SUMMARY
Background
While the deleterious psychosocial and mental health effects of dementia caregiving are firmly established, very little is known about the burdens or psychiatric outcomes of providing care to a spouse with less severe cognitive impairment, such as mild cognitive impairment (MCI). We characterized the nature and level of caregiver burden and psychiatric morbidity in spouses of persons diagnosed with MCI.
Methods
Interview assessments were completed on a cohort of 27 spouses of persons with a recent diagnosis of MCI. Patient medical records were reviewed to collect information regarding the MCI patient’s medical history.
Results
Respondents endorsed elevated levels of both task-related responsibilities and subjective caregiver burden. Depression and anxiety symptom levels also showed some elevations. Measures of caregiver burden were significantly associated with depression and anxiety levels. In particular, even after controlling for demographic risk factors for distress, nursing task burden was correlated with elevated depressive symptoms, and greater lifestyle constraints were correlated with higher anxiety levels.
Conclusion
Although caregiver burden and psychiatric morbidity levels were lower than those typically observed in family dementia caregiving samples, our findings suggest that MCI caregivers have already begun to experience distress in association with elevated caregiving burden. These individuals may be ideal targets for selective preventive interventions to maximize their psychological well-being as caregiving burdens related to their spouses’ cognitive impairment increase.
Keywords: mild cognitive impairment, MCI caregiving, caregiver burden
INTRODUCTION
As the prevalence of cognitively impaired older adults increases in the near future, the number of family members who adopt caregiving roles is also expected to rise (Alzheimer’s Association, 1998). The insidious nature of symptom onset and progression in persons with dementing disorders often necessitates that family members assume the role of dementia caregiver without identifying themselves as such until the middle stages of the disease. In support of this, studies show that dementing disorders (e.g. Alzheimer’s disease) are typically diagnosed from two to five years after the emergence of cognitive impairment (Schulz, 2001). The subtle onset and progression of caregiving responsibilities may be especially salient for spousal caregivers (vs adult child care-givers) of persons with dementia because caregiving grows naturally out of prior patterns of support and assistance that are exchanged between married individuals (Montgomery and Kosloski, 1994; Seltzer and Li, 2000).
While the stressors or burden associated with dementia caregiving are well-established (Zarit et al., 1986; Chappell and Penning, 1996; Dempsey and Baago, 1998; Donaldson and Burns, 1999; Burns and Rabins, 2000), little is known about the range of stressors inherent in living with or providing care to a spouse with mild cognitive impairment (MCI). Yet, empirical identification of key MCI caregiver stressors is important because the population of elderly individuals with MCI is growing, in part, due to better diagnostic tools for this condition (Kiovisto et al., 1995; Hanninen et al., 1996; Schroder et al., 1998). Information regarding the burden and the potential mental health impact on spousal caregivers of MCI patients will help us understand if these outcomes are distinct from those observed in spouses of persons with diagnosable dementia syndromes such as Alzheimer’s disease. Unique aspects of an MCI diagnosis that may provoke distress and perceptions of burden include the prognostic uncertainty of the diagnosis and the caregiver’s need to anticipate their spouse’s future care needs, ensure the health and safety of their spouse in light of their mild cognitive deficits, and provide emotional support to their spouse. Individuals who may have long provided daily support and advice to their spouse may experience significant emotional upheaval as their ‘normal’ care for the individual evolves to that required in the face of increasing cognitive limitations.
In the absence of empirical data, however, it is not possible to know whether these caregivers require assistance adjusting to this potential stressor and whether perceived caregiver burden is associated with emotional distress in these caregivers. Therefore, the purpose of this study was to systematically describe the level and nature of perceived caregiver burden, as well as the extent to which depressive and anxiety symptoms are experienced by spouses of persons recently diagnosed with MCI. Drawing on conceptual models of the chronic stress process linked to caregiving, and its impact on caregiver morbidity in the context of patient dementia (Pruchno and Resch, 1989; Neundorfer, 1991; Schulz et al., 1995; Wijeratne, 1997), we hypothesized that the subjects would endorse both objective and subjective caregiver burden and that both mean distress levels and the proportions of subjects exceeding cut-points for potentially clinically significant distress would be elevated in spouses of patients with MCI, relative to normative population values. To the extent that caregiver burden is associated with heightened risk for caregiver psychiatric morbidity (Schulz et al., 1995), this information may contribute to our understanding of the evolution of psychiatric morbidity among elderly caregivers of persons with cognitive impairment.
METHOD
Design
This was a cross-sectional, descriptive correlational study.
Respondents
Eligible respondents were spousal caregivers of individuals diagnosed with MCI (both MCI-amnestic and MCI-other, based on ICD-10 diagnostic criteria) in the six months prior to the start of data collection, and new cases diagnosed during the subsequent seven months of data collection. All were recruited from the University of Pittsburgh Alzheimer’s Disease Research Center (ADRC) patient and informant Registry. To be entered in the Registry, a patient must have a memory complaint and be accompanied by an informant. Potential Registry members may have been referred by their own physician or may have contacted the ADRC as a result of media presentations or word-of-mouth. To be eligible for the current study, the informants had to be spouses or non-married cohabitating partners of the person with MCI, live with the MCI patient in a community (non-institutional and non-assisted) setting, and be able to understand English. A decision was made to enroll spouses of individuals who had been diagnosed with MCI within the six months prior to study inception because it was unlikely these individuals would have progressed to a dementing disorder within this period of time (Petersen et al., 1999) and their inclusion enhanced the sample size (in fact, none had progressed to dementia by the time data were collected in the present study). Also, subjects in the study were limited to spouses/partners (rather than offspring) because these individuals are most likely to retain the caregiving role in the event that MCI progresses to dementia (Stone et al., 1987).
Over a 13-month time frame (including the six months prior to study start-up), a total of 41 persons were diagnosed with MCI during the ADRC Diagnostic Consensus Conferences. All of these individuals and their spouses or partners were eligible to participate. Of those, 20% (n = 8) did not have a spouse/partner and 14% (n = 6) refused participation. Thus, a total of 27 patients and their spouses provided the data. For purposes of discussion, we refer to the sample as ‘spousal caregivers’, although it is recognized that these individuals do not necessarily identify themselves as caregivers.
Procedure
During the regularly scheduled meeting to discuss outcomes of the ADRC diagnostic evaluation, the ADRC social worker asked spouses of persons with MCI if they were willing to be interviewed about their role as a spouse of a person with MCI. Once written informed consent was given, a trained research associate gathered data in a structured interview format in the subject’s home or other convenient location. Participants were compensated for their time. Information regarding the patient’s status at the time of diagnosis was gathered by extracting data from the ADRC medical records.
Measures
Standard caregiver demographic information (age, gender, race, level of education, employment status, income, number of cohabitants in household) was gathered, along with several measures of objective and subjective MCI caregiver burden and psychiatric symptomatology.
Objective caregiver burden and stressors related to MCI
Three measures assessed elements of the care-givers’ objective tasks and responsibilities.
Responsibilities
Caregivers indicated which of 11 household and personal management tasks (e.g. preparing meals, running errands) and which of eight nursing-related tasks (e.g. helping with medications) they performed for their spouse. This set of items was adapted from the task burden list of Montgomery and associates (Montgomery et al., 1985). If the care-giver endorsed a specific task, follow-up items ascertained if they had ‘always done the task’ or ‘only since their spouse’s memory problems developed’. The total number of household task performed was summed, as was the total number of nursing tasks. Additionally, the proportion of the sample endorsing new responsibility for a specific task (e.g. since their spouse began to show signs of memory impairment) was computed.
Lifestyle constraints (LC)
Montgomery’s Objective Caregiver Burden Scale (Montgomery et al., 1985) was used to determine perceptions of lifestyle constraint related to caregiving responsibilities (e.g. time, privacy, money, and leisure activities). This scale demonstrates sound psychometric properties (Cronbach’s alpha from 0.80 to 0.90) when used with a wide array of family caregiver populations (Sisk, 1999). In the current study, respondents rated eight items concerning the degree to which aspects of their life were affected by their spouse’s memory impairment (1 = little or no restriction; 5 = a large degree of restriction). Internal consistency reliability was adequate only after the elimination of one item (amount of time you have for yourself). The remaining seven items were therefore averaged to create an objective burden index (alpha = 0.80) and individual responses were explored to describe which aspects of life are most affected by their role as caregiver.
MCI-behavioral stressors
Respondents reported on their spouse’s behavioral status with the Memory and Behavior Problem Checklist (MBPC; Zarit and Zarit, 1987). The MBPC inquires about the frequency of 30 behavioral problems commonly exhibited by individuals with dementia, as well as the extent of the caregiver’s reaction to such behaviors (discussed under Subjective Caregiver Burden section below). Reliability alphas of the RMBPC range from 0.84 to 0.90 when used with older adults (Teri et al., 1992). Item responses on the frequency component of the MBPC are rated on a six-point scale (0 = never occurred to 5 = occurs daily or more often). An index was computed to reflect the average frequency of behavioral problems exhibited by the care recipient. Individual item responses were also explored to determine which behaviors were most frequently encountered by MCI caregivers.
Subjective caregiver burden related to MCI
Two measures were used to assess elements of the respondents’ burden associated with the samples’ new care-giving responsibilities.
Subjective caregiver burden
We utilized the Subjective Caregiver Burden Scale (SBS et al., 1980) as an index of subjective burden because it demonstrates sound psychometric properties when used in a variety of caregiving contexts (reliability alphas ranging from 0.80 to 0.95, Sisk, 1999). The SBS utilizes a series of 13 questions focused on caregiver perceptions of the caregiving role (e.g. ‘I feel it is painful to watch my spouse develop memory problems’, ‘I feel that giving help to my spouse has enabled me to learn new skills’) with responses ranging from 1 = never to 5 = almost always. As with the index of Objective Caregiver Burden, the reliability alpha was adequate only after eliminating one item (‘I feel that I don’t do as much for my spouse as I could or should’). The remaining 12 items were therefore averaged to create a subjective burden index (α = 85). In addition, individual responses were explored to more fully understand the nature of subjective burden in the sample.
Burden associated with MCI-related behaviors
As noted above, the MBPC evaluated the caregiver’s reaction to specific behaviors exhibited by the care recipient (Zarit et al., 1980) and has acceptable reliability when used with older adults (Teri et al., 1992). Caregivers noted how much each specific behavior ‘bothered or upset’ them (0 = not at all, 4 = extremely). This portion of the MBPC demonstrated an internal consistency alpha of 0.82. An index was computed to determine how bothersome, on average, the behaviors were for the spousal caregivers. Individual items were also examined to explore which behaviors were most bothersome.
Psychiatric morbidity
Depression
Depressive symptoms were measured with Center for Epidemiological Studies the–Depression Scale (CES-D; Radloff, 1977). The CES-D was designed to measure depressive symptoms in nonpsychiatric subjects and has been used with spousal dementia caregiving populations (Pruchno and Resch, 1989; Robinson, 1989). Its 20 items are each rated on a four-point response scale corresponding to the frequency of the symptom in the preceding week. A higher total CES-D score indicates a higher level of depressive symptoms. A cutoff score of 16 or greater is indicative of individuals at high risk for clinical depression. We chose the CES-D because of its relatively high internal consistency (Cronbach’s alpha = 0.87 in the present sample) and predictive validity for the diagnosis of depression in family caregiving samples (Bergmanevans, 1994). For each subject, we considered their continuous score, as well as whether they exceeded threshold scores for potentially clinically significant depressive symptomatology.
Anxiety
Symptoms of anxiety were measured using the State portion of the State Trait Anxiety Inventory (STAI; Spielberger, 1983). The STAI-S is a 20-item questionnaire with response options ranging from 1 (not at all or never) to 4 (very much or almost always). Higher scores indicate higher anxiety symptom levels. A cutoff score of 44 or greater is indicative of individuals at high risk for clinically significant anxiety symptoms (Spielberger, 1983). The STAI has been widely used as an anxiety measure in psychological research (Buros, 1978; Schulz and Williamson, 1994) and has acceptable psychometric properties when used with older adults (McDonald and Spielberger, 1983). Directions for the STAI indicate that the inventory may be modified to encompass the time interval of interest to the researcher, so long as the interval is sufficiently recent (Spielberger, 1983). Thus, the directions for administration accommodated administration by the interviewer (rather than self), and the duration of time being probed was changed from ‘right now’ to ‘during the past week’. In this study, the State subscale of the STAI had an internal consistency reliability of 0.90. Continuous STAI State scores were considered for each subject, as well as whether they exceeded threshold scores for potentially clinically significant anxiety symptoms.
Analyses
We initially performed simple descriptive analyses of study variables. We examined not only summary and average scores on the caregiver burden scales, but individual item responses as well, since little is known about their distribution in this particular population. ‘Potential cases’ of clinically significant depression and anxiety were established through predetermined cutoff scores. Product-moment correlation coefficients (Pearson’s r) were computed to determine the direction and the strength of associations between the subjects’ level of depressive or anxiety symptoms and the measures of caregiver burden. Lastly, multivariable linear regression analyses were performed to examine whether burden variables were associated with depression and anxiety symptom levels, after controlling for known demographic correlates of psychiatric morbidity. Given the limited sample size, we used a conservative strategy of including burden variables in the regression analyses only if they showed a moderate to large bivariate association (r ≥ 0.40) with the dependent variable in question (Cohen, 1988). The distribution of variables were evaluated for normality prior to the bivariate and multivariate analyses. Because data related to nursing responsibilities showed a bimodal distribution, these data were dichotomized to indicate responsibility for any (versus none) of the nursing tasks. Depression and anxiety symptom scores were also skewed and, therefore, these data were logarithmically transformed.
RESULTS
Background characteristics of the study group
As shown in Table 1, participants were predominantly cognitively intact Caucasian, women, married (100% heterosexual) for almost five decades, and living alone with their spouse. Respondents ranged in age from age 54 to 82 (M = 70.7, SD = 7.63). Although respondents were relatively highly educated, and one-half of the sample continued to work on a part-time basis (both paid and volunteer work), almost one-fifth (18.5%, n = 5) of the sample reported financial strain.
Table 1.
Sample characteristics (n = 27)
| Characteristic | Caregiver | Spouse with MCI |
|---|---|---|
| Age in years, range (M ± SD) | 54–82 (70.7 ± 7.6) | 59–87 (73.8 ± 7.0) |
| MMSE score, range (M ± SD) | 28–30 (29.7 ± 0.5) | 21–30 (26.4 ± 2.7) |
| Gender, % female (n) | 85.2 (23) | 14.8 (4) |
| Race, % Caucasian (n) | 92.6 (25) | 92.6 (25) |
| Marital Status, % Married (n) | 100 (27) | |
| Years in relationship, range (M ± SD) | 11–58 (44.3 ± 11.4) | |
| Educational Level; % (n) | ||
| ≤High school education | 22.2 (6) | 29.6 (8) |
| ≤Bachelor’s degree | 55.5 (15) | 29.6 (8) |
| >Bachelor’s degree | 22.2 (6) | 40.7 (11) |
| Employment; % (n) | ||
| Homemaker | 22.2 (6) | 3.7 (1) |
| Full time employment | 11.1 (3) | 7.4 (2) |
| Retired/not working | 22.2 (6) | 85.2 (23) |
| Retired/working part time | 40.7 (11) | 0 (0) |
| Disabled | 3.7 (1) | 3.7 (1) |
| Household Income; % (n) | ||
| $10,000 to $19,999/year | 11.1 (3) | |
| $20,000 to $29,999/year | 14.8 (4) | |
| $30,000 to $39,999/year | 25.9 (7) | |
| $40,000 to $49,999/year | 11.1 (3) | |
| >$60,000/year | 33.3 (9) | |
As expected, care recipients were very similar to their spousal caregivers, with the exception of lower scores on the Mini Mental Status Exam. At the ADRC, patients are either diagnosed with ‘amnestic MCI’ [(i.e. deficit in the memory domain) or ‘other MCI’ (deficit noted in one or more domain(s) other than memory, Petersen et al. (2001)]. Almost half (44.4%) of the sample cared for a spouse with amnestic MCI. In addition to a diagnosis of MCI, one-third of the care recipients (33.3%) had a concomitant psychiatric disorder noted in their medical record (e.g. Major Depressive Disorder, in remission) and 7.4% had a concomitant medical diagnosis of cerebrovascular disease (CVD). Care recipients with a concomitant psychiatric diagnosis were almost evenly distributed between the two types of MCI (five had amnestic MCI, four had other MCI), although only care recipients with amnestic MCI had a concomitant medical diagnosis of CVD. Participant characteristics did not vary as a function of the type of MCI diagnosed, or the presence of concomitant psychiatric disorder or CVD in their spouse.
Caregiver burden
As shown in Table 2, caregivers regularly performed an average of four household and 0–1 nursing tasks for the care recipient. The proportion of the total sample endorsing responsibility for a specific task, as well as the proportion of the sub-sample acknowledging that the task was a new responsibility (i.e. since their spouse began to show signs of cognitive impairment), are illustrated in Table 3. All caregivers who reported responsibility for transportation, running errands, and managing business affairs, also stated that these were new responsibilities. Nearly one-half of the sample endorsed being responsible for administering their spouses’ medication and a relatively large proportion of that sub-sample reported that this was a new responsibility. Overall, very few caregivers were responsible for other nursing tasks. However, when such tasks were endorsed, they were new responsibilities. The only tasks not endorsed by the sample were nursing tasks commonly associated with severe functional disability.
Table 2.
Caregiver burden and psychiatric symptoms (n = 27)
| Measure | Scale range | Sample range | Sample Mean (SD) | n (%) Above threshold for potential caseness |
|---|---|---|---|---|
| Objective burden | ||||
| Household management Tasks | 0–11 | 0–10 | 4.07 (2.84) | |
| Nursing tasks | 0–8 | 0–4 | 0.69 (1.12) | |
| Lifestyle constraints | 1–5 | 2.86–4.0 | 3.23 (0.35) | |
| MCI-behavioral stressors | 0–5 | 0.03–2.0 | 0.87 (0.48) | |
| Subjective burden | ||||
| Subjective caregiver burden | 1–5 | 1.25–4.0 | 2.22 (0.68) | |
| Reaction to MCI-related behaviors | 0–4 | 0–1.27 | 0.47 (0.34) | |
| Psychiatric morbidity | ||||
| Depression symptoms | 0–60 | 0–34 | 8.48 (8.87) | 3 (11%) |
| Anxiety symptoms | 20–80 | 23–56 | 29.40 (8.42) | 3 (11%) |
Table 3.
Nature and level of household and nursing responsibilities (n = 27)
| Task | % (n) Endorsing responsibility for task | Of those completing task, % (n) endorsing new responsibility since MCI |
|---|---|---|
| Household | ||
| Laundry | 63.0 (n = 17) | 5.9 (n = 1) |
| Meal preparation | 59.3 (n = 16) | 0 |
| Manage finances | 44.4 (n = 12) | 75 (n = 9) |
| House cleaning | 44.4 (n = 12) | 8.3 (n = 1) |
| Medical decisions | 40.7 (n = 11) | 72.7 (n = 8) |
| Home repairs | 33.3 (n = 9) | 55.6 (n = 5) |
| Transportation | 29.6 (n = 8) | 100 (n = 8) |
| Business affairs | 22.2 (n = 6) | 100 (n = 6) |
| Running errands | 22.2 (n = 6) | 100 (n = 6) |
| Making telephone calls | 22.2 (n = 6) | 66.6 (n = 4) |
| Yard work | 18.5 (n = 5) | 0 |
| Nursing | ||
| Medication administration | 40.7 (n = 11) | 81.8 (n = 9) |
| Bathing | 11.1 (n = 3) | 100 (n = 3) |
| Dressing | 7.4 (n = 2) | 100 (n = 2) |
| Toileting | 3.7 (n = 1) | 100 (n = 1) |
| Walking | 3.7 (n = 1) | 100 (n = 1) |
| Help in/out of bed | 0 | |
| Help in/out of chairs | 0 | |
| Eating | 0 | |
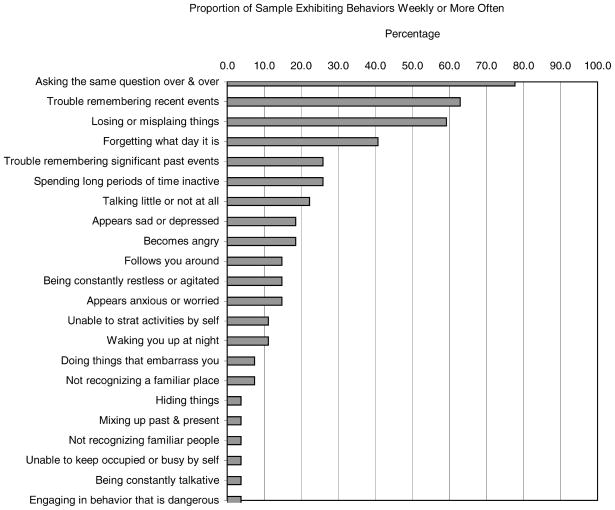
Among the other measures of caregiver burden shown in Table 2, respondents scored above the midpoint on the index of lifestyle constraints. With regards to individual lifestyle constraint items, ‘amount of time to self’ received the highest burden rating (3.63 ± 0.79) and ‘amount of privacy’ and ‘amount of vacation time’ received the lowest burden raring (3.15 ± 0.53), both above the midpoint. Although the mean frequency of MCI-related behavioral stressors was relatively low, Figure 1 shows that certain MCI related behaviors were considerably more common than others. In the Figure, MCI-related behaviors were categorized according to whether they occurred at least weekly. The most commonly endorsed behaviors were asking the same questions over and over again, having trouble remembering recent events, and losing or misplacing things. Respondents did not endorse behaviors indicative of severe cognitive impairment such as ‘not recognizing a familiar object’, ‘unable to find way about outdoors’, or ‘wandering or getting lost easily’. Nor did the sample endorse delusional behaviors (i.e. being ‘suspicious or accusative’) or catastrophic behavioral reactions such as ‘destroying property’ ‘striking out or trying to hit’.
Figure 1.
Proportion of sample endorsing behaviors exhibited by care recipient, weekly or more often
Respondents’ ratings of subjective caregiver burden averaged near to below the midpoint of the scales. However, on the Zarit subjective burden measure (Zarit et al., 1980), some items were rated as more burdensome than others. For example, the statement, ‘I feel it is painful to watch my spouse develop memory problems’ received the highest average burden rating (3.41 ± 0.97), while ‘I feel pleased with my relationship with my spouse’ received the lowest burden rating (1.59 ± 0.89). Concerning respondents’ ratings of their reactions to MCI-related behaviors in their spouse, examination of individual items shows that the most frequently occurring behaviors were also the most distressing (although the mean levels of distress were relatively low). ‘Losing or misplacing things’ received the highest mean rating of stressfulness (1.48 ± 1.16), followed by ‘asking the same questions over and over’ (1.19 ± 0.96) and ‘trouble remembering recent events’ (1.0 ± 0.92).
Psychiatric morbidity
The severity of depressive and anxiety symptoms, as well as the proportion of the sample exceeding cut-points for clinically significant symptoms are also reported in Table 2. Three caregivers (11.1%) exceeded the cut-point for potentially clinically significant depressive symptoms and an equal number surpassed the cut-point for potentially clinically significant anxiety symptoms. Of these highly distressed caregivers, one scored above the cut-point on both the depression and anxiety symptom measures while the additional four potential cases experienced either only high levels of depression (n = 2) or only anxiety (n = 2) symptoms. As a whole, the mean level of depression symptoms in the sample fell within the range for sub-threshold depression in older adults (i.e. operationally defined as a CES-D score of 6–8; Blazer et al., 1991). Closer examination of the CES-D summary scores indicated that 48.1% of the sample (n = 13) endorsed low levels of depressive symptoms (i.e. a summary score ≤ 7), 40.8% (n = 11) endorsed sub-threshold depressive symptom levels, and 11.1% (n = 3) reported potentially clinically significant levels of depressive symptoms (cut score of ≥ 16). While similar cut-scores for sub-threshold anxiety symptoms have not been described in the literature, a relatively large proportion of the sample (88.8%, n = 24) scored ≤ the cut-score of 44, suggesting generally low levels of anxiety symptoms for most respondents.
Associations between psychiatric morbidity, caregiver responsibilities, and caregiver burden
Simple correlations among the measures of caregiver burden, and psychiatric morbidity are displayed in Table 4. Only a higher level of nursing responsibilities was significantly correlated with higher levels of depressive symptoms. In contrast, a number of variables were significantly correlated with symptoms of anxiety, including higher ratings of household responsibilities, lifestyle constraints, and subjective caregiver burden. Neither depression nor anxiety symptoms were significantly correlated with the frequency of MCI-related behaviors in the care recipient or the respondents’ reactions to such behaviors. Further exploration of the bivariate correlations showed that while many of the caregiver burden measures used in this study were interrelated, they were far from completely overlapping.
Table 4.
Pearson Correlations of Key Variables (n = 27)
| Nursing tasks a |
Lifestyle constraint |
MCI-behavioral stressors |
Subjective caregiver burden |
Reaction to MCI-behaviors |
Depression symptomsb |
Anxiety symptomsb |
|
|---|---|---|---|---|---|---|---|
| Household management tasks | 0.473* | 0.677** | 0.609** | 0.421* | 0.536** | 0.171 | 0.393* |
| Nursing tasksa | 0.310 | 0.675** | 0.177 | 0.231 | 0.440* | 0.217 | |
| Lifestyle constraint | 0.550** | 0.566** | 0.501** | 0.201 | 0.432* | ||
| MCI-behavioral stressors | 0.453* | 0.730** | 0.348 | 0.237 | |||
| Subjective caregiver burden | 0.579** | 0.351 | 0.449* | ||||
| Reaction to MCI-behaviors | 0.337 | 0.207 | |||||
| Depression symptomsb | 0.380 | ||||||
| Anxiety symptomsb |
Note: p values are two-tailed.
p <0.05;
p <0.01.
Dichotomized as responsibility for any nursing tasks (yes/no) for bivariate descriptive analyses.
Log10 transformed data used in the analyses.
Unique contributions of demographic variables and caregiver burden to psychiatric morbidity
Demographic variables including gender, age, and education are known to be associated with depression and anxiety (Yee and Schulz, 2000; Blazer, 2003; Dew et al., 2003). Thus, to determine whether the association of caregiver burden with psychiatric morbidity was independent of known demographic risk factors, a separate linear regression analysis was performed for each of the two psychiatric morbidity variables. Each regression analysis included age and education (the majority of our sample were women, precluding our ability to examine gender in more detail). Furthermore, because of our small sample and the exploratory nature of our work, we adopted a very conservative approach to the inclusion of care-giver burden variables in the regression models. Specifically, in order to quality for inclusion in a given regression analysis, a given caregiver burden variable was required to show at least a moderately large zero-order relationship (r ≥ 0.4) with the specific dependent variable. Thus, age, educational level, and nursing responsibilities were entered into the model for depression while age, educational level, lifestyle constraints and subjective caregiver burden were entered into the model for anxiety.
Results of the regression analyses are presented in Table 5. After controlling for the impact of age and education, respondents with nursing responsibilities were significantly more likely to report higher levels of depression symptoms and those with higher levels of lifestyle constraints were more likely to report higher levels of anxiety symptoms.
Table 5.
Regression of psychiatric morbidity on demographic, responsibility, and burden variables
| Dependent variable | Independent variables | β | t statistic | p value |
|---|---|---|---|---|
| Depression symptomsa | Caregiver age | − 0.00 | − 0.02 | 0.985 |
| Caregiver education | − 0.36 | − 2.23 | 0.036 | |
| Nursing responsibilitiesb | 0.65 | 4.03 | 0.001 | |
| r = 0.660, F(3,23) = 5.92, p = 0.004 | ||||
| Anxiety symptomsa | Caregiver age | − 0.44 | − 2.47 | 0.022 |
| Caregiver education | 0.22 | − 1.37 | 0.185 | |
| Lifestyle constraints | 0.47 | 2.27 | 0.033 | |
| Subjective burden | 0.03 | 0.141 | 0.889 | |
| r = 0.663, F(4,22) = 4.32, p = 0.01 |
Note:
Log10 transformed data used in the analyses.
Dichotomized as responsibility for any nursing tasks (yes/no) for multiple regression analyses.
DISCUSSION
As far as we know, this investigation is the first examination of perceived burden and psychiatric morbidity among family caregivers of persons with MCI. Results suggest that even at early stages of cognitive impairment, husbands and wives assume the role of family caregiver and experience both caregiver burden and psychiatric morbidity associated with the role. The areas of caregiving responsibilities were diverse, and included household tasks and some nursing tasks (primarily regarding medications). The fact that many of these responsibilities were new suggests that the tasks may have been directly related to the onset of MCI in their spouse. Indeed, respondents endorsed a variety of lifestyle constraints that they themselves attributed to the cognitive changes in their spouse, and they reported the onset of a variety of behaviors in their spouse that represented changes in functioning. These changes were distressing, although respondents’ subjective perceptions of feeling burdened remained generally low.
Considered in the context of the levels of objective and subjective burden reported by caregivers to persons with dementia (Hooyman et al. 1985; Zarit et al., 1987; Robinson, 1988; Montgomery, 1989; Neundorfer, 1991; Gitlin et al., 2001), it is clear that the present sample’s overall level of burden is much more mild. This is perhaps most obvious in the types of behavioral problems that they reported among their spouses. Many of the severely disruptive behaviors such as waking up at night or an inability to initiate various activities by themselves were not present in spouses with MCI. However, it is striking that even at the stage of MCI, certain behaviors were already prevalent and were sources of concern, including asking the same question repeatedly, difficulty remembering recent events, and losing or misplacing things.
The emotional well-being of the present sample of MCI caregivers appears to lie midway between that observed in community samples of otherwise healthy older adults and that seen in dementia caregivers. Specifically, while mean levels of depressive and anxiety symptoms were lower than the typical levels observed in dementia caregivers (e.g. CES-D scores from 14 to 16, Schulz et al., 1990; Baumgarten et al., 1992; Schulz et al., 1995; Wijeratne, 1997), they were only slightly higher than levels seen in healthy non-caregiving elderly cohorts (e.g. CES-D scores from 7.4 to 9.4, Blazer et al., 1991).
We observed significant associations between several elements of caregiver burden and both depressive and anxiety symptoms in the sample. These associations persisted even after controlling for known demographic correlates of symptomatology, and they suggest important linkages to be explored in future research. First, having greater responsibility for nursing tasks was associated with higher depression levels. The most commonly endorsed nursing task was medication administration. We speculate that it was uniquely linked to depression in these caregivers because this task may have served as a daily reminder of the fact that their spouse had MCI. This is supported by additional evidence of high ratings, on average, on the item indicating that respondents found it painful to watch their spouse develop memory problems.
Second, a higher level of lifestyle constraint was uniquely associated with elevated anxiety symptoms. Since the item, ‘amount of time to self’ received the highest rating in this area; it is possible that respondents experienced a heightened state of arousal (e.g. vigilance) in light of their spouses’ cognitive impairment.
Several limitations of the present study must be borne in mind. First, the size and composition of the sample introduce potential limitations on generalizability to the population of spousal MCI caregivers. Our small sample, although recruited from a clearly defined sampling frame, was predominantly white and the caregiver spouses were mostly female. This reflects the population of patients and spouses in the ADRC subject Registry. Our use of the ADRC subject Registry enhanced the study’s internal validity because it ensured that all individuals were carefully diagnosed with MCI. However, reliance on the Registry also potentially limits the external validity of the results since such individuals (i.e. spouses of individuals who have sought a medical diagnosis and treatment for their memory complaints) may differ from individuals and their spousal MCI caregivers who have not sought care from an ADRC. In addition, the generalizability of our findings may also be reduced by our focus on spousal caregivers, as opposed to other types of family caregivers. However, we chose to focus on spouses because the majority of caregivers to persons with cognitive impairment (including dementia) are female spouses (Grant et al., 1992; Rabins, 1998).
Second, our study’s cross-sectional design limits any conclusions regarding the causal or predictive direction of the caregiver burden-psychiatric morbidity relationship. It is possible that the associations we observed were due to some underlying characteristics of the caregivers or care recipients. It will clearly be important in future work with larger, more heterogeneous samples, to longitudinally examine these relationships in order to more fully characterize the direction of effects and the extent to which the effects are independent of or are influenced by other characteristics of the caregiver and care recipient (e.g. co-morbidities in patient or the patient’s perceptions of his/her own roles and responsibilities) over time and with more heterogeneous samples.
In conclusion, the present study provides three types of evidence that spousal caregivers to persons with MCI are already providing care typically associated with dementia caregiving and are at high risk (12% annual probability) for increasing caregiver burden with concomitant increases in subsequent psychiatric morbidity. First, even at this early stage of cognitive impairment, respondents endorsed elevated caregiving burden levels, although these levels are clearly lower than those endorsed by family dementia caregiving samples. Second, this sample endorsed depressive and anxiety symptoms midway between community and dementia caregiving samples. And third, measures of caregiver burden are significantly associated with depression and anxiety symptoms in this sample.
Given the heightened risk for dementia in persons with MCI, it is likely that many of these MCI care-givers will eventually become dementia caregivers. They may thus be ideal targets for selective preventive interventions to reduce the psychiatric morbidity that is so commonly observed in individuals with dementia caregiving responsibilities. These interventions could be specifically designed to strengthen intrapersonal skills and resources in order to prevent the worsening of psychiatric symptoms as some of these caregivers progress to become dementia caregivers. For example, to the extent that caregiver burden contributes to psychiatric morbidity, interventions designed to promote affective self-management and adaptive coping skills training, implemented very early in the caregiving trajectory, hold promise for contributing to more positive mental health outcomes in these spousal caregivers (Areán et al., 1993; Hosaka and Sugiyama, 1999; Gallagher-Thompson et al., 2000; Hepburn et al., 2001; Alexopoulos, et al., 2003). Self-management interventions, with their focus on promoting self-efficacy (i.e. confidence in one’s ability to undertake behavior in order to accomplish certain goals (Taal et al., 1996) have already been found to improve emotional well-being in a variety of distressed samples of older adults (Tableman, 1987; Rokke et al., 2000; Cutler, 2001). They may be even more useful if administered prophylactically, especially among individuals just entering the caregiver role, as they attempt to adjust to the changing cognitive status of their spouse.
Acknowledgments
This work was supported by the University of Pittsburgh Alzheimer’s Disease Research Center (P50 AG05133) and grants from the National Institute of Mental Health (P30 MH52247, T32 MH19986 and R25 MH60473).
Contract/grant sponsor: University of Pittsburgh Alzheimer’s Disease Research Center; contract/grant number: P50 AG05133.
Contract/grant sponsor: National Institute of Mental Health; contract/grant numbers: P30 MH52247, T32 MH19986 and R25 MH60473.
References
- Alexopoulos GS, Raue P, Areán PA. Problem-solving therapy versus supportive therapy in geriatric major depression with executive dysfunction. Am J Geriatr Psychiatry. 2003;11:46–52. [PubMed] [Google Scholar]
- Alzheimer’s Association. Alzheimer’s Association Releases State Estimates of Boomers by 2050. Aging Res Training News. 1998;21:38–39. [Google Scholar]
- Areán PA, Perri M, Nezu A, Schein R, Christopher F, Joseph T. Comparative effectiveness of social problem-solving therapy as treatments of depression in older adults. J Consult Clin Psychol. 1993;61:1003–1010. doi: 10.1037//0022-006x.61.6.1003. [DOI] [PubMed] [Google Scholar]
- Baumgarten M, Battista RN, Infante-Rivard C, Hanley JA, Becker R, Gauthier S. The psychological and physical health of family members caring for an elderly person with dementia. J Clin Epidemio. 1992;45:61–70. doi: 10.1016/0895-4356(92)90189-t. [DOI] [PubMed] [Google Scholar]
- Bergman-Evans B. A health profile of spousal Alzheimer’s caregivers: depression and physical health characteristics. J Psychosocial Nurs Men Health Serv. 1994;32:25–30. doi: 10.3928/0279-3695-19940901-10. [DOI] [PubMed] [Google Scholar]
- Blazer DG. Depression in late life: review and commentary. J Gerontol. 2003;58:M249–M265. doi: 10.1093/gerona/58.3.m249. [DOI] [PubMed] [Google Scholar]
- Blazer DG, Burchett B, Service C, George L. The association of age and depression among the elderly: an epidemiologic exploration. J Gerontol. 1991;46:M210–M215. doi: 10.1093/geronj/46.6.m210. [DOI] [PubMed] [Google Scholar]
- Burns A, Rabins P. Carer burden in dementia. Int J Geriar Psychiatry. 2000;15:S9–S13. doi: 10.1002/1099-1166(200007)15:1+<::aid-gps160>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Buros OK. The Eighth Mental Measurement Yearbook. Gryphon Press; Hyde Park, NJ: 1978. [Google Scholar]
- Chappell NL, Penning M. Behavioral problems and distress among caregivers of people with dementia. Ageing Soc. 1996;16:57–73. [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Erlbaum Associates; Hillside, NJ: 1988. [Google Scholar]
- Cutler CG. Self-care agency and symptom management in patients treated for mood disorders. Arch Psychiatr Nurs. 2001;15:24–31. doi: 10.1053/apnu.2001.20578. [DOI] [PubMed] [Google Scholar]
- Dempsey M, Baago S. Latent grief: the unique and hidden grief of carers of loved ones with dementia. Am J Alzheimer’s Dis. 1998;13:84–91. [Google Scholar]
- Dew M, Martire L, Hall M. Depression: epidemiology and risk factors. In: Thase M, Potokar J, editors. Advances in the Management and Treatment of Depression. Martin Dunitz/Taylor Francis; London: 2003. pp. 1–39. [Google Scholar]
- Donaldson C, Burns A. Burden of Alzheimer’s disease: helping the patient and caregiver. J Geriatr Psychiatry Neurol. 1999;12:21–28. doi: 10.1177/089198879901200106. [DOI] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Lovett S, Rose J, et al. Impact of psychoeducational interventions on distressed family caregivers. J Clin Geropsychol. 2000;6:91–110. [Google Scholar]
- Gitlin LN, Cocoran M, Winter L, Boyce A, Hauck WW. A randomized, controlled trial of a home environmental intervention: effect on efficacy and upset in caregivers and on daily function of persons with dementia. Gerontologist. 2001;41:4–14. doi: 10.1093/geront/41.1.4. [DOI] [PubMed] [Google Scholar]
- Grant I, Patterson T, Hauger R, Irwin M. Special Issue: current research on dementia & Alzheimer’s disease. Arch Psychiatry. 1992;4:77–80. [Google Scholar]
- Hanninen T, Koivisto K, Reinikainen K, et al. Prevalence of ageing-associated cognitive decline in an elderly population. Age Ageing. 1996;25:201–205. doi: 10.1093/ageing/25.3.201. [DOI] [PubMed] [Google Scholar]
- Hepburn KW, Tornatore J, Benter B, Ostwald SW. Dementia family caregiver training: Affecting beliefs about caregiving and caregiver outcomes. J Am Geriatr Soc. 2001;49:450–457. doi: 10.1046/j.1532-5415.2001.49090.x. [DOI] [PubMed] [Google Scholar]
- Hooyman N, Gonyea J, Montgomery R. The impact of in-home services termination of family caregivers. Gerontologist. 1985;25:141–145. doi: 10.1093/geront/25.2.141. [DOI] [PubMed] [Google Scholar]
- Hosaka T, Sugiyama Y. A structured intervention for family caregivers of dementia patients: a pilot study. Tokai J Exp Clin Med. 1999;24:35–39. [PubMed] [Google Scholar]
- Kiovisto K, Reinikainen K, Hanninen T, et al. Prevalence of age-associated memory impairment in a randomly selected population from eastern Finland. Neurology. 1995;45:741–747. doi: 10.1212/wnl.45.4.741. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Spielberger CD. Measuring anxiety in hospitalized geriatric patients. Series in Clinical & Community Psychology: Stress & Anxiety. 1983;2:135–143. [Google Scholar]
- Montgomery RJV. Investigating caregiver burden. In: Markides KS, Cooper CL, editors. Aging, Stress and Health. Wiley; New York: 1989. pp. 201–218. [Google Scholar]
- Montgomery RJV, Gonyea JG, Hooyman NR. Caregiving and the experience of subjective and objective burden. Fam Relat. 1985;34:19–26. [Google Scholar]
- Montgomery RJV, Kosloski K. A longitudinal analysis of nursing home placement for disabled elders cared for by spouses vs. adult children. J Gerontol. 1994;49:S62–S74. doi: 10.1093/geronj/49.2.s62. [DOI] [PubMed] [Google Scholar]
- Neundorfer MM. Coping and health outcomes in spouse care-givers of persons with dementia. Nurs Res. 1991;40:260–265. [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neuro. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: Early detection of dementia: Mild cognitive impairment (an evidence-based review) Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- Pruchno RA, Resch NL. Husbands and wives as caregivers: antecedents of depression and burden. Gerontologist. 1989;29:159–165. doi: 10.1093/geront/29.2.159. [DOI] [PubMed] [Google Scholar]
- Rabins PV. The caregiver’s role in Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9:25–28. doi: 10.1159/000051200. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Robinson KM. A social skills training program for adult caregivers. Adv Nurs Sci. 1988;10:59–72. doi: 10.1097/00012272-198801000-00010. [DOI] [PubMed] [Google Scholar]
- Robinson KM. Predictors of depression among wife caregivers. Nurs Res. 1989;38:359–363. [PubMed] [Google Scholar]
- Rokke PD, Tomhave JA, Jocic Z. Self-management therapy educational group therapy for depressed elders. Cognit Ther Res. 2000;24:99–119. [Google Scholar]
- Schroder J, Kratz B, Pantel J, Minnemann E, Lehr U, Sauer H. Prevalence of mild cognitive impairment in an elderly community sample. J Neural Transm. 1998;54:51–59. doi: 10.1007/978-3-7091-7508-8_5. [DOI] [PubMed] [Google Scholar]
- Schulz R. Some critical issues in caregiver intervention research. Aging Ment Health. 2001;5:S112–S115. doi: 10.1080/13607860120044882. [DOI] [PubMed] [Google Scholar]
- Schulz R, O’Brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates, and causes. Gerontologist. 1995;35:771–791. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- Schulz R, Visintainer P, Williamson GM. Psychiatric and physical morbidity effects of caregiving. J Gerontol. 1990;45:P181–P191. doi: 10.1093/geronj/45.5.p181. [DOI] [PubMed] [Google Scholar]
- Schulz R, Williamson GM. Health effects of caregiving: prevalence of mental and physical illness in Alzheimer’s caregivers. In: Light E, Niederehe G, Lebowitz BD, editors. Stress Effects on Family Caregivers of Alzheimer’s Patients: Research and Interventions. Springer; New York: 1994. pp. 38–63. [Google Scholar]
- Seltzer MM, Li LW. The dynamics of caregiving: transitions during a three-year prospective study. Gerontologist. 2000;40:165–178. doi: 10.1093/geront/40.2.165. [DOI] [PubMed] [Google Scholar]
- Sisk RJ. Caregiver burden and health promotion. Int J Nursing Studies. 1999;37:37–43. doi: 10.1016/s0020-7489(99)00053-x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Consulting Psychologist Press; Palo Alto, CA: 1983. [Google Scholar]
- Stone R, Cafferata GL, Sangl J. Caregivers of the frail elderly: a national profile. Gerontologist. 1987;27:616–626. doi: 10.1093/geront/27.5.616. [DOI] [PubMed] [Google Scholar]
- Taal E, Rasker JJ, Wiegman O. Patient education and self-management in the rheumatic diseases: a self-efficacy approach. Arthritis Care Res. 1996;9:229–238. doi: 10.1002/1529-0131(199606)9:3<229::aid-anr1790090312>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Tableman B. Stress management training: an approach to prevention of depression in low-income populations. In: Munoz RF, editor. Depression Prevention. Hemisphere Publishing Corporation; San Francisco, CA: 1987. pp. 38–63. [Google Scholar]
- Teri L, Truax P, Logsdon R, Uomoto J, Zarit S, Vitaliano PP. Assessment of behavioral problems in dementia: the revised memory and behavior problem checklist. Psychology and Aging. 1992;7:622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- Wijeratne C. Review: pathways to morbidity in carers of dementia sufferers. Int Psychogeriatr. 1997;9:69–79. doi: 10.1017/s1041610297004225. [DOI] [PubMed] [Google Scholar]
- Yee JL, Schulz R. Gender differences in psychiatric morbidity among family caregivers: a review and analysis. Gerontologist. 2000;40:147–164. doi: 10.1093/geront/40.2.147. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Anthony CR, Boutselis M. Interventions with caregivers of dementia patients: comparison of two approaches. Psychol Aging. 1987;2:225–232. doi: 10.1037//0882-7974.2.3.225. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Reever K, Bach-Peterson J. Relatives of the impaired elderly: correlates and feelings of burden. Gerontologist. 1980;20:649–655. doi: 10.1093/geront/20.6.649. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Todd P, Zarit J. Subjective burden of husbands and wives as caregivers: a longitudinal study. Gerontologist. 1986;32:665–672. doi: 10.1093/geront/26.3.260. [DOI] [PubMed] [Google Scholar]
- Zarit SH, Zarit KE. The Memory and Behavior Problems Checklist–1987R. Pennsylvania State University; University Park, PA: 1987. [Google Scholar]