact test).
Figure 5.
Papilloma outgrowth after peptide priming/ booster immunization in HLA-A2.1 transgenic rabbits. Nineteen HLA-A2.1 transgenic rabbits were divided into four groups (N=5/per group except four animals for chimeric PADRE -HPVE7/82–90). 100µl of TT helper or PADRE fused peptides together with 25µg of CpG2007 were delivered to HLA-A2.1 transgenic rabbits by intranasal and ocular routes for three times with a two-week interval between immunizations. One week after the final booster immunization, the rabbits were challenged with wild type CRPV and a hybrid CRPV containing HPV16E7/82–90 DNA at left and right back sites. Three days after DNA challenge, the rabbits were given 6 shots of DNA vaccine (half of the normal dose of DNA). The rabbits were monitored for papilloma outgrowth at week 2 after the infection. Significantly smaller papillomas were found in CRPVE1/303–311 peptide immunized rabbits with both adjuvants when compared with corresponding control peptide HPV16E7/82–90(P<0.05, unpaired student t test). No significant difference was found between TT helper vs. PADRE fused CRPVE1/303–311 as found previously noted (P>0.05, unpaired student t test).
3.6 Stronger protective immunity was induced in HLA-A2.1 transgenic rabbits by CRPVE1/303–311 than by HPV16E7/82–90 peptide when mucosally delivered chimeric peptide immunization was followed by a single DNA booster vaccination
CRPV DNA containing HPV16E7/82–90 has two HLA-A2.1 epitopes present in the genome (CRPVE1/303–311 and HPV16E7/82–90). Both CRPVE1/303–311 and HPV16E7/82–90 peptide immunization should provide immunity against this hybrid DNA infection. Consistent with previous results [19, 21], significantly more infection sites (95% sites) on TThelper fused CRPVE1/303–311 immunized rabbits were protected than those on TThelper fused HPV16E7/82–90 immunized rabbits (Table 5, P<0.05, Fischer’s exact test). However, both PADRE chimeric peptides provided comparative protection against this hybrid DNA challenge when compared with TT helper chimeric peptides (Table 5, P>0.05, Fischer’s exact test). This implies that different epitopes on the same genome can be recognized and targeted differentially and also that the choice of adjuvant could contribute significantly to the outcome.
4. Discussion
Gene-gun based DNA vaccination has been very successful in different animal models[13, 18, 36]. Because the delivery route plays such a critical role in outcome of vaccination [37–39], alternative delivery methods have been pursued and tested [22, 40–42]. We have used gene-gun delivery to achieve strong and specific protective and therapeutic immunity in our rabbit papillomavirus model [10, 43]. Our recently established HLA-A2.1 transgenic model has allowed us to target a single epitope that promotes complete protective immunity in animals following DNA vaccination [20]. However, the gene-gun delivery system requires pure gold particles which are expensive. A low-cost vaccine delivery method is desired. Most vaccines are delivered by injection, which increases the risk of infection from HIV, hepatitis, and other serious diseases in areas where sterilization is problematic. Moreover, injecting vaccines can be a complex process, and used syringes and needles create a major waste disposal problem. Therefore, a noninvasive and effective vaccine delivery method is highly desirable for clinical use [44]. Peptide immunization in mice has shown promising results [24, 27, 45]. Mucosal delivery of peptide has been shown to be effective in the ocular HSV/rabbit model [28, 29]. To achieve an optimal result, we tested different adjuvants to augment the protection. Our data suggest that delivery route plays a critical role in the vaccination outcome. Mucosal peptide delivery in combination with DNA vaccination achieved the best result.
Adjuvants are added to many vaccines to increase their immunogenicity and efficacy [22, 46]. Many adjuvants help to trigger innate immunity or to direct Th-1 immunity in the host. While some of the adjuvants show universal effects in all species, others work in a more species-restricted manner [47–49]. In this study, adjuvant (HBV core) that worked well in HHD mice was found not to be effective in rabbits. On the other hand, TT helper and PADRE adjuvants worked much better in rabbits with TT helper showing more potent response in peptide immunization. Previous studies demonstrated that strong Th1 response was generated by mucosally delivery of HSV D-1 multi epitope peptides together with CpG2007 in an ocular HSV/ rabbit model [29]. In our study, we did not test CpG2007 with peptide alone. However, we found that stronger immunity was generated in rabbits when CpG2007 was used together with TT helper motif than with PADRE motif. Therefore, knowledge as to how to formulate more than two adjuvants for a vaccine could impact the outcome significantly. We have also shown that the delivery route used for peptide immunization is important for the outcome. The ocular HSV/rabbit model has demonstrated that mucosal delivery of lipopeptide without adjuvant stimulated strong immunity [28] indicating the mucosal routes are optimal delivery routes for this animal. Our study with the rabbit papillomavirus model further confirmed this observation. In this study, we combined intranasal and ocular routes together for delivery. It would be interesting to find if these two mucosal delivery routes are equivalent in stimulating immune responses for peptide immunization in our animals. We demonstrated that intradermal injection of peptide emulsion did not initiate an immune response in rabbits although it did in mice. However, when we delivered CRPVE1/303–311 peptide together with TT helper peptide mucosally, significant and specific protection was found. Therefore, both adjuvant and delivery route play important roles in the outcome of immune response in vivo.
Long peptides that include CD4 and CD8 epitopes have been demonstrated to be powerful for therapeutic vaccination [50, 51]. Other adjuvants such as CpG-ODN and Th-fusion have also been reported to enhance the immunotherapy effect [24, 52]. In this study, we fused our epitope with TT helper motif or PADRE and tested whether the chimeric products could provide improved immunity in animals. Although these chimeric peptides failed to stimulate a T cell response in HHD mice and rabbits after in vitro stimulation, they did prime strong protective immunity in A2 rabbits when combined with CpG2007. The difference on the immunogenicity of these chimeric peptides in HHD mice and HLA-A2.1 transgenic rabbits might result from the constitution of these two transgenic animals. HHD mice express a chimeric form of HLA-A2.1 with alpha 1 and alpha 2 domains derived from the human HLA-A2 and alpha 3 derived from mouse H-2Db and human beta-2 microglobulin covalently attached [6]. The transgenic rabbits, on the other hand, contain the whole human HLA-A2.1 heavy chain together with rabbit beta-2 microglobulin [19]. The compatibility of the peptide/MHCI complex from these two transgenic animals might influence outcome in terms of immunogenicity. In other words, the A2 rabbit has a better chance to recognize these epitopes when compared with A2 mice. We have demonstrated that some epitopes that are missed by A2 mice can be detected by A2 rabbits. This further confirms the advantage of this novel transgenic rabbit model. In addition, rabbits show higher genetic homology to humans and thus, results generated from this humanized rabbits could be more relevant to the human situation. In summary, chimeric peptide is an additional avenue for peptide based vaccine in rabbits.
Gene gun-delivered DNA vaccination has been shown to provide superior immunity when compared to biojector and syringe injection in mice [18]. Our studies have demonstrated that gene gun- delivered DNA vaccination is effective in protecting rabbits from subsequent viral infection [13, 14, 53–55]. Three immunizations were usually administered to achieve the best protection in our previous studies [45–47]. In one of our most recent studies, we showed that a single DNA immunization failed to provide any protection [10]. In the study presented here, peptide priming followed by a single half-dose DNA booster immunization provided strong and specific protective immunity in rabbits. This suggests that the peptide immunization primed a strong immunity for DNA vaccination because no such strong protection was seen with peptide immunization alone. Because DNA vaccination by gene-gun is more expensive and time consuming than peptide immunization, the combination of peptide priming and low dose gene gun boosting could provide a satisfactory strategy. By combining both delivery methods, we achieved strong protective immunity comparable to that of DNA vaccination alone. Different laboratories have tested numerous prime/boost strategies in attempts to find the best combinations. DNA prime-protein booster has been shown to induce high titers of neutralizing antibody [56]; A DNA priming/ vaccinia booster regime stimulated strong T cell response in vivo [57]. In our study, we found priming with peptide and boosting with DNA could stimulate strong protective immunity in animals. Because both peptide and DNA delivery methods are noninvasive, this combined immunization strategy can be potentially adapted for future clinical trials.
In this study, we also compared two HLA-A2.1 restricted epitopes located on the mutant genome (CRPV containing HPV16E7/82–90) to see how the host immune system responded to each of them. CRPVE1/303–311 has been shown to be a very strong target for host immunity in an earlier study while HPV16E7/82–90 was found to be relatively weaker [19, 21]. We immunized HLA-A2.1 rabbits with either CRPVE1/303–311 or HPV16E7/82–90 and challenged them with CRPV DNA mutant containing HPV16E7/82–90. Our data further confirmed that CRPVE1/303–311 showed significantly stronger protection when compared with HPV16E7/82–90. Therefore, our HLA-A2.1 transgenic rabbit model can not only be used to screen immunogenicity of a certain epitope but can also be used to compare the strength of different epitopes.
In summary, peptides delivered mucosally can prime an immune response in rabbits that, when boosted by a low dose of gene-gun delivered DNA, can provoke strong and specific protective immunity in our HLA-A2.1 transgenic rabbit model. This noninvasive vaccination regime will be attractive for clinical application.
Acknowledgements
This work was supported by the National Cancer Institute grant RO1 CA47622 from the National Institutes of Health and the Jake Gittlen Memorial Golf Tournament.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Carbone FR, Bevan MJ. Induction of ovalbumin-specific cytotoxic T cells by in vivo peptide immunization. J.Exp.Med. 1989;169:603–612. doi: 10.1084/jem.169.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou X, Berg L, Motal UMA, Jondal M. In vivo primary induction of virus-specific CTL by immunization with 9-mer synthetic peptides. J.Immunol.Meth. 1992;153:193–200. doi: 10.1016/0022-1759(92)90322-k. [DOI] [PubMed] [Google Scholar]
- 3.Himoudi N, Abraham JD, Fournillier A, et al. Comparative vaccine studies in HLA-A2.1-transgenic mice reveal a clustered organization of epitopes presented in hepatitis C virus natural infection. J.Virol. 2002;76(24):12735–12746. doi: 10.1128/JVI.76.24.12735-12746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shirai M, Arichi T, Nishioka M, et al. CTL responses of HLA-A2.1-transgenic mice specific for hepatitis C viral peptides predict epitopes for CTL of humans carrying HLA-A2.1. J.Immunol. 1995;154(6):2733–2742. [PubMed] [Google Scholar]
- 5.Le AX, Bernhard EJ, Holterman MJ, et al. Cytotoxic T cell responses in HLA-A2.1 transgenic mice. Recognition of HLA alloantigens and utilization of HLA-A2.1 as a restriction element. J.Immunol. 1989;142(4):1366–1371. [PubMed] [Google Scholar]
- 6.Bernhard EJ, Le AX, Barbosa JA, Lacy E, Engelhard VH. Cytotoxic T lymphocytes from HLA-A2 transgenic mice specific for HLA-A2 expressed on human cells. J.Exp.Med. 1988;168(3):1157–1162. doi: 10.1084/jem.168.3.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kast WM, Brandt RMP, Drijfhout JW, Melief CJM. Human-Leukocyte Antigen-A2.1 Restricted Candidate Cytotoxic T-Lymphocyte Epitopes of Human Papillomavirus Type-16 E6-Protein and E7-Protein Identified by Using the Processing-Defective Human Cell Line-T2. J.Immunother. 1993;14(2):115–120. doi: 10.1097/00002371-199308000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Meng WS, Butterfield LH, Ribas A, et al. Fine specificity analysis of an HLA-A2.1-restricted immunodominant T cell epitope derived from human alpha-fetoprotein. Mol.Immunol. 2000;37(16):943–950. doi: 10.1016/s0161-5890(01)00017-7. [DOI] [PubMed] [Google Scholar]
- 9.Huang YH, Tao MH, Hu CP, Syu WJ, Wu JC. Identification of novel HLA-A*0201-restricted CD8+ T-cell epitopes on hepatitis delta virus. J.Gen.Virol. 2004;85(Pt 10):3089–3098. doi: 10.1099/vir.0.80183-0. [DOI] [PubMed] [Google Scholar]
- 10.Hu J, Cladel N, Peng X, Balogh K, Christensen ND. Protective immunity with an E1 multivalent epitope DNA vaccine against cottontail rabbit papillomavirus (CRPV) infection in an HLA-A2.1 transgenic rabbit model 1. Vaccine. 2008;26(6):809–816. doi: 10.1016/j.vaccine.2007.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mateo L, Gardner J, Chen Q, et al. An HLA-A2 polyepitope vaccine for melanoma immunotherapy. J.Immunol. 1999;163(7):4058–4063. [PubMed] [Google Scholar]
- 12.Wang B, Chen H, Jiang X, et al. Identification of an HLA-A*0201-restricted CD8+ T-cell epitope SSp-1 of SARS-CoV spike protein. Blood. 2004;104(1):200–206. doi: 10.1182/blood-2003-11-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han R, Reed CA, Cladel NM, Christensen ND. Immunization of rabbits with cottontail rabbit papillomavirus E1 and E2 genes: protective immunity induced by gene gun-mediated intracutaneous delivery but not by intramuscular injection. Vaccine. 2000;18(26):2937–2944. doi: 10.1016/s0264-410x(00)00110-9. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Han R, Cladel NM, Pickel MD, Christensen ND. Intracutaneous DNA vaccination with the E8 gene of cottontail rabbit papillomavirus induces protective immunity against virus challenge in rabbits. J.Virol. 2002;76(13):6453–6459. doi: 10.1128/JVI.76.13.6453-6459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu J, Cladel NM, Wang Z, Han R, Pickel MD, Christensen ND. GM-CSF enhances protective immunity to cottontail rabbit papillomavirus E8 genetic vaccination in rabbits. Vaccine. 2004;22(9–10):1124–1130. doi: 10.1016/j.vaccine.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Sundaram P, Tigelaar RE, Xiao W, Brandsma JL. Intracutaneous vaccination of rabbits with the E6 gene of cottontail rabbit papillomavirus provides partial protection against virus challenge. Vaccine. 1998;16(6):613–623. doi: 10.1016/s0264-410x(97)84510-0. [DOI] [PubMed] [Google Scholar]
- 17.Christensen ND, Han R, Cladel NM, Pickel MD. Combination treatment with intralesional cidofovir and viral-DNA vaccination cures large cottontail rabbit papillomavirus-induced papillomas and reduces recurrences. Antimicrob.Agents Chemother. 2001;45(4):1201–1209. doi: 10.1128/AAC.45.4.1201-1209.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trimble C, Lin CT, Hung CF, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21(25–26):4036–4042. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 19.Hu J, Peng X, Schell TD, et al. An HLA-A2.1-Transgenic Rabbit Model to Study Immunity to Papillomavirus Infection. J.Immunol. 2006;177(11):8037–8045. doi: 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- 20.Chentoufi AA, Dasgupta G, Christensen ND, et al. A Novel HLA (HLA-A*0201) Transgenic Rabbit Model for Preclinical Evaluation of Human CD8+ T Cell Epitope-Based Vaccines against Ocular Herpes. J.Immunol. 2010;184(5):2561–2571. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Schell TD, Peng X, Cladel NM, Christensen ND. Strong and Specific Protective and Therapeutic Immunity Induced by Single HLA-A2.1 Restricted Epitope DNA Vaccine in Rabbits. Procedia in Vaccinology. :4–14. 1-1-2009. [Google Scholar]
- 22.Pichichero ME. Improving Vaccine Delivery Using Novel Adjuvant Systems. Hum.Vaccin. 2008;4(4) doi: 10.4161/hv.4.4.5742. [DOI] [PubMed] [Google Scholar]
- 23.Rosa DS, Tzelepis F, Cunha MG, Soares IS, Rodrigues MM. The pan HLA DR-binding epitope improves adjuvant-assisted immunization with a recombinant protein containing a malaria vaccine candidate. Immunol.Lett. 2004;92(3):259–268. doi: 10.1016/j.imlet.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Daftarian P, Ali S, Sharan R, et al. Immunization with Th-CTL fusion peptide and cytosine-phosphate-guanine DNA in transgenic HLA-A2 mice induces recognition of HIV-infected T cells and clears vaccinia virus challenge. J.Immunol. 2003;171(8):4028–4039. doi: 10.4049/jimmunol.171.8.4028. [DOI] [PubMed] [Google Scholar]
- 25.Malyala P, Chesko J, Ugozzoli M, et al. The potency of the adjuvant, CpG oligos, is enhanced by encapsulation in PLG microparticles. J.Pharm.Sci. 2008;97(3):1155–1164. doi: 10.1002/jps.21065. [DOI] [PubMed] [Google Scholar]
- 26.Kim TG, Kim CH, Won EH, et al. CpG-ODN-stimulated dendritic cells act as a potent adjuvant for E7 protein delivery to induce antigen-specific antitumour immunity in a HPV 16 E7-associated animal tumour model 543. Immunology. 2004;112(1):117–125. doi: 10.1111/j.1365-2567.2004.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawana K, Kawana Y, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Nasal immunization of mice with peptide having a cross-neutralization epitope on minor capsid protein L2 of human papillomavirus type 16 elicit systemic and mucosal antibodies. Vaccine. 2001;19(11–12):1496–1502. doi: 10.1016/s0264-410x(00)00367-4. [DOI] [PubMed] [Google Scholar]
- 28.BenMohamed L, Belkaid Y, Loing E, Brahimi K, Gras-Masse H, Druilhe P. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur.J.Immunol. 2002;32(8):2274–2281. doi: 10.1002/1521-4141(200208)32:8<2274::AID-IMMU2274>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine. 2005;23(7):873–883. doi: 10.1016/j.vaccine.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 30.BenMohamed L, Krishnan R, Auge C, Primus JF, Diamond DJ. Intranasal administration of a synthetic lipopeptide without adjuvant induces systemic immune responses. Immunology. 2002;106(1):113–121. doi: 10.1046/j.1365-2567.2002.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu J, Peng X, Budgeon LR, Cladel NM, Balogh KK, Christensen ND. Establishment of a Cottontail Rabbit Papillomavirus/HLA-A2.1 Transgenic Rabbit Model. J.Virol. 2007;81(13):7171–7177. doi: 10.1128/JVI.00200-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schell TD, Lippolis JD, Tevethia SS. Cytotoxic T lymphocytes from HLA-A2.1 transgenic mice define a potential human epitope from simian virus 40 large T antigen. Cancer Res. 2001;61(3):873–879. [PubMed] [Google Scholar]
- 33.Cladel NM, Hu J, Balogh K, Mejia A, Christensen ND. Wounding prior to challenge substantially improves infectivity of cottontail rabbit papillomavirus and allows for standardization of infection. J.Virol.Methods. 2008;148(1–2):34–39. doi: 10.1016/j.jviromet.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klinman DM, Yamshchikov G, Ishigatsubo Y. Contribution of CpG motifs to the Immunogenicity of DNA Vaccines. J.Immunol. 1998;158:3635–3639. [PubMed] [Google Scholar]
- 35.Gupta K, Cooper C. A review of the role of CpG oligodeoxynucleotides as toll-like receptor 9 agonists in prophylactic and therapeutic vaccine development in infectious diseases. Drugs R.D. 2008;9(3):137–145. doi: 10.2165/00126839-200809030-00001. [DOI] [PubMed] [Google Scholar]
- 36.Pertmer TM, Eisenbraun MD, McCabe D, Prayaga SK, Fuller DH, Haynes JR. Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine. 1995;13(15):1427–1430. doi: 10.1016/0264-410x(95)00069-d. [DOI] [PubMed] [Google Scholar]
- 37.Fynan EF, Webster RG, Fuller DH, Haynes JR, Santoro JC, Robinson HL. DNA vaccines - protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc.Natl.Acad.Sci.USA. 1993;90(24):11478. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat.Biotechnol. 2000;18(1):33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 39.Han R, Reed CA, Cladel NM, Christensen ND. Intramuscular injection of plasmid DNA encoding cottontail rabbit papillomavirus E1, E2, E6 and E7 induces T cell-mediated but not humoral immune responses in rabbits. Vaccine. 1999;17(11–12):1558–1566. doi: 10.1016/s0264-410x(98)00356-9. [DOI] [PubMed] [Google Scholar]
- 40.Simerska P, Moyle PM, Olive C, Toth I. Oral vaccine delivery - new strategies and technologies. Curr.Drug Deliv. 2009;6(4):347–358. doi: 10.2174/156720109789000537. [DOI] [PubMed] [Google Scholar]
- 41.Christensen D, Agger EM, Andreasen LV, Kirby D, Andersen P, Perrie Y. Liposome-based cationic adjuvant formulations (CAF): past, present, and future. J.Liposome Res. 2009;19(1):2–11. doi: 10.1080/08982100902726820. [DOI] [PubMed] [Google Scholar]
- 42.Liu XS, Abdul-Jabbar I, Qi YM, Frazer IH, Zhou J. Mucosal immunisation with papillomavirus virus-like particles elicits systemic and mucosal immunity in mice. Virology. 1998;252(1):39–45. doi: 10.1006/viro.1998.9442. [DOI] [PubMed] [Google Scholar]
- 43.Christensen ND. Cottontail rabbit papillomavirus (CRPV) model system to test antiviral and immunotherapeutic strategies. Antivir.Chem.Chemother. 2005;16(6):355–362. doi: 10.1177/095632020501600602. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan VJ, Mikszta JA, Laurent P, Huang J, Ford B. Noninvasive delivery technologies: respiratory delivery of vaccines. Expert.Opin.Drug Deliv. 2006;3(1):87–95. doi: 10.1517/17425247.3.1.87. [DOI] [PubMed] [Google Scholar]
- 45.Itoh T, Celis E. Transcutaneous immunization with cytotoxic T-cell peptide epitopes provides effective antitumor immunity in mice. J.Immunother. 2005;28(5):430–437. doi: 10.1097/01.cji.0000171289.78495.b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell MF, Newman MJ, editors. Vaccine Design: the subunit and adjuvant approach. New York: Plenum Press; 1995. [Google Scholar]
- 47.Stanley MA. Imiquimod and the imidazoquinolones: mechanism of action and therapeutic potential. Clin.Exp.Dermatol. 2002;27(7):571–577. doi: 10.1046/j.1365-2230.2002.01151.x. [DOI] [PubMed] [Google Scholar]
- 48.Velders MP, Weijzen S, Eiben GL, et al. Defined flanking spacers and enhanced proteolysis is essential for eradication of established tumors by an epitope string DNA vaccine. J.Immunol. 2001;166(9):5366–5373. doi: 10.4049/jimmunol.166.9.5366. [DOI] [PubMed] [Google Scholar]
- 49.Sokolovska A, Hem SL, HogenEsch H. Activation of dendritic cells and induction of CD4(+) T cell differentiation by aluminum-containing adjuvants. Vaccine. 2007;25(23):4575–4585. doi: 10.1016/j.vaccine.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 50.Welters MJ, Kenter GG, Piersma SJ, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin.Cancer Res. 2008;14(1):178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 51.Kenter GG, Welters MJ, Valentijn AR, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin.Cancer Res. 2008;14(1):169–177. doi: 10.1158/1078-0432.CCR-07-1881. [DOI] [PubMed] [Google Scholar]
- 52.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Van der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur.J.Immunol. 2008;38(4):1033–1042. doi: 10.1002/eji.200737995. [DOI] [PubMed] [Google Scholar]
- 53.Han R, Cladel NM, Reed CA, Peng X, Christensen ND. Protection of rabbits from viral challenge by gene gun-based intracutaneous vaccination with a combination of cottontail rabbit papillomavirus E1, E2, E6, and E7 genes. J.Virol. 1999;73(8):7039–7043. doi: 10.1128/jvi.73.8.7039-7043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Han R, Cladel NM, Reed CA, et al. DNA vaccination prevents and/or delays carcinoma development of papillomavirus-induced skin papillomas on rabbits. J.Virol. 2000;74:9712–9716. doi: 10.1128/jvi.74.20.9712-9716.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu J, Cladel NM, Budgeon LR, Reed CA, Pickel MD, Christensen ND. Protective cell-mediated immunity by DNA vaccination against Papillomavirus L1 capsid protein in the Cottontail Rabbit Papillomavirus model. Viral Immunol. 2006;19(3):492–507. doi: 10.1089/vim.2006.19.492. [DOI] [PubMed] [Google Scholar]
- 56.Wang S, Pal R, Mascola JR, et al. Polyvalent HIV-1 Env vaccine formulations delivered by the DNA priming plus protein boosting approach are effective in generating neutralizing antibodies against primary human immunodeficiency virus type 1 isolates from subtypes A, B, C, D and E. Virology. 2006;350(1):34–47. doi: 10.1016/j.virol.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 57.Chen C, Wang T, Hung C, Pardoll DM, Wu T. Boosting with recombinant vaccinia increases HPV-16 E7-specific T cell precursor frequencies of HPV-16 E7-expressing DNA vaccines. Vaccine. 2000;18(19):2015–2022. doi: 10.1016/s0264-410x(99)00528-9. [DOI] [PubMed] [Google Scholar]