Abstract
Deep brain stimulation of the subthalamic nucleus (STN-DBS) is efficacious in treating the motor symptoms of Parkinson’s disease (PD). However, the impact of STN-DBS on the progression of PD is unknown. Previous preclinical studies have demonstrated that STN-DBS can attenuate the degeneration of a relatively intact nigrostriatal system from dopamine (DA)-depleting neurotoxins. The present study examined whether STN-DBS can provide neuroprotection in the face of prior significant nigral DA neuron loss similar to PD patients at the time of diagnosis. STN-DBS between two and four weeks after intrastriatal 6-hydroxydopamine (6-OHDA) provided significant sparing of DA neurons in the SN of rats. This effect was not due to inadvertent lesioning of the STN and was dependent upon proper electrode placement. Since STN-DBS appears to have significant neuroprotective properties, initiation of STN-DBS earlier in the course of PD may provide added neuroprotective benefits in addition to its ability to provide symptomatic relief.
Keywords: Deep Brain Stimulation, Subthalamic Nucleus, Parkinson’s disease, Neuroprotection, 6-hydroxydopamine, Stereology
Introduction
Deep brain stimulation of the subthalamic nucleus (STN-DBS) is now the most frequently practiced surgical therapy for the treatment of Parkinson’s disease (PD). The efficacy of STN-DBS in the relief of the cardinal motor symptoms of PD is well documented with recent reports indicating that symptomatic relief is both long-lasting and comparable to best medical therapy (Krack, et al. 2003, Schupbach, et al. 2005, Weaver, et al. 2009). However, the clinical use of this treatment has proceeded without systematic study of the impact of STN-DBS on the progression of the disease itself. Currently, PD patients are not considered candidates for STN-DBS unless symptoms have been present for a minimum of five years (Chang and Chou 2006, Neimat, et al. 2006). The average PD patient has a mean disease duration of 14 years before STN-DBS is performed (Volkmann 2004) during which time it is likely that the disease has progressed significantly (Goetz, et al. 2000). Therefore, by the time patients initiate DBS therapy they are well into the later stages of PD, which makes it difficult to determine whether STN-DBS can preserve remaining DA neurons. Positive indications of STN-DBS’ neuroprotective efficacy in parkinsonian animal models would suggest that expansion of this treatment to PD patients in early and moderate stages of the disease should be seriously considered.
Preclinical studies in both rats and monkeys have demonstrated that STN-DBS can prevent the degeneration of nigral dopamine (DA) neurons from the insult produced by DA-depleting neurotoxins (Harnack, et al. 2008, Maesawa, et al. 2004, Temel, et al. 2006, Wallace, et al. 2007). While these studies are a promising preliminary indication of STN-DBS’ neuroprotective potential, the overwhelming majority of nigral DA neurons were present when STN stimulation was initiated either immediately prior to, or soon after toxin administration. While this experimental paradigm provides the best opportunity to observe neuroprotection, it is confounded by the possibility that the reduction in nigral DA neuron degeneration associated with STN-DBS is due to prevention of toxin uptake/metabolism and does not accurately model the DA neuron loss that already exists in PD patients who undergo the procedure.
In the present study, we utilized unbiased stereology and analysis of striatal DA to characterize the time course and magnitude of nigral DA neuron and dopaminergic terminal loss following unilateral intrastriatal 6-OHDA injection to rats. Our goal was to precisely define the rate of progression of the relatively protracted nigrostriatal degeneration induced by intrastriatal 6-OHDA and then utilize these same lesion parameters to establish partial nigrostriatal degeneration prior to initiating long-term high frequency STN-DBS. The impact of STN stimulation on SN DA neuron number was evaluated. Additionally, we examined effects on the degree of striatal dopaminergic innervation and levels of striatal DA and DA metabolites, parameters that prior studies did not examine. Our results demonstrate that STN-DBS initiated after significant nigral DA neuron loss can prevent further DA neuron degeneration, but did not preserve striatal DA innervation and levels of striatal DA, effects attributed to limitations of the 6-OHDA model employed here.
Materials and Methods
Animals
Male, Sprague Dawley rats (Harlan, 200-250 g) were used in these studies. For Experiment 1: Time Course of Nigrostriatal Degeneration Following Intrastriatal 6-OHDA, a total of 24 rats were divided amongst 4 groups (2 weeks post 6-OHDA, 4 weeks post 6-OHDA, 6 weeks post 6-OHDA, and 6 weeks post intrastriatal vehicle injection). In the final analysis there were a total of 5 rats in the 2 week group, 4 rats in the 4 week group, 6 rats in the 6 week group, and 6 rats in the vehicle injected group. For Experiment 2: Impact of STN DBS after 6-OHDA Lesion, a total of 30 rats were divided into either the ACTIVE stimulator group (n=19) or the INACTIVE stimulator control group (n = 11). Animals were only included in the final analyses if they completed the full two week stimulation interval. All animals were given food and water ad libitum, and housed in reversed light-dark cycle conditions in the University of Cincinnati Vontz Center vivarium, which is fully AAALAC approved.
Experimental Overview
Time Course of Nigrostriatal Degeneration Following Intrastriatal 6-OHDA
Rats were either unilaterally lesioned via intrastriatal injections of 6-OHDA or injected with an equal volume of vehicle into the striatum. Rats were culled into three separate groups for sacrifice at either 2 weeks, 4 weeks, or 6 weeks after surgery. Forelimb akinesia was assessed via the cylinder task (details on the procedure are presented in the following text) prior to surgery and at each of these timepoints in the 6-week post surgery 6-OHDA and vehicle groups. Amphetamine-induced rotational asymmetry was quantified for the 6-OHDA rats at these same time points. After sacrifice stereological cell counts of the tyrosine hydroxylase immunoreactive (THir) and the neuronal nuclei immunoreactive (NeuN) neurons were performed in both the ipsilateral lesioned and contralateral unlesioned substantia nigra of the 6-OHDA injected rats. Stereological counts of THir neurites within the striatum also were made in the ipsilateral lesioned striatum and contralateral unlesioned striatum.
Stimulation of the STN after Intrastriatal 6-OHDA
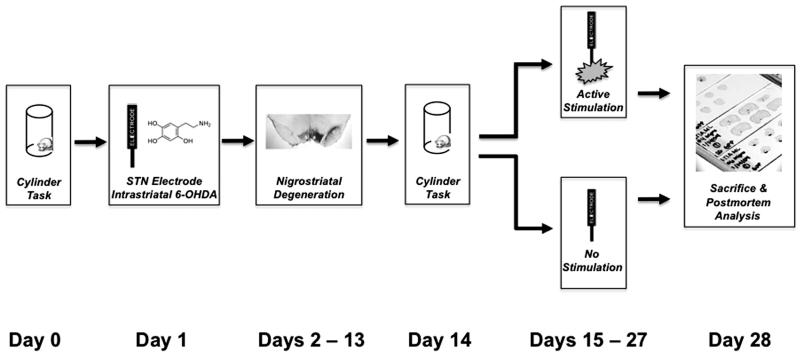
Prior to 6-OHDA injection and stimulating electrode implantation surgery, all rats were assessed for forelimb akinesia utilizing the cylinder task. The following day all rats were unilaterally lesioned via injections of 6-OHDA into the striatum and implanted unilaterally (ipsilateral to 6-OHDA) with stimulating electrodes into the STN during the same surgical session. All rats were allowed to recover from surgery for exactly two weeks. At two weeks all rats were reassessed for the degree of contralateral forelimb akinesia via the cylinder task. Rats that displayed contralateral forelimb akinesia were divided into two separate groups: ACTIVE (n = 19) or INACTIVE (n = 11) stimulation. Rats in the ACTIVE group had their stimulators connected to an external stimulation source and received STN stimulation continuously for two weeks. Rats in the INACTIVE group received no stimulation during the two-week interval. At the end of the two weeks all rats were sacrificed and their brains processed for stereological cell counts of THir neurons in the SN, THir neurites in the striatum or levels of DA and DA metabolites in the striatum. Placement of the stimulating electrode was analyzed histologically utilizing Kluver-Barrera staining. This experimental design is illustrated in Figure 1.
Figure 1. Experimental Design for STN-DBS After Intrastriatal 6-OHDA.
On Day 0 forelimb akinesia was assessed in all rats via the cylinder task. On Day 1 all rats were unilaterally lesioned via injections of 6-OHDA into the striatum and implanted unilaterally (ipsilateral to 6-OHDA) with stimulating electrodes into the STN during the same surgical session. During Days 2-13 all rats were allowed to recover from surgery. On Day 14 all rats were reassessed for degree of contralateral forelimb akinesia via the cylinder task. Rats that displayed a 20% reduction in contralateral forelimb use were divided into two separate groups: ACTIVE (n = 19) or INACTIVE (n = 11) stimulation. Rats in the ACTIVE stimulation group had their stimulators connected to an external stimulation source and received STN stimulation during Days 15-27 twenty-four hours a day. Rats in the INACTIVE group received no stimulation during Days 15-27. On Day 28 all rats were sacrificed and their brains processed for stereological cell counts of THir neurons in the SN, THir neurites in the striatum or levels of DA and DA metabolites in the striatum and frontal cortex. Appropriate placement of the stimulating electrode was confirmed histologically utilizing Kluver-Barrera staining.
Intrastriatal 6-OHDA Injections
Rats were anesthetized prior to surgery with Equi-Thesin (0.3 ml/100 g body weight i.p.; chloral hydrate 42.5 mg/ml + sodium pentobarbital 9.72 mg/ml) and rats injected in two sites in the striatum with either 6-OHDA (MP Biomedicals, Solon, OH; 5 μg/μl 6-OHDA in 0.02% ascorbic acid, 0.9% saline solution) or vehicle. The coordinates for these injections were AP +1.6 mm, ML +2.4 mm, DV −4.2 mm and AP −0.2 mm, ML + 2.6 mm, DV −7.0 mm. A total of 2 μl 6-OHDA (total dose of 6-OHDA = 20 μg over two sites) or vehicle was injected at 0.5 μl/minute.
Behavioral Testing
Non-drugged, spontaneous use of the forepaws was measured in rats as described by Schallert (Schallert 2006). A 20% reduction in contralateral forelimb use was utilized to confirm 6-OHDA lesion prior to enrollment in either the ACTIVE or INACTIVE groups. Amphetamine-induced rotational asymmetry was assessed as described previously (Terpstra et al., 2007). Forelimb akinesia and rotations were not assessed in rats connected to stimulator cables as our external hardware connections interfered with rat mobility and paw placement and are incompatible with the rapid rotations induced by amphetamine.
Extracellular microelectrode recordings
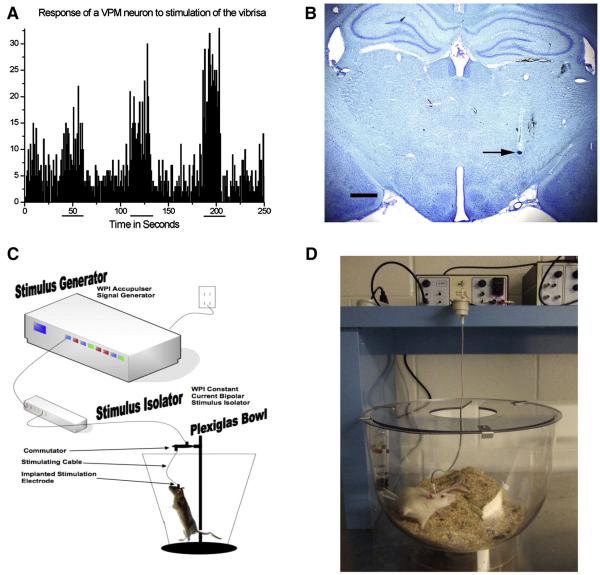
Extracellular microelectrode recording was used to provide STN targeting guidance as described previously (Maesawa, et al. 2004). Potentials of the electrode were amplified 10,000x, conducted through a band pass filter, and monitored with the aid of an oscilloscope display, audio monitor and an online computer. Bregma was carefully delineated and marked. Initial coordinates for electrode implantation were at AP −3.6 mm and L +2.5 mm according to the atlas of Paxinos and Watson (Paxinos, G. and Watson, R. 2005). The electrode was then lowered to 5.0 mm ventral (using a micromanipulator) where the approximate dorsal border of the ventral posterior medial nucleus of the thalamus (VPM) is located. Upon entering the vicinity of the VPM and proceeding ventrally, sensory responses to stimulation of the contralateral vibrissae were confirmed (Friedberg, et al. 2004, Maesawa, et al. 2004). As the electrode was advanced ventrally a relatively silent area representing the zona incerta (ZI) was encountered which spans approximately 0.5-1.0 mm. Immediately ventral to the ZI, the STN was readily distinguishable by a sudden increase of irregular spikes firing at a high rate. Located immediately ventral to the STN is the cerebral peduncle, which also is a strikingly silent structure. The STN spans approximately 100-200 μm along its dorsal ventral (DV) axis (Paxinos, G. and Watson, R. 2005). In sessions where the zone of sudden irregular spikes was found to be wider in DV span (> 300 μm) the electrode position was assumed to be within the SN and therefore too posterior. If this happened, the electrode was withdrawn and repositioned 200 μm in the rostral direction and recording was resumed. The precise coordinates of the dorsal border of the STN were then carefully recorded to guide subsequent electrode placement. This procedure is illustrated in Figure 2A-B.
Figure 2. Extracellular Recording-Guided Electrode Placement in STN and Long Term STN DBS Platform.
A. Response of a neuron within the ventral posterior medial nucleus of the thalamus (VPM) to stimulation of the contralateral vibrissa, stimulation period indicated by horizontal lines beneath x axis. This VPM landmark is used to determine appropriate AP and ML electrode placement, the STN is located 0.5 – 1.0 mm ventral to this site and is readily distinguishable by a sudden increase of irregular spikes firing at a high rate. B. Dye infusion within STN recording site (arrow). After the coordinates of the STN were identified a bipolar concentric microelectrode was implanted with the electrode fixed in place using dental acrylic and bone screws. Scale Bar = 1000 μM. C. Schematic of an individual stimulation setup, including an Accupulser Signal Generator (World Precision Instruments, WPI) connected to a Constant Current Bipolar Stimulus Isolator (WPI) that is connected to the concentric bipolar stimulating electrode implanted into the rat STN and secured using dental cement and bone screws. The rat is housed in a Raturn System Bowl (BioAnalytical Systems, Inc., BASi). The stimulator cable is routed through a commutator to allow the rat to move freely during the two week stimulation interval. D. Photograph of an individual rat undergoing STN-DBS during the two week stimulation interval.
Estimation of Current Spread
In order to ensure we were not stimulating the SN during STN stimulation, we estimated the distance of current spread associated with STN stimulation by utilizing simultaneous STN stimulation and extracellular recordings.
Concentric bipolar stimulating electrodes identical to the electrodes we ultimately used for chronic STN stimulation (inner electrode projection 1 mm, inner insulated electrode diameter 0.15 mm, outer electrode gauge 26, Plastics One, Roanoke, VA) were placed within the STN (AP −3.8 mm, L +2.5 mm, approximately DV −7.6 from dura) using the extracellular guided recording techniques described previously. A tungsten-recording electrode was then used to record from varying sites between STN and SN (Site 1: AP - 6.12, ML +2.0, DV −4.5 from dura; Site 2: AP −5.88, ML +2.4, DV −8.0 from dura; Site 3: AP −5.2, ML +2.4, DV −8.0 from dura; Site 4: AP −4.00, ML +2.5, DV −8.0 from dura). Locations of the recording sites were verified histologically at the conclusion of the experiment utilizing Kluver Barrera staining. At each site a single neuron was isolated and the response of the cell to STN stimulation (frequency 130 Hz; pulse duration 60 μsec, amplitude 100 μA) was recorded by constructing a peristimulus time histogram (PSTH) using 100 sweeps and bin width of one msec, prestimulation of 40 msec and post stimulation of 200 msec. At this current intensity the stimulus artifact was between 2 and 3 msec. During the recording, the occurrence of antidromic activation of the cell was tested by measuring the response jitter. If the PSTH showed a distinct peak with latency between 5 msec and 8 msec, it was concluded that the cell was activated orthodromically. It was assumed that the cell was activated by spread of current if 1) no distinct peak in PSTH was observed during the first 10 msec after the stimulus artifact, 2) the cell was not antidromically activated, and 3) the number of spikes in bins within the 10 msec following the onset of the stimulation current was more than 3 times the number of spikes during the prestimulation period. Using this technique current spread was not detected when the cell recorded from was located in the caudal mesencephalon, either outside the SN (Site 1) or within the SN (Sites 2 and 3), all of these sites were at minimum 1.4 mm caudal to the stimulating electrode. In contrast, current spread was detected when the recording electrode was placed in the rostral SN (Site 4), approximately 0.25 mm caudal to the stimulating electrode. Based on these experiments we estimate that under our experimental stimulation parameters current spread impacts not only the STN but also a sphere immediately surrounding the STN with an approximate radius of at least 250 μM. In order to minimize the possibility that our STN stimulation generated current that spread to any portion of the SN all stimulating electrodes were placed in the anterior portion of the STN in subsequent experiments (Bregma – 3.6 mm), a full 900 μM rostral to the rostral border of the SN.
Electrode Implantation
Stimulating electrode implantation took place immediately following STN recording and intrastriatal 6-OHDA injection. A bipolar concentric microelectrode (as described in previous section) was lowered to the dorsal border of the STN with coordinates for each rat predetermined by extracellular guided recordings. The electrode was then fixed in place using dental acrylic and bone screws. The dorsal border of the STN was chosen as the implantation location in order to minimize any damage resulting from electrode implantation. At the conclusion of all experiments stimulating electrode placement was verified morphologically utilizing Kluver Barrerra staining.
Long-Term Stimulation
Rats assigned to the ACTIVE group were individually housed in Plexiglas Raturn bowls (BASi, Inc., West Lafayette, IN) and connected to a commutator (Plastics One, Roanoke, VA) via a stimulating cable connected to the external plug of the STN microelectrode. The commutators were connected to an Accupulser Signal Generator (World Precision Instruments, Sarasota, FL) via a battery-powered Constant Current Bipolar Stimulus Isolator (World Precision Instruments). This is illustrated in Figure 3. Rats were stimulated at frequency of 130 Hz, 60 μs pulse width, and an intensity of 30-50 μA, which was below the threshold of contralateral forepaw and orofacial dyskinesia, thereby preventing problems with feeding or locomotion during the stimulus interval. In subsequent studies we have confirmed that these identical STN stimulation parameters can provide functional improvements in contralateral forelimb akinesia (Spieles-Engemann et al., 2009). Stimulation commenced two weeks following 6-OHDA/electrode implantation surgery and was active twenty-four hours/day for a two week period. INACTIVE animals were housed individually in standard shoebox cages for the identical two-week interval. Our long-term STN-DBS stimulation platform is depicted in Figure 2 C-D.
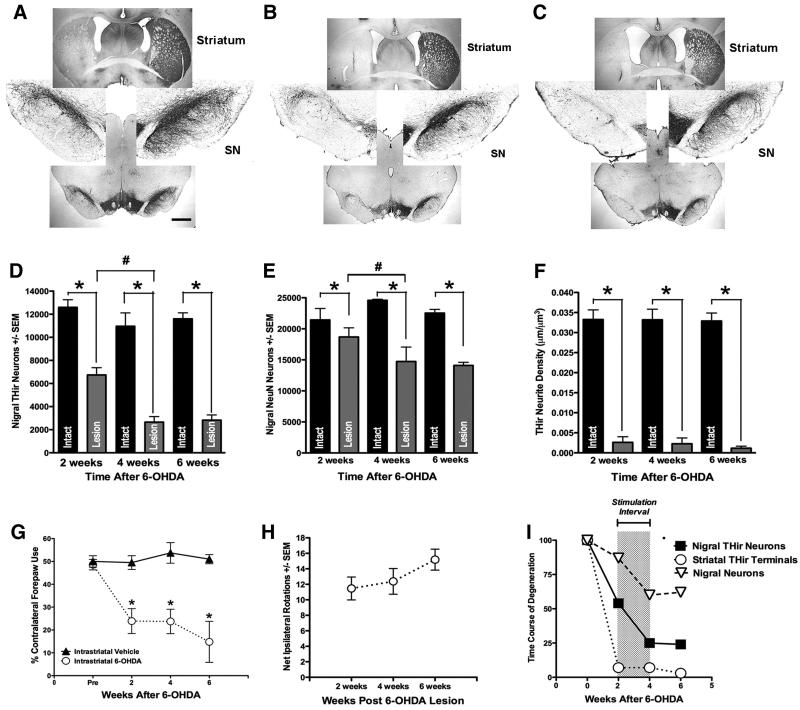
Figure 3. Time Course, Magnitude and Behavioral Impact of Nigrostriatal Degeneration Following Intrastriatal 6-OHDA.
Micrographs of THir neurons in the SN and THir terminals in the striatum at 2 (A), 4 (B) and 6 (C) weeks after unilateral intrastriatal 6-OHDA (left). Progressive degeneration of nigral THir neurons is illustrated by comparing nigral the number of THir neurons evident in the left hemisphere with the normal complement of THir nigral neurons in the contralateral mesencephalon. Note that almost complete absence of THir neurites at the level of the striatum starting at 2 weeks (A) after 6-OHDA. Scale Bar = 1000 μM. D. Stereological counts of THir neurons in the SN reveal significantly fewer THir neurons in the lesioned SN (grey bars) relative to the intact SN (black bars) at all time points examined (*, p<0.001). In addition, significantly fewer THir neurons were present in the SN ipsilateral to 6-OHDA at 4 weeks compared to the ipsilateral SN at 2 weeks (#, p<0.001). There were no significant differences between the number of THir neurons in the SN ipsilateral to 6-OHDA between four and six weeks after lesion (p ≥ 0.05). E. Stereological counts of NeuNir neurons in the SN reveal significantly fewer NeuNir neurons in the lesioned SN relative to the intact SN at all time points examined (*, p<0.001). Further, significantly fewer NeuNir neurons are present in the SN ipsilateral to 6-OHDA at 4 weeks compared to the ipsilateral SN at 2 weeks (#, p< 0.005). There were no significant differences between the number of NeuNir neurons in the SN ipsilateral to 6-OHDA between four and six weeks after lesion (p ≥ 0.05). F. THir neurite density in the striatum was significantly reduced at 2, 4 and 6 weeks following lesion (*, p<0.0001) with no progression observed after two weeks. G. Contralateral forelimb akinesia in the cylinder task at 2, 4 and 6 weeks after intrastriatal vehicle injection (black solid triangles) or 6-OHDA injection (white open circles). All rats receiving 6-OHDA exhibited a significant reduction in contralateral forepaw use at each time point examined compared to both pre-6-OHDA baseline contralateral forepaw use (*, p<0.002). No significant differences in contralateral forepaw use were observed between two, four and six weeks after 6-OHDA (p ≥ 0.05). H. Amphetamine-induced rotational asymmetry after intrastriatal 6-OHDA. All rats receiving 6-OHDA exhibited ipsilateral rotations at all post-6-OHDA time points examined. No significant differences in rotational asymmetry were observed between two, four and six weeks after 6-OHDA (p ≥ 0.05). I. Schematic illustrating the time course of degeneration of nigral THir neurons (black squares), nigral NeuNir neurons (white triangles) and striatal THir neurites (white circles) in the ipsilateral hemisphere following intrastriatal 6-OHDA. Specifically, our lesion parameters lead to loss of 46% THir and 13% NeurNir neurons in the SN at two weeks, progressing further to a loss of 75% THir and 31% NeurNir nigral neurons at four weeks. Nigral DA neuron loss is essentially completed by four weeks under these lesion parameters as analysis of SN neurons at six weeks post 6-OHDA is essentially identical to numbers of THir and NeuN ir nigral neurons at four weeks. In contrast to the progressive loss of nigral neurons that we observe over the course of four weeks after 6-OHDA, the loss of striatal THir and the behavioral manifestations of this loss occurs over a much more condensed time frame and is essentially complete within two weeks post 6-OHDA. The interval of STN-DBS used in subsequent experiments is indicated by the grey box.
Sacrifice
At the conclusion of the two week stimulation interval rats for morphological analysis were deeply anesthetized (60 mg/kg, pentobarbital, i.p.) and perfused intracardially with saline. Brains were removed, post fixed for 24 hours in 4% paraformaldehyde and transferred to 30% sucrose in 0.1M PO4 buffer. Rats utilized for HPLC analysis of striatal and frontal cortex DA and metabolites were perfused intracardially with heparinized saline at 37 °C fol lowed by ice-cold saline in order to remove endogenous circulating catecholamines from blood vessels. The brains were immediately removed and flash frozen in 3-methyl butane. Brains were stored at −80 °C until analysis.
Tyrosine Hydroxylase and Neuronal Nuclei Immunohistochemistry for SN Neurons and Striatal Neurites
Paraformaldehyde perfused brains were frozen on dry ice and sectioned at a 35 μm thickness using a sliding microtome. Every sixth section was processed for labeling with antisera against TH or NeuN using the free-floating method . Following blocking in serum, tissue was incubated in primary antisera directed against TH (SN: Chemicon MAB318, mouse anti TH 1:4,000; Striatum: Immunostar 22941, mouse anti-TH 1:8,000) or NeuN (Chemicon, mouse anti NeuN Clone A60 1:1,000), overnight at room temperature. Triton-X (0.3 %) was added to the 0.1M Tris buffer during incubations and rinses to permeabilize cell membranes. Following primary incubation, sections were incubated in biotinylated secondary antisera against mouse IgG (Chemicon AP124B, 1:400) followed by the Vector ABC detection kit employing horseradish peroxidase (Vector Laboratories, Burlingame, CA). Antibody labeling was visualized by exposure to 0.5 mg/ml 3,3′ diaminobenzidine (DAB) and 0.03% H2O2 in Tris buffer. Sections were mounted on subbed slides, dehydrated to xylene and coverslipped with Cytoseal (Richard-Allan Scientific, Waltham, MA).
Kluver-Barrera Histology
Kluver-Barrera staining was used to visualize electrode placement and quantification of the STN cell number. Staining was performed in every sixth section throughout the STN according to previously described methods (Kluver and Barrera 1953). Stimulating electrode placement was considered to be appropriately targeted to the STN if the tip of the stimulating electrode was observed to be within or at the border of the STN within any of the sections examined.
Stereology
Stereology was performed using a BX52 Olympus microscope (Olympus America Inc.) equipped with Stereo Investigator stereological software (Microbrightfield Bioscience, Williston, VT) and a Microfire CCD camera (Optronics, Goleta, CA) as described previously (Kordower, et al. 2006). Using the optical fractionator principle, the SN or the STN was individually outlined on each section under a low magnification (1.25X) and cell counts of THir (SN) or Kluver-Barrera stained (STN) neurons were made according to stereological principles while focusing down through the z-axis at 60X. In order to assure that only the SN was included in the NeuNir analysis, the Virtual Slices Module for Stereo Investigator (Microbrightfield Bioscience) was used to superimpose the midbrain sections immunolabeled for TH over adjacent midbrain sections immunostained for NeuN to delineate the region of interest. Analysis of THir neurite density was performed utilizing the Space Balls probe in Stereo Investigator. This hemispheric probe was used to obtain an unbiased estimate of THir neurite length in the striatum using previously reported methods (Koprich, et al. 2003). Every sixth coronal section through the entire rostral-caudal span of the dorsolateral striatum was analyzed. This lateral area of the striatum was chosen for analysis based on its involvement in paw reaching and other sensorimotor tasks (Brown, et al. 2002, Kelley, et al. 1988, Pisa 1988). CEs for all analyses were ≤ 0.10.
Dissection of Striatum and Frontal Cortex for HPLC Analysis
Frozen brains were equilibrated at a temperature of −18 °C prior to di ssection. 1-2 mm coronal slabs were blocked from each brain utilizing a brain blocker (Zivic, Pittsburg, PA) and striatal and frontal cortex tissues from both hemispheres were microdissected while being held at a constant −12 °C on a cold plate (Teca, Chicago,IL) . Frozen dissected structures were placed individually in vials and stored at – 80 °C until analysis.
High Protein Liquid Chromatography (HPLC)
Homogenized samples were analyzed as described previously (Koprich, et al. 2003). Samples were separated on a Microsorb MV C-18 column (5 Am, 4.6_250 mm, Varian, Palo Alto, CA) and simultaneously examined for DA, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA). Compounds were detected using a 12-channel coulometric array detector (CoulArray 5200, ESA, Chelmsford, MA) attached to a Waters 2695 Solvent Delivery System (Waters, Milford, MA) under the following conditions: flow rate of 1 ml/min; detection potentials of 50, 175, 350, 400 and 525 mV, and; scrubbing potential of 650 mV. The mobile phase consisted of a 10% methanol solution in distilled H2O containing 21 g/L (0.1 M) citric acid, 10.65 g/L (0.075 M) Na2HPO4, 176 mg/L (0.8 M) heptanesulfonic acid and 36 mg/L (0.097 mM) EDTA at a pH of 4.1. Data are expressed as ng/mg protein.
Statistical Analysis
For the experiment Time Course of Nigrostriatal Degeneration, a one-way analysis of variance (ANOVA) was performed to look at between group differences in THir and NeuNir neuron numbers, and THir terminal density. Significant differences in main effects were determined by Fisher LSD post hoc analysis. To determine significant differences between the lesioned and non-lesioned side within each group, a one-way repeated measures ANOVA (RM-ANOVA) was performed, followed by a Holm-Sidak (THir neurons and terminal density) or Dunn’s (NeuN) post-hoc test. A one-way RM-ANOVA followed by a Holm-Sidak post-hoc test was also used to look at group differences in behavioral tasks.
For the experiment Effects of Long-Term STN Stimulation on Nigrostriatal Neurons and Biochemistry, two-way RM-ANOVAS followed by a Tukeys post-hoc test were used to determine significant differences in THir neuron number and terminal density as well as DA and DA metabolite levels in the striatum and cortex. A one-way ANOVA followed by a Fisher LSD post-hoc test was used to determine differences between groups with good stimulator placement and those with poor stimulator placement. SigmaStat 3.0 software (Systat Software, San Jose, CA) was used for all statistical analyses and the level of statistical significance was set at 0.05.
Results
Time Course of Nigrostriatal Degeneration After Intrastriatal 6-OHDA
Reductions in THir Neurons in the SN
At the two week, four week and six week post 6-OHDA intervals there were significantly fewer THir neurons in the SN ipsilateral to intrastriatal 6-OHDA injection as compared to the contralateral SN ( 2 week: F(1, 5) = 34.641, p = 0.004; 4 week: F(1, 4) = 42.486, p = 0.007; 6 week: F(1, 6) = 329.345, p ≤ 0.001). No significant differences were observed between the number THir neurons in the SN contralateral to 6-OHDA injection at any of the time points examined (p ≥ 0.05). Significant loss of THir neurons in the ipsilateral SN occurred between two and four weeks and two and six weeks post intrastriatal 6-OHDA (F(2, 15) = 13.292, p ≤ 0.001). There were no significant differences between the number of THir neurons in the SN ipsilateral to 6-OHDA between four and six weeks after lesion (p ≥ 0.05). Therefore, our intrastriatal 6-OHDA lesion parameters caused a 46 ± 6% decrease in THir neurons in the SN at two weeks, progressing significantly further to a 75 ± 4% reduction at four weeks, and a 76 ± 3% decrease at six weeks. These results are depicted in Figure 3A-D, and 4I.
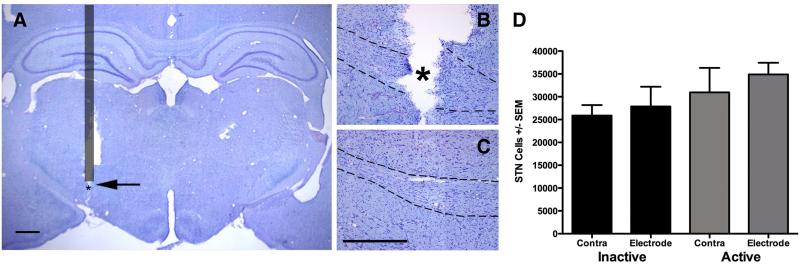
Figure 4. Verification of Stimulating Electrode Placement and Impact on Cell Survival in the STN.
A-C. Representative micrographs of Kluver Barrera stained coronal sections through the STN. A. Schematic Overlay of Electrode Position. The nonactive portion of the electrode is indicated by the grey bar (400 μM diameter), the area where the active tip of the electrode was placed is indicated by an asterisk (active electrode tip diameter = 150 μM). Tissue damage is unavoidable during the detachment of the electrode from the dental acrylic and the removal of the electrode from the brain. B. Desired placement is verified within the STN (arrow, outline) and damage appears contained as it is not apparent in a coronal section approximately 175 μM caudal (C). The rat STN spans approximately 1 mm in the rostral caudal dimension. Scale bar in A = 1000 μM, B and C = 500 μM. D. Stereological assessment of the number of cells within the STN reveals that neither electrode implantation nor stimulation significantly decreases STN cell number (p<0.05).
Loss of NeuNir Neurons in the SN
In order to distinguish between loss of TH phenotype and actual nigral neuronal death, we quantified NeuNir neurons within the SN of these same rats. There were significantly fewer NeuN positive cells in the SN ipsilateral to intrastriatal 6-OHDA injection as compared to the contralateral SN at all time points examined (2 week: F(1, 4) = 22.319, p = 0.018; 4 week: F(1, 3) = 20.697, p = 0.045; 6 week: F(1,6) = 105.906, p ≤ 0.001). No significant differences were observed between the numbers of NeuNir neurons in the SN contralateral to 6-OHDA injection at any of the time points examined (p ≥ 0.05). Significant loss of NeuNir neurons in the ipsilateral SN occurred between two and four weeks and two and six weeks post intrastriatal 6-OHDA (F(2, 13)=11.579, p = 0.002). There were no significant differences between the number of NeuNir neurons in the SN ipsilateral to 6-OHDA between four and six weeks after lesion (p ≥ 0.05). Therefore, our intrastriatal 6-OHDA lesion parameters caused a 13 ± 1.9% decrease in NeuNir neurons in the SN at two weeks, progressing significantly further to a 31 ± 9.1% reduction at four weeks, with no further significant neuronal loss observed at six weeks (37 ± 2.9%). Importantly, when we compared the numbers of total neurons lost between the THir counts and the NeuNir counts at the three time points after intrastriatal 6-OHDA it was evident that at 2 weeks post 6-OHDA a loss of TH phenotype occurred prior to overt neuronal loss in the SN (5,854 THir neurons lost compared to 2,748 NeuNir neurons lost). However, at four and 6 weeks post 6-OHDA reductions in both THir and NeuNir neurons ipsilateral to lesion appeared to be relatively identical (Four weeks: 8,292 THir neurons, 9,848 NeuNir neurons. Six weeks: 8,776 THir neurons, 8,433 NeuNir neurons). These numbers of nigral THir and NeuNir neurons, arrived at using unbiased stereology, indicate that during the first two weeks after 6-OHDA a mixture of TH phenotype loss and cell death of SN neurons occurred, whereas between two and four weeks after 6-OHDA further overt neuronal loss took place that remained stable at six weeks post 6-OHDA. These results are illustrated in Figures 3E and 3I.
Reductions in THir Neurites in the Striatum
We also determined the impact of intrastriatal 6-OHDA injection on THir neurite density in the striatum. Again, there were significantly fewer THir positive neurites in the striatum ipsilateral to 6-OHDA compared to the contralateral striatum at all time points examined (2 week: F(1, 6) = 272.84, p ≤ 0.001; 4 week: F(1, 5) = 267.159, p ≤ 0.001; 6 week: F(1, 5) = 122.675, p ≤ 0.001). However, in contrast to the gradual reduction in THir and NeuNir nigral neurons that we observed at two and four weeks post lesion, marked reductions in striatal THir neurite density were observed in the ipsilateral striatum at the two week post 6-OHDA time point that were maintained over the course of four and six weeks. No significant differences were observed between the number of THir neurites in the striatum contralateral to 6-OHDA injection at any of the time points examined (p ≥ 0.05). There were no significant differences in striatal THir neurites in the ipsilateral striatum between the three different post 6-OHDA time points (p ≥ 0.05). Therefore, our intrastriatal 6-OHDA lesion parameters caused a 93 ± 4.2% decrease in striatal THir neurite density at two weeks, with no significant further neurite loss observed at four (93 ± 3.6%) and six weeks (97 ± 1.4%). These results are depicted in Figure 3F and 3I.
Induction of Contralateral Forelimb Akinesia and Rotational Asymmetry
In order to determine the impact of our lesioning protocol on motor performance we analyzed both forelimb akinesia via the cylinder task and amphetamine-induced rotational asymmetry. In the cylinder task the affected paw is contralateral to the side of the 6-OHDA injection. For the cylinder task, all rats receiving 6-OHDA exhibited a significant reduction in contralateral forepaw use at all time points examined compared to both pre-6-OHDA baseline contralateral forepaw use (F(3, 6) = 9.726, p ≤ 0.001), as well as to vehicle injected control rats (F(1,12) = 18.652, p = 0.002). However, no significant differences in contralateral forepaw use were observed between two, four and six weeks after 6-OHDA (p ≥ 0.05). These results are illustrated in Figure 3G.
Similarly, all rats receiving 6-OHDA exhibited ipsilateral amphetamine-induced rotations at all post-6-OHDA time points examined. No significant differences in ipsilateral rotations were observed between two, four and six weeks after 6-OHDA (p ≥ 0.05). These results are illustrated in Figure 3H.
Effects of Long-Term STN Stimulation on Nigrostriatal Neurons and Striatal DA
Stimulator Placement
Examination of coronal sections through the level of the STN revealed postmortem tissue damage that occurred during the detachment of the electrode from the dental acrylic and the removal of the electrode from the brain (Figure 4A). Approximately six out of the nineteen rats in the ACTIVE group had stimulating electrode tips that were determined to be located outside of the STN during postmortem histological analysis. In general, the majority of poorly placed stimulating electrodes were observed to be located caudal to the STN, in between the STN and the SN. Rats in the ACTIVE group with misplaced STN stimulating electrodes were subsequently separated into a separate group from henceforth referred to as ACTIVE MISPLACED.
STN Cell Number
In order to determine whether either electrode implantation or stimulation resulted in significant cell loss in the STN, we utilized unbiased stereology of Kluver-Barrera stained sections to quantify the number of cresyl violet stained cells in the STN on the side ipsilateral to the electrode, as well as the contralateral STN in both the ACTIVE and INACTIVE groups. Damage that occurred during postmortem electrode did not prevent the quantification of cells within the STN and neighboring sections (Figure 4B-C). There were no significant differences observed in the number of cellular profiles in the STN between the ACTIVE and INACTIVE groups nor the ipsilateral implanted side versus the contralateral intact side (p ≥ 0.05). These results are illustrated in Figure 4D. Therefore, neither implantation nor electrical stimulation impacted total cell number in the STN.
Survival of THir Neurons in the SN following STN Stimulation
After two weeks of stimulation (4 weeks post lesion) there were significantly more THir neurons remaining in the ipsilateral SN of ACTIVE rats compared to the number of THir neurons in the ipsilateral SN of both the INACTIVE and the ACTIVE MISPLACED rats (F(2, 19) = 32.261, p ≤ 0.001). Further, rats in the ACTIVE MISPLACED group possessed significantly fewer THir neurons in the contralateral SN compared to the number of THir neurons in the contralateral SN of both the ACTIVE and INACTIVE groups (F(2, 19) = 5.586, p = 0.014). In the most rostral sections through the SN of rats in the ACTIVE MISPLACED group, THir neurons appeared smaller with shorter neurites, often with lighter staining intensity. Therefore, it appears that 2 weeks of stimulation of the STN can halt the nigral THir neuron degeneration that normally would be expected to occur between two and four weeks after intrastriatal 6-OHDA using our lesion parameters. Further, improper placement of active stimulating electrodes outside of the STN not only will not halt the nigral degeneration, but it also can cause nigral neuron degeneration by itself. These results are illustrated in Figs. 5A-I.
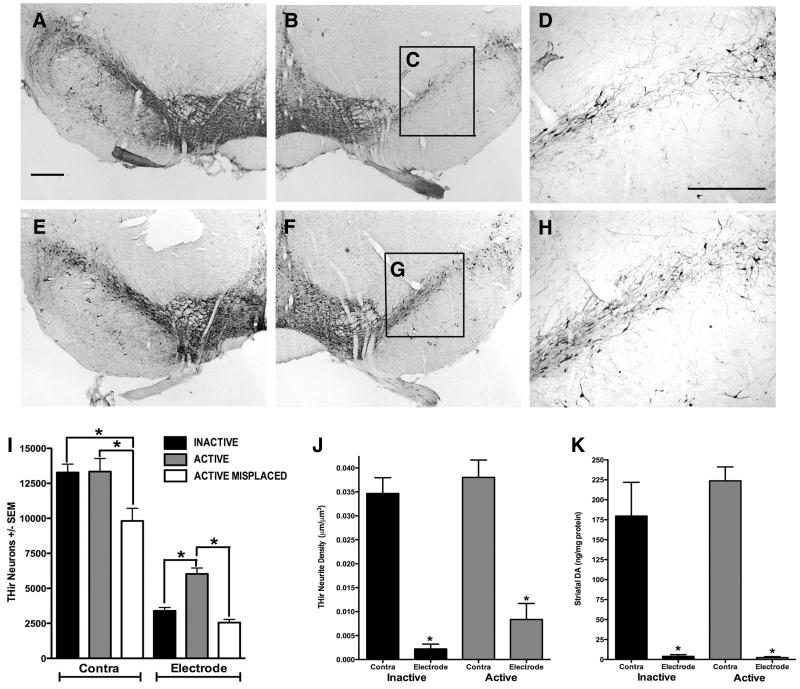
Figure 5. STN-DBS Initiated Two Weeks After Intrastriatal 6-OHDA Halts Ongoing Nigral DA Neuron Degeneration.
Rats received unilaterally intrastriatal injections of 6-OHDA and stimulation was initiated 2 weeks later (corresponding to 50% nigral DA neuron loss) and continued for a period of 2 weeks (associated with significant nigral degeneration to 75% loss). A-D. No stimulation (INACTIVE). Representative micrographs of THir neurons four weeks after 6-OHDA in the contralateral (A) and ipsilateral (B) SN. Note higher magnification (D) of nigral THir neurons from the area delineated by the black box in C demonstrating striking degeneration. Scale Bar in A = 500 μM, D = 325 μM. E-H. STN stimulation (ACTIVE). THir nigral neurons four weeks after 6-OHDA in the contralateral (E) and the ipsilateral (F). Note the presence of more THir nigral neurons (G, H) in rats that received two weeks of STN-DBS. I. Stereological counts of THir neurons in the SN of rats implanted with inactive electrodes in the STN (INACTIVE, black bars), rats receiving two weeks of STN stimulation (ACTIVE, grey bars) and rats receiving two weeks of stimulation outside of and caudal to the STN (ACTIVE MISPLACED, white bars). THir SN neurons in the mesencephalon contralateral to 6-OHDA/stimulating electrode (Contra) and ipsilateral to 6-OHDA/stimulating electrode (Electrode) were quantified. Unilateral stimulation of the STN (ACTIVE) completely halted the progression of nigral DA neuron degeneration normally observed between two and four weeks after 6-OHDA (*, p < 0.05). In contrast, lesion progression continued to expected levels of approximately 75% loss in rats implanted with INACTIVE STN stimulators. ACTIVE MISPLACED stimulation did not confer neuroprotection exhibiting similar numbers of THir neurons in the SN ipsilateral to 6-OHDA/electrode as rats in the INACTIVE group and significantly . fewer THir neurons in the contralateral SN compared to the number of THir neurons in the contralateral SN of both the ACTIVE and INACTIVE groups (*, p<0.02). J. and K. Two weeks of ACTIVE STN stimulation did not restore striatal THir neurite density (J) or levels of striatal DA (K). Rats in both the ACTIVE stimulation group and the INACTIVE control group exhibited a significant loss of striatal THir neurite density and DA levels within the ipsilateral striatum as a result of intrastriatal 6-OHDA (*, p<0.001).
Impact of STN-DBS on Striatal THir Neurites
We also examined the impact of long-term STN-DBS on THir neurite survival in the striatum. Rats in both the ACTIVE and the INACTIVE groups exhibited significant loss of ipsilateral striatal THir neurite density as a result of intrastriatal 6-OHDA (F(1, 9) = 88.175, P ≤ 0.001). Two weeks after stimulation, there were no significant differences in the density of THir neurites in the striatum ipsilateral to STN stimulation in rats in the ACTIVE group as compared to the density of THir neurites in the ipsilateral striatum of rats in the INACTIVE control group (p ≥ 0.05). These results are depicted in Figure 5J.
DA and DA Metabolite Levels
We examined the impact of long-term STN-DBS on levels of DA, 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the striatum and the frontal cortex. These results are listed in Table 1. Rats in both the ACTIVE stimulation group and the INACTIVE control group exhibited a significant loss of striatal DA within the ipsilateral striatum as a result of intrastriatal 6-OHDA (F(1, 9) = 111.239, P ≤ 0.001). Two weeks after stimulation, there were no significant differences in the levels of striatal DA in the ipsilateral striatum of rats in the ACTIVE stimulation group as compared to the levels of DA in the ipsilateral striatum of rats in the INACTIVE control group (p ≥ 0.05). This is depicted in Figure 5K. Similar results were observed for levels of DOPAC and HVA in the ipsilateral striatum of both the ACTIVE and INACTIVE groups with both groups displaying significant loss of both DA metabolites due to 6-OHDA compared to the contralateral striatum (DOPAC: F(1, 9) = 57.801, P ≤ 0.001; HVA: F(1, 9) = 52.796, P ≤ 0.001) with no significant differences observed in ipsilateral DOPAC or HVA in the ipsilateral striatum of ACTIVE and INACTIVE rats (p > 0.05) as a result of two weeks of stimulation. Rats in both the ACTIVE and INACTIVE groups also exhibited a significant loss of DA and HVA in the cortex ipsilateral to 6-OHDA injection (DA: F(1, 9) = 8.331, P = 0.014; HVA: F(1, 9) = 23.872, P ≤ 0.001), but not DOPAC (p ≥; 0.05). There were no significant differences observed in DA, DOPAC or HVA levels in either the ipsilateral or contralateral frontal cortex as a result of two weeks of stimulation (p > 0.05).
Table 1. Levels of DA, DOPAC and HVA in the Striatum and Frontal Cortex.
| Hemisphere | Stimulation | DA | HVA | DOPAC | |
|---|---|---|---|---|---|
| Striatum | Contra | Inactive Stim | 179.6 ± 42.2 | 14.4 ± 3.3 | 16.6 ± 4.2 |
| Contra | Active Stim | 223.7 ± 17.4 | 14.7 ± 1.2 | 19.6 ± 2 | |
| Ipsi | Inactive Stim | 3.9 ± 2.1* | 4.0 ± 2.3* | 2.5 ± 1.5* | |
| Ipsi | Active Stim | 2.4 ± 1.1* | 0.8 ± 0.8* | 1.7 ± 0.6* | |
| Cortex | Contra | Inactive Stim | 3.1 ± 0.3 | 3.6 ± 2.0 | 2.2 ± 1.2 |
| Contra | Active Stim | 3.6 ± 3.4 | 4.8 ± 0.7 | 3.0 ± 0.6 | |
| Ipsi | Inactive Stim | 2.4 ± 0.5* | b.d.l.* | 1.6 ± 0.7 | |
| Ipsi | Active Stim | 2.6 ± 0.3* | b.d.l.* | 0.7 ± 0.7 |
Rats in both the ACTIVE stimulation group and the INACTIVE control group exhibited a significant loss of DA, DOPAC and HVA within the ipsilateral striatum and a significant loss of DA and HVA in the frontal cortex as a result of intrastriatal 6-OHDA (*, p<0.001). No significant differences were observed in the levels of any of the catecholamines examined in either structure as a result of ACTIVE stimulation of the STN (p>0.05). Values are expressed in units of ng/mg protein ± S.E.M. Below detectable limits = b.d.l.
Discussion
The present experiments characterize an intrastriatal 6-OHDA lesioning paradigm resulting in relatively protracted nigral DA neuron degeneration that is expressed first as a loss of TH phenotype and ultimately leads to frank cell loss of nigral DA neurons. Specifically, our lesion parameters lead to loss of 46% of THir neurons in the SN at two weeks, progressing further to a loss of 75% of THir neurons at four weeks. In contrast, the loss of striatal dopaminergic (DAergic) innervation and the behavioral manifestations of this loss occur over a much more condensed time frame; specifically within two weeks after 6-OHDA lesioning. Subsequent studies utilizing these lesion parameters indicate striatal DAergic denervation may be complete even sooner than two weeks after lesion (within days, unpublished observations). Interestingly, this initial loss of terminals followed by nigral neuronal loss may recapitulate an important feature of the sequence of events in PD, albeit over a shorter period of time. The changes in motor function symptomatic of PD are not usually apparent until DA levels in the striatum have dropped to less than 20% of normal (Hornykiewicz 1988). In the post mortem putamen of PD patients with disease duration ≤ 5 years a substantial loss of both TH and dopamine transporter immunoreactivity was observed while numerous melanin-containing neurons in the SN were still present (Dodiya, et al. 2009).
A thorough examination of the time course and magnitude of both nigral DA neuron and striatal terminal loss was an essential first experiment in order to understand the impact of STN DBS initiated at two weeks following toxin injection. Our intrastriatal 6-OHDA lesion strategy was based on the work of Sauer and Oertel (Sauer and Oertel 1994) who were the first to inject 6-OHDA into the striatum (instead of nigral cell bodies or proximal axons). Since that study intrastriatal 6-OHDA has been utilized extensively to evaluate the therapeutic potential of numerous agents (Garcia-Arencibia, et al. 2007, Madhavan, et al. 2009, Sortwell and Kowdower 2006, Xue, et al. 2007). Sauer and Oertel reported an initial downregulation of DA phenotype followed by nigral neuronal loss that continued to significantly impact the nigral DA neuron population for up to 4 weeks. No observations regarding the time course of striatal terminal loss were reported. In the present study, with the benefit of modern stereological quantitation methods, we have been able to confirm and refine the original findings of Sauer and Oertel as well as to demonstrate the time course of striatal terminal loss in this model. Specifically: 1) DA phenotype loss precedes frank neuronal loss of nigral DA neurons, 2) significant nigral neuron loss occurs over four weeks, and 3) striatal terminal loss is not protracted, occurring within days of intrastriatal 6-OHDA injection. While other laboratories have characterized the extent of nigrostriatal DA neuron loss after intrastriatal 6-OHDA (Aponso, et al. 2008, Blandini, et al. 2007, Kirik, et al. 1998, Lee, et al. 1996) this is the first published report to utilize stereological methods to document the magnitude and time course of loss of nigral DA neuron phenotype, nigral neurons and striatal terminals.
The critical finding of the present study is that STN-DBS initiated two weeks post intrastriatal 6-OHDA, at a time when approximately 50% SN DA cell loss has already occurred, halts continued DA neuron death. This neuroprotective effect of STN-DBS was not due to lesion of the STN and was dependent upon proper electrode placement. Previous studies have examined whether STN-DBS can slow or halt the progression of PD in preclinical animal models. However, significant nigral neuron loss was not confirmed prior to the initiation of stimulation, nor could it be expected based on the experimental paradigm. In rats STN-DBS administered within a few hours to seven days after intrastriatal 6-OHDA injection significantly increases the number of surviving THir nigral neurons (Harnack, et al. 2008, Maesawa, et al. 2004, Temel, et al. 2006). STN-DBS given to non-human primates either before or six days after MPTP treatment also results in protection of SN DA neurons (Wallace, et al. 2007).
Despite these encouraging findings, the question of whether STN-DBS can provide neuroprotection when applied following considerable nigrostriatal degeneration was unknown. Further, no previous study reported on the impact of STN-DBS at the level of the striatum. The present study examined the effects of STN-DBS at a critical time point when approximately 50% of DA neuron loss in the SN had occurred. Here we have shown that although STN-DBS can provide neuroprotection for the DA neurons in the SN, this protection does not extend to the THir neurites or DA levels in the striatum. Given that 93% of striatal DAergic terminals had already succumbed to the 6-OHDA at the time that stimulation was initiated, this is not surprising. Experimental treatment strategies that seek to evaluate neuroprotection at the level of the striatum should appreciate the limitations of the intrastriatal 6-OHDA model in this regard. While it is possible that lower concentrations of 6-OHDA may lead to protracted loss of striatal DA terminals, this would need to be specifically determined. While we observed no increase in THir striatal neurites following two weeks of STN stimulation it is possible that longer periods of stimulation may induce significant compensatory sprouting of remaining DAergic neurites. Previous work in which viral vectors have provided continuous delivery of trophic factors (Eslamboli, et al. 2005, Sortwell, et al. 2008) indicates that this compensatory process can take many weeks.
The timing of STN-DBS in this study relative to 6-OHDA intrastriatal injection rules out for the first time the possibility that STN-DBS provides its neuroprotective benefits by preventing 6-OHDA uptake/metabolism. Previously it has been hypothesized that STN-DBS may provide neuroprotection via inhibition of the overactive STN resulting from striatal DA denervation, thus preventing excitotoxic cell death in the SN. However, growing evidence suggests that STN-DBS drives and synchronizes the STN instead of inhibiting it (Boulet, et al. 2006, Ceballos-Baumann, et al. 1999, Hershey, et al. 2003, Hilker, et al. 2005, Windels, et al. 2000, Windels, et al. 2003, Zhang, et al. 2008). Therefore, it is unlikely that decreased excitotoxicity is involved in the STN-DBS-mediated neuroprotection that we observe. While we did not directly investigate the mechanism of STN-DBS-mediated neuroprotection in this study additional experiments in our laboratory have documented that under these same lesion and stimulation parameters a significant increase in nigrostriatal brain derived neurotrophic factor (BDNF) is associated with STN-DBS (Spieles-Engemann, et al. 2009). It is possible that increased BDNF induced by the STN stimulation may be the mechanism responsible for this neuroprotection. Augmentation of BDNF in the nigrostriatal system by either exogenous protein infusion or vector-mediated delivery can similarly protect from 6-OHDA (Altar, et al. 1994, Klein, et al. 1999, Shults, et al. 1995, Singh, et al. 2006, Sun, et al. 2005). Future investigations will directly examine this issue.
Investigating the neuroprotective effects of STN-DBS in a clinical population is difficult. However, Hilker and colleaues (Hilker, et al. 2005) conducted a prospective study in advanced PD patients using 18F-fluorodopa PET to measure disease progression. The authors concluded that their findings did not support a neuroprotective effect of clinically effective STN-DBS. However, they also acknowledged that neuroprotective effects may be observable in patients in earlier stages of PD. Our present results illustrate the limitations of intervening too late in the disease process: it is by definition impossible to protect what has already been lost. We appreciate that the intrastriatal 6-OHDA model is not PD however the exact cause of DA neuron degeneration in sporadic PD is currently unknown. Decades of research have indicated that the disease is likely due to cumulative effects of genetic and environmental factors (Elbaz, et al. 2007, Migliore and Coppede 2009, Mouradian 2002, Poirier, et al. 1991). Oxidative stress has been consistently implicated in contributing to the pathology of PD (Henchcliffe and Beal 2008, Jenner 2007, Przedborski, et al. 2001). Similarly, oxidative stress has been implicated as the mechanism of cell death following intrastriatal 6-OHDA (Joglar, et al. 2009, Rodriguez-Pallares, et al. 2007, Rodriguez-Pallares, et al. 2009, Sanchez-Iglesias, et al. 2007, Smith and Cass 2007). Until the exact causes of PD are elucidated the intrastriatal 6-OHDA model provides a useful tool to screen potential neuroprotective therapies.
Recent reports conclude that DBS is as effective or more effective than best medical therapy in alleviating disability in moderate to severe PD patients (Krack, et al. 2003, Schupbach, et al. 2005, Weaver, et al. 2009). The conclusion that STN-DBS is neuroprotective in parkinsonian animal models would suggest that this treatment strategy holds potential to delay the progression of PD and that this therapy should be expanded and offered to PD patients in early stages of the disease, a time when a greater complement of DA neurons and terminals remain to be influenced. As illustrated by our inability to protect striatal THir neurites, the neuroprotective potential of STN DBS appears unavoidably linked to the magnitude of nigrostriatal degeneration that has already occurred. Under present treatment protocols PD patients are not considered for STN-DBS until symptoms have been present for a minimum of five years (Chang and Chou 2006, Neimat, et al. 2006) and the typical PD patient has a mean disease duration of 14 years before STN-DBS is performed (Volkmann 2004) during which time the disease has progressed significantly. Therefore, by the time patients initiate DBS they are well into the later stages of PD with STN DBS often considered as a treatment option of last resort. However, the possibility of STN DBS-mediated neuroprotection must be carefully weighed against the risks associated with the procedure as adverse events are significantly higher in DBS compared to best medical therapy in patients with advanced PD (Weaver et al. 2009). An ongoing clinical trial seeks to determine the safety and tolerability of STN DBS in early PD and to compare these results to optimal drug therapy (Vanderbilt University). Careful consideration of the results from this trial, and previous trials, will allow patients to determine what treatment option represents their best course of action.
Acknowledgments
We are thankful for the excellent technical assistance of Mr. James Lee in the execution of this project. We are also grateful for the input and insight of Dr. P. David Charles and Dr. Helen Bronte-Stewart. This work was supported by the University of Cincinnati, the Davis Phinney Foundation for Parkinson’s Research, NS064696 (ASE), and the Udall Parkinson’s Disease Research Center of Excellence at the University of Cincinnati NS058830 (TJC)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altar CA, Boylan CB, Fritsche M, Jones BE, Jackson C, Wiegand SJ, Lindsay RM, Hyman C. Efficacy of brain-derived neurotrophic factor and neurotrophin-3 on neurochemical and behavioral deficits associated with partial nigrostriatal dopamine lesions. J. Neurochem. 1994;63:1021–1032. doi: 10.1046/j.1471-4159.1994.63031021.x. [DOI] [PubMed] [Google Scholar]
- Aponso PM, Faull RL, Connor B. Increased progenitor cell proliferation and astrogenesis in the partial progressive 6-hydroxydopamine model of Parkinson’s disease. Neuroscience. 2008;151:1142–1153. doi: 10.1016/j.neuroscience.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Blandini F, Levandis G, Bazzini E, Nappi G, Armentero MT. Time-course of nigrostriatal damage, basal ganglia metabolic changes and behavioural alterations following intrastriatal injection of 6-hydroxydopamine in the rat: new clues from an old model. Eur. J. Neurosci. 2007;25:397–405. doi: 10.1111/j.1460-9568.2006.05285.x. [DOI] [PubMed] [Google Scholar]
- Boulet S, Lacombe E, Carcenac C, Feuerstein C, Sgambato-Faure V, Poupard A, Savasta M. Subthalamic stimulation-induced forelimb dyskinesias are linked to an increase in glutamate levels in the substantia nigra pars reticulata. J. Neurosci. 2006;26:10768–10776. doi: 10.1523/JNEUROSCI.3065-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LL, Feldman SM, Smith DM, Cavanaugh JR, Ackermann RF, Graybiel AM. Differential metabolic activity in the striosome and matrix compartments of the rat striatum during natural behaviors. J. Neurosci. 2002;22:305–314. doi: 10.1523/JNEUROSCI.22-01-00305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Boecker H, Bartenstein P, von Falkenhayn I, Riescher H, Conrad B, Moringlane JR, Alesch F. A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease: enhanced movement-related activity of motor-association cortex and decreased motor cortex resting activity. Arch. Neurol. 1999;56:997–1003. doi: 10.1001/archneur.56.8.997. [DOI] [PubMed] [Google Scholar]
- Chang VC, Chou KL. Deep brain stimulation for Parkinson’s disease: patient selection and motor outcomes. Med. Health R. I. 2006;89:142–144. [PubMed] [Google Scholar]
- Dodiya HB, Chu Y, Beach TG, Adler CH, Olanow CW, Bartus RT, Kordower JH. The Status Dopaminergic Putamenal Innervation in Parkinson’s Disease as a Function of Disease Duration: Relevance to Trophic Factor Therapy. Society for Neurscience Abstracts. 2009 [Google Scholar]
- Elbaz A, Dufouil C, Alperovitch A. Interaction between genes and environment in neurodegenerative diseases. C. R. Biol. 2007;330:318–328. doi: 10.1016/j.crvi.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Annett L, Kirik D. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson’s disease. J. Neurosci. 2005;25:769–777. doi: 10.1523/JNEUROSCI.4421-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg MH, Lee SM, Ebner FF. The contribution of the principal and spinal trigeminal nuclei to the receptive field properties of thalamic VPM neurons in the rat. J. Neurocytol. 2004;33:75–85. doi: 10.1023/B:NEUR.0000029649.28599.a5. [DOI] [PubMed] [Google Scholar]
- Garcia-Arencibia M, Gonzalez S, de Lago E, Ramos JA, Mechoulam R, Fernandez-Ruiz J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007;1134:162–170. doi: 10.1016/j.brainres.2006.11.063. [DOI] [PubMed] [Google Scholar]
- Goetz CG, Stebbins GT, Blasucci LM. Differential progression of motor impairment in levodopa-treated Parkinson’s disease. Mov. Disord. 2000;15:479–484. doi: 10.1002/1531-8257(200005)15:3<479::AID-MDS1009>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Harnack D, Meissner W, Jira JA, Winter C, Morgenstern R, Kupsch A. Placebo-controlled chronic high-frequency stimulation of the subthalamic nucleus preserves dopaminergic nigral neurons in a rat model of progressive Parkinsonism. Exp. Neurol. 2008;210:257–260. doi: 10.1016/j.expneurol.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 2008;4:600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- Hershey T, Revilla FJ, Wernle AR, McGee-Minnich L, Antenor JV, Videen TO, Dowling JL, Mink JW, Perlmutter JS. Cortical and subcortical blood flow effects of subthalamic nucleus stimulation in PD. Neurology. 2003;61:816–821. doi: 10.1212/01.wnl.0000083991.81859.73. [DOI] [PubMed] [Google Scholar]
- Hilker R, Portman AT, Voges J, Staal MJ, Burghaus L, van Laar T, Koulousakis A, Maguire RP, Pruim J, de Jong BM, Herholz K, Sturm V, Heiss WD, Leenders KL. Disease progression continues in patients with advanced Parkinson’s disease and effective subthalamic nucleus stimulation. J. Neurol. Neurosurg. Psychiatry. 2005;76:1217–1221. doi: 10.1136/jnnp.2004.057893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. Neurochemical pathology and the etiology of Parkinson’s disease: basic facts and hypothetical possibilities. Mt. Sinai J. Med. 1988;55:11–20. [PubMed] [Google Scholar]
- Jenner P. Oxidative stress and Parkinson’s disease. Handb. Clin. Neurol. 2007;83:507–520. doi: 10.1016/S0072-9752(07)83024-7. [DOI] [PubMed] [Google Scholar]
- Joglar B, Rodriguez-Pallares J, Rodriguez-Perez AI, Rey P, Guerra MJ, Labandeira-Garcia JL. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: relevance to progression of the disease. J. Neurochem. 2009;109:656–669. doi: 10.1111/j.1471-4159.2009.05999.x. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Lang CG, Gauthier AM. Induction of oral stereotypy following amphetamine microinjection into a discrete subregion of the striatum. Psychopharmacology (Berl) 1988;95:556–559. doi: 10.1007/BF00172976. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp. Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Klein RL, Lewis MH, Muzyczka N, Meyer EM. Prevention of 6-hydroxydopamine-induced rotational behavior by BDNF somatic gene transfer. Brain Res. 1999;847:314–320. doi: 10.1016/s0006-8993(99)02116-2. [DOI] [PubMed] [Google Scholar]
- Kluver H, Barrera E. A method for the combined staining of cells and fibers in the nervous system. J. Neuropathol. Exp. Neurol. 1953;12:400–403. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Kanaan NM, Chu Y, Suresh Babu R, Stansell J, 3rd, Terpstra BT, Sortwell CE, Steece-Collier K, Collier TJ. Failure of proteasome inhibitor administration to provide a model of Parkinson’s disease in rats and monkeys. Ann. Neurol. 2006;60:264–268. doi: 10.1002/ana.20935. [DOI] [PubMed] [Google Scholar]
- Koprich JB, Campbell NG, Lipton JW. Neonatal 3,4-methylenedioxymethamphetamine (ecstasy) alters dopamine and serotonin neurochemistry and increases brain-derived neurotrophic factor in the forebrain and brainstem of the rat. Brain Res. Dev. Brain Res. 2003;147:177–182. doi: 10.1016/s0165-3806(03)00219-0. [DOI] [PubMed] [Google Scholar]
- Koprich JB, Chen EY, Kanaan NM, Campbell NG, Kordower JH, Lipton JW. Prenatal 3,4-methylenedioxymethamphetamine (ecstasy) alters exploratory behavior, reduces monoamine metabolism, and increases forebrain tyrosine hydroxylase fiber density of juvenile rats. Neurotoxicol. Teratol. 2003;25:509–517. doi: 10.1016/s0892-0362(03)00091-6. [DOI] [PubMed] [Google Scholar]
- Krack P, Batir A, Van Blercom N, Chabardes S, Fraix V, Ardouin C, Koudsie A, Limousin PD, Benazzouz A, LeBas JF, Benabid AL, Pollak P. Five-year follow-up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N. Engl. J. Med. 2003;349:1925–1934. doi: 10.1056/NEJMoa035275. [DOI] [PubMed] [Google Scholar]
- Lee CS, Sauer H, Bjorklund A. Dopaminergic neuronal degeneration and motor impairments following axon terminal lesion by instrastriatal 6-hydroxydopamine in the rat. Neuroscience. 1996;72:641–653. doi: 10.1016/0306-4522(95)00571-4. [DOI] [PubMed] [Google Scholar]
- Madhavan L, Daley BF, Paumier KL, Collier TJ. Transplantation of subventricular zone neural precursors induces an endogenous precursor cell response in a rat model of Parkinson’s disease. J. Comp. Neurol. 2009;515:102–115. doi: 10.1002/cne.22033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesawa S, Kaneoke Y, Kajita Y, Usui N, Misawa N, Nakayama A, Yoshida J. Long-term stimulation of the subthalamic nucleus in hemiparkinsonian rats: neuroprotection of dopaminergic neurons. J. Neurosurg. 2004;100:679–687. doi: 10.3171/jns.2004.100.4.0679. [DOI] [PubMed] [Google Scholar]
- Migliore L, Coppede F. Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases. Mutat. Res. 2009;667:82–97. doi: 10.1016/j.mrfmmm.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Mouradian MM. Recent advances in the genetics and pathogenesis of Parkinson disease. Neurology. 2002;58:179–185. doi: 10.1212/wnl.58.2.179. [DOI] [PubMed] [Google Scholar]
- Neimat JS, Hamani C, Lozano AM. Neural stimulation for Parkinson’s disease: current therapies and future directions. Expert Rev. Neurother. 2006;6:101–109. doi: 10.1586/14737175.6.1.101. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson R. The Rat Brain in Stereotaxic Coordinates. Elsevier Academic Press; Burlington, MA: 2005. [Google Scholar]
- Pisa M. Motor somatotopy in the striatum of rat: manipulation, biting and gait. Behav. Brain Res. 1988;27:21–35. doi: 10.1016/0166-4328(88)90106-4. [DOI] [PubMed] [Google Scholar]
- Poirier J, Kogan S, Gauthier S. Environment, genetics and idiopathic Parkinson’s disease. Can. J. Neurol. Sci. 1991;18:70–76. doi: 10.1017/s0317167100031334. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J. Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pallares J, Parga JA, Joglar B, Guerra MJ, Labandeira-Garcia JL. The mitochondrial ATP-sensitive potassium channel blocker 5-hydroxydecanoate inhibits toxicity of 6-hydroxydopamine on dopaminergic neurons. Neurotox Res. 2009;15:82–95. doi: 10.1007/s12640-009-9010-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pallares J, Parga JA, Munoz A, Rey P, Guerra MJ, Labandeira-Garcia JL. Mechanism of 6-hydroxydopamine neurotoxicity: the role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J. Neurochem. 2007;103:145–156. doi: 10.1111/j.1471-4159.2007.04699.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Iglesias S, Rey P, Mendez-Alvarez E, Labandeira-Garcia JL, Soto-Otero R. Time-course of brain oxidative damage caused by intrastriatal administration of 6-hydroxydopamine in a rat model of Parkinson’s disease. Neurochem. Res. 2007;32:99–105. doi: 10.1007/s11064-006-9232-6. [DOI] [PubMed] [Google Scholar]
- Sauer H, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupbach WM, Chastan N, Welter ML, Houeto JL, Mesnage V, Bonnet AM, Czernecki V, Maltete D, Hartmann A, Mallet L, Pidoux B, Dormont D, Navarro S, Cornu P, Mallet A, Agid Y. Stimulation of the subthalamic nucleus in Parkinson’s disease: a 5 year follow up. J. Neurol. Neurosurg. Psychiatry. 2005;76:1640–1644. doi: 10.1136/jnnp.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shults CW, Kimber T, Altar CA. BDNF attenuates the effects of intrastriatal injection of 6-hydroxydopamine. Neuroreport. 1995;6:1109–1112. doi: 10.1097/00001756-199505300-00009. [DOI] [PubMed] [Google Scholar]
- Singh S, Ahmad R, Mathur D, Sagar RK, Krishana B. Neuroprotective effect of BDNF in young and aged 6-OHDA treated rat model of Parkinson disease. Indian. J. Exp. Biol. 2006;44:699–704. [PubMed] [Google Scholar]
- Smith MP, Cass WA. Oxidative stress and dopamine depletion in an intrastriatal 6-hydroxydopamine model of Parkinson’s disease. Neuroscience. 2007;144:1057–1066. doi: 10.1016/j.neuroscience.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sortwell CE, Collier TJ, Terpstra BT, Thompson VB, O’Malley J, Steece-Collier K, Wohlgenant SM, Daley BF, Mandel RJ, Lipton JW. The potential role for the trophic factor pleitrophin (PTN) to protect the parkinsonian brain. Cell Transplantation. 2008;17:481. [Google Scholar]
- Sortwell CE, Kowdower JH. In vivo gene therapy as a potential treatment for Parkinson’s disease. In: Olanow W, Brundin P, editors. Restorative Therapies in Parkinson’s Disease. Kluwer Press; New York, NY: 2006. pp. 317–344. [Google Scholar]
- Spieles-Engemann AL, Behbehani MM, Collier TJ, Steece-Collier K, Wohlgenant SL, Thompson VB, Lipton JW, Sortwell CE. Deep Brain Stimulation in a Rodent Model of Parkinson’s Disease Upregulates BDNF in Subthalamic Nucleus Target Structures. Society for Neurscience Abstracts. 2009 [Google Scholar]
- Sun M, Kong L, Wang X, Lu XG, Gao Q, Geller AI. Comparison of the capability of GDNF, BDNF, or both, to protect nigrostriatal neurons in a rat model of Parkinson’s disease. Brain Res. 2005;1052:119–129. doi: 10.1016/j.brainres.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel Y, Visser-Vandewalle V, Kaplan S, Kozan R, Daemen MA, Blokland A, Schmitz C, Steinbusch HW. Protection of nigral cell death by bilateral subthalamic nucleus stimulation. Brain Res. 2006;1120:100–105. doi: 10.1016/j.brainres.2006.08.082. [DOI] [PubMed] [Google Scholar]
- Terpstra BT, Collier TJ, Marchionini DM, Levine ND, Paumier KL, Sortwell CE. Increased cell suspension concentration augments the survival rate of grafted tyrosine hydroxylase immunoreactive neurons. J Neurosci. Meth. 2007;166(1):13–9. doi: 10.1016/j.jneumeth.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderbilt University . ClinicalTrials.gov [Internet] National Library of Medicine (US); Bethesda (MD): 2000. Deep brain stimulation (DBS) for early stage Parkinson’s disease.ClinicalTrials.gov [cited 2010 Feb 20]. Available from: http://clinicaltrials.gov/ct2/show/NCT00282152 NLM Identifier: NCT00282152. [Google Scholar]
- Volkmann J. Deep brain stimulation for the treatment of Parkinson’s disease. J. Clin. Neurophysiol. 2004;21:6–17. doi: 10.1097/00004691-200401000-00003. [DOI] [PubMed] [Google Scholar]
- Wallace BA, Ashkan K, Heise CE, Foote KD, Torres N, Mitrofanis J, Benabid AL. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain. 2007;130:2129–2145. doi: 10.1093/brain/awm137. [DOI] [PubMed] [Google Scholar]
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, Rothlind J, Sagher O, Reda D, Moy CS, Pahwa R, Burchiel K, Hogarth P, Lai EC, Duda JE, Holloway K, Samii A, Horn S, Bronstein J, Stoner G, Heemskerk J, Huang GD, CSP 468 Study Group Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windels F, Bruet N, Poupard A, Feuerstein C, Bertrand A, Savasta M. Influence of the frequency parameter on extracellular glutamate and gamma-aminobutyric acid in substantia nigra and globus pallidus during electrical stimulation of subthalamic nucleus in rats. J. Neurosci. Res. 2003;72:259–267. doi: 10.1002/jnr.10577. [DOI] [PubMed] [Google Scholar]
- Windels F, Bruet N, Poupard A, Urbain N, Chouvet G, Feuerstein C, Savasta M. Effects of high frequency stimulation of subthalamic nucleus on extracellular glutamate and GABA in substantia nigra and globus pallidus in the normal rat. Eur. J. Neurosci. 2000;12:4141–4146. doi: 10.1046/j.1460-9568.2000.00296.x. [DOI] [PubMed] [Google Scholar]
- Xue YQ, Zhao LR, Guo WP, Duan WM. Intrastriatal administration of erythropoietin protects dopaminergic neurons and improves neurobehavioral outcome in a rat model of Parkinson’s disease. Neuroscience. 2007;146:1245–1258. doi: 10.1016/j.neuroscience.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Zhang B, Chu J, Zhang J, Ma Y. Change of extracellular glutamate and gamma-aminobutyric acid in substantia nigra and globus pallidus during electrical stimulation of subthalamic nucleus in epileptic rats. Stereotact. Funct. Neurosurg. 2008;86:208–215. doi: 10.1159/000131657. [DOI] [PubMed] [Google Scholar]