In this issue of the Journal of Allergy and Clinical Immunology, Amu and colleagues demonstrate a significant role for interleukin-10 (IL-10)-producing regulatory B (Breg) cells during mouse models of allergic airway inflammation1. The authors identified a Breg cell subpopulation that expands in vivo and in vitro in response to parasitic Schistosoma mansoni worm infection. The adoptive transfer of these Breg cells into allergen-sensitized mice suppresses anaphylaxis and allergen-induced airway hyper-responsiveness through IL-10-dependent mechanisms1, 2. These important findings expand the clinical significance of studies showing that IL-10-competent Breg cells dramatically regulate inflammation and autoimmunity in mouse models of contact hypersensitivity3, experimental autoimmune encephalomyelitis (EAE)4, 5, collagen induced arthritis6, and inflammatory bowel disease7.
These studies focus on a relatively rare IL-10-competent mouse B cell subset that represents only 1–2% of spleen B cells in naïve wild type mice8. We call these cells “B10 cells” because IL-10 secretion is universally recognized as their mechanism of regulatory function, they only produce IL-10 transcripts3, 9, and multiple other B cell subsets with regulatory properties are likely to exist. B10 cells are predominantly contained within a phenotypically unique CD1dhiCD5+CD19hi B cell subpopulation that normally represents only 2–7% of spleen B cells3. B10 cells appear to be functionally mature since they can be identified by cytoplasmic IL-10 expression following only 5 hours (h) of in vitro stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin. B10 progenitor (B10pro) cells have also been functionally identified within the spleen CD1dhiCD5+ B cell subpopulation, but these cells require 48 h of in vitro stimulation through CD40 or with LPS before they acquire the ability to express cytoplasmic IL-10 after 5 h PMA and ionomycin stimulation9, 10. Thereby, purifying spleen CD1dhi or CD1dhiCD5+ B cells enriches for functionally potent B10 and B10pro cells that can be adoptively transferred into recipient mice to shift the normal balance of regulatory networks towards a more immunosuppressive phenotype.
Parasitic infections with S. mansoni worms in the current study drives both B cell and B10 cell expansion in mice1. Transferring spleen CD1dhi B cells from worm-infected mice into ovalbumin (allergen)-challenged recipients inhibits both acute and established airway inflammation. Most likely, B10 cells expand more than other B cells in vivo because they proliferate more vigorously in response to polyclonal mitogens when compared with non-B10 cells9. Since antigen-specific B10 cells are required to inhibit contact hypersensitivity and autoimmunity3, 5, it is unlikely that worm antigen-specific B10 cells would inhibit ovalbumin-driven disease. Thus, it will be important to determine whether S. mansoni worms are driving polyclonal, antigen-specific or cross-reactive B10 and B10pro cell expansion/maturation. It will also be important to determine whether worm-driven B10 cell expansion can regulate contact hypersensitivity and autoimmunity. Regardless, helminth-driven B10 cell expansion supports the “hygiene hypothesis,” whereby a decrease in helminth infections within a population is proposed to increase allergic disease incidence11. Thus, B10 cell function and their relative frequencies may also be important factors contributing to human allergic diseases.
The authors propose to have identified a distinct IL10+CD1dhiCD21hiCD23+IgD+ IgMhiCD19+ spleen Breg cell subpopulation. However, there are currently no cell surface markers that uniquely delineate all IL-10-competent B10 cells or B10pro cells. Rather, the ability of B10 cells to produce IL-10 is the single functional marker that unifies most current studies and identifies a population of cells with a fairly homogenous cell surface phenotype3. Isolating B cells based on IL-10 expression alone is technically problematic as this selects for either IL-10 secreting cells or cytoplasmic IL-10+ cells that must be permeabilized, while functionally important B10pro cells are lost using these methods. Moreover, IL-10 competence is most frequently measured after PMA and ionophore stimulation in vitro. As shown by Amu and colleagues, single markers such as CD1dhi, CD21hi, CD23+, IgDlow, or IgMhi could be used to enrich for B10 cells, but they also exclude a substantial proportion of functionally competent B10 cells that are then diluted within the remaining non-selected B cell population. IL-10 competent B cells found within other mouse tissues also differently express some of these cell surface markers9. Despite these technical issues, most studies within the evolving Breg cell field are likely to be examining the same rare and functionally unique B10 and B10pro cell subset that regulates immune responses through the production of IL-10.
B cells contribute to asthma pathogenesis by producing IgE12. However, Amu and colleagues also identified B cell subsets that either exacerbated or regulated allergic airway inflammation. While most B cells express CD1d, a spleen CD1dlow B cell subset was expanded in helminth-infected mice. Asthma was exacerbated when these CD1dlow B cells were adoptively transferred into allergen-sensitized mice. Although it was not determined whether these B cells contributed to IgE production, the future characterization of these cells may reveal a novel B cell subset that preferentially contributes to disease pathogenesis through unknown mechanisms. Distinct B cell subsets with opposing pathogenic and negative regulatory functions have also been observed during EAE pathogenesis in mice5. Mature B cell depletion using CD20 monoclonal antibody before EAE induction exacerbates subsequent disease, while B cell depletion during EAE progression dramatically reduces disease symptoms. Exacerbated autoimmune disease results from B10 cell depletion before disease initiation, which is ameliorated by the adoptive transfer of spleen CD1dhiCD5+ B cells. B10 and other B cells have also been found to have opposing protective and pathogenic functions during mouse models of systemic lupus erythematosus, respectively13, 14. Thereby, different B cell subsets may display opposing protective and pathogenic functions during human asthma, since B cell depletion may improve atopic eczema15. Thus, future mouse and patient studies are needed to further uncover the likely complexities of B cell function during different stages of airway immunopathology.
Amu and colleagues demonstrate that IL-10 production by CD1dhi B cells is required to observe their regulatory effects, and they show that S. mansoni infected CD1d-deficient mice are highly susceptible to allergic airway inflammation. However, it remains essential to determine whether B cell CD1d expression is required for B10 or B10 pro cell function since CD1d expression is not required for B10 cell development9. Multiple leukocyte lineages and subsets produce IL-10, and the mechanisms by which IL-10 can inhibit or augment immune responses are equally complex. However, Amu and colleagues propose that adoptively transferred CD1dhi B cells suppress airway inflammation by inducing natural FoxP3+ CD4+ regulatory T (Treg) cell recruitment into the lungs, where they suppress lung inflammation. Whether B10 cells must enter the lung to induce these changes or exert these effects distally remains unknown. It is also unknown if B10 cells actually control Treg cell migration or whether enhanced Treg emigration into the lung is an indirect consequence of reduced inflammation. Nonetheless, Treg cell numbers are significantly decreased in CD19-deficient NZB/W mice that have few B10 cells, while wild type CD1dhiCD5+ B cell transferred into CD19−/− NZB/W mice induces Treg cell expansion14. These independent studies suggest a potential link between B10 cell function and Treg cell frequencies that needs to be explored.
Multiple laboratories have demonstrated that Breg cells are functionally significant in diverse diseases. It will be important to determine whether other parasites and infectious agents also drive B10 cell expansion as a potential mechanism for reducing host immune responses. This will further open the door for identifying B10 cell-directed therapies. Turning these laboratory observations into therapeutic targets for modulating immune responses and pathology will be a significant, but important challenge for the future.
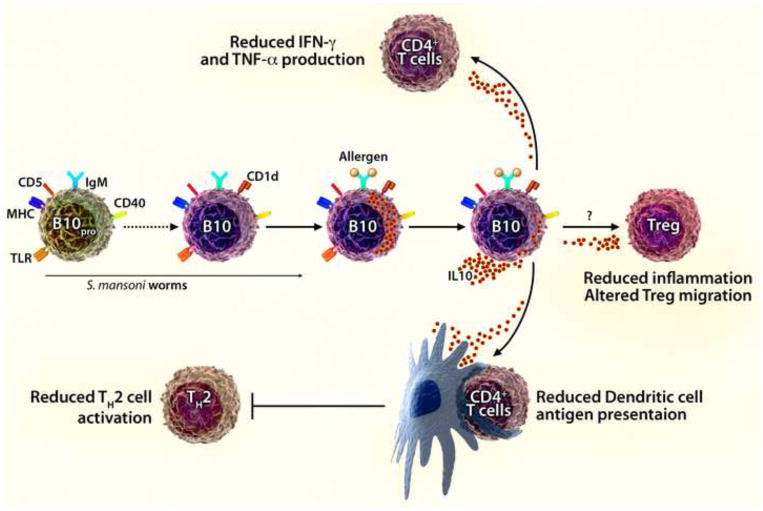
Figure 1.
IL-10-producing regulatory B10 cells inhibit allergic airway disease. Spleen B10pro cells mature into antigen-specific CD1dhiCD5+ regulatory B10 cells that are competent to produce and secrete IL-10 in response to allergen (antigen) challenge. Amu and colleagues show that spleen B10pro/B10 cells are induced to mature/expand in response to S. mansoni worm infection. The subsequent adoptive transfer of CD1dhi B cells purified from infected mice into allergen-sensitized recipients suppressed the induction of acute and allergic airway inflammation, which is proposed to result from the recruitment of Treg cells into the lungs of challenged mice. From our studies, B10 cells are also known to inhibit CD4+ T cell production of IFN-γ and TNF-α, to reduce the antigen-presenting capacity of DCs, and to reduce inflammatory responses through the production of IL-10. Thereby, B10 cells are likely to inhibit lung inflammation through multiple IL-10-dependent mechanisms of negative regulation.
Acknowledgments
Acknowledgments, and financial & competing interests disclosure
We thank Drs. Cynthia Magro, David DiLillo, Michelle Schweitzer, Jonathan Poe, and Susan Smith for comments. This work was supported by NIH grants (AI057157 and AI56363). T. F. T. is a paid consultant for MedImmune, Inc. and Angelica Therapeutics, Inc. and shareholder of Angelica Therapeutics. The authors have no other financial conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amu S, Saunders SP, Kronenberg M, Mangan NE, Atzberger A, Fallon PG. B regulatory cells prevent and reverse allergic airway inflammation via FoxP3+ Treg cells in a murine model. J Allergy Clin Immunol. 2010 doi: 10.1016/j.jaci.2010.01.018. in press. [DOI] [PubMed] [Google Scholar]
- 2.Mangan NE, Fallon RE, Smith P, van Rooijen N, McKenzie AN, Fallon PG. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J Immunol. 2004;173:6346–56. doi: 10.4049/jimmunol.173.10.6346. [DOI] [PubMed] [Google Scholar]
- 3.Yanaba K, Bouaziz J-D, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–50. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 4.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–50. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita T, Yanaba K, Bouaziz J-D, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–30. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–30. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 8.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 9.Yanaba K, Bouaziz J-D, Matsushita T, Tasubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–72. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–99. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 11.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–4. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 12.Boyce JA, Broide D, Matsumoto K, Bochner BS. Advances in mechanisms of asthma, allergy, and immunology in 2008. J Allergy Clin Immunol. 2009;123:569–74. doi: 10.1016/j.jaci.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas KM, Watanabe R, Matsushita T, Nakashima H, Ishiura N, Okochi H, et al. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol. 2010 doi: 10.4049/jimmunol.0902391. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe R, Ishiura N, Nakashima H, Kuwano Y, Okochi H, Tamaki K, et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010 doi: 10.4049/jimmunol.0902385. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon D, Hösli S, Kostylina G, Yawalkar N, Simon HU. Anti-CD20 (rituximab) treatment improves atopic eczema. J Allergy Clin Immunol. 2008;121:122–8. doi: 10.1016/j.jaci.2007.11.016. [DOI] [PubMed] [Google Scholar]