Abstract
Background
Patients with total knee arthroplasty (TKA) have impaired balance and movement control. Exercise interventions have not targeted these impairments in this population.
Objectives
The purposes of this study were: (1) to determine the feasibility of applying a balance exercise program in patients with TKA, (2) to investigate whether a functional training (FT) program supplemented with a balance exercise program (FT+B program) could improve physical function compared with an FT program alone in a small group of individuals with TKA, and (3) to test the methods and calculate sample size for a future randomized trial with a larger study sample.
Design
This study was a double-blind, pilot randomized clinical trial.
Setting
The study was conducted in the clinical laboratory of an academic center.
Participants
The participants were 43 individuals (30 female, 13 male; mean age=68 years, SD=8) who underwent TKA 2 to 6 months prior to the study.
Interventions
The interventions were 6 weeks (12 sessions) of a supervised FT or FT+B program, followed by a 4-month home exercise program.
Measurements
Feasibility measures included pain, stiffness, adherence, and attrition. The primary outcome measure was a battery of physical performance tests: self-selected gait speed, chair rise test, and single-leg stance time. Secondary outcome measures were the Western Ontario and McMaster Universities Osteoarthritis Index and the Lower Extremity Functional Scale.
Results
Feasibility of the balance training in people with TKA was supported by high exercise adherence, a relatively low dropout rate, and no adverse events. Both groups demonstrated clinically important improvements in lower-extremity functional status. The degree of improvement seemed higher for gait speed, single-leg stance time, and stiffness in the FT+B group compared with the FT group.
Limitations
Due to the pilot nature of the study, differences between groups did not have adequate power to show statistical significance.
Conclusions
There is a need for conducting a larger randomized controlled trial to test the effectiveness of an FT+B program after TKA.
By the year 2020, it is estimated that more than 3 million total knee arthroplasty (TKA) surgeries will be performed in the United States for end-stage arthritis in the knee joint.1 Although there is a rapid and substantial improvement in knee pain after TKA, 37% of patients have limited functional improvement 1 year after the surgery.2 The most common limitations of these patients are diminished walking speed, difficulty ascending and descending stairs, and inability to return to sports played prior to the surgery.3–5 In light of these limitations, researchers are continually testing the benefits of exercise therapy to improve the outcomes of patients after TKA.
Studies that investigated the effectiveness of exercises after hospital discharge have used traditional exercise programs consisting of range of motion, stretching, strengthening, and endurance exercises and have shown a small beneficial effect on pain and function.6–9 Some studies have tested the effectiveness of functional training (FT).6,10 Functional training programs consist of more dynamic exercises and require the performance of activities generally limited by patients with TKA, such as stair ascending and descending, walking, and chair stands.6,10 Although these trials have shown better outcomes for pain, physical function, and quality of life, the effects tend to fade at follow-ups longer than 3 to 4 months,11 suggesting that exercise programs could be refined to produce long-lasting functional improvements in these patients.
In refining exercise therapy approaches, it is important to consider factors that may contribute to functional deficits of patients with TKA. One such factor may be impaired movement control and balance. Although during TKA surgery several tendons, capsule, and remaining ligaments are retightened to restore the joint spaces deteriorated by the arthritis, in order to restore the intra-articular geometry, some of the knee ligaments are removed or released. These alterations may affect the function of several mechanoreceptors and impair movement control and balance.12 Several studies have identified deficits in components of the balance system, such as decreased ability to detect joint position and motion, delayed muscle latency, altered amplitude of muscle activity, and decreased postural control, in patients after TKA.12–19 Therefore, exercises aimed at improving the impaired movement control and balance of patients after TKA should be considered.
We are not aware of exercise interventions that attempted to specifically target patients’ deficits related to balance and movement control. Although the FT programs used so far require patients to perform functional activities such as chair rises, bilateral and unilateral knee flexion in standing, climbing stairs, and walking in place, these activities do not seem to fully challenge patients’ balance and movement control. To improve these deficits, exercises should expose patients to activities that challenge their stability and propose movement problems that mimic more skilled abilities of everyday life such as twisting, turning, sudden starts and stops, standing over unstable surfaces, walking while changing speed and direction, using narrow paths, overcoming obstacles, and so on. If patients improve their ability to maintain stability and solve more challenging movement problems, it could help to decrease further the functional limitations and increase their confidence to safely engage in a more physically active lifestyle, ultimately resulting in a longer-term benefit in individuals with TKA. Before a more challenging balance exercise program is recommended in patients with TKA, its feasibility should be tested, including its safety and tolerability by these patients.20 If feasibility is demonstrated and the exercise program shows a beneficial effect, it will support the need for trials with larger sample sizes to test the effectiveness of the exercise program to return patients with TKA to higher levels of physical function. This study of a small sample of patients is the initial step toward that end.
The aims of this pilot study were: (1) to determine the feasibility of applying a balance exercise program in patients with TKA, (2) to investigate whether an FT program supplemented with a balance exercise program (FT+B program) could improve physical function compared with an FT program alone in a small group of patients with TKA, and (3) to test the methods and calculate a sample size for a future randomized trial with a larger study sample to further the research agenda on improving functional outcome in patients with TKA.
Method
Design Overview
This study was a double-blind, pilot randomized clinical trial.
Setting and Participants
Study implementation took place from January 2007 to May 2008 in the Department of Physical Therapy at the University of Pittsburgh. Participants were recruited from one orthopedic surgeon. Patients who had a TKA performed in the previous months were mailed study information. Interested participants were screened for eligibility over the telephone. All participants gave written consent prior to the study.
Patients were eligible if they were at least 50 years of age and had a unilateral TKA in the previous 2 to 6 months. A minimum of 2 months after TKA was specified to avoid knee pain, effusion, or limitations in motion restricting the implementation of the exercise programs. Individuals were excluded if they reported 2 or more falls within the previous year, were unable to ambulate a distance of 30.48 m (100 ft) without an assistive device or a rest period, had an acute illness or cardiovascular disease, had high blood pressure not controlled by medication, had severe visual impairment, had a lower-extremity amputation, had a progressive neurological disorder, or were pregnant. All participants underwent a tricompartmental, cemented TKA, using a minimally invasive technique, with a quadriceps muscle–sparing incision. The same surgeon performed all surgeries. Participants received the same rehabilitation while in the hospital. After hospital discharge, participants received inpatient, home, or outpatient physical therapy, as needed. This information was not recorded because there is no evidence to support differential TKA outcomes across rehabilitation settings.8,9,21
Randomization and Interventions
A statistician who was not aware of the study aims generated the randomization plan and added it into the computer program used for the paperless data collection in this study. A research assistant who was not involved with recruitment or testing performed the randomization after the baseline session. Participants were randomly assigned to receive either the FT program or the FT+B program. Participants were not aware of what type of exercises the other group received until the end of the study.
Supervised exercise programs.
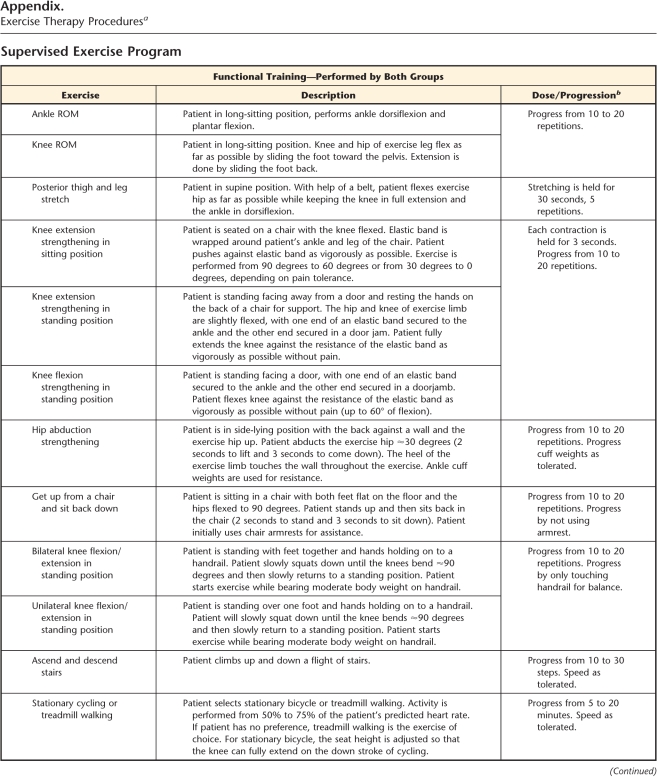
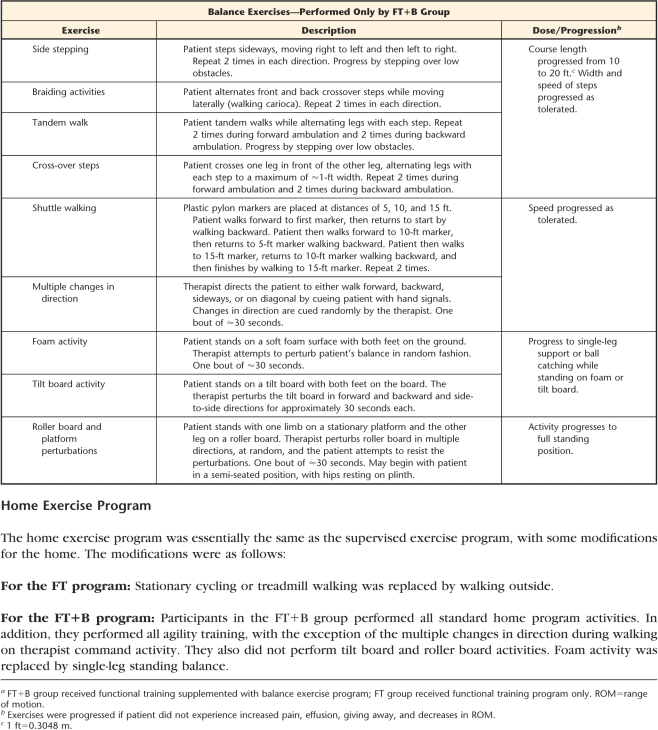
During the trial, 5 physical therapists were involved in delivering the 12 sessions of supervised exercise over 6 weeks. Sessions were individualized. The FT program consisted of warm-up, strengthening exercises, functional task-oriented exercises, endurance exercises, and cool-down. It was based on the protocol published by Moffet and colleagues.10 The FT+B program included all of the above components plus balance exercises (agility and perturbation techniques) and was based on the protocol published by Fitzgerald and colleagues22 (Appendix).
Home exercise programs.
At the end of supervised program, participants were asked to continue exercising at home 2 times per week for 4 months. Adherence to the home exercise program was recorded in an exercise log. Participants were called once a month to encourage adherence.
Outcomes and Follow-up
Trained study personnel who were unaware of group assignment performed all outcome assessments. At the end of the trial, each tester was asked to guess group assignment. The testers correctly guessed group assignment 56% of the time, which is close to chance guessing and suggests appropriate masking. Outcome data were collected at baseline, after the completion of the supervised program (2 months), and at the completion of the 4-month home exercise program period (6 months after randomization). The 6-month follow-up was the endpoint of major interest.
Feasibility was assessed by pain, stiffness, adherence to the supervised programs, adherence to the home exercises, attrition, and adverse events. Pain was measured in 2 ways. Pain during activities was measured by the 5-item Western Ontario and McMaster Universities Osteoarthritis Index pain subscale (WOMAC-PN).23–25 Intensity of knee pain was measured using an 11-point numeric pain scale, which was anchored on the left with the phrase “no pain” and on the right with the phrase “worst imaginable pain.”26–28 Although pain intensity was measured at all measurement points in the knee that had undergone surgery (surgical knee), it was measured only at 6 months in the knee that had not undergone surgery (nonsurgical knee). We did not collect data on pain in the nonsurgical knee prior to the 6-month point because this measure was proposed during a data safety meeting after starting study implementation. Stiffness was measured by the 2-item WOMAC stiffness subscale (WOMAC-ST).23–25 Adherence to the supervised programs was calculated as the number of exercise sessions performed over the 12 sessions prescribed. Adherence to the home exercises was calculated as the number of exercises performed over the number of exercises prescribed. Attrition was calculated as the number of participants at the end of the trial over the participants originally randomized to each group.
As per the recommendation of experts in arthritis, lower-extremity functional status was measured with performance-based and self-report measures.29 Performance-based measures rather than self-report measures were chosen as the primary outcome measure because patients with TKA tend to self-report their outcome as good even when they experience difficulty performing daily tasks.30,31 Moreover, self-reports of physical function are more influenced by pain.32 We chose a battery of tests easily done in the clinical setting. The battery of tests included self-selected gait speed, a timed chair rise test, and single-leg stance time. Self-selected gait speed was measured by recording the time each participant needed to pass 2 infrared beams 4 m apart, located in the central part of a longer path of 7 m to avoid measurement during gait acceleration or deceleration. Participants were timed twice, and the faster speed was recorded. For the timed chair rise test, participants were seated in a chair without armrests with their arms crossed over their chest. They were timed during 5 repetitions of rising to a full upright position and sitting back down in the chair without assistance. The single-leg stance test was a measure of balance that consisted of recording the length of time participants balanced on one leg while keeping their hands on their hips. The test lasted up to 30 seconds and was stopped if: (1) the swing leg touched the floor, (2) the tested foot displaced on the floor, (3) the swing lower leg touched the tested limb, or (4) the arms swung away from the hips. These tests cover important domains of lower-extremity physical function such as walking ability, dynamic and static balance, muscle strength and power, and movement control. They have been shown to be reliable and responsive to interventions and to have the ability to discriminate from low to high functional ability in individuals of various ages and functional levels.33–38
Secondary functional outcome measures were the condition-specific WOMAC physical function subscale (WOMAC-PF) and the region-specific Lower Extremity Functional Scale (LEFS). The WOMAC-PF has 17 items (each scored from 0 to 4), with a total score of up to 68 points. Larger scores indicate worse function. Evidence for the validity of the WOMAC is well established.23–25 The WOMAC version LK3.1 was used. The LEFS was used as a measure of lower-extremity function. The scale consists of 20 items (each scored from 0 to 4), with the total score ranging from 0 to 80 (larger scores indicate better function). Reliability, validity, and responsiveness have been established for this measure.39
Data Analysis
Because the goal of the study was parameter estimation rather than hypotheses testing, we calculated 95% confidence intervals (CIs) around our observed point estimates of effect. Baseline characteristics were compared by visual observation of the absolute differences between the 2 groups. Adherence and attrition rates were visually compared between the groups.
Point estimates and 95% CIs were compared for changes in pain, stiffness, and physical function in the FT and FT+B groups at the 2- and 6-month time periods. As a measure of relative improvement, we calculated the percentage of change for each group at 2 and 6 months. To test whether the within-patient changes (within-group differences) were clinically important, we compared the minimal important difference (MID) of each outcome against either the 95% CI or the percentage changes. The MID for a scale is the smallest change score associated with a patient's perception of an important change in health status.40 Differences were considered small but clinically important when the 95% CI around the estimated size of the within-group effect included the MID.41 The MIDs for the measures were obtained from the literature and were as follows: a difference of 0.05 m/s in self-selected gait speed,42 a difference of 9 points in WOMAC-PF scores,43 a difference of 9 points in LEFS scores,39 and a difference of 2 points in numeric pain rating scale scores.44 Between-group differences at 2 and 6 months were estimated by calculating the difference in mean change between the groups and their 95% CI.
The SPSS version 16.0* was used to compute point estimates and 95% CI. To estimate sample size, we used the mean difference between groups at 6 months and the standard deviation of such differences. Sample sizes were estimated using Sample Power 2.0.*
Role of the Funding Source
This study was funded by the Central Research Development Fund; the University of Pittsburgh Medical Center Health System Competitive Medical Research Fund; the Claude D. Pepper Older American Independence Center (P30-AG024827); the National Center for Research Resources, a component of the National Institutes of Health, and NIH Roadmap for Medical Research (KL2 RR024154-02); and the American College of Rheumatology Research and Education Foundation New Investigator Award. The funding sources played no role in the design, conduct, or reporting of the study. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official view of the funding sources.
Results
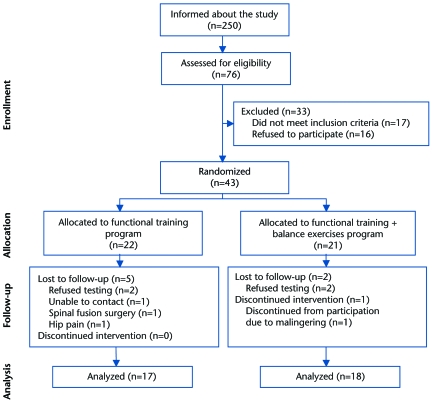
Of the 76 participants assessed for eligibility, 43 underwent baseline testing and randomization. From the 22 participants assigned to the FT group and the 21 participants assigned to the FT+B group, 17 and 18 participants, respectively, completed the study and were included in the analysis (Fig. 1).
Figure 1.
Participant flow during the study.
Baseline Comparisons
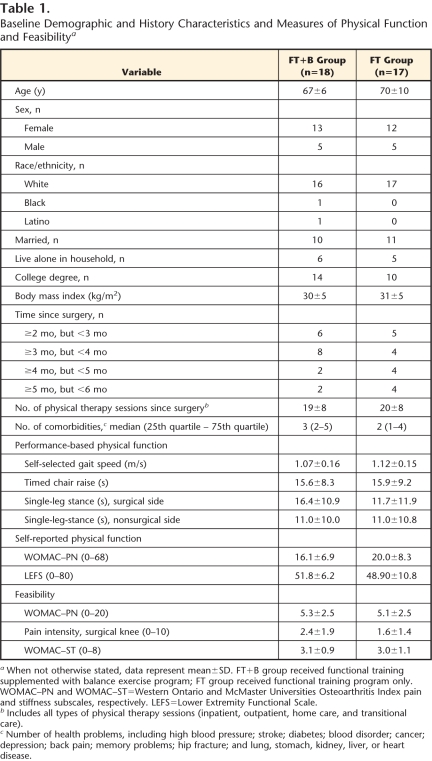
Table 1 presents baseline participant characteristics for both treatment groups. Visual observation of the differences indicated that the groups were well matched for the majority of the variables. Participants in the FT+B group had slightly better function (3.9 points lower on the WOMAC-PF and 2.9 points higher on the LEFS) than participants in the FT group. These differences were likely not relevant, as they represent less than half of the MID for the WOMAC-PF and LEFS. For the performance-based measures, participants in the FT+B group balanced 4.7 seconds longer on the surgical leg and were 0.05 m/s slower than the FT group. Differences in single-leg stance and gait speed seemed clinically meaningful and supported accounting for baseline scores when observing the change in both groups. The baseline differences between the groups were taken into consideration by calculating the point estimates and 95% CI of change scores, rather than using the exit scores (follow-up).
Table 1.
Baseline Demographic and History Characteristics and Measures of Physical Function and Feasibility a
When not otherwise stated, data represent mean±SD. FT+B group received functional training supplemented with balance exercise program; FT group received functional training program only. WOMAC–PN and WOMAC–ST=Western Ontario and McMaster Universities Osteoarthritis Index pain and stiffness subscales, respectively. LEFS=Lower Extremity Functional Scale.
b Includes all types of physical therapy sessions (inpatient, outpatient, home care, and transitional care).
c Number of health problems, including high blood pressure; stroke; diabetes; blood disorder; cancer; depression; back pain; memory problems; hip fracture; and lung, stomach, kidney, liver, or heart disease.
Feasibility
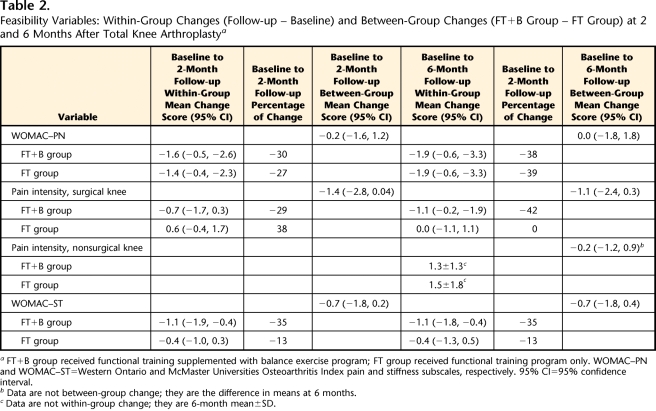
Adherence to the supervised exercises was 100% in both groups. Adherence to the home exercise program was similar: the FT+B group performed a mean of 64% (SD=38%) of the prescribed exercises, whereas the FT group performed a mean of 67% (SD=21%) of the prescribed exercises. The between-group difference was 3% (95% CI=−24, 19). The overall attrition rate was 16%: 10% (2/21) in the FT+B group and 23% (5/22) in the FT group. There were no adverse events or clinical complications related to the study interventions. The balance exercise program did not exacerbate pain or stiffness (Tab. 2). The WOMAC-PN scores decreased similarly in both groups. In the FT+B group, pain intensity in the surgical knee decreased minimally at 2 months (0.72 points) and slightly more at 6 months (1.1 points). For the FT group, knee pain increased slightly at 2 months and returned to baseline level at 6 months. Mean pain intensity in the nonsurgical knee at 6 months was similar in both groups (we did not collect these data at baseline and at 2 months). The WOMAC-ST scores decreased 35% in the FT+B group and 13% in the FT group.
Table 2.
Feasibility Variables: Within-Group Changes (Follow-up − Baseline) and Between-Group Changes (FT+B Group − FT Group) at 2 and 6 Months After Total Knee Arthroplasty a
FT+B group received functional training supplemented with balance exercise program; FT group received functional training program only. WOMAC–PN and WOMAC–ST=Western Ontario and McMaster Universities Osteoarthritis Index pain and stiffness subscales, respectively. 95% CI=95% confidence interval.
b Data are not between-group change; they are the difference in means at 6 months.
c Data are not within-group change; they are 6-month mean±SD.
Primary and Secondary Outcomes
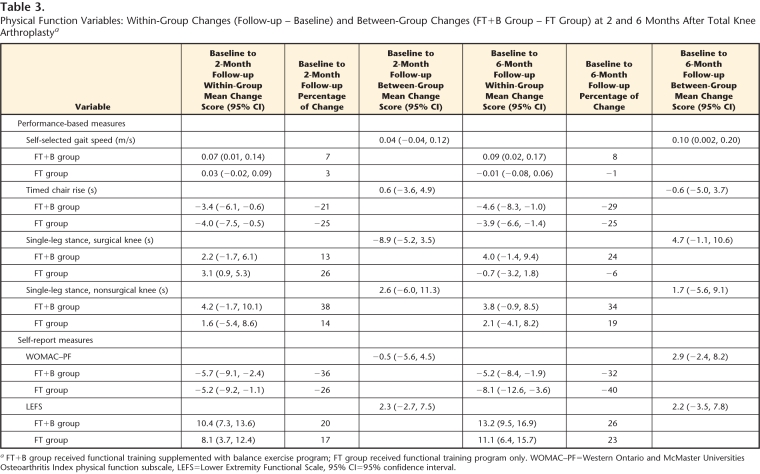
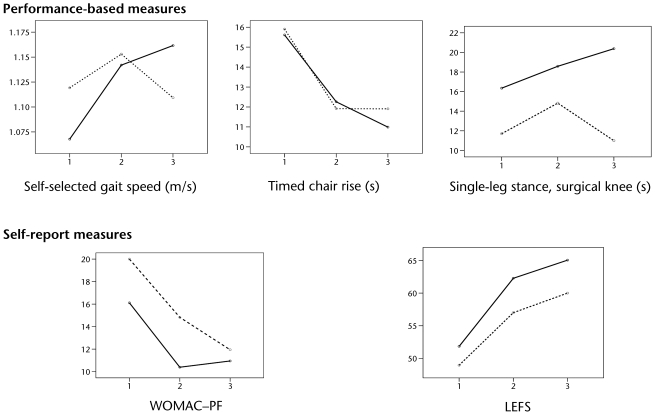
Table 3 displays the changes in physical function. Within-group increases in self-selected gait speed seemed clinically important in the FT+B group. Differences between groups favored the FT+B group (between-group mean difference=0.10 m/s). Both groups had similar decreases in chair rise times (>20%). In the FT+B group, single-leg stance on the surgical side decreased minimally at 2 months (13%) and kept decreasing at 6 months (24%). For the FT group, single-leg stance on the surgical side decreased 26% at 2 months and returned to baseline level at 6 months. Between-group differences in single-leg stance on the surgical side at 6 months favored the FT+B group by 25%. The decrease in single-leg stance time on the nonsurgical side was clinically important only in the FT+B group (38% at 2 months and 34% at 6 months). Figure 2 shows that both groups had improved scores on the performance-based tests from the baseline to the 2 follow-ups. Participants in the FT+B group continued to improve from 2 to 6 months, whereas the participants in the FT group maintained the same scores or had worse scores on these measures. Participants in both groups had similar improvements in WOMAC-PF and LEFS scores at the 2- and 6-month follow-ups (Tab. 3 and Fig. 2).
Table 3.
Physical Function Variables: Within-Group Changes (Follow-up − Baseline) and Between-Group Changes (FT+B Group − FT Group) at 2 and 6 Months After Total Knee Arthroplasty a
FT+B group received functional training supplemented with balance exercise program; FT group received functional training program only. WOMAC–PF=Western Ontario and McMaster Universities Osteoarthritis Index physical function subscale, LEFS=Lower Extremity Functional Scale, 95% CI=95% confidence interval.
Figure 2.
Lower-extremity functional status across time. Y axis represents outcome, and X axis represents time (1=baseline, 2=2-month follow-up, and 3=6-month follow-up). Solid and dashed lines represent the participants who received functional training supplemented with balance exercise program (FT+B group) and the participants who received functional training program only (FT group), respectively. WOMAC–PF=Western Ontario and McMaster Universities Osteoarthritis Index physical function subscale, LEFS=Lower Extremity Functional Scale.
Sample Size Estimations
The sample size for future trials was estimated based on the 3 primary outcomes (gait speed, chair rise time, and single-leg stance time), pain, and stiffness. Using an alpha level of .01 to account for 5 comparisons and a 2-sample t test to compare the groups, 88 participants per study arm will provide: 96% power to detect a difference in gait speed of 0.10 m/s (SD=0.155); 3% power to detect a difference in chair rise time of 0.62 second (SD=6.28) (would need 2,400 participants per arm to have 80% power); 91% power for a difference in single-leg stance time in the surgical side of 4.74 second (SD=7.91); 80% power for a difference in pain intensity of 1.1 second (SD=2.02); and 98% power to detect a difference in stiffness of 0.70 points (SD=0.98). Thus, 88 participants per study arm will allow differences in self-selected gait speed, single-leg stance balance, pain, and stiffness to be detected, although the power will be too low to detect differences in chair rise time or patient-reported physical function. Power was estimated using the Sample Power 2.0 equation for 2 independent-samples t test.
Discussion
This is the first study that demonstrated the feasibility and potential efficacy of combining FT with a balance exercise program in patients after TKA. The findings demonstrated potential clinically important benefits in walking speed, single-leg stance, stiffness, and pain intensity. The lack of adverse events, the high rate of adherence during the supervised and home programs, and the acceptable attrition rate underscore the need for further studies with larger samples sizes and longer follow-ups to draw more definitive conclusions about whether the FT+B program should be incorporated into clinical practice.
Although the pilot nature of the study does not allow conclusions to be drawn, the mean difference between groups in self-selected gait speed at 6 months was 0.1 m/s (approximately 0.2 mph), which is considered substantial meaningful change.42 The change observed in self-selected gait speed in the FT+B group was larger than the changes reported in a meta-analysis that examined the effect of therapeutic exercise on changing self-selected gait speed in community-dwelling older adults.45 Results of the meta-analysis indicated that traditional exercise training resulted in an overall gait speed change of 0.01 m/s, whereas specific exercises, such as those of high-intensity and high-dosage exercise programs, yielded gait speed changes on the magnitude of 0.02 m/s.45 With regard to the single-leg stance test, participants in the FT+B group could balance an average of 4 seconds longer than the baseline measurement in each leg at the 6-month follow-up. The ability to balance longer probably indicates improved balance and more confidence in the use of the surgical lower extremity and may have important clinical implications. If balancing longer associates with longer single-leg support time on the surgical side during gait, participation in the FT+B program may help decrease the gait asymmetry commonly reported after TKA,46 which ultimately may help protect the other weight-bearing joints.
To our knowledge, we delivered the intervention later after surgery than any other study. We enrolled participants from 2 to 6 months after TKA. Thus, the 6-week exercise program was initiated from the 9th week after TKA (for the participants enrolled 2 months after TKA) to the 23th week after TKA (for the participants enrolled 6 months after TKA) and terminated 6 weeks later (14–28 weeks after TKA). Thus, an interesting observation of the pilot study was that participants in the FT+B group continued to show improved functional performance from the 2-month follow-up to the 6-month follow-up (Fig. 2), a period in which previous studies have shown a plateau in functional improvement.6,10,47,48 Mizner et al49 described that functional plateau around 3 months after surgery. A meta-analysis on the effectiveness of exercise therapy after TKA concluded that most improvements in physical function take place within 3 to 4 months postoperatively.11 Kennedy et al48 reported that the greatest improvement in functional status occurred in the first 3 months after TKA, some improvement occurred at a lower rate from 3 to 6 months, and almost no improvement occurred from 6 to 12 months. Thus, in future trials, if the FT+B program is demonstrated to be effective when implemented several months after surgery, a time when supervised exercises generally are no longer prescribed, it may serve as the first step to justify later implementation of exercise programs following TKA.
The results of this pilot work seem to indicate that the patient-reported measures of function (WOMAC-PF and LEFS) do not capture the same information as the performance-based measures. This observation seems to support the use of performance-based measures as primary outcome measures in future trials and is in agreement with previous literature in TKA suggesting that performance-based measures are more sensitive to change50 and less influenced by pain32 than self-reported measures. Moreover, scores obtained with patient-report and performance-based measures seem particularly divergent in patients with TKA.31,51 In a sample of patients tested before and 8 weeks after TKA, Parent and Moffet31 reported that WOMAC-PF scores improved after 8 weeks compared with the preoperative values, whereas performance-based scores became worse. Another study showed that within 16 days after TKA, WOMAC-PF scores did not change, whereas LEFS scores changed moderately (function worsened) and performance-based scores markedly worsened.51
A limitation of our study is that we cannot interpret the WOMAC-PN and WOMAC-ST scores as representing symptoms specific to the surgical side. Because we did not ask the participants to focus on a particular knee during completion of the questionnaire, the WOMAC-PN and WOMAC-ST scores may have been influenced by their perception of pain and stiffness in the nonsurgical knee. Another limitation is not having collected information about the severity of osteoarthritis in the nonsurgical knee. In a future trial with a larger sample size, we intend to collect information specifically about pain and stiffness in the nonsurgical knee, as well as ascertain the degree of nonsurgical knee osteoarthritis using radiography,52 to account for their potential effects on outcome.
Some of the methods tested during the pilot work included feasibility of recruitment and appropriateness of inclusion and exclusion criteria. Recruiting participants at least 2 months after TKA by mailing study information was shown not to be difficult. From the 250 individuals informed about the study, 30% (76/250) were willing to participate and were assessed for eligibility, and from this group, 56% (43/76) were found to be eligible and were randomly assigned to treatment groups. In addition, because we enrolled an average of 3 to 4 participants a month while recruiting from a single surgeon, it seems that timely recruitment in a trial with a larger sample can be achieved by extending recruitment to several surgeons. We noticed that the attrition rate could have been minimized if we had had more stringent exclusion criteria. For example, if we had excluded individuals with musculoskeletal conditions that affected lower-extremity function and those who were unable to bear weight on the surgical knee, the participants who were lost to follow-up due to spinal or hip surgery or to malingering would not have been included. In a future trial, we plan to have more thorough exclusion criteria to minimize attrition even further.
A lesson learned during the implementation of the pilot study related to the choice of the primary outcome measure. When we originally designed the pilot study, we planned to use the Short Physical Performance Battery (SPPB) as the primary outcome measure. The SPPB has 3 components: self-selected gait speed during a 4-m walk, the Five-Times-Sit-to-Stand Test, and standing balance. Each component is scored from 0 (not able) to 4 (good function) and summed for a total score ranging from 0 to 12. The SPPB was chosen due to its good validity as a measure of lower-extremity function in older adults.53,54 However, when testing started, we observed a ceiling effect in the SPPB scores. The first participants tested reached the maximum score of 4 on gait speed (gait speed of ≥0.83 m/s) and standing balance (held a tandem stance for 10 seconds). This observation made us change the primary outcome measure. For gait speed and the Five-Times-Sit-to-Stand Test, we avoided a ceiling effect by recording the scores for speed (in meters per second) and time (in seconds) as continuous measures, rather than using a score ranging from 0 to 4. The tandem condition was replaced by a more challenging test of balance: the single-leg stance test.35 Therefore, the use of the SPPB and its scoring system does not seem appropriate for patients after TKA due to its potential ceiling effect (at the end of the baseline testing, 94% of the participants had a gait speed of ≥0.83 m/s, and 79% could hold the tandem stance for 10 seconds).
In a future randomized trial, although we plan to administer the same 3 performance-based tests used in the pilot study, self-selected gait speed will be the primary outcome measure. The rationale is that gait speed has been shown to predict functional decline, nursing home placement, and mortality.33,55–58 Specifically, a decrease in gait speed of 0.1 m/s has been associated with a 10% decrease in the ability to perform instrumental activities of daily living.59 In older adults, slowed gait speed has been related to an increased risk for falls.60–62 Combining this information with the fact that a change in self-selected gait speed of 0.1 m/s is considered substantial meaningful change,42 in a future trial we may want to dichotomize the outcome measure to calculate the proportion of participants in each study arm who increase their gait speed above 0.1 m/s. If we do so, the sample size estimation also seems adequate. Based on the pilot study results that 50% (9/18) of participants in the FT+B group and 24% (4/17) of the participants in the FT group increased their gait speed above 0.1 m/s, if we use a chi-square test to compare proportions between groups, having 88 participants per study arm (α=.01) will provide 81% power to detect clinically important between-group differences. Power was estimated using the Sample Power 2.0 equation to test proportions for 2 independent samples (chi-square test).
An additional concern with the sample size estimation is accounting for the attrition rate. As we intend in a future trial to determine the long-term effectiveness of the FT+B program (24-month follow-up), we will need to estimate the long-term attrition rate. As we anticipate ≅12% attrition at 6-month follow-up (lower than the attrition observed in the pilot work due to the more stringent exclusion criteria planned for a future trial) and we found patients with TKA to be committed study participants, we expect attrition to be 20% at 24 months. Consequently, we will need to randomize 114 participants per study arm to warrant 88 participants per group at the end of the trial.
An additional limitation of this pilot study is that, although we theorized that the balance exercise program would increase participants’ confidence to safely engage in a more physically active lifestyle, we did not measure physical activity. Anecdotal observation during trial implementation supports the need to measure physical activity. We received thank-you notes from 4 participants in this study. Coincidently, all 4 individuals participated in the FT+B group. The common theme of these notes was the patient's report of being much more active than prior to the exercise program. Lastly, although the performance-based tests used as the primary outcome measure cover important domains such as walking ability, balance, muscle power, and movement control, they may not be sufficiently sensitive to capture improvements due to challenging exercises that target balance and movement control. Future trials should include performance tasks with more skilled movement such as walking over obstacles and changing speeds and directions while walking.
Conclusions
The results of this pilot study indicated that the FT+B program is safe, is well tolerated, and has the potential to decrease functional limitations in patients with TKA. In future trials, we recommend that the exclusion criteria be more stringent to minimize attrition, that the signs and symptoms of the nonsurgical lower extremity be recorded and potentially controlled in the analyses, and that more challenging performance-based tasks be included to capture improvements in more skilled movements. A randomized trial with a larger sample size to test the effectiveness of the FT+B program to return patients with TKA to higher levels of physical function is warranted.
The Bottom Line
What do we already know about this topic?
Although exercise programs have been the mainstay of treatment for the functional deficits of patients after total knee arthroplasty (TKA), their effectiveness has been limited. To date, exercise programs have not targeted the deficits in balance and movement control of these patients.
What new information does this study offer?
This small study demonstrated that exercise programs that target balance and movement control are safe, well tolerated, and appear to improve functional performance, stiffness, and pain of patients after TKA.
If you're a patient, what might these findings mean for you?
Exercises that challenge a patient's balance may be beneficial. There is a need for conducting larger studies to confirm these results before incorporating these exercises into rehabilitation after TKA.
Appendix.
Appendix.
Exercise Therapy Procedures a
a FT+B group received functional training supplemented with balance exercise program; FT group received functional training program only. ROM=range of motion.
b Exercises were progressed if patient did not experience increased pain, effusion, giving away, and decreases in ROM.
c 1 ft=0.3048 m.
Footnotes
This study was approved by the University of Pittsburgh Institutional Review Board.
This research was presented at the Combined Sections Meeting of the American Physical Therapy Association; February 9–12, 2009; Las Vegas, Nevada.
This study was funded by the Central Research Development Fund; the University of Pittsburgh Medical Center Health System Competitive Medical Research Fund; the Claude D. Pepper Older American Independence Center (P30-AG024827); the National Center for Research Resources, a component of the National Institutes of Health, and NIH Roadmap for Medical Research (KL2 RR024154-02); and the American College of Rheumatology Research and Education Foundation New Investigator Award.
SPSS Inc, 233 S Wacker Dr, Chicago, IL 60606.
Dr Piva and Dr Fitzgerald provided concept/idea/research design and fund procurement. Dr Piva provided writing. Ms Gil and Mr Almeida provided data collection and clerical support. Dr Fitzgerald provided data analysis. Ms Gil, Mr Almeida, and Mr Levison provided project management. Dr DiGioia and Mr Levison provided participants and facilities/equipment. Ms Gil, Dr DiGioia, Mr Levison, and Dr Fitzgerald provided institutional liaisons. All authors provided consultation (including review of manuscript before submission).
References
- 1.Kurtz SM, Ong KL, Schmier J, et al. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty 2009;24:195–203 [DOI] [PubMed] [Google Scholar]
- 2.Franklin PD, Li W, Ayers DC. The Chitranjan Ranawat Award: functional outcome after total knee replacement varies with patient attributes. Clin Orthop Relat Res 2008;466:2597–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradbury N, Borton D, Spoo G, Cross MJ. Participation in sports after total knee replacement. Am J Sports Med 1998;26:530–535 [DOI] [PubMed] [Google Scholar]
- 4.Konig A, Walther M, Kirschner S, Gohlke F. Balance sheets of knee and functional scores 5 years after total knee arthroplasty for osteoarthritis: a source for patient information. J Arthroplasty 2000;15:289–294 [DOI] [PubMed] [Google Scholar]
- 5.Walsh MB, Woodhouse LJ, Thomas SG, Finch E. Physical impairments and functional limitations: a comparison of individuals 1 year after total knee arthroplasty with control subjects. Phys Ther 1998;78:248–258 [DOI] [PubMed] [Google Scholar]
- 6.Frost H, Lamb SE, Robertson S. A randomized controlled trial of exercise to improve mobility and function after elective knee arthroplasty: feasibility, results and methodological difficulties. Clin Rehabil 2002;16:200–209 [DOI] [PubMed] [Google Scholar]
- 7.Shepperd S, Harwood D, Jenkinson C, et al. Randomised controlled trial comparing hospital at home care with inpatient hospital care, I: three month follow up of health outcomes. BMJ 1998;316:1786–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer JF, Speechley M, Bourne R, et al. Comparison of clinic- and home-based rehabilitation programs after total knee arthroplasty. Clin Orthop Relat Res 2003;410:225–234 [DOI] [PubMed] [Google Scholar]
- 9.Walsh MB, Herbold J. Outcome after rehabilitation for total joint replacement at IRF and SNF: a case-controlled comparison. Am J Phys Med Rehabil 2006;85:1–5 [DOI] [PubMed] [Google Scholar]
- 10.Moffet H, Collet JP, Shapiro SH, et al. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: a single-blind randomized controlled trial. Arch Phys Med Rehabil 2004;85:546–556 [DOI] [PubMed] [Google Scholar]
- 11.Minns Lowe CJ, Barker KL, Dewey M, Sackley M. Effectiveness of physiotherapy exercise after knee arthroplasty for osteoarthritis: systematic review and meta-analysis of randomised controlled trials. BMJ 2007;335:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attfield SF, Wilton TJ, Pratt DJ, Sambatakakis A. Soft-tissue balance and recovery of proprioception after total knee replacement. J Bone Joint Surg Br 1996;78:540–545 [PubMed] [Google Scholar]
- 13.Barrett DS, Cobb AG, Bentley G. Joint proprioception in normal, osteoarthritic and replaced knees. J Bone Joint Surg Br 1991;73:53–56 [DOI] [PubMed] [Google Scholar]
- 14.Swanik CB, Lephart SM, Rubash HE. Proprioception, kinesthesia, and balance after total knee arthroplasty with cruciate-retaining and posterior stabilized prostheses. J Bone Joint Surg Am 2004;86:328–334 [DOI] [PubMed] [Google Scholar]
- 15.Viton JM, Atlani L, Mesure S, et al. Reorganization of equilibrium and movement control strategies after total knee arthroplasty. J Rehabil Med 2002;34:12–19 [DOI] [PubMed] [Google Scholar]
- 16.Wada M, Kawahara H, Shimada S, et al. Joint proprioception before and after total knee arthroplasty. Clin Orthop Relat Res 2002;403:161–167 [DOI] [PubMed] [Google Scholar]
- 17.Gage WH, Frank JS, Prentice SD, Stevenson P. Organization of postural responses following a rotational support surface perturbation, after TKA: sagittal plane rotations. Gait Posture 2007;25:112–120 [DOI] [PubMed] [Google Scholar]
- 18.Gage WH, Frank JS, Prentice SD, Stevenson P. Postural responses following a rotational support surface perturbation, following knee joint replacement: frontal plane rotations. Gait Posture 2008;27:286–293 [DOI] [PubMed] [Google Scholar]
- 19.Mandeville D, Osternig LR, Chou LS. The effect of total knee replacement surgery on gait stability. Gait Posture 2008;27:103–109 [DOI] [PubMed] [Google Scholar]
- 20.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307–312 [DOI] [PubMed] [Google Scholar]
- 21.Rajan RA, Pack Y, Jackson H, et al. No need for outpatient physiotherapy following total knee arthroplasty: a randomized trial of 120 patients. Acta Orthop Scand 2004;75:71–73 [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald GK, Childs JD, Ridge TM, Irrgang JJ. Agility and perturbation training for a physically active individual with knee osteoarthritis. Phys Ther 2002;82:372–382 [PubMed] [Google Scholar]
- 23.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–1840 [PubMed] [Google Scholar]
- 24.Bellamy N, Kean WF, Buchanan WW, et al. Double blind randomized controlled trial of sodium meclofenamate (Meclomen) and diclofenac sodium (Voltaren): post validation reapplication of the WOMAC Osteoarthritis Index. J Rheumatol 1992;19:153–159 [PubMed] [Google Scholar]
- 25.Hawker G, Melfi C, Paul J, et al. Comparison of a generic (SF-36) and a disease-specific (WOMAC) (Western Ontario and McMaster Universities Osteoarthritis Index) instrument in the measurement of outcomes after knee replacement surgery. J Rheumatol 1995;22:1193–1196 [PubMed] [Google Scholar]
- 26.Jensen MP, Turner JA, Romano JM. What is the maximum number of levels needed in pain intensity measurement? Pain 1994;58:387–392 [DOI] [PubMed] [Google Scholar]
- 27.Marx RG, Jones EC, Allen AA, et al. Reliability, validity, and responsiveness of four knee outcome scales for athletic patients. J Bone Joint Surg Am 2001;83:1459–1469 [DOI] [PubMed] [Google Scholar]
- 28.Katz J, Melzack R. Measurement of pain. Surg Clin North Am 1999;79:231–252 [DOI] [PubMed] [Google Scholar]
- 29.Bellamy N, Kirwan J, Boers M, et al. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip, and hand osteoarthritis: consensus development at OMERACT III. J Rheumatol 1997;24:799–802 [PubMed] [Google Scholar]
- 30.Woolhead GM, Donovan JL, Dieppe PA. Outcomes of total knee replacement: a qualitative study. Rheumatology (Oxford) 2005;44:1032–1037 [DOI] [PubMed] [Google Scholar]
- 31.Parent E, Moffet H. Comparative responsiveness of locomotor tests and questionnaires used to follow early recovery after total knee arthroplasty. Arch Phys Med Rehabil. 2002;83:70–80 [DOI] [PubMed] [Google Scholar]
- 32.Stratford PW, Kennedy DM, Woodhouse LJ. Performance measures provide assessments of pain and function in people with advanced osteoarthritis of the hip or knee. Phys Ther 2006;86:1489–1496 [DOI] [PubMed] [Google Scholar]
- 33.Hardy SE, Perera S, Roumani YF, et al. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc 2007;55:1727–1734 [DOI] [PubMed] [Google Scholar]
- 34.Jette AM, Jette DU, Ng J, et al. Are performance-based measures sufficiently reliable for use in multicenter trials? J Gerontol A Biol Sci Med Sci 1999;54:M3–M6 [DOI] [PubMed] [Google Scholar]
- 35.Curb JD, Ceria-Ulep CD, Rodriguez BL, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc 2006;54:737–742 [DOI] [PubMed] [Google Scholar]
- 36.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. J Am Geriatr Soc 2009;57:251–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci 2001;56:M644–M649 [DOI] [PubMed] [Google Scholar]
- 38.Seeman TE, Charpentier PA, Berkman LF, et al. Predicting changes in physical performance in a high-functioning elderly cohort: MacArthur studies of successful aging. J Gerontol 1994;49:M97–M108 [DOI] [PubMed] [Google Scholar]
- 39.Binkley JM, Stratford PW, Lott SA, Riddle DL; North American orthopaedic Rehabilitation Research Network The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther 1999;79:371–383 [PubMed] [Google Scholar]
- 40.Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407–415 [DOI] [PubMed] [Google Scholar]
- 41.Jones B, Jarvis P, Lewis JA, Ebbutt AF. Trials to assess equivalence: the importance of rigorous methods. BMJ 1996;313:36–39; erratum in BMJ. 1996;313:550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical perfomance measures in older adults. J Am Geriatr Soc 2006;54:743–749 [DOI] [PubMed] [Google Scholar]
- 43.Pua YH, Cowan SM, Wrigley TV, Bennell KL. The Lower Extremity Functional Scale could be an alternative to the Western Ontario and McMaster Universities Osteoarthritis Index physical function scale. J Clin Epidemiol 2009;62:1103–1111 [DOI] [PubMed] [Google Scholar]
- 44.Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine 2005;30:1331–1334 [DOI] [PubMed] [Google Scholar]
- 45.Lopopolo RB, Greco M, Sullivan D, et al. Effect of therapeutic exercise on gait speed in community-dwelling elderly people: a meta-analysis. Phys Ther 2006;86:520–540 [PubMed] [Google Scholar]
- 46.Andriacchi TP, Galante JO, Fermier RW. The influence of total knee-replacement design on walking and stair-climbing. J Bone Joint Surg Am 1982;64:1328–1335 [PubMed] [Google Scholar]
- 47.Kennedy DM, Stratford PW, Hanna SE, et al. Modeling early recovery of physical function following hip and knee arthroplasty. BMC Musculoskelet Disord 2006;7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kennedy DM, Stratford PW, Riddle DL, et al. Assessing recovery and establishing prognosis following total knee arthroplasty. Phys Ther 2008;88:22–32 [DOI] [PubMed] [Google Scholar]
- 49.Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther 2005;35:424–436 [DOI] [PubMed] [Google Scholar]
- 50.Stratford PW, Kennedy DM, Riddle DL. New study design evaluated the validity of measures to assess change after hip or knee arthroplasty. J Clin Epidemiol 2009;62:347–352 [DOI] [PubMed] [Google Scholar]
- 51.Stratford PW, Kennedy DM. Performance measures were necessary to obtain a complete picture of osteoarthritic patients. J Clin Epidemiol 2006;59:160–167 [DOI] [PubMed] [Google Scholar]
- 52.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guralnik JM, Seeman TE, Tinetti ME, et al. Validation and use of performance measures of functioning in a non-disabled older population: MacArthur studies of successful aging. Aging (Milano) 1994;6:410–419 [DOI] [PubMed] [Google Scholar]
- 54.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–M231 [DOI] [PubMed] [Google Scholar]
- 55.Brach JS, Vanswearingen JM, Newman AB, Kriska AM. Identifying early decline of physical function in community-dwelling older women: performance-based and self-report measures. Phys Ther 2002;82:320–328 [PubMed] [Google Scholar]
- 56.Gill TM, Williams CS, Tinetti ME. Assessing risk for the onset of functional dependence among older adults: the role of physical performance. J Am Geriatr Soc 1995;43:603–609 [DOI] [PubMed] [Google Scholar]
- 57.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spirduso WW, Cronin DL. Exercise dose-response effects on quality of life and independent living in older adults. Med Sci Sports Exerc 2001;33:S598–S608 [DOI] [PubMed] [Google Scholar]
- 59.Judge JO, Schechtman K, Cress E; the FICSIT (Frailty and Injury: Cooperative Studies of Intervention Trials) Group The relationship between physical performance measures and independence in instrumental activities of daily living. J Am Geriatr Soc 1996;44:1332–1341 [DOI] [PubMed] [Google Scholar]
- 60.Daley MJ, Spinks WL. Exercise, mobility and aging. Sports Med 2000;29:1–12 [DOI] [PubMed] [Google Scholar]
- 61.Rubenstein LZ, Powers CM, Maclean CH. Quality indicators for the management and prevention of falls and mobility problems in vulnerable elders. Ann Intern Med 2001;135:686–693 [DOI] [PubMed] [Google Scholar]
- 62.Sadeghi H, Prince F, Zabjek KF, et al. Knee flexors/extensors in gait of elderly and young able-bodied men (II). Knee 2002;9:55–63 [DOI] [PubMed] [Google Scholar]