Abstract
In the brains of male zebra finches (Taeniopygia guttata), the nuclei that direct song learning and production are larger than the corresponding regions in females, who do not sing. The dimorphism in Area X of the medial striatum (MSt), an area important for song learning, is even more dramatic in that it is identifiable in males but not females by Nissl stain. In the present study, conspecific song, but not other auditory stimuli, induced expression of the immediate early gene ZENK in the MSt surrounding but not within Area X in juvenile males (30 and 45 days post-hatch). ZENK immunoreactivity following conspecific songs was homogeneous throughout the MSt of females at the same ages. Little to no FOS immunoreactivity was observed in Area X or the rest of the MSt, and levels were not influenced by the type of auditory stimulus presented. Thus, the clear morphological difference in the lateral MSt (Area X) of males and females is mirrored by a specific functional one, and the data suggest a role for ZENK expression in the MSt outside of Area X in responding to relevant song stimuli.
Keywords: Song perception, Immediate early gene, Area X, Basal ganglia, Song learning, Sex difference
Nottebohm and Arnold [23] first described dramatic sexual dimorphisms in the brains of adult zebra finches and canaries wherein regions that control song learning and production are larger in males that sing than in females that do not. One of these regions, Area X of the medial striatum (MSt), is not identifiable via Nissl stains in females. Area X plays an integral role in male song learning and possibly song production in adulthood. For example, juvenile males with Area X ablated produce abnormal song in adulthood, although in adult males lesions of the nucleus have no detectable effect on song production or quality [26,31]. Interestingly, neurons in Area X show singing-related electrophysiological activity that declines slowly after song stops being produced [11], suggesting a function for the region outside of the sensitive period for song learning. Cells in this region in both juveniles and adults also display long-term potentiation [7]. In birds that hear song and sing in response, ZENK expression is increased in Area X compared to birds that hear song only or birds that hear no song [13–15,24]. Additionally, when the tracheosyringeal nerve is axotomized in developing males, the electrophysiological activity of some neurons within Area X is then tuned to the abnormal, axotomized bird’s own song as well as the tutor’s song, suggesting that neurons within Area X are important for vocal practice [32]. In males, the Nissl-defined volume of Area X increases between d10 and d40, when the adult volume is reached [20], and this change in volume is due in part to the addition of neurons during that period in males but not females [16,21].
While it is clear that Area X is highly sexually dimorphic and important for the development of song in males, it was unknown whether the corresponding lateral region of MSt in females is responsive to auditory stimuli. Therefore, in the present study, birds were presented with auditory stimuli at two periods in development: when males [8,12] and perhaps females [6,19] are beginning to learn song (d30), and when males commence practicing what they have learned (d45 [8,12]). Densities of ZENK and FOS immunoreactive cells were quantified within Area X in males and the corresponding area (lateral MSt, lMSt) in females. In addition, because examination of the tissue indicated that the rest of the MSt showed increased ZENK expression under some conditions in both sexes, a medial portion (mMSt) was analyzed too, which also allowed a comparison between sexes and across groups in a “control” region neighboring the one of primary interest.
Male and female juvenile zebra finches were used (d30 and d45, with day of hatch as d1; number of birds per group indicated in Fig. 2). Birds were housed in communal aviaries with their parents until removal for exposure to stimuli. Each aviary was maintained with a constant supply of finch seed and water, and fruit or vegetables were provided weekly. Lights in the aviary were set to a 12:12 cycle, with lights on at 07:00. This experiment was conducted according to guidelines of the National Institutes of Health detailed in Guide for the Care and Use of Laboratory Animals. Data from the caudomedial nidopallium (NCM), caudomedial mesopallium (CMM) and hippocampus from these animals were previously reported [3,4].
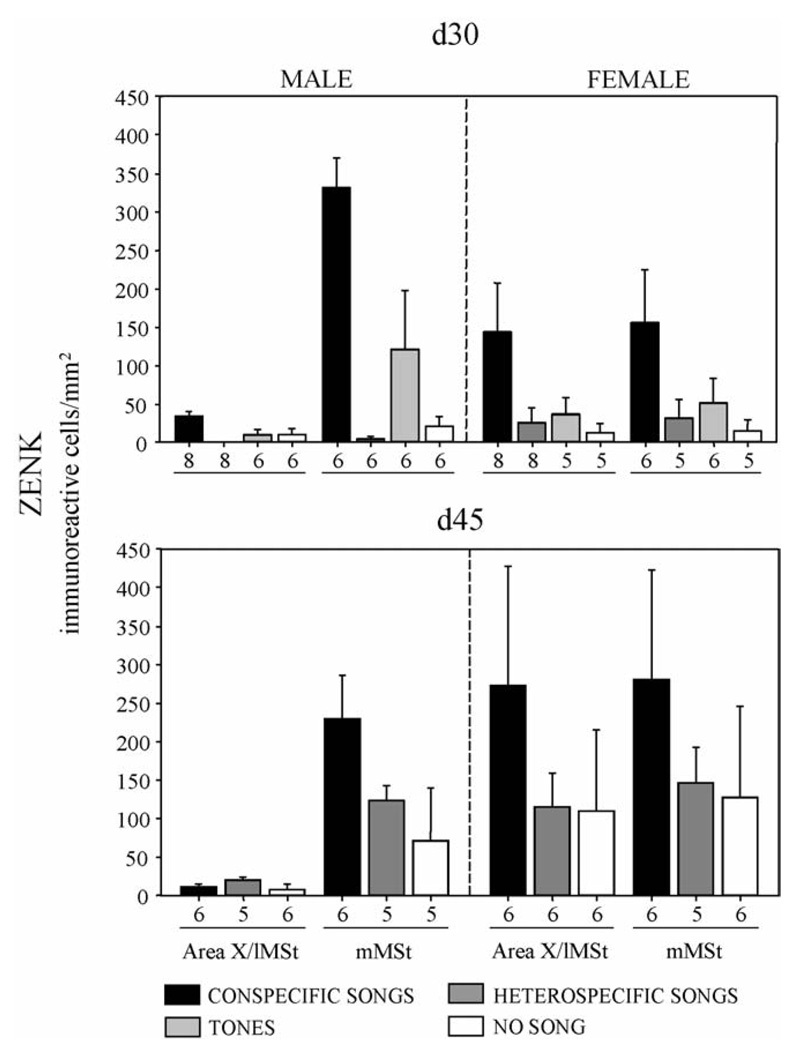
Fig. 2.
Mean (+S.E.M.) density of ZENK-immunoreactive neurons in Area X or the corresponding region in females (the lateral portion of the medial striatum; lMSt) and the medial portion of the medial striatum (mMSt) in both sexes. Analyses were conducted in birds 30 (top panels) or 45 (bottom panels) days post-hatch (d30 or d45) following presentations of conspecific songs, heterospecific songs, randomly generated tones (d30 only) or no song. The number of birds per group is indicated below each x-axis.
At the beginning of each session, an individual bird was removed from an aviary, placed in a stainless steel cage and immediately transported to a sound isolated room. Following a 60-min acclimation period, a birdwas presented with either conspecific songs, heterospecific songs, tones (d30 only) or no song for 30 min followed by a 60-min period of silence. For d30 birds, song/tone files or an empty sound file were played using Cool Edit (Syntrillium Software; now Adobe Audition, Adobe Systems) through a speaker connected to a PC [3]; for the d45 birds, files were burned onto compact disc and delivered via a Sony CD Walkman (#D-E220) connected to the same speaker [4]. Ten conspecific songs were obtained from males from our colony. Heterospecific songs (American robin, Baird’s sparrow, Bell’s vireo, Cassin’s finch, Connecticut warbler, marsh wren, Scott’s oriole, summer tanager, western meadowlark and white breasted nuthatch) were acquired from the National Geographic Society/Cornell Laboratory of Ornithology’s Guide to Bird Sounds and were selected based on some similarities in frequency and bout length to zebra finch song. Ten tone files were generated using Cool Edit, and these tone sets were created with characteristics (frequency range, note length, silent intervals between notes) similar to zebra finch song. All auditory stimuli were 30 s in length, were novel to the birds, and were the same as indicated in prior published reports [2–4]. Three conspecific songs, heterospecific songs or tone sets were randomly picked from their banks of ten and played in a fixed order, separated by 30 s of silence, and repeated for the 30-min stimulation period. Acclimation, song exposure and the post-auditory stimulus period were carried out in the dark, replicating the methods from our prior study in adult female zebra finches [2].
Following the 60-min post-auditory stimulus period, birds were given an overdose of Equithesin in the dark and perfused with 0.1M phosphate buffered saline (PBS) followed by 4% paraformaldehyde in PBS. Brains were removed and the sex of each bird was confirmed by inspecting its gonad(s). Following a 1-h post-fix in paraformaldehyde, brains were sunk overnight in 30% sucrose in PBS at 4 °C. Alternate series of 30 µm coronally cut sections were collected and stored in cryoprotectant overnight at 4 °C then at −20 °C until immunohistochemical processing.
As we did not intend to directly compare across ages, tissue from d30 and d45 animals were not processed concurrently for immunohistochemistry. However, within each age group, sections from at least one bird of each sex and stimulus condition underwent the immunohistochemical procedure simultaneously to limit confounds due to variations in the processing of the tissue. Cryoprotected sections were rinsed for one hr in PBS. Immunohistochemistry for c-FOS and ZENK was performed in alternate sets of tissue by means of a chicken primary antibody to detect c-FOS (1:20,000) and a commercially available antibody forZENK(Santa Cruz Biotech; catalog #sc-189; 0.1µg/ml [3,4]). Following primary antibody incubation, protein visualization was completed with the addition of a donkey anti-rabbit secondary antibody (1:500; Jackson ImmunoResearch Laboratories), Elite ABC kit solution (Vector Laboratories) and diaminobenzidine (Sigma). Sections were then mounted on gelatin-coated slides, dehydrated, cleared in xylene and coverslipped with Permount.
The densities of FOS- and ZENK-immunoreactive nuclei were determined by hand using a 0.49×0.49mm ocular grid. Cells with distinct nuclear staining were counted in the lMSt in four sections by an experimenter blind to sex and type of auditory stimuli presented. Either the left or right side of the brain was randomly selected between the region where the border of the MSt becomes nearly perpendicular with the ventral surface of the brain to where the height of the overlying nidopallium at its medial edge is relatively narrow and the portion of the lateral ventricle neighboring the nidopallium begins to curve. This region included nearly the entire rostral-to-caudal extent of Area X in males. Counts were also taken in the adjacent mMSt, in which the ocular grid was placed 0.049mm from the midline and 0.196mm below the pallial–subpallial lamina separating MSt from the overlying nidopallium, approximately 0.2mm below the nidopallial border. The four densities of immunoreactive neurons were averaged within each brain region for each animal. Analyses of variance were used to evaluate effects of sex (between birds), auditory stimulus condition (between birds) and MSt region (within birds) independently for FOS and ZENK, and separately for the two ages. When warranted, Fisher’s PLSD was used post hoc to further examine significant effects of auditory stimulus exposures, and planned comparisons (paired t-tests) were used to determine differences in immunoreactivity between the two regions of the MSt within animals.
In d30 birds (Figs. 1 and 2), significant differences in ZENK immunoreactivity were found across auditory stimulus conditions (F(3, 42) = 8.54, p = 0.0002) and between the lateral (Area X in males) and medial portions of MSt (F(1, 42) = 27.98, p < 0.0001). Region within MSt also interacted significantly with auditory stimulus condition (F(3, 42) = 12.00, p < 0.0001). However, the most intriguing results stem from the interactions involving the two sexes. Area within MSt interacted with sex (F(1, 42) = 20.03, p < 0.0001), and a significant three-way interaction involving region of MSt, sex and auditory stimulus condition was also detected (F(1, 42) = 27.98, p < 0.0001). In both the lMSt (F(3, 42) = 3.18, p = 0.034) and mMSt (F (3, 42) = 11.66, p < 0.0001), conspecific song produced an increased density of immunoreactive cells compared to all other manipulations (Fisher’s PLSD all p < 0. 041); no significant differences were found among the other auditory stimuli (all p > 0.201). Importantly, following conspecific song presentations, the density of ZENK immunoreactive neurons in males was very low in Area X compared to the surround (Fig. 1C; t(7) = 7.82, p = 0.0001), whereas it was uniform throughout the MSt in females (Fig. 1D; t(7) = 1.36, p = 0.216). Levels of FOS immunoreactivity were approximately 1/10th those of ZENK (Fig. 1), and no significant main effects or interactions were uncovered in the MST in the FOS analysis in these same d30 birds (data not shown).
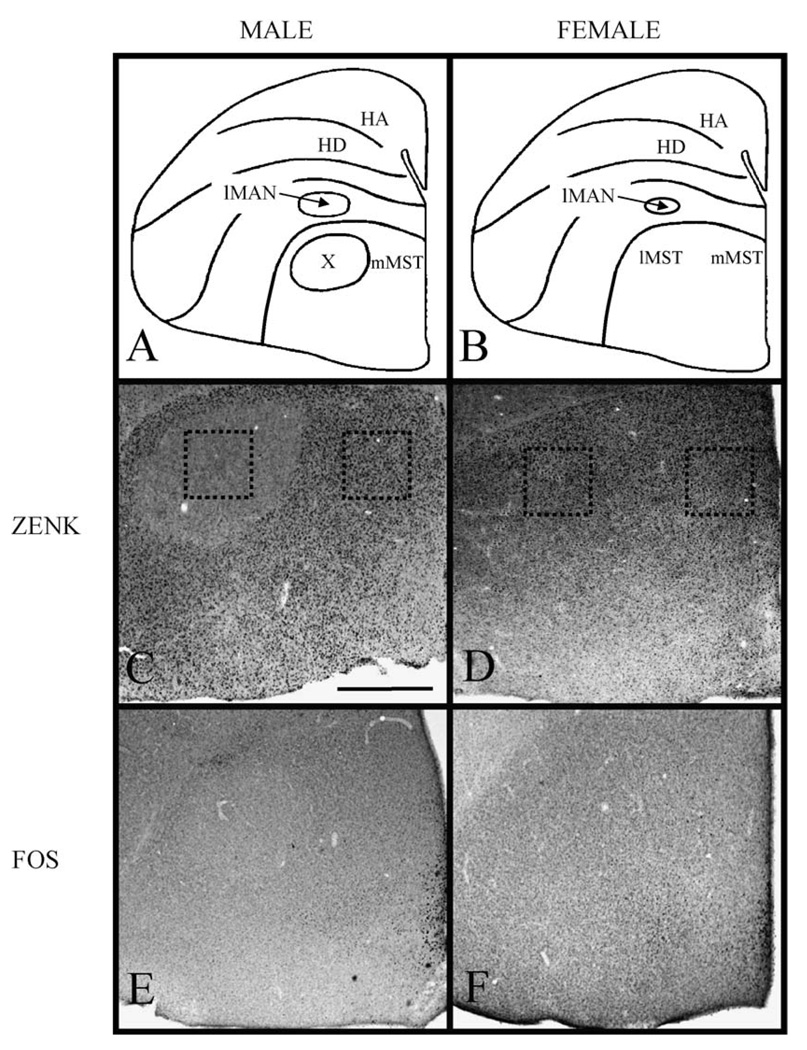
Fig. 1.
Drawings of coronal sections through the zebra finch brain detailing the structures visible via Nissl stain (panels A and B). In males (A), Area X is evident as a large nucleus distinct from the surrounding medial striatum (MSt). Area X is not detectable in the female MSt (B). The remaining panels are photomicrographs of coronal sections through the MSt of juvenile (d30) zebra finches detailing ZENK and FOS immunoreactivity following conspecific song presentations. Note the almost complete absence of ZENK immunoreactivity in Area X in males (panel C, dotted box on left) compared to the relatively homogeneous immunoreactivity throughout the MSt in females (dotted boxes in panel D). Few or no FOS immunoreactive neurons were detected in either the male (E) or female (F) MSt. The midline is at the right in each section. HA, hyperpallium apicale; HD, hyperpallium densocellulare; lMAN, lateral portion of the magnocellular nucleus of the anterior nidopallium; X, Area X of the medial striatum (MSt); mMST, medial portion of the medial striatum, lMSt; lateral portion of the medial striatum. Scale bar = 1.0 mm.
Similar to d30, at d45 (Fig. 2, bottom) significant differences in ZENK immunoreactivity were found between the lMSt and mMSt (F (1, 29) = 23.27, p < 0.0001), and this variable interacted significantly with sex (F(1, 29) = 12.81, p = 0.0012). No main effect of auditory stimulus condition or interaction with sex was found within mMST or lMST (all F < 1.71, all p > 0.199). A sex difference in ZENK positive cells was uncovered in lMST (F(1, 29) = 5.32, p = 0.028) but not mMST (F (1, 29) = 0.37, p = 0.551), replicating the lack of a ZENK response within Area X in males and the homogeneity of immunoreactivity throughout the MSt in females. Like the d30 results, the density of ZENK immunoreactive neurons in Area X was significantly lower than that in the mMSt of males that heard conspecific songs (t(5) = 3.82, p = 0.012), and no difference in ZENK immunoreactivity was found between the female lMSt and mMSt(t(5) = 0.41, p = 0.697). Also similar to those data from d30 birds, no significant main effects or interactions were uncovered in d45 birds using the FOS analysis, and the levels were quite low.
The present data on ZENK labeling parallel the sex difference observed in the MSt with Nissl staining, in that Area X is distinct from the surrounding MSt only in males (reviewed in [34]) and, interestingly, following only conspecific song presentations. The morphological difference is therefore mirrored by a functional one: ZENK is not induced in Area X in males with song presentations but is up-regulated within the corresponding region in females similar to the rest of the MSt. These patterns of ZENK immunoreactivity suggest differential roles for cells in the lMSt in males and females based perhaps on demand for the learning and production of vocal communication signals in males, and highlight a potential role for the mMSt in song behavior that warrants further investigation.
Similar patterns within the MSt are seen in neurochemical data from other studies. FOXP1 and FOXP2, genes of a family involved in human language, are differentially expressed in male and female zebra finches. In developing males, Area X expresses considerably more FOXP2 mRNA than the surrounding MSt [9], but in adulthood, expression within Area X is not discernable from other portions of the region [9] or is only slightly higher [33]. In adult females, mRNA for FOXP2 [9] and FOXP1 [33] is homogenously and highly expressed throughout the MSt. Further, neurons immunoreactive for an antibody to the NMDA receptor subunit NR1 are not detected within Area X in adult males but are found in the surrounding striatum. Interestingly, NR1-immunoreactive cells are found throughout the striatum in females [25]. These patterns suggest that an interaction between ZENK and the other genes is possible. For example, ZENK induced by hearing conspecific song could influence the expression of NR1 in the MSt outside of Area X. ZENK is integral in the modulation of neuronal excitability (reviewed in [17]), and expression of zif-268 (of which ZENK is an avian homolog) in rodents plays a role in maintaining plastic changes associated with long-term potentiation (LTP) [17]. Glutamatergic receptors are important in LTP as well [22], and activation of them during song template acquisition is vital to song learning and perhaps even the opening of the sensitive period for it [1,5,10,22,27,29]. Evidence from slice preparations from adult males and those as young as d47 shows that neurons within Area X exhibit activity-dependent LTP that is not induced between d24 and d37 [7] and can be blocked by the NMDA receptor antagonist AP5 [7]. An analysis of whether ZENK and these other genes are co-expressed at various developmental stages in cells within Area X and the rest of the MSt following directed singing and presentations of conspecific song can lead to more of an understanding of their roles in influencing song learning and behavior.
That ZENK but little if any FOS is induced in the MSt in response to song at the ages studied suggests markedly different roles for these immediate early genes in the responses of neurons in this region at these developmental stages. Interestingly, in these same d30 birds, neurons within auditory areas in females showed specificity for conspecific songs with FOS but not ZENK expression, and the same regions in males showed specific ZENK but not FOS induction [3]. In the d45 animals analyzed in this study, cells within auditory perceptual regions did not exhibit this differential effect; both sexes produced increased ZENK and FOS expression [4]. Differential responses between ZENK and FOS are also seen in other songbirds. Experience with long-bout songs increases the ZENK response in the CMM and NCM of European starlings when exposed to novel long-bout compared to short-bout songs. In contrast, experience with short-bout songs results in an increased FOS response to long-bout songs when novel types of both song stimuli are presented to separate groups of females [30]. Based on some similarities in the signal transduction cascades of these immediate early genes, differences in the function of ZENK and FOS are difficult to interpret. Data from the present study and that of others reinforces the need to quantify levels of multiple immediate early genes in studies of neural responses to auditory signals.
An exciting possibility is that the mMSt in both male and female zebra finches is involved somewhat generally in motivated responses, not just those induced by conspecific song. While it is clear that Area X is important for song learning in juvenile males [31], the MSt outside of this region may serve functions likely to be more common in the two sexes. Alternatively, the ZENK response in d30 and d45 birds in the present study could result from a specialization of neurons within the MSt during the song-learning period that is not conserved into adulthood. A similar analysis should be undertaken in adult males and females (>d100) to determine whether the expression pattern observed is solely a feature of developing neurons within the MSt/Area X. For example, if ZENK is no longer expressed in this region in adult males and females, it would be consistent with a role for this gene in the mMSt on processes related to the development of both sexes, including possibly song memorization [6,8,12,19].
Perhaps even more interesting is the dramatic sex difference in immunoreactivity seen between the lMST in females and Area X in males following conspecific song stimuli. Data from a few studies indicate the potential for a structure resembling Area X to be present in females. For example, injections of 3H-thymidine between d20 and d30 [21] and an anterograde tracer in adulthood [28] into HVC result in some labeling in the area of the female MSt where Area X is located in males. Additionally, retrograde tracer injections into the lMSt of female birds revealed labeled cell bodies in the lateral portion of the magnocellular nucleus of the anterior nidopallium (lMAN; [18]); this finding was confirmed by anterograde tracer injection into lMAN, which resulted in a network of labeled fibers in the lMSt [18]. Although the present data do not rule out the possibility that particular functions of Area X in males may be achieved by a small subset of cells dispersed in the lMSt in females, the current findings and those of others are more consistent with the idea that a functional Area X is not present in females. Together with reported sex differences in the neurochemistry of the cells in this region (see above), it appears that the phenotype and not just the gross morphology of cells in this region is sexually differentiated.
Acknowledgements
We thank Stephanie Fuehring, Katie Grausam, Nancy Oberg and Malik Williams for help with tissue collection and processing and Dr. Els D’Hondt from the lab of Dr. Frans Vandesande, Catholic University of Leuven, Belgium, for the FOS antibody. This work was supported by NIH grants RO1 MH55488, K02 065907 (JW) and F31 MH64982 (DJB).
References
- 1.Aamodt SM, Nordeen EJ, Nordeen KW. Blockade of NMDA receptors during song model exposure impairs song development in juvenile zebra finches. Neurobiol. Learn. Mem. 1996;65:91–98. doi: 10.1006/nlme.1996.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bailey DJ, Rosebush JC, Wade J. The hippocampus and caudomedial neostriatum show selective responsiveness to conspecific song in the female zebra finch. J. Neurobiol. 2002;52:43–51. doi: 10.1002/neu.10070. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DJ, Wade J. Differential expression of the immediate early genes FOS and ZENK following auditory stimulation in the juvenile male and female zebra finch. Mol. Brain Res. 2003;116:147–154. doi: 10.1016/s0169-328x(03)00288-2. [DOI] [PubMed] [Google Scholar]
- 4.Bailey DJ, Wade J. FOS and ZENK responses in 45 day-old zebra finches vary with auditory stimulus and brain region, but not sex. Behav. Brain. Res. 2005;162:108–115. doi: 10.1016/j.bbr.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Basham ME, Nordeen EJ, Nordeen KW. Blockade of NMDA receptors in the anterior forebrain impairs sensory acquisition in the zebra finch (Poephila guttata) Neurobiol. Learn. Mem. 1996;66:295–304. doi: 10.1006/nlme.1996.0071. [DOI] [PubMed] [Google Scholar]
- 6.Clayton NS. Song discrimination learning in zebra finches. Anim. Behav. 1988;36:1016–1024. [Google Scholar]
- 7.Ding L, Perkel DJ. Long-term potentiation in an avian basal ganglia nucleus essential for vocal learning. J. Neurosci. 2004;24:488–494. doi: 10.1523/JNEUROSCI.4358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eales LA. Song learning in zebra finches: some effects of song model availability on what is learnt and when. Anim. Behav. 1985;33:1293–1300. [Google Scholar]
- 9.Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C. FoxP2 expression in avian vocal learners and non-learners. J. Neurosci. 2004;24:3164–3175. doi: 10.1523/JNEUROSCI.4369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinrich JE, Singh TD, Sohrabji F, Nordeen KW, Nordeen EJ. Developmental and hormonal regulation of NR2A mRNA in forebrain regions controlling avian vocal learning. J. Neurobiol. 2002;51:149–159. doi: 10.1002/neu.10046. [DOI] [PubMed] [Google Scholar]
- 11.Hessler NA, Doupe AJ. Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J. Neurosci. 1999;19:10461–10481. doi: 10.1523/JNEUROSCI.19-23-10461.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Immelmann K. Song development in the zebra finch and other estrildid finches. In: Hinde R, editor. Bird Vocalizations. Cambridge: University Press; 1969. pp. 61–74. [Google Scholar]
- 13.Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- 15.Jin H, Clayton DF. Localized changes in immediate-early gene regulation during sensory and motor learning in zebra finches. Neuron. 1997;19:1049–1059. doi: 10.1016/s0896-6273(00)80396-7. [DOI] [PubMed] [Google Scholar]
- 16.Kirn JR, DeVoogd TJ. Genesis and death of vocal control neurons during sexual differentiation in the zebra finch. J. Neurosci. 1989;9:3176–3187. doi: 10.1523/JNEUROSCI.09-09-03176.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGF1-A/Krox-24/TIS8/ZENK? Prog. Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Kreck G, Nixdorf-Bergweiler BE. Evidence for a cortical-basal ganglia projection pathway in female zebra finches. Neuroreport. 2005;16:21–24. doi: 10.1097/00001756-200501190-00006. [DOI] [PubMed] [Google Scholar]
- 19.Miller DB. Long-term recognition of father’s song by female zebra finches. Nature. 1979;280:389–391. [Google Scholar]
- 20.Nixdorf-Bergweiler BE. Divergent and parallel development in volume sizes of telencephalic song nuclei in male and female zebra finches. J. Comp. Neurol. 1996;375:445–456. doi: 10.1002/(SICI)1096-9861(19961118)375:3<445::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Nordeen EJ, Nordeen KW. Sex and regional differences in the incorporation of neurons born during song learning in zebra finches. J. Neurosci. 1988;8:2869–2874. doi: 10.1523/JNEUROSCI.08-08-02869.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nordeen KW, Nordeen EJ. Synaptic and molecular mechanisms regulating plasticity during early learning. Ann. N. Y. Acad. Sci. 2004;1016:416–437. doi: 10.1196/annals.1298.018. [DOI] [PubMed] [Google Scholar]
- 23.Nottebohm F, Arnold A. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- 24.Phillmore LS, Bloomfield LL, Weisman RG. Effects of song and calls on ZENK expression in the auditory telencephalon of field- and isolate-reared black capped chickadees. Behav. Brain. Res. 2003;147:125–134. doi: 10.1016/s0166-4328(03)00155-4. [DOI] [PubMed] [Google Scholar]
- 25.Saldanha CJ, Schlinger BA, Micevych PE, Horvath TL. Presynaptic N-methyl-d-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. J. Comp. Neurol. 2004;469:522–534. doi: 10.1002/cne.11035. [DOI] [PubMed] [Google Scholar]
- 26.Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: Implications for vocal learning. J. Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott LL, Singh TD, Nordeen EJ, Nordeen KW. Developmental patterns of NMDAR expression within the song system do not recur during adult vocal plasticity in zebra finches. J. Neurobiol. 2004;58:442–454. doi: 10.1002/neu.10300. [DOI] [PubMed] [Google Scholar]
- 28.Simpson HB, Vicario DS. Early estrogen treatment of female zebra finches masculinizes the brain pathway for learned vocalizations. J. Neurobiol. 1991;22:777–793. doi: 10.1002/neu.480220711. [DOI] [PubMed] [Google Scholar]
- 29.Singh TD, Basham ME, Nordeen EJ, Nordeen KW. Early sensory and hormonal experience modulate age-related changes in NR2B mRNA within a forebrain region controlling avian vocal learning. J. Neurobiol. 2000;44:82–94. doi: 10.1002/1097-4695(200007)44:1<82::aid-neu8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 30.Sockman KW, Gentner TQ, Ball GF. Complemetary neural systems for the experience-dependent integration of mate-choice cues in European starlings. J. Neurobiol. 2005;62:72–81. doi: 10.1002/neu.20068. [DOI] [PubMed] [Google Scholar]
- 31.Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav. Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 32.Solis MM, Doupe AJ. Compromised neural selectivity for song in birds with impaired sensorimotor learning. Neuron. 2000;25:109–121. doi: 10.1016/s0896-6273(00)80875-2. [DOI] [PubMed] [Google Scholar]
- 33.Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA. Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J. Neurosci. 2004;24:3152–3163. doi: 10.1523/JNEUROSCI.5589-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wade J. Zebra finch sexual differentiation: the aromatization hypothesis revisited. Microsc. Res. Tech. 2001;54:354–363. doi: 10.1002/jemt.1148. [DOI] [PubMed] [Google Scholar]