Abstract
A new synthetic method has been developed to prepare fluorescent silica nanoparticles without employing isothiocyanated dye molecules and (3-aminopropyl)triethoxysilane (APS) for the thiourea linkage formation; the resulting fluorescent silica nanoparticles show excellent photochemical, thermal and pH stabilities and a good biocompatibility with over 85% viability from various cell types.
Since the preparation method of silica nanoparticles was developed by Werner Stöber,1 many researchers have focused on attempts to control the size and uniformity, as well as to utilize them to various fields including biological application. Silica has several advantages including its ease of preparation through the hydrolysis–condensation reaction from relatively inexpensive precursor molecules such as tetraethyl orthosilicate (TEOS) in the presence of acid- or base-catalysts, the possibility of surface modification with various well-studied organosilicon compounds,2 and its non-acute toxicity.3a,b As the surface silanol groups can be easily modified by various functional groups and modified silica nanoparticles can effectively penetrate the cell membrane, there has been a great amount of research effort to use them as carriers for the delivery of drugs or genes.3a,c,d,4 To improve the application of silica nanoparticles to biological research, fluorescent dye molecules were introduced into silica nanoparticles by using a thiourea-linkage forming reaction through amino-terminated alkyltrialkoxysilane compounds such as (3-aminopropyl)triethoxysilane (APS) and dye molecules having an isothiocyanate functional group, i.e. Rhodamine isothiocyanate (RITC) and Fluorescein isothiocyanate (FITC).5 This photostable fluorescent property has become one of the most appreciated functionalities of fluorescent silica nanoparticles and has opened a new era of bioimaging.6–8a Although this thiourea-linkage formation reaction is simple and well studied as a useful bioconjugation method,9 it has several drawbacks in preparing fluorescent silica nanoparticles. First, an excess amount of APS is usually used in order to incorporate all the possible dye molecules into silica nanoparticles (about two times excess compared to the amount of isothiocyanated dye molecule such as RITC or FITC), and it is co-condensed with TEOS during the silica formation process. Although the amino terminal groups on the nanoparticle surface could be used for the further chemical linkage reactions such as conjugation with drugs or specific binding antibodies,8b,d the amount of amino terminal groups was not reproducibly controllable and the excess amount of APS sometimes adversely affected the size uniformity.10a Second, it is reported that primary amine groups on the surface make silica nanoparticles stable and dispersible only in acidic condition, but lead to precipitation in neutral and basic conditions, even in the pH range 7.0–7.5, where most biological experiments are carried out.3c,10b Third and most importantly, a cytotoxicity of terminal amine groups from the APS treated nanomaterials has been recently reported.11a Therefore, it would be worthwhile to develop a new synthetic method to prepare fluorescent silica nanoparticles, which have covalently linked fluorescent dye molecules in the silica matrix without using APS for the thiourea linkage formation.
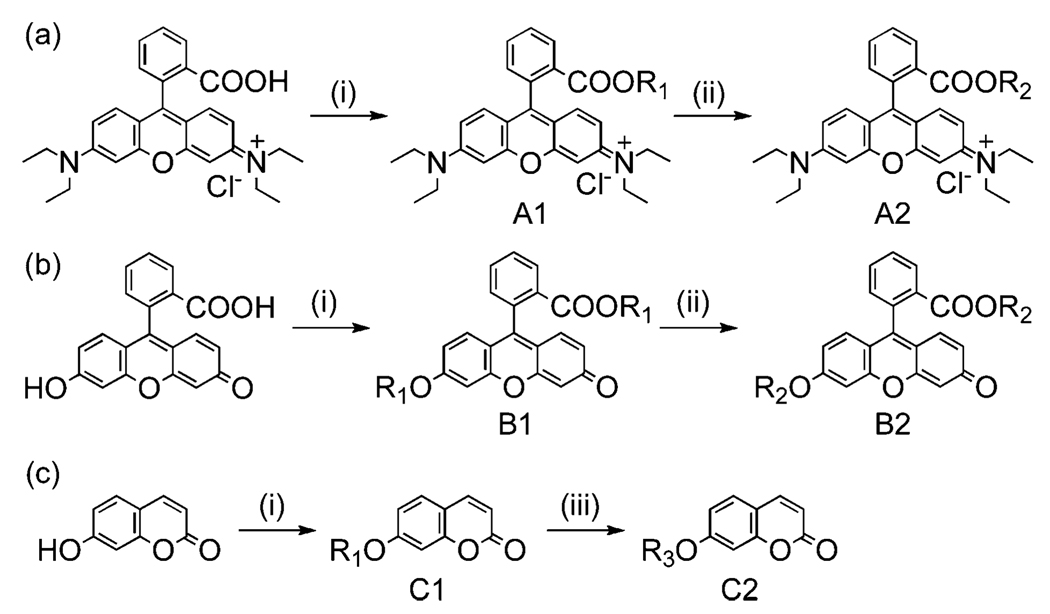
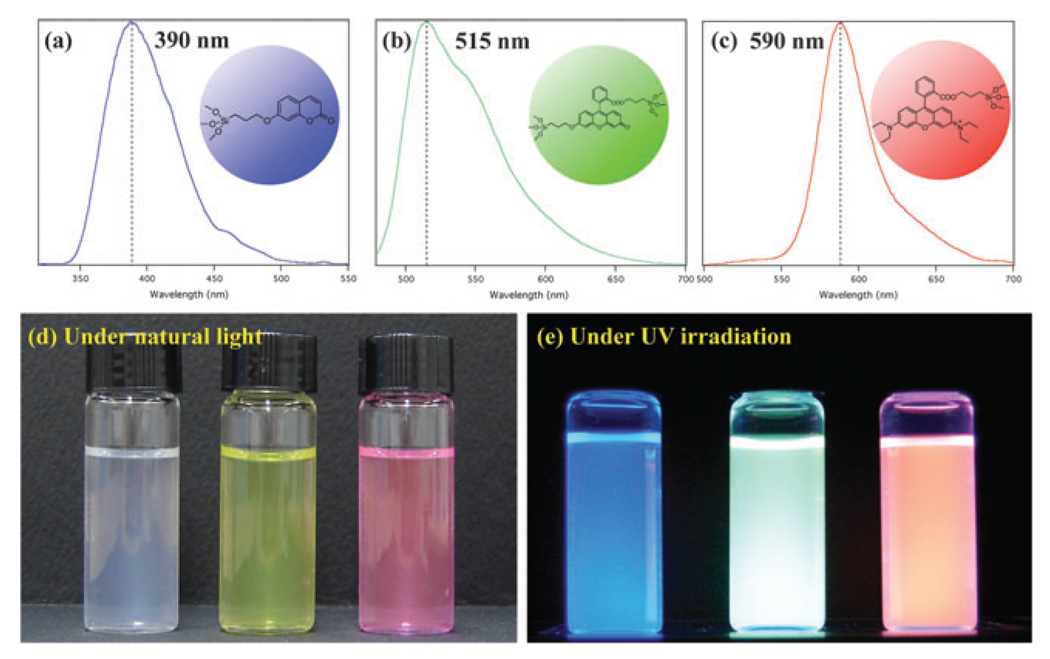
Trialkoxysilyl terminal groups could be directly introduced into fluorescent organic dye molecules by the consecutive allylation and hydrosilation as described in Scheme 1. Carboxylic acid and hydroxy groups in dye molecules could be deprotonated with a base in DMF solvent, and coupled with allyl iodide in a reasonably high yield (>90%). These allyl terminal groups in derivatized dye molecules could be quantitatively converted into trialkoxysilyl groups by hydrosilation reaction in the presence of Pt/C catalyst. All of these derivatized dye molecules were successfully incorporated into silica nanoparticles by co-condensation with TEOS in basic ethanol solution and aqueous NH3(Fig. 1). The incorporation ratio of fluorescent dye molecules was optimized at ~11% (molar ratio of dye to TEOS), and the size of the silica nanoparticles could be controlled in the range from 30 to 500 nm by varying the concentration of TEOS, water and ammonia in ethanol solution (see ESI†). As shown in Fig. 1, the emission wavelengths from the fluorescent silica nanoparticles are similar to those of the parent dye molecules (blue for Coumarin at 390 nm, green for Fluorescein at 515 nm, and red for Rhodamine B at 590 nm), and silica nanoparticles in the aqueous solution produce a very bright photoluminescence. Since these dye molecules are chemically bound to the silica matrix, they cannot be easily removed by extracting with a large amount of solvent or applying sonication, and furthermore they are not easily photo-bleached as reported in the literature due to the restricted movement of dye molecules after being embedded in the silica matrix.3a,6a,10a These properties make fluorescent silica nanoparticles very useful for consecutive monitoring with multiple-colour staining.
Scheme 1.
Derivatization of organic dyes for incorporation into silica matrices. Reagents and conditions: (i) allyl iodide, Cs2CO3, DMF; (ii) trimethoxysilane, Pt/C, methanol; (iii) triethoxysilane, Pt(dvs), toluene; R1 = –CH2–CH==CH2, R2 = –(CH2)3Si(OMe)3, R3 = –(CH2)3Si(OEt)3
Figure 1.
Photoluminescence spectra and photographs of 50 nm-sized, dye-incorporated silica nanoparticle in water.
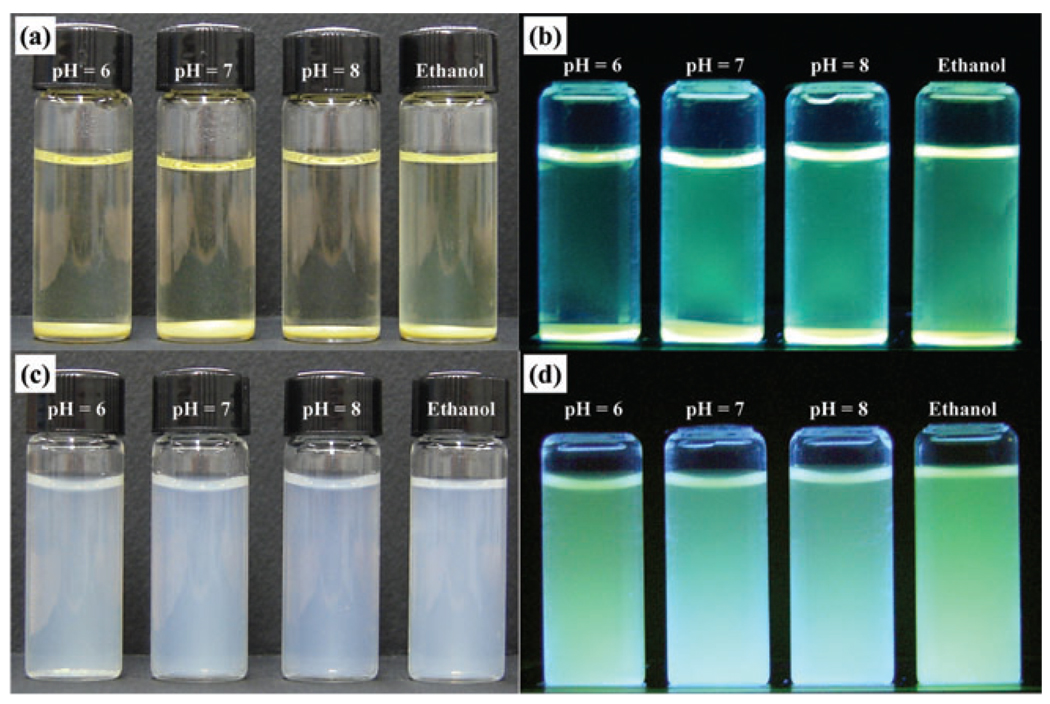
Unlike fluorescent silica nanoparticles prepared from APS and isothiocyanated dye molecules for the thiourea linkage formation, which precipitates in ethanol and water at neutral and basic pH, no amine terminal groups are present on the nanoparticle surface. This results in nanoparticles that are highly dispersible in ethanol and water at a wide pH range from acidic to basic as clearly shown in Fig. 2. They also demonstrated a good thermal stability even after refluxing in water or ethanol for 1 day.
Figure 2.
Stability of 50 nm-sized, fluorescent silica nanoparticles in water at various pH and in ethanol; (a) and (b) precipitated SiO2 prepared by FITC-APS conjugated method, (c) and (d) dispersed SiO2 prepared by a direct modification of fluorescein molecule as described in Scheme 1(b). Images (b) and (d) were taken under UV irradiation.
The Si–OH groups on the surface of silica nanoparticles could be successfully modified with silicon coupling reagents such as [methoxy(polyethyleneoxy)propyl]trimethoxysilane (PEG-Si(OMe)3) for enhancing the biocompatibility and N-trimethoxysilylpropyl-N,N,N-trimethylammonium chloride ((MeO)3Si-PTMA) for introducing positive charges. After the modification, their size and emission wavelength were not significantly affected as confirmed by absorption/emission spectra and TEM measurements. As expected, the surface-modified silica nanoparticles also showed an excellent thermal stability even after refluxing in water or ethanol for 3 days.
Although several studies reported the cytotoxicity of various silica nanoparticles with different opinions,3b,6a,8a,c,11b this has not been fully investigated for effects of the actual size of aggregated silica nanoparticles and the surface ligands. In order to determine the potential use of fluorescent silica nanoparticles for bio-applications, therefore, the biochemical properties related to cell growth and differentiation have been analyzed with a number of different cell types including A549 (human lung cancer), HEK 293 (human embryonic kidney), and MC3T3-E1 (murine preosteoblast). The viability of a normal cell line such as MC3T3-E1 has not been studied much for nanoparticles, while cancer cell lines such as A549 have been studied much more extensively for the cytotoxicity testing of nanoparticles and therefore can be used as a reference. Cell viability was measured in all cell types using a well- known MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfonyl)-2H-tetrazolium, inner salt) assay as a function of the surface ligand and the dosage of nanoparticle. A549, HEK 293 and MC3T3-E1 cells were treated with silica nanoparticles prepared by direct modification of Rhodamine B (Scheme 1(a)) and successive surface modification, SiO2(RhB)-OH, SiO2(RhB)-PEG and SiO2(RhB)-PTMA, respectively, with various dosages of 10, 50 and 100 µg ml−1 of fluorescent silica nanoparticles for 3 days. In these conditions, the cell viability was maintained at >85% in all groups, corroborating the fact that the fluorescent silica nanoparticles prepared from the present method did not show any acute cytotoxicity to various cell types at the level up to 100 µg ml−1 over 72 h (see ESI†).
The rate of nanoparticle uptake into the cell was very sensitive towards the surface functionalities. Positively charged SiO2(RhB)-PTMA had the fastest rate of uptake, showing a bright fluorescence from the cytoplasm of MC3T3-E1 cells after only 1 h incubation and almost neutral SiO2(RhB)–OH had a reasonably efficient uptake within ~2 h. However, SiO2(RhB)-PEG having neutral surface charges with biocompatible PEG groups showed a very slow uptake rate when compared to positively charged SiO2(RhB)-PTMA and it took about 1 day for MC3T3-E1 cells incubated with them to show bright fluorescence. This difference seems to be a result of the charge interaction between the positive charges of the nanoparticle’s surface and partial negative charges on the cell membrane.3c None of the silica nanoparticles of 50 nm size with various surface charges could penetrate the nuclear membrane, with a dark nucleus clearly shown in fluorescent images even after a few days of incubation.
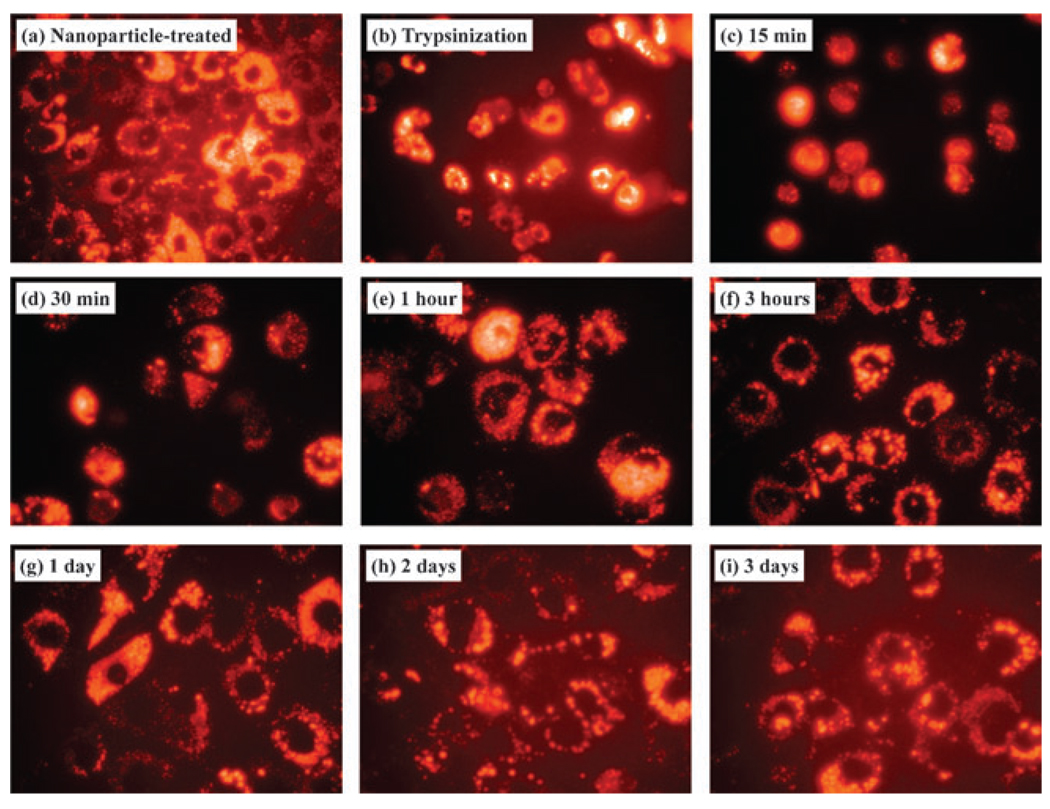
Since the fluorescent dye molecules are chemically embedded in the solid silica matrix have an excellent photostability without susceptibility to photobleaching,3a,6a,9a biocompatible and stable fluorescent silica nanoparticles prepared in the present method by the direct modification of fluorescent molecules could be used for biological experiments which require the monitoring of multiple time points to investigate the differentiation and/or development of cells. As shown in Fig. 3(a) and (b), MC3T3-E1 cells incubated with SiO2(RhB)-PTMA for 24 h showed a bright orange fluorescence from the cytoplasm, and this was also clearly observed from the floating cells after trypsinization (Fig. 3(b)), treating with the trypsin enzyme commonly found in the digestive tract which can be used to digest the proteins that facilitate adhesion to the container and inter-cellularly, in order to detach cells from the surface and divide into other plates for subculturing. During the settlement of cells onto new culture media (Fig. 3(c)–(f)), the movement of cell spreading was easily monitored by fluorescent imaging. Fluorescence could still be observed for several days as shown in Fig. 3(g), (h) and (i), corroborating that our fluorescent nanoparticles did not affect the proliferation of MC3T3-E1 cells or show photobleaching; which makes them very useful as bio-imaging and tagging materials for long-term and consecutive multiple-time monitoring experiments.
Figure 3.
Fluorescent images of MC3T3-E1 cells incubated with 50 nm-sized, positively charged, orange fluorescent silica nanoparticles; 100 µg ml−1 of nanoparticles were added to the medium.
In conclusion, new size-controllable fluorescent silica nanoparticles having red, green and blue fluorescent colours were successfully synthesized by the modified Stöber method from TEOS and directly derivatized fluorescent organic dye molecules. They are highly dispersible in ethanol and water at a range of pH from acidic to basic, along with a good thermal stability. The surface-modified silica nanoparticles also showed an excellent thermal stability even after refluxing in water or ethanol for 3 days. Silica nanoparticles prepared by direct modification of Rhodamine B and successive surface modification, SiO2(RhB)–OH, SiO2(RhB)–PEG and SiO2(RhB)–PTMA, respectively, showed a cell viability >85% and did not show any acute cytotoxicity to various cell types at a level up to 100 µg ml−1 over 72 h. The continuous monitoring experiments with MC3T3-E1 cells cultured with SiO2(RhB)-PTMA clearly showed that our fluorescent nanoparticles did not affect the proliferation of MC3T3-E1 and they could be used for long-term and multiple-time bio-imaging systems.
Supplementary Material
Acknowledgments
This work was supported by the National Core Research Center program of the Korea Science and Engineering Foundation (KOSEF) through the NANO System Institute at Seoul National University and grants from the NIAMS (AR056090) and Emory University (G. R. B. Jr and C. E. C.).
Footnotes
Electronic supplementary information (ESI) available: Detailed experimental section. See DOI: 10.1039/b902195g
Notes and references
- 1.Stöber W, Fink A, Bohn E. J. Colloid Interface Sci. 1968;26:62. [Google Scholar]
- 2.Vansant EF, Voort PVD, Vrancken KC. Characterization and Chemical Modification of the Silica Surface. Amsterdam: Elsevier; 1995. [Google Scholar]
- 3.(a) Tan w, Wang K, He X, Zhao XJ, Drake T, Wang L, Bagwe RP. Med. Res. Rev. 2004;24:621. doi: 10.1002/med.20003. [DOI] [PubMed] [Google Scholar]; (b) Lin W, Huang Y-W, Zhou X-D, Ma Y. Toxicol. Appl. Pharmacol. 2006;217:252. doi: 10.1016/j.taap.2006.10.004. [DOI] [PubMed] [Google Scholar]; (c) Xu ZP, Zeng QH, Lu GQ, Yu AB. Chem. Eng. Sci. 2006;61:1027. [Google Scholar]; (d) Watson P, Jones AT, Stephens DJ. Adv. Drug Delivery Rev. 2005;57:43. doi: 10.1016/j.addr.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 4.(a) Salgueiriño-Maceira V, Correa-Duarte MA. Adv. Mater. 2007;19:4131. [Google Scholar]; (b) Salgueiriño-Maceira V, Correa-Duarte MA, Spasova M, Liz-Marzán LM, Farle M. Adv. Funct. Mater. 2006;16:509. [Google Scholar]
- 5.(a) Verhaegh NAM, Blaaderen Av. Langmuir. 1994;10:1427. [Google Scholar]; (b) Blaaderen Av, Vrij A. Langmuir. 1992;8:2921. [Google Scholar]; (c) Santra S, Zhang P, Wang K, Tapec R, Tan W. Anal. Chem. 2001;73:4988. doi: 10.1021/ac010406+. [DOI] [PubMed] [Google Scholar]
- 6.(a) Ow H, Larson DR, Srivastava M, Baird BA, Webb WW, Wiesner U. Nano Lett. 2005;5:113. doi: 10.1021/nl0482478. [DOI] [PubMed] [Google Scholar]; (b) Choi J, Burns AA, Williams RM, Zhou Z, Flesken-Nikitin A, Zipfel WR, Wiesner U, Nikitin AY. J. Biomed. Opt. 2007;12:064007-1. doi: 10.1117/1.2823149. [DOI] [PubMed] [Google Scholar]
- 7.(a) Fuller JE, Zugates GT, Ferreira LS, Ow HS, Nguyen NN, Wiesner UB, Langer RS. Biomaterials. 2008;29:1526. doi: 10.1016/j.biomaterials.2007.11.025. [DOI] [PubMed] [Google Scholar]; (b) Roy I, Ohulchanskyy TY, Bharali DJ, Pudavar HE, Mistretta RA, Kaur N, Prasad PN. Proc. Natl. Acad. Sci. U. S. A. 2005;102:279. doi: 10.1073/pnas.0408039101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Yoon T-J, Kim JS, Kim BG, Yu KN, Cho M-H, Lee J-K. Angew. Chem., Int. Ed. 2005;44:1068. doi: 10.1002/anie.200461910. [DOI] [PubMed] [Google Scholar]; (b) Yoon T-J, Yu KN, Kim E, Kim JS, Kim BG, Yun S-H, Sohn B-H, Cho M-H, Lee J-K, Park SB. Small. 2006;2:209. doi: 10.1002/smll.200500360. [DOI] [PubMed] [Google Scholar]; (c) Kim JS, Yoon T-J, Yu KN, Kim BG, Park SJ, Kim HW, Lee KH, Park SB, Lee J-K, Cho MH. Toxicol. Sci. 2006;89:338. doi: 10.1093/toxsci/kfj027. [DOI] [PubMed] [Google Scholar]; (d) Gemeinhart RA, Luo D, Saltzman WM. Biotechnol. Prog. 2005;21:532. doi: 10.1021/bp049648w. [DOI] [PubMed] [Google Scholar]
- 9.Hermanson GT. Bioconjugate Technique. San Diego, CA: Academic Press, Inc.; 1996. [Google Scholar]
- 10.(a) Nakamura M, Shono M, Ishimura K. Anal. Chem. 2007;79:6507. doi: 10.1021/ac070394d. [DOI] [PubMed] [Google Scholar]; (b) Earhart C, Jana NR, Erathodiyil N, Ying JY. Langmuir. 2008;24:6215. doi: 10.1021/la800066g. [DOI] [PubMed] [Google Scholar]
- 11.(a) Nan A, Bai X, Son SJ, Lee SB, Ghandehari H. Nano Lett. 2008;8:2150. doi: 10.1021/nl0802741. [DOI] [PubMed] [Google Scholar]; (b) Chen M, Mikecz Av. Exp. Cell Res. 2005;305:51. doi: 10.1016/j.yexcr.2004.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.