Abstract
Although many studies have indicated that fish oil (FO) improves cardiovascular risk factors and reduces histopathologic manifestations of injury in experimental renal injury models, potential mechanisms underlying this protective effect have not been adequately defined. The objective of this study was to identify potential signaling pathways that confer protection in the Dahl rat model of salt-sensitive hypertension. Male Dahl salt-sensitive rats (n=10 per group) were provided with formulated diets containing 8% NaCl, 20% protein, and 25% FO or 25% corn oil (CO) for 28 days. FO reduced blood pressure (-11% at 4 weeks, P<0.05), urine protein excretion (-45% at 4 weeks, P<0.05), plasma cholesterol and triglyceride levels (-54%, P<0.001 and -58%, P<0.05), and histopathologic manifestations of renal injury, including vascular hypertrophy, segmental and global glomerular sclerosis, interstitial fibrosis, and tubular atrophy. Interstitial inflammation was significantly reduced by FO (-32%, P<0.001), as assessed by quantitative analysis of ED1-positive cells in sections of renal cortex. FO reduced tubulointerstitial proliferative activity, as assessed by Western blot analysis of cortical homogenates for PCNA (-51%, P<0.01) and quantitative analysis of Mib-1 stained sections of renal cortex (-42%, P<0.001). Decreased proliferative activity was associated with reduced p-ERK expression (-37%, P<0.005) and NF-κB activation (-42%, P<0.05). FO reduced COX-2 expression (-63%, P<0.01) and membrane translocation of the NADPH oxidase subunits p47phox and p67phox (-26% and -34%, P<0.05). We propose that FO ameliorates renal injury in Dahl salt-sensitive rats through inhibition of ERK, decreased NF-κB activation, inhibition of COX-2 expression, and decreased NADPH oxidase activation.
Keywords: Fibrosis, glomerulosclerosis, DHA, EPA, rat
Introduction
Hypertensive nephrosclerosis is the second most common cause of end-stage renal disease in the United States (98). Over 70% of the 5.6 million individuals in the United States with elevated serum creatinine levels are hypertensive (16). The risk of end-stage renal disease is directly related to the degree of blood pressure elevation. Hypertension, which occurs in as many as 43 million individuals, is clearly a multi-factorial disorder resulting from a variety of environmental and genetic factors. Of the environmental factors, excess dietary salt intake appears to be one of the most important (93, 103). Of note, salt sensitivity is an independent cardiovascular risk factor in patients with hypertension (69).
Given the prevalence of hypertension, nontoxic, easily-tolerated therapeutic agents to complement standard antihypertensive therapy may play a critical role in reducing the prevalence of cardiovascular and renal disease secondary to hypertension. A number of studies have provided evidence that dietary fish oil (FO) supplementation ameliorates cardiovascular and renal disease in humans (12, 21-23, 38, 51, 68, 78, 92) and a variety of experimental model systems, including spontaneously hypertensive rats (SHR) (44, 50, 91) and Dahl salt-sensitive (SS) rats, which provide a model of human salt-sensitive hypertension (45, 83, 85, 86, 94, 101). Despite extensive study, potential mechanisms whereby dietary FO supplementation protects against the development of renal disease have not been adequately defined (33, 34).
The protective effects of FO in renovascular hypertension are likely to be at least in part related to reduction of blood pressure (10, 27, 30, 66, 70) and improvement of serum lipid profiles (39, 50, 59, 82). However, recent studies have indicated that high salt intake promotes renal disease through mechanisms which are independent of blood pressure (89), and there is evidence that FO may have protective effects which are independent of blood pressure reduction alone (91). In vitro studies have uncovered several potential mechanisms whereby FO may play a critical role in ameliorating renal injury. In cultured cells, FO suppresses growth factor-induced proliferation through cell cycle arrest in G1 phase (14, 32, 95, 107). In vitro studies of cultured cells and ex vivo studies of inflammatory cells have shown that FO has potent anti-inflammatory effects, by reducing cytokine production and NF-κB activation (13, 46, 48, 49, 102), COX-2 expression (60), and NADPH oxidase activation (90). There have been few studies to address the potential in vivo relevance of these findings in models of renal injury.
To address this issue, we used an experimental model of salt-sensitive hypertension to identify potential pathway(s) through which FO may prevent or ameliorate renal disease. In accord with previous descriptive studies, we demonstrate that FO reduces blood pressure, serum lipids, and histopathologic manifestations of renal injury. Reduction of blood pressure by treating SS rats with hydralazine failed to prevent renal injury, indicating that other pathways may be at least in part responsible for the protective effects of FO. We demonstrate that FO inhibits ERK signaling and NF-κB activation, reduces interstitial inflammation, and decreases the renal proliferative response to injury. The anti-inflammatory effects of FO are related to decreased cyclooxygenase-2 (COX-2) expression and NADPH oxidase assembly. Our studies have identified several key pathways modulated by FO which may underlie its protective effect in renovascular hypertension.
Materials and Methods
Animal model
All animal procedures were performed according to institutional animal care guidelines established by the National Institutes of Health, and the study protocol was approved by the Mayo Clinic College of Medicine Institutional Animal Care and Use Committee. Studies were conducted using 30 male Dahl SS rats and 10 male Dahl salt-resistant (SR) rats purchased from Harlan Sprague Dawley (Indianapolis, IN) at 5 to 6 weeks of age. The rats were housed under standard conditions with access to a normal-salt diet (NSD; 0.45% NaCl, 20% protein, 5% corn oil) and water ad libitum for 2 weeks prior to experiments. At 7 to 8 weeks of age, the SS rats were randomly divided into three treatment groups (n=10 per group) and switched to one of three high-salt diet formulations prepared by Purina TestDiet® (Richmond, IN). The diets, derived from Basal Diet #5755, contained 8% NaCl, 20% protein, and one of the following: 5% corn oil (HSD), 25% corn oil (HSD+CO), or 25% fish oil (HSD+FO). The FO (160g/kg eicosapentaenoic acid [EPA] and 100g/kg docosahexaenoic acid [DHA]) was kindly provided by Pronova Biocare (Lysaker, Norway). All diets delivered the same amount of vitamins, minerals, and fiber per calorie. The SR rats received the HSD, and served as the negative control. In a separate experiment, hydralazine was administered to SS rats on the HSD to determine the effect lowering blood pressure has on renal damage and signaling pathways. Animals were divided into three groups and given the NSD (n = 3), the HSD (n = 3), or the HSD + hydralazine (HYD; 5 mg/kg body weight/day) in drinking water (n = 6).
Systolic blood pressure in conscious rats was measured weekly by the tail cuff method using the XBP1000 Noninvasive Blood Pressure System (Kent Scientific Corp., Torrington, CT). Each week, rats were weighed and placed in metabolic cages to monitor food intake and urine output, and to collect 24 hour urine samples. Urine protein was measured using the Lowry method (58). After 28 days, the rats were anesthetized with ketamine/xylazine, blood samples were collected for lipid analysis, and the kidneys and hearts were harvested. Portions of the kidneys were fixed for histopathologic analysis and immunohistochemical staining. Additional portions were snap frozen in liquid nitrogen and stored at -80° C for Western blot analysis.
Histology and immunohistochemistry
Renal tissue was fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin per standard techniques. Sections were cut at a thickness of 4 μm and stained with H&E, Masson's trichrome, or Sirius red. Immunostains were performed using the following antibodies: α-smooth muscle actin (α-SMA; DakoCytomation, Carpenteria, CA), Mib-1 (Ki67; NovoCastra Laboratories, UK), and ED1 (Fitzgerald Industries, Concord, MA). Slides were deparaffinized, followed by antigen retrieval via heat treatment in EDTA for 30 minutes using a vegetable steamer. Trypsin treatment was performed for the ED1 stain. Commercially available kits (Vectastain ABC Kit, Vector Laboratories, Burlingame, CA; Envision Plus HRP Kit, DakoCytomation) were used for the blocking, secondary antibody and amplification steps. NovaRed (Vector Labs) was used for color development, followed by hematoxylin counterstain. To facilitate consistency between staining batches, the slides were stained on the Dako Autostainer (DakoCytomation), an automated staining machine.
Assessment of histopathologic features
Quantitative analysis of histopathologic manifestations of renal injury was performed, in a blinded fashion, with the MetaVue Image Analysis System (Universal Imaging Corporation, Downington, PA). Glomerular, interstitial, tubular, and vascular features of renal tissue, including glomerular size and vascular lumen to wall thickness ratios, were analyzed on H&E stained slides. Interstitial fibrosis was determined by quantitative assessment of extracellular matrix in trichrome stained slides and in Sirius red stained slides observed with both white and polarized light, the latter being an index of fibrillar collagen deposition (19). Glomerular and tubulointerstitial staining for α-SMA, a marker of epithelial to mesenchymal transformation, were expressed as a percentage of their respective surface areas. The proliferation marker Mib-1 and the macrophage marker ED1 were assessed as the number of positively staining glomerular or interstitial cells per 200× field.
Western blot analysis
For preparation of whole cell lysates, renal cortical tissue was homogenized in 1X Lysis Buffer (Cell Signaling Technology, Inc., Beverly, MA) with protease inhibitors (Protease Inhibitor Cocktail, Roche Applied Science, Indianapolis, IN). Homogenates were then centrifuged at 10,000 × g for 10 minutes at 4° C, and the resulting supernatants used for Western blot analysis. Microsomal fractions were prepared as previously described (15). In brief, tissues were homogenized in a buffer containing 0.25 M sucrose and 20 mM Tris-HCl, pH 7.2. Homogenates were centrifuged at 2,000 × g for 10 min to remove insoluble debris, then ultracentrifuged at 100,000 × g for 60 min. The pellets were resuspended in homogenizing buffer, and used for detection of levels of COX-2, p47phox, p67phox, and HO-1. Protein concentrations were determined using the Lowry method. Equal amounts of whole cell lysate or microsomal protein (∼100 μg) were denatured for 5 min at 100° C in SDS loading buffer (BioRad Laboratories, Inc., Burlingame, CA) and subjected to SDS-PAGE in the Criterion System (BioRad) at a constant current (200 mA/gel), followed by transfer to PVDF membranes (BioRad). The membranes were blocked with 1X casein (Vector Laboratories) in Tris-buffered saline (TBS) containing 0.5% Tween 20, and incubated with primary antibodies for phospho (p)-ERK and PCNA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), HO-1 (Stressgen Biotechnologies, Ann Arbor, MI), COX-2 (Cayman Chemical, Ann Arbor, MI), p47phox and p67phox (BD Transduction Laboratories, Franklin Lakes, NJ), followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (Southern Biotech, Birmingham, AL and Stressgen). The blots were then visualized by exposure to x-ray film using the ECL Western blotting detection reagents and analysis system (Amersham Biosciences Corp., Piscataway, NJ). The blots were stripped and reprobed with antibodies for total ERK (Santa Cruz) or GAPDH (Novus Biologicals, Inc., Littleton, CO) to normalize for gel loading and transfer efficiency; microsomal blots were stained with a MemCode Reversible Protein Stain Kit (Pierce, Rockford, IL). Densitometric analyses were performed with the Kodak ds Image Station 440.
Electrophoretic mobility shift assay (EMSA)
Tissue nuclear extracts were prepared and assessed for activation of NF-κB. by EMSA, as previously described by Rangan et al (84) and modified by Nath et al (73). Approximately 75 mg of rat renal cortex was homogenized on ice in 400 μL of buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 2 mM MgCl2, 0.1 mM EDTA, and protease inhibitors [0.5 mM DTT + Roche Protease Inhibitor Cocktail]), followed by the addition of 50 μL of 10% Nonidet-P40. The samples were vortexed extensively and centrifuged at 13,000 × g for 5 min at 4°C. The pellets were resuspended in 60 μL of buffer B (7.5 mM HEPES, 300 mM NaCl, 50 mM KCl, 10% glycerol, and protease inhibitors), vortexed, and centrifuged at 13,000 × g for 10 min. The extracted nuclear protein in the supernatants was measured using the Lowry method. The NF-κB probe used in the EMSA, a double stranded consensus oligonucleotide from Promega (Madison, WI), was labeled with [32P] γATP; unincorporated nucleotide was removed using a BioSpin6 column (BioRad). Five micrograms of nuclear extract was combined with binding buffer containing 7.5 mM HEPES, pH 8.0, 100 mM NaCl, 1 mM MgCl2, 0.05 mM EDTA, 0.5 mM DTT, 3% glycerol, 0.2 mg/mL poly(dI+dC), and 50,000 CPM of labeled NF-κB probe, and incubated for 30 min at room temperature. The binding reactions were analyzed by electrophoresis on a 5% non-denaturing polyacrylamide gel and visualized by autoradiography. Supershift analysis was performed using a concentrated anti-p65 antibody (Santa Cruz): 1 μL of antibody was added to 5 μg nuclear extract and allowed to incubate on ice for 15 min before the binding reaction.
Statistical analysis
Data are expressed as mean ± SEM. Statistical significance was evaluated by one-way analysis of variance, followed by Bonferroni multiple comparisons test and Mann-Whitney test for unpaired observations. Differences were considered to be significant when P<0.05.
Results
Fish oil improves serum lipid profiles in SS+HSD rats
Male Dahl SS rats (n=10 per group) were provided with one of the following formulated diets: 0.45% NaCl, 5% CO (SS+NSD); 8% NaCl, 5% CO (SS+HSD); 8% NaCl, 25% CO (SS+HSD+CO); or 8% NaCl, 25% FO (SS+HSD+FO). SR rats were provided with the 8% NaCl, 5% CO diet (SR+HSD). No significant differences in daily food intake between the CO- and FO-treated rats were observed, and total weight gain during the experimental period was similar in all groups (data not shown). At the end of the experimental period, serum triglyceride and total cholesterol levels were obtained in non-fasted rats. As previously reported, serum cholesterol levels increased by two-fold in SS rats fed the HSD compared to SR rats fed the HSD (Table 1). Whereas the 25% CO diet had no significant effect on serum cholesterol or triglyceride levels in SS rats, the 25% FO diet reduced serum cholesterol and triglyceride levels more than 50% (Table 1).
Table 1.
Serum lipids, heart/body weight ratio, and histopathologic manifestations of renal injury.
| SR+HSD (n=10) | SS+HSD (n=10) | SS+HSD+CO (n=10) | SS+HSD+HYD (n=6) | SS+HSD+FO (n=10) | |
|---|---|---|---|---|---|
| Cholesterol (mg/dL) | 52 ± 2 | 103 ± 5# | 116 ± 6# | 122 ± 16# | 49 ± 4*+§ |
| Triglycerides (mg/dL) | 123 ± 10 | 129 ± 14 | 141 ± 25 | 92 ± 17 | 59 ± 6#*+ |
| Heart/body weight ratio (×10-3) | 3.86 ± 0.08 | 4.86 ± 0.11# | 4.67 ± 0.11# | 4.01 ± 0.06*+ | 4.18 ± 0.15*+ |
| Segmental sclerosis (% of glomeruli) | 0 | 4.87 ± 0.77# | 4.38 ± 0.98# | 5.30 ± 0.52# | 1.46 ± 0.27*+§ |
| Glomerular area (μm2) | 10,723 ± 380 | 16,416 ± 316# | 17,238 ± 613 | 15,085 ± 369#*+ | 12,591 ± 382#*+§ |
| Blood Vessels (lumen/wall thickness) | |||||
| Arcuate arteries | 0.98 ± 0.07 | 0.80 ± 0.05 | 0.94 ± 0.04* | 0.93 ± 0.03 | 1.03 ± 0.07* |
| Interlobular arteries | 0.83 ± 0.05 | 0.50 ± 0.04# | 0.45 ± 0.04 | 0.40 ± 0.03# | 0.84 ± 0.08*+§ |
| Arterioles | 0.61 ± 0.03 | 0.35 ± 0.03# | 0.32 ± 0.03 | 0.35 ± 0.03# | 0.63 ± 0.02*+§ |
| Tubular casts (% of cortical surface area) | 0.25 ± 0.10 | 13.23 ± 1.27# | 11.36 ± 1.41# | 13.46 ± 0.86# | 6.06 ± 0.47#*+§ |
Values are mean ± SEM. SR, salt-resistant rats; SS, salt-sensitive rats; HSD, high-salt diet (8% NaCl); CO, corn oil (25%); HYD, hydralazine (5 mg/kg/d); FO, fish oil (25%).
P<0.05 vs. SR+HSD,
P<0.05 vs. SS+HSD,
P<0.05 vs. SS+HSD+CO,
P<0.05 vs. SS+HSD+HYD.
Fish oil reduces blood pressure and heart weight in SS+HSD rats
There were no significant differences in systolic blood pressure prior to administration of formulated diets containing high salt (Figure 1A). As expected, systolic blood pressure was significantly increased in SS+HSD rats, whereas the systolic blood pressure did not significantly increase throughout the experimental period in SR+HSD rats (Figure 1A). Blood pressure elevation in SS rats on the high-salt diet containing 25% CO (SS+HSD+CO) was similar to that observed in SS rats on the high-salt diet containing 5% CO (SS+HSD, data not shown). FO supplementation significantly reduced systolic blood pressure, compared to SS+HSD rats (Figure 1A). An additional group of SS+HSD rats was treated with hydralazine (HYD; 5 mg/kg body weight/d), and blood pressure was measured after 4 weeks. This treatment (SS+HSD+HYD) significantly reduced systolic blood pressure compared to SS+HSD (141 mmHg vs. 193 mmHg, P<0.05; Figure 1B). This reduction of blood pressure was greater than that observed in SS+HSD+FO rats (141 mmHg vs. 172 mmHg, P<0.05). As expected, systolic blood pressure of SR+HSD and SS+NSD rats remained at basal levels (Figure 1B). Heart weight is the best measure of myocardial hypertrophy in response to systemic hypertension. The mean heart weight/body weight ratio of SS+HSD+FO rats was found to be significantly lower than that of SS+HSD or SS+HSD+CO rats (Table 1). SS+HSD+HYD rats had ratios similar to those of SS+HSD+FO rats (Table 1).
Figure 1. Fish oil decreases salt-sensitive hypertension in Dahl SS rats.
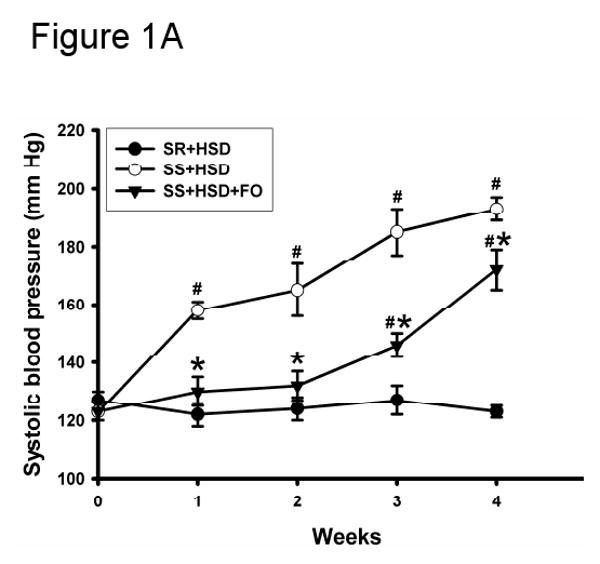
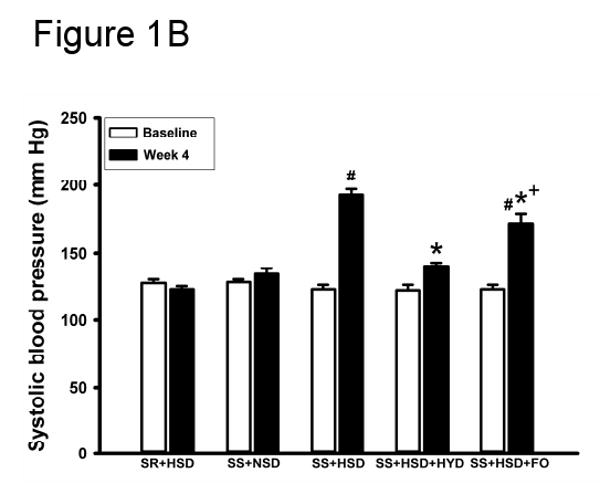
(A) Systolic blood pressure was measured in SR and SS rats at baseline and at weekly intervals during administration of high-salt diet alone (HSD, 8% NaCl; SR+HSD and SS+HSD) or supplemented with fish oil (FO, 25%; SS+HSD+FO). Values are mean ± SEM (n=10). #P<0.05 vs. SR+HSD, *P<0.05 vs. SS+HSD. (B) Rats were treated with normal-salt diet (NSD, 0.45% NaCl; SS+NSD, n=3), high-salt diet alone (SR+HSD and SS+HSD, n=10), or high-salt diet supplemented with hydralazine (HYD, 5 mg/kg/d; SS+HSD+HYD, n=6) or fish oil (SS+HSD+FO, n=10). Blood pressure was measured at baseline and after 4 weeks on diet. Values are mean ± SEM. #P<0.05 vs. SS+NSD, *P<0.05 vs. SS+HSD, +P<0.05 vs. SS+HSD+HYD.
Fish oil decreases urinary protein excretion in SS+HSD rats
Urine protein excretion is a well-recognized marker of glomerular damage. In accordance with previous observations, the development of renovascular hypertension in SS+HSD rats was associated with the development of proteinuria (Figure 2A). Although blood pressure was significantly reduced in SS+HSD+HYD rats, urine protein excretion progressively increased with time (Figure 2A). Urine protein excretion in SS+HSD and SS+HSD+HYD rats was not significantly different. SS rats on a normal-salt diet (SS+NSD) did not develop significant proteinuria (Figure 2A). FO treatment (SS+HSD+FO) significantly decreased urine protein excretion compared to SS+HSD+CO rats (Figure 2B). SR+HSD rats did not develop significant proteinuria (Figure 2B).
Figure 2. Fish oil reduces proteinuria in Dahl SS rats.
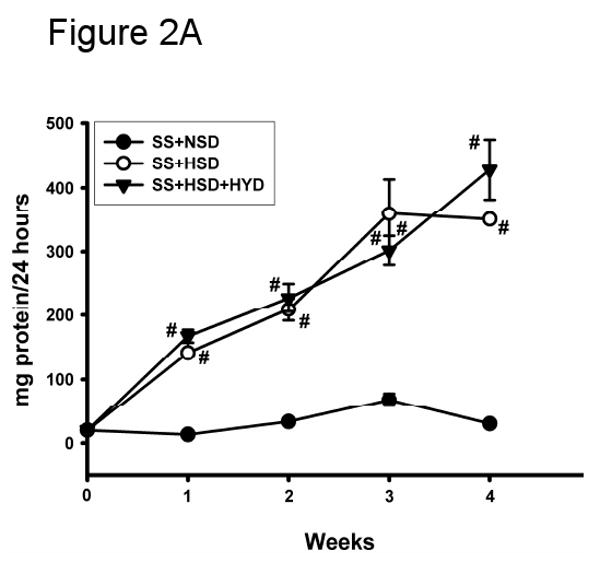
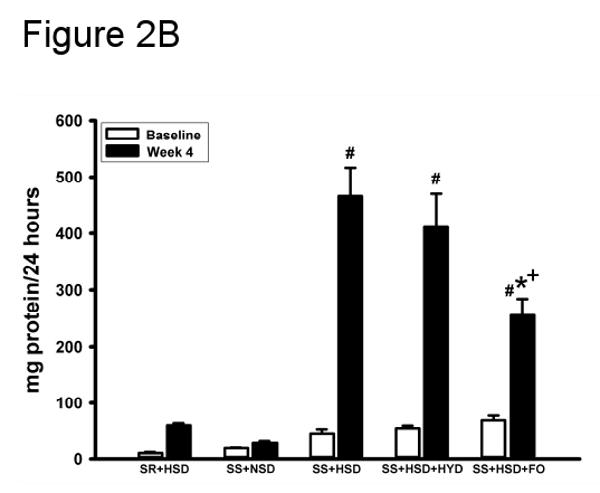
(A) 24 hour urine protein excretion was assessed at baseline and at weekly intervals in SS rats fed diets containing normal salt (NSD, 0.45% NaCl; SS+NSD, n=3), high salt (HSD, 8% NaCl; SS+HSD, n=10), or high salt with hydralazine (HYD, 5 mg/kg/d; SS+HSD+HYD, n=6). Values are mean ± SEM. #P<0.05 vs. SS+HSD. (B) 24 hour urine protein was measured at baseline and after 4 weeks in rats on normal-salt diet (SS+NSD, n=3), high-salt diet alone (SR+HSD and SS+HSD, n=10), or high-salt diet supplemented with hydralazine (SS+HSD+HYD, n=6) or fish oil (SS+HSD+FO, n=10). Values are mean ± SEM. #P<0.05 vs. SS+NSD, *P<0.05 vs. SS+HSD, +P<0.05 vs. SS+HSD+HYD.
Fish oil decreases histopathologic manifestations of renal injury in SS+HSD rats
A summary of histopathologic alterations identified in hypertensive SS+HSD rats is provided in Table 1. SS+HSD rats developed segmental and global glomerular sclerosis with glomerular enlargement, as assessed by quantitative evaluation of glomerular planar surface area with the MetaVue Image Analysis System (Table 1, Figure 3). Addition of 25% CO to the diet had no effect on the extent of glomerular sclerosis and hypertrophy (SS+HSD+CO vs. SS+HSD). However, addition of 25% FO to the diet significantly reduced glomerular sclerosis and hypertrophy (SS+HSD+FO vs. SS+HSD+CO) (Table 1, Figure 3). Arcuate-sized arteries from SS+HSD showed a modest increase in diameter and reduction in lumen/wall thickness ratio. FO, but not CO, prevented the development of fibromuscular hyperplasia in SS rats (SS+HSD+FO vs. SS+HSD+CO) (Table 1, Figure 3). Both interlobular sized arteries and arterioles showed striking fibromuscular proliferation in SS+HSD rats, leading to a significant increase in vessel diameter and a significant decrease in the lumen/wall thickness ratio (Table 1, Figure 3). Although hydralazine reduced blood pressure to levels significantly lower than that observed in SS+HSD+FO rats, the extent of glomerular and vascular hyperplasia was similar to that observed in hypertensive rats (SS+HSD+HYD vs. SS+HSD and SS+HSD+CO; Table 1). However, hydralazine appeared to limit the extent of glomerular enlargement that developed in hypertensive rats (SS+HSD+HYD vs. SS+HSD and SS+HSD+CO; Table 1). SS+HSD and SS+HSD+CO rats developed tubular atrophy, with dilated tubules containing proteinaceous casts involving up to 13% of the cortical surface area (Table 1, Figure 3). FO significantly arrested the development of these tubular lesions (SS+HSD+FO vs. SS+HSD+CO). SS+HSD rats developed interstitial fibrosis, as determined by quantitative assessment of extracellular matrix in trichrome- and Sirius red-stained slides (Figure 4). The extent of interstitial fibrosis was significantly lower in SS+HSD+FO rats compared to SS+HSD and SS+HSD+CO rats (Figure 4). As expected, the SR+HSD rats did not develop any significant glomerular enlargement or sclerosis, vascular hyperplasia, interstitial fibrosis, or tubular atrophy (Table 1, Figures 3 and 4). α-SMA is a marker of mesangial cell activation and interstitial myofibroblast transformation (104). Both glomerular and tubulointerstitial α-SMA expression were markedly increased in SS+HSD and SS+HSD+CO rats (Figure 5A, B). This response was significantly blunted in SS+HSD+FO rats (Figure 5A, B).
Figure 3. Fish oil decreases histopathologic changes brought about by a high-salt diet in Dahl SS rats.
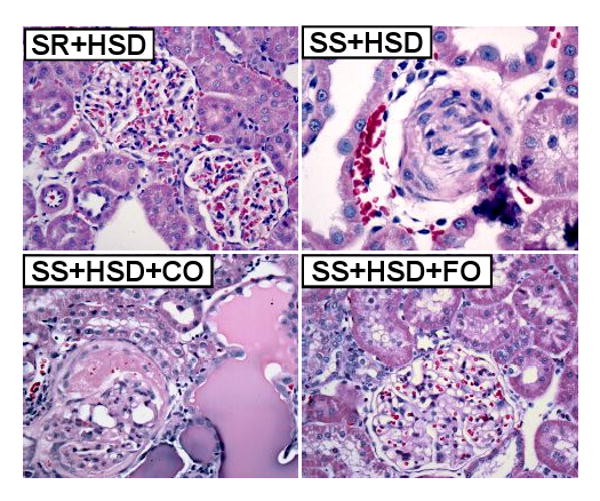
Representative H&E stains of renal tissue from rats after 4 weeks on high-salt diet alone (SR+HSD and SS+HSD), or supplemented with corn oil (SS+HSD+CO) or fish oil (SS+HSD+FO) as indicated.
Figure 4. Fish oil reduces interstitial fibrosis in Dahl SS rats on a high-salt diet.
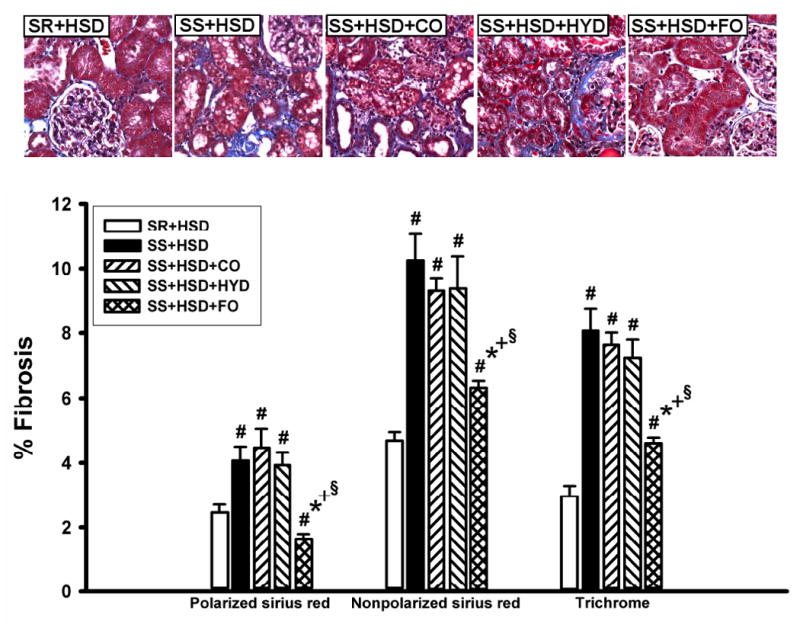
SR and SS rats were maintained for 4 weeks on high-salt diet alone (SR+HSD and SS+HSD), or supplemented with corn oil (SS+HSD+CO), hydralazine (SS+HSD+HYD), or fish oil (SS+HSD+FO) as indicated. Histologic sections of renal tissue were stained with Sirius red or Masson's trichrome; staining was quantitated, relative to cortical surface area, with the MetaVue Image Analysis System. Values are mean ± SEM (n=10). #P<0.05 vs. SR+HSD, *P<0.05 vs. SS+HSD, +P<0.05 vs. SS+HSD+CO, §P<0.05 vs. SS+HSD+HYD. Images = representative trichrome stains.
Figure 5. Fish oil decreases salt-dependent α-SMA expression in Dahl SS rats.
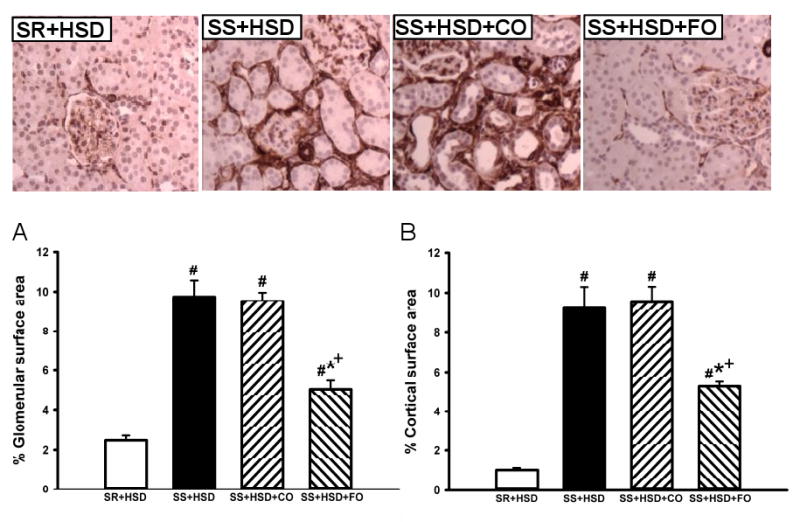
Glomerular (A) and interstitial (B) staining for α-SMA in kidneys of SR and SS rats maintained for 4 weeks on high-salt diet alone (SR+HSD and SS+HSD), or supplemented with corn oil (SS+HSD+CO) or fish oil (SS+HSD+FO) as indicated. Staining was analyzed with the MetaVue Image Analysis System. Values are mean ± SEM (n=10). #P<0.05 vs. SR+HSD, *P<0.05 vs. SS+HSD, +P<0.05 vs. SS+HSD+CO. Images = representative α-SMA stains.
Fish oil decreases tubular epithelial cell proliferation in SS+HSD rats
The development of hypertension in SS+HSD rats was associated with a 2.4-fold increase in proliferation of tubular epithelial cells, as assessed by quantitative analysis of histologic sections stained with the proliferation marker Mib-1. Tubular epithelial cell proliferation was 42% lower in SS+HSD+FO rats, compared to SS+HSD or SS+HSD+CO rats (Figure 6A). The number of glomerular Mib-1 positive cells was increased in SS+HSD or SS+HSD+CO rats, but FO did not significantly reduce the number of positive cells. To confirm these findings, Western blots prepared from homogenates of renal cortex were probed with an antibody directed against the proliferation marker PCNA. As expected, proliferative activity was increased in cortical homogenates obtained from SS+HSD and SS+HSD+CO rats, but not SS+HSD+FO rats (Figure 6B). Although hydralazine significantly reduced blood pressure in SS+HSD rats, it did not decrease PCNA expression in SS+HSD rats (Figure 6C). In our previous in vitro studies, we demonstrated that the antiproliferative effect of DHA was associated with reduced ERK activity (107). To test the in vivo relevance of this observation, we assessed p-ERK expression in renal cortical homogenates from the rats in the present study. The development of hypertension in SS+HSD rats was associated with increased expression of pERK. Dietary administration of FO, but not CO, completely abolished this effect (SS+HSD+FO vs. SS+HSD+CO; Figure 6D).
Figure 6. Fish oil has an antiproliferative effect in Dahl SS rats on a high-salt diet.
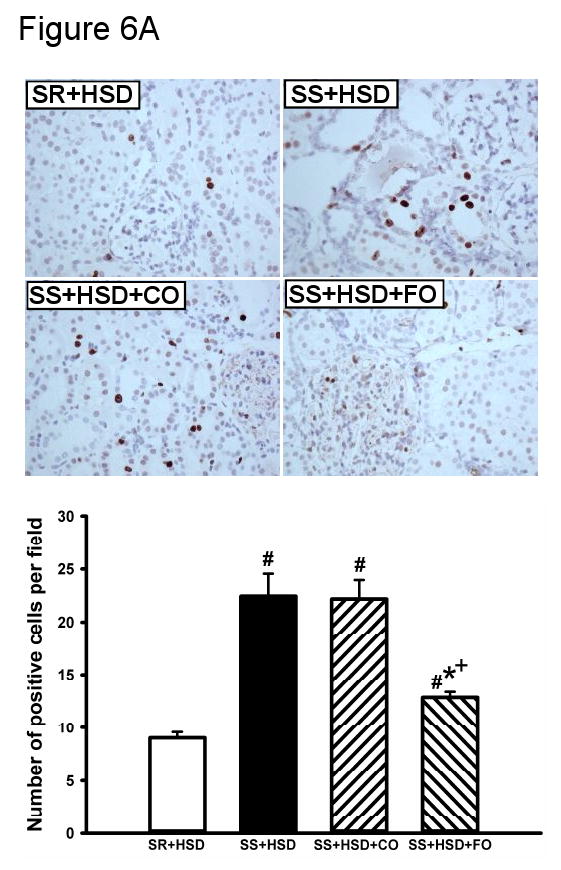
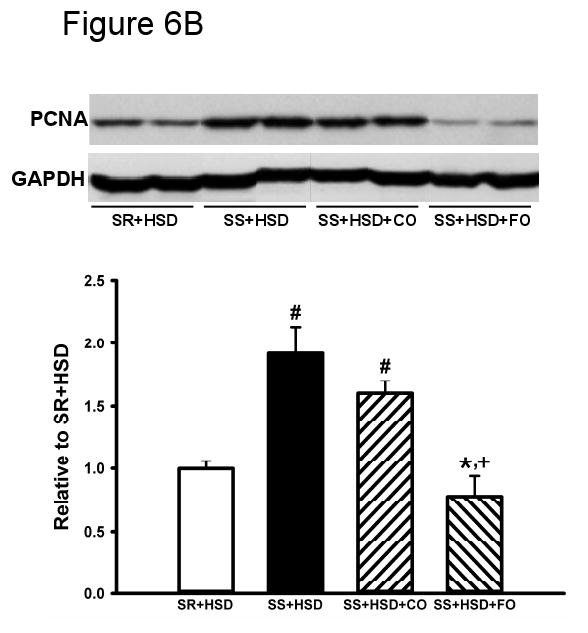
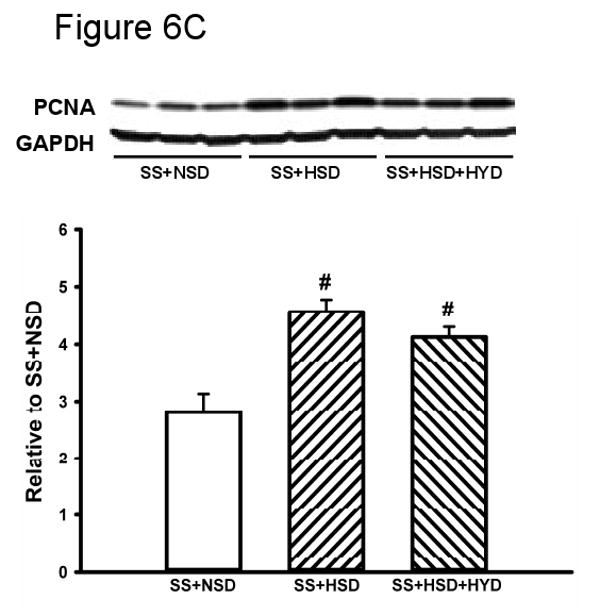
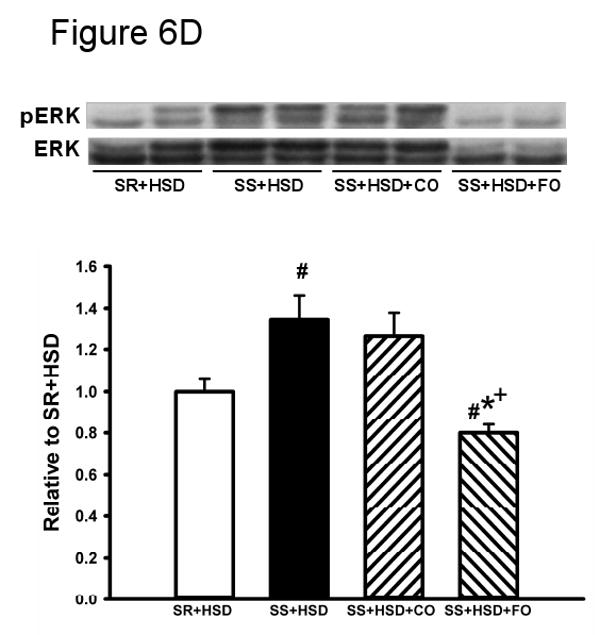
(A) Mib-1 stains of SR and SS rats after 4 weeks on high-salt diet alone (SR+HSD and SS+HSD), or supplemented with corn oil (SS+HSD+CO) or fish oil (SS+HSD+FO) as indicated. Values are mean ± SEM (n=10). (B) Western blot of PCNA expression in renal cortical homogenates from above rats, normalized to GAPDH. Values are mean ± SEM (n=10). (C) Comparison of PCNA expression in SS rats on normal-salt diet (SS+NSD, n=3), high-salt diet alone (SS+HSD, n=10) or supplemented with hydralazine (SS+HSD+HYD, n=6), normalized to GAPDH. Values are mean ± SEM. (D) Western blot of pERK expression in renal cortical homogenates from rats in (A), normalized to total ERK. Values are mean ± SEM (n=10). For (A), (B), and (D): #P<0.05 vs. SR+HSD, *P<0.05 vs. SS+HSD, +P<0.05 vs. SS+HSD+CO. For (C): #P<0.05 vs. SS+NSD. Insets = representative Mib-1 stains (A), PCNA Western blots (B, C), and pERK Western blot (D).
FO reduces inflammation in SS+HSD rats
Interstitial inflammation is a well recognized feature of renal lesions in SS+HSD rats. Interstitial influx of macrophages, lymphocytes, and plasma cells may directly contribute to the chronic histopathologic lesions associated with this model. In vitro and ex vivo studies have shown that FO has potent anti-inflammatory effects (2, 13, 24, 102). We therefore sought to determine whether macrophage influx was lower in SS+HSD+FO rats, as compared to SS+HSD+CO rats. Histologic sections were stained with the macrophage marker ED1, and the number of ED1-positive interstitial cells per 200× field was quantitated. SS+HSD rats had nearly 6-times more ED1-positive cells, compared to SR+HSD rats (Figure 7A). The number of positive cells in SS+HSD+FO rats was 23% lower than those in SS+HSD or SS+HSD+CO rats (P<0.05). Administration of 25% CO did not reduce the number of ED1-positive cells (Figure 7A).
Figure 7. Fish oil reduces salt-induced inflammation in Dahl SS rats.
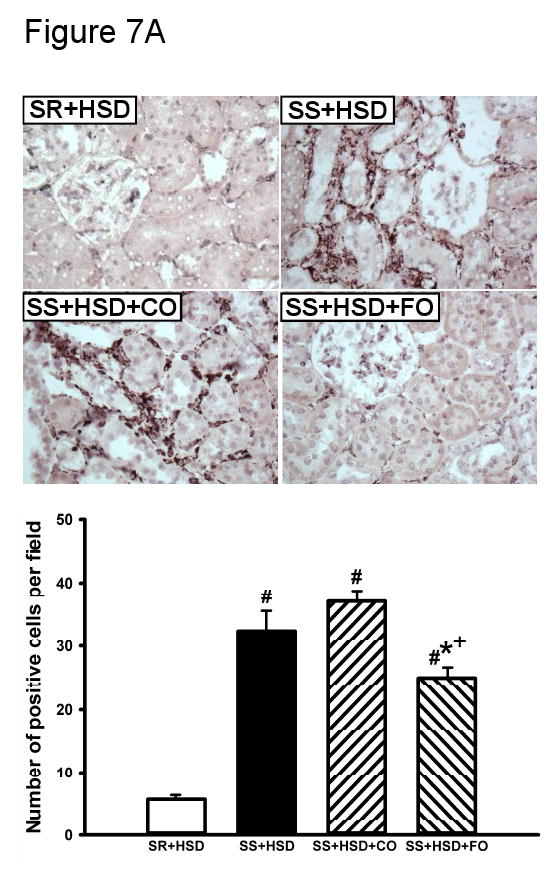
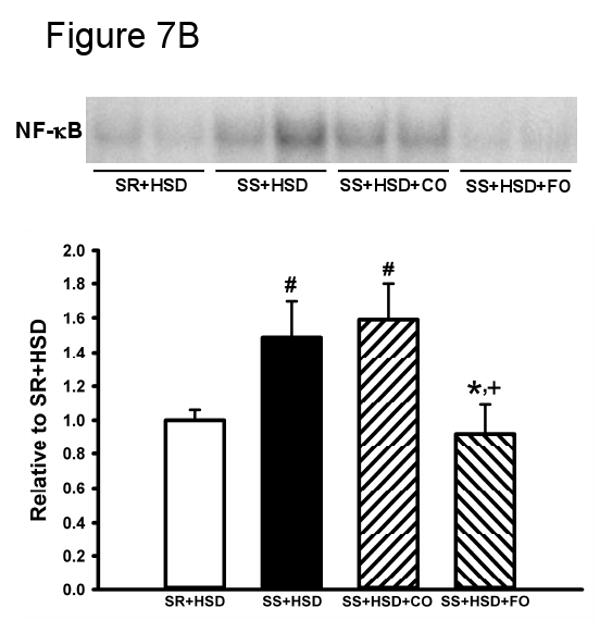
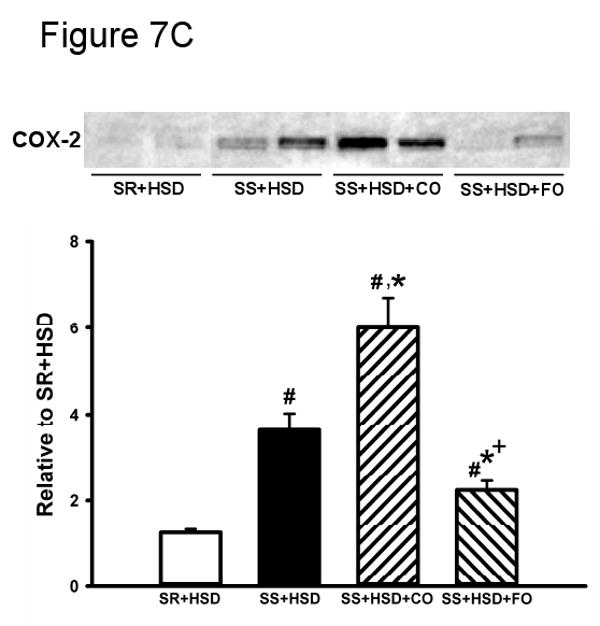
SR and SS rats were maintained for 4 weeks on high-salt diet alone (SR+HSD and SS+HSD), or supplemented with corn oil (SS+HSD+CO) or fish oil (SS+HSD+FO) as indicated. (A) ED1 stains of kidney sections. (B) NF-κB EMSA of renal nuclear extracts. (C) COX-2 expression of renal microsomal fractions by Western blot analysis. Values are mean ± SEM (n=10); #P<0.05 vs. SR+HSD, *P<0.05 vs. SS+HSD, +P<0.05 vs. SS+HSD+CO. Insets = representative ED1 stain (A), NF-κB EMSA (B), and COX-2 Western blot (C).
FO reduces NF-κB activity in SS+HSD rats
A variety of pro-inflammatory cytokines promote the activation of effector cells through activation of the NF-κB signaling pathway. We sought to test the hypothesis that the anti-inflammatory effects of FO were associated with reduced NF-κB activation. Nuclear extracts prepared from renal cortex were subjected to EMSA using a 32P-labeled NF-κB probe (73). Extracts from SS+HSD and SS+HSD+CO rats showed increased binding to the NF-κB probe compared to SR+HSD rats, as evidenced by increased band density on autoradiographs (Figure 7B). The band was “supershifted” following incubation with a p65 antibody (data not shown). Binding to the NF-κB probe was significantly decreased in SS+HSD+FO rats, indicative of reduced NF-κB activity (Figure 7B).
FO reduces COX-2 production in SS+HSD rats
In vitro studies indicate that NF-κB activates the COX pathway, leading the production of proinflammatory mediators including prostaglandins, leukotrienes, and HETEs (81). We therefore sought to test the hypothesis that FO-mediated reduction in NF-κB activation is associated with reduced COX-2 expression, as assessed by Western blot analysis of microsomal preparations of rat renal cortex. As expected, COX-2 was induced in hypertensive SS+HSD rats. FO, but not CO, prevented induction of COX-2 (SS+HSD+FO vs. SS+HSD+CO; Figure 7C).
FO prevents membrane translocation of the NADPH oxidase components p47phox and p67phox in SS+HSD rats
The development of hypertension in SS+HSD rats is associated with increased NADPH oxidase activity and oxidative stress. To test the hypothesis that FO prevents membrane translocation of the NADPH oxidase subunits p47phox and p67phox, we compared the relative abundance of each in renal cortical membranes from SS+HSD vs. SS+HSD+CO and SS+HSD+FO rats by Western blot. The development of hypertension in SS+HSD rats was associated with membrane translocation of p47phox and p67phox (Figure 8). FO, but not CO, significantly decreased translocation of both subunits (SS+HSD+FO vs. SS+HSD+CO; Figure 8).
Figure 8. Fish oil prevents membrane translocation of NADPH oxidase subunits p47phox and p67phox in Dahl SS rats on a high-salt diet.
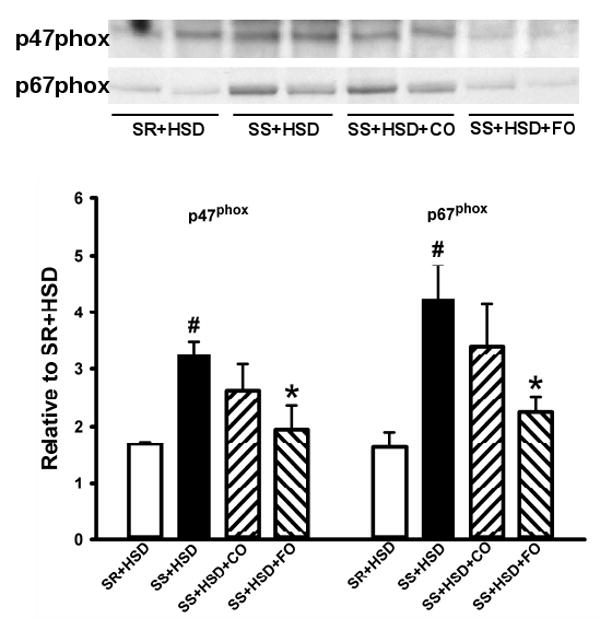
Western blots for p47phox and p67phox in renal microsomal fractions of SR or SS rats maintained on high-salt diet alone (SR+HSD and SS+HSD), or supplemented with corn oil (SS+HSD+CO) or fish oil (SS+HSD+FO) as indicated. Values are mean ± SEM (n=10); #P<0.05 vs. SR+HSD, *P<0.05 vs. SS+HSD. Inset = representative Western blots.
FO does not induce HO-1 expression in SS+HSD rats
HO is the major enzyme responsible for the degradation of heme. HO-1, an inducible isozyme of HO, has been shown to have potent anti-inflammatory effects in a variety of injury models (1, 5). We therefore sought to determine whether the protective effect of FO in SS+HSD rats is associated with induction of HO-1, as assessed by Western blot analysis of microsomal preparations of rat renal cortex. We found that the level of HO-1 expression appeared to reflect the extent of renal damage, in that the highest level of HO-1 expression was observed in SS+HSD and SS+HSD+CO rats, and was reduced in SS+HSD+FO rats (Figure 9).
Figure 9. Fish oil does not induce HO-1 expression in hypertensive Dahl SS rats.
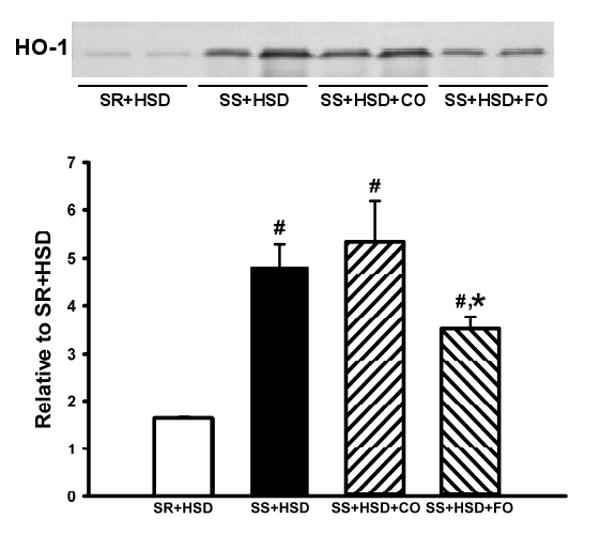
Western blot for HO-1 in renal microsomal fractions of SR and SS rats on high-salt diet alone (SR+HSD and SS+HSD), or supplemented with corn oil (SS+HSD+CO) or fish oil (SS+HSD+FO) as indicated. Values are mean ± SEM (n=10); #P<0.05 vs. SR+HSD, *P<0.05 vs. SS+HSD. Inset = representative Western blot.
Discussion
Although a number of in vitro studies have identified several pathways through which FO may prevent the development of renal disease, the in vivo relevance of these observations has not been established. The primary objective of this study was to determine whether pathways modulated by FO in vitro may provide a mechanistic basis for its renoprotective effect in salt-sensitive hypertension. In accordance with previous studies in hypertensive rats (6, 50, 91), we found that FO partially reduces blood pressure, improves lipid profiles, and decreases proteinuria.
In human studies, reduction of blood pressure is thought to underlie at least some of the cardioprotective effects of FO (66, 68). We found that FO partially, but not completely, reduced the elevated blood pressure that develops in SS+HSD rats. The reduction of blood pressure observed in this study was associated with decreased left ventricular hypertrophy, as assessed by heart weight. Similar effects of FO on left ventricular hypertrophy have been identified in SHR (99). EPA and DHA, the predominant fatty acid constituents in fish oil, exert vasorelaxant effects in aortas from hypertensive animals (26, 27) through a nitric oxide-independent mechanism that involves modulation of intracellular calcium release (25, 67).
Although FO reduced blood pressure in Dahl SS rats fed the high-salt diet, the blood pressure remained significantly elevated compared to SR controls. This incomplete reduction of blood pressure has been observed in SHR treated with DHA (50, 86). Additionally, SHR treated with menhaden oil for 12 weeks showed less renal fibrosis, but no differences in systolic blood pressure (91). Although hydralazine was more effective than FO in reducing blood pressure in SS+HSD rats, this treatment failed to prevent the development of glomerular, interstitial, and vascular alterations that are characteristic of SS+HSD rats. Based on these considerations, we propose that the protective effects of FO in SS+HSD rats are largely independent of blood pressure reduction.
We demonstrate that FO significantly reduces serum cholesterol and triglyceride levels in SS+HSD rats. Similar lipid lowering effects of FO have been reported in SHR (50, 91, 99). Hydralazine treatment had no effect on serum lipid levels. Lipids are recognized risk factors for the development of progressive renal disease (80). It is thought that reduction of serum lipid levels is one of the mechanisms underlying the cardioprotective effects of FO in humans (68). A meta-analysis of 21 clinical trials has shown that FO promotes a dose-dependent beneficial effect on serum triglycerides (8). Studies indicate that FO lowers serum triglyceride levels through suppression of hepatic lipogenesis, upregulation of fatty acid oxidation, and promoting glycogen synthesis from glucose (8, 17). However, in apoE (-/-) mice, FO reduced the formation of atherosclerotic lesions through a mechanism that did not involve a lipid lowering effect (100). The effect appeared to be through induction of antioxidant enzyme activities (100). Based on these considerations, it is likely that lipid lowering effects do not account for all of the renoprotective effects of FO in salt-sensitive hypertension.
We found that FO reduces urine protein excretion in SS+HSD rats. This finding is similar to the reported effects of FO in SHR (44, 87). However, as with blood pressure, urine protein excretion in SS+HSD+FO remained significantly elevated compared to SR+HSD rats. Of note, reduction of blood pressure with hydralazine failed to decrease urine protein excretion compared to SS+HSD rats.
Histopathologic manifestations of chronic renal injury in Dahl SS rats include glomerular hypertrophy, glomerulosclerosis, interstitial inflammation, interstitial fibrosis, tubular atrophy, and fibromuscular thickening of interstitial arteries (63, 86, 94, 97, 106). In accord with several studies in SHR (6, 44, 62, 91), we demonstrate that FO prevents the development of chronic glomerular, tubulointerstitial, and vascular alterations in SS+HSD rats. These extensive histopathologic lesions were also observed in rats treated with hydralazine to lower blood pressure (SS+HSD+HYD). We found that FO prevented the development of glomerular hypertrophy and segmental sclerosis. Similar effects of FO on glomerular hypertrophy have been reported in SHR (6). Tubulointerstitial expression of α-SMA is a marker of epithelial to mesenchymal transformation and a marker of “pro-fibrotic” state (31, 47, 55). Tubulointerstitial expression of α-SMA was significantly reduced in SS+HSD+FO rats. By quantitative analysis of trichrome- and Sirius red-stained slides, we demonstrate that FO significantly reduces interstitial fibrosis. Arteriopathic alterations, characterized by vascular smooth muscle hyperplasia and luminal constriction are characteristic histopathologic features of renovascular hypertension in Dahl SS rats and in SHR (88, 97, 101, 106). We found that FO reduced vascular fibromuscular hyperplasia in SS+HSD rats, as evidenced by an increased lumen/wall thickness ratio of interlobular sized arteries and arterioles.
Although we, and others (6, 44, 62, 91), have shown that FO decreases the development of renal injury in Dahl SS+HSD rats, relatively few studies have addressed potential signaling pathways whereby FO confers a protective effect. In vitro studies of cultured cells treated with DHA or EPA have provided evidence for several potential signaling pathways. We have previously demonstrated that low-dose DHA inhibits proliferation of renal cells in vitro through inhibition of growth factor-stimulated ERK activity and suppression of cyclin E kinase activity (107). In the ATS model of mesangial proliferative glomerulonephritis, we have previously demonstrated that FO suppresses the mesangial proliferative response to injury (32).
In hypertensive SS+HSD and SS+HSD+CO rats, we demonstrate that tubulointerstitial expression of the proliferation marker Mib-1 is markedly increased. Tubulointerstitial Mib-1 expression was significantly decreased in SS+HSD+FO rats. Furthermore, expression of the proliferation marker PCNA in cortical homogenates was also increased in SS+HSD and SS+HSD+CO, whereas FO significantly reduced PCNA expression to levels observed in SR+HSD rats. We found that the antiproliferative effect of FO in SS+HSD rats was associated with significant inhibition of ERK activity. In SS+HSD rats, the development of hypertension is associated with chronic ERK activation (37, 77). The effect of FO on ERK activity in Dahl SS rats has not been previously defined. However, the ANG II type 1 receptor antagonist candesartan decreased ERK activity and renal injury in Dahl SS rats through a mechanism that appeared to be independent of blood pressure reduction (77). In a murine model of type 2 diabetes, EPA ameliorates renal damage through suppression of the proinflammatory cytokine MCP-1 and ERK (36). In the RAW 264.7 murine macrophage cell line, FO reduced LPS-stimulated TNFα production through inhibition of ERK activity (56). These studies indicate that FO-mediated inhibition of ERK activity may lead to suppression of renal proliferation as well as inflammation in Dahl SS rats.
We found that interstitial inflammation was significantly reduced in SS+HSD+FO rats, as assessed by immunostaining for the macrophage marker ED1. Both in vitro studies of cultured cells and ex vivo studies of leukocytes have shown that DHA and/or EPA decrease the production of proinflammatory cytokines (2, 11, 13, 18, 24, 41, 48, 102). These studies have provided the rationale for clinical trials of FO in patients with a variety of inflammatory/autoimmune diseases (13, 38, 75). Of note, reduction of interstitial inflammation by mycophenolate mofetil alone significantly reduced blood pressure in Dahl SS rats (61).
Activation of NF-κB may play an important role in mediating interstitial monocyte infiltration (84), and may thereby provide an important target of FO in treatment of glomerulonephritis (57). The development of hypertension in Dahl SS rats is associated with marked upregulation of NF-κB (35). We found that NF-κB activation was significantly suppressed in SS+HSD+FO rats, as evidenced by decreased NF-κB binding of nuclear extracts subjected to EMSA. The relevance of this observation is underscored by a study of adriamycin nephrosis, in which inhibition of NF-κB with pyrrolidine dithiocarbamate reduced interstitial inflammation and the extent of renal injury (84). Our findings are in accord with a number of in vitro studies which demonstrate that FO decreases the production of proinflammatory cytokines through downregulation of NF-κB signaling (11, 54, 79, 108). Sodium directly stimulates NF-κB activation in cultured renal proximal tubular epithelial cells (35). In cultured endothelial cells (60), rat vascular smooth muscle cells (11) and renal tubular epithelial cells (54), the EPA- and/or DHA-mediated decrease in NF-κB signaling appears to occur through inhibition of ERK activity. Based on these considerations, we hypothesize that FO decreases NF-κB activation and renal inflammation through downregulation of ERK. However, we cannot exclude the possibility that the effect of FO on NF-κB activation is due to reduced inflammation in SS+HSD+FO rats.
In Dahl SS rats, we demonstrate that FO significantly decreases COX-2 expression. It is well recognized that arachidonic acid metabolites, produced through the action of COX-2, lead to the production of a variety of metabolites which promote inflammation and increase vascular tone. In SHR, the development of hypertension is associated with increased production of the COX-2 product thromboxane A2, a potent vasoconstrictor. In this model, FO reduces blood pressure and vascular reactivity (87, 105). In Dahl SS rats, the COX-2 inhibitor celecoxib reduces interstitial inflammation and histopathologic manifestations of renal injury (42). In vitro studies indicate that FO, in addition to competing with arachidonic acid as a substrate for COX, can directly suppress COX-2 induction (60). The inhibitory effect of DHA on COX-2 expression was due to decreased NF-κB activation, as assessed by EMSA, and decreased binding of NF-κB to the COX-2 promoter (60). In this study, DHA decreased NF-κB activation by decreasing cytokine-stimulated reactive oxygen species generation and ERK activation through effects on NADPH oxidase activity (60).
We found that FO prevented the assembly of the NADPH oxidase complex, as assessed by decreased membrane translocation of the NADPH oxidase subunits p47phox and p67phox in hypertensive SS+HSD+FO rats. Other investigators have shown that FO decreases lipid peroxidation and enhances antioxidant status in SHR (29). The development of hypertension in SS rats is associated with increased oxidative stress (9, 52, 65, 96) and increased NADPH oxidase-mediated superoxide production (3, 40). The importance of NADPH oxidase activation in the development of hypertension is underscored by studies employing p47 phox (-/-) mice. In these animals, ANG II infusion fails to elicit hypertension and superoxide generation is markedly attenuated (53). The antioxidant α-tocopherol prevents the development of hypertension and renal dysfunction in SS rats (7, 28, 96). The superoxide dismutase mimetic tempol reduces glomerular injury (43, 64) and reduces MAPK activity in Dahl SS rats (76). DHA attenuates the development of hypertension by decreasing NADPH oxidase activity in rats infused with ANG II (20). Based on these observations, we propose that FO ameliorates renal injury in salt-sensitive hypertension at least in part by inhibiting the assembly of NADPH oxidase subunits. However, we cannot exclude the possibility that the effect of FO on membrane translocation of p47 phox and p67 phox in SS+HSD rats is due to the fact that FO prevents the influx of circulating inflammatory cells into the kidney. Further studies are needed to address this issue.
HO has been shown to have a number of critical anti-oxidant, anti-inflammatory, and cytoprotective functions (5, 71-73). Induction of HO-1 occurs as an adaptive response to a variety of injurious stimuli, including ischemia, cytokines, and oxidative stress (4, 74). We found that HO-1 expression was decreased in SS+HSD+FO rats, compared to SS+HSD or SS+HSD+CO rats. The lack of induction of HO-1 in FO-treated rats suggests that FO does not directly regulate HO-1 expression, and that the level of HO-1 expression in SS+HSD rats reflects the underlying extent of renal injury.
In summary, we demonstrate that FO improves lipid profiles and reduces histopathologic manifestations of renal injury in salt-sensitive hypertensive rats. This effect appears to be largely independent of decreases in blood pressure in SS+HSD+FO rats. We have identified several potential targets which underlie these therapeutic effects of FO in preventing renal damage. FO markedly decreases the proliferative response to renal injury through downregulation of ERK activity. Complementary in vitro studies previously performed in our laboratory demonstrate that the FO-mediated decrease in ERK activity is associated with reduction in cyclin E kinase activity (107). FO exerts potent anti-inflammatory effects through downregulation of NF-κB. This reduction in NF-κB signaling may underlie the reduction in COX-2 expression, as the proximal promoter region of the COX-2 gene contains an NF-κB binding site (60). Finally, FO inhibits assembly of NADPH oxidase subunits (p47phox, p67phox), an effect which may underlie the previously-reported antioxidant effects of FO in renal injury models (20, 29). FO-mediated modulation of these pathways may underlie the reported protective effects of FO in other experimental renal disease models and in human renal disease.
Acknowledgments
These studies were supported by a grant from the Bryan Dyson Foundation and the National Institutes of Health DK16105. The authors gratefully acknowledge the expert technical contributions of Dr. Ping Yin and the expert secretarial assistance of Ms. Cherish Grabau. Dr. Montserrat Diaz Encarnacion is presently affiliated with Fundacion Puigvert, Barcelona, Spain.
References
- 1.Abraham N, Lutton J, Drummond G, Kappas A. The biological significance and physiological role of heme oxygenase. Cell Physiol Biochem. 1996;6:129–168. [Google Scholar]
- 2.Adam O. Dietary fatty acids and immune reactions in synovial tissue. Eur J Med Res. 2003;8:381–387. [PubMed] [Google Scholar]
- 3.Adler S, Huang H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Renal Physiol. 2004;287:F907–913. doi: 10.1152/ajprenal.00060.2004. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal A, Balla J, Alam J, Croatt AJ, Nath KA. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney Int. 1995;48:1298–1307. doi: 10.1038/ki.1995.414. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 GeneAblation and expression. J Am Soc Nephrol. 2000;11:965–973. doi: 10.1681/ASN.V115965. [DOI] [PubMed] [Google Scholar]
- 6.Aguila MB, Pinheiro AR, Aquino JC, Gomes AP, Mandarim-de-Lacerda CA. Different edible oil beneficial effects (canola oil, fish oil, palm oil, olive oil, and soybean oil) on spontaneously hypertensive rat glomerular enlargement and glomeruli number. Prostaglandins Other Lipid Mediat. 2005;76:74–85. doi: 10.1016/j.prostaglandins.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Atarashi K, Ishiyama A, Takagi M, Minami M, Kimura K, Goto A, Omata M. Vitamin E ameliorates the renal injury of Dahl salt-sensitive rats. Am J Hypertens. 1997;10:116S–119S. [PubMed] [Google Scholar]
- 8.Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J. Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis. 2006;189:19–30. doi: 10.1016/j.atherosclerosis.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Bayorh MA, Ganafa AA, Socci RR, Silvestrov N, Abukhalaf IK. The role of oxidative stress in salt-induced hypertension. Am J Hypertens. 2004;17:31–36. doi: 10.1016/j.amjhyper.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Bonaa KH, Bjerve KS, Straume B, Gram IT, Thelle D. Effect of eicosapentaenoic and docosahexaenoic acids on blood pressure in hypertension. A population-based intervention trial from the Tromso study. N Engl J Med. 1990;322:795–801. doi: 10.1056/NEJM199003223221202. [DOI] [PubMed] [Google Scholar]
- 11.Bousserouel S, Brouillet A, Bereziat G, Raymondjean M, Andreani M. Different effects of n-6 and n-3 polyunsaturated fatty acids on the activation of rat smooth muscle cells by interleukin-1 beta. J Lipid Res. 2003;44:601–611. doi: 10.1194/jlr.M200092-JLR200. [DOI] [PubMed] [Google Scholar]
- 12.Breslow JL. n-3 fatty acids and cardiovascular disease. Am J Clin Nutr. 2006;83:1477S–1482S. doi: 10.1093/ajcn/83.6.1477S. [DOI] [PubMed] [Google Scholar]
- 13.Calder PC. n-3 polyunsaturated fatty acids and cytokine production in health and disease. Ann Nutr Metab. 1997;41:203–234. doi: 10.1159/000177997. [DOI] [PubMed] [Google Scholar]
- 14.Chen ZY, Istfan NW. Docosahexaenoic acid, a major constituent of fish oil diets, prevents activation of cyclin-dependent kinases and S-phase entry by serum stimulation in HT-29 cells. Prostaglandins Leukot Essent Fatty Acids. 2001;64:67–73. doi: 10.1054/plef.2000.0239. [DOI] [PubMed] [Google Scholar]
- 15.Cheng J, Yusufi AN, Thompson MA, Chini EN, Grande JP. Nicotinic Acid Adenine Dinucleotide Phosphate: A New Ca2+ Releasing Agent in Kidney. J Am Soc Nephrol. 2001;12:54–60. doi: 10.1681/ASN.V12154. [DOI] [PubMed] [Google Scholar]
- 16.Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, Klag MJ. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988-1994) Arch Intern Med. 2001;161:1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 17.Davidson MH. Mechanisms for the hypotriglyceridemic effect of marine omega-3 fatty acids. Am J Cardiol. 2006;98:27i–33i. doi: 10.1016/j.amjcard.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 18.de La Puerta Vazquez R, Martinez-Dominguez E, Sanchez Perona J, Ruiz-Gutierrez V. Effects of different dietary oils on inflammatory mediator generation and fatty acid composition in rat neutrophils. Metabolism. 2004;53:59–65. doi: 10.1016/j.metabol.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Diaz Encarnacion M, Griffin M, Slezak J, Bergstralh E, Stegall M, Velosa J, Grande J. Correlation of quantitative digital image analysis with glomerular filtration rate in CAN. Am J Transplantation. 2004;4:248–256. doi: 10.1046/j.1600-6143.2003.00311.x. [DOI] [PubMed] [Google Scholar]
- 20.Diep QN, Amiri F, Touyz RM, Cohn JS, Endemann D, Neves MF, Schiffrin EL. PPARalpha activator effects on Ang II-induced vascular oxidative stress and inflammation. Hypertension. 2002;40:866–871. doi: 10.1161/01.hyp.0000037969.41360.cc. [DOI] [PubMed] [Google Scholar]
- 21.Donadio J, Grande J, Bergstralh E, Dart R, Larson T, Spencer D. The long-term outcome of patients with IgA nephropathy treated with fish oil in a controlled trial. J Am Soc Nephrol. 1999;10:1772–1777. doi: 10.1681/ASN.V1081772. [DOI] [PubMed] [Google Scholar]
- 22.Donadio JV, Bergstralh EJ, Offord KP, Spencer DC, Holley KE. A controlled trial of fish oil in IgA nephropathy. N Engl J Med. 1994;331:1194–1199. doi: 10.1056/NEJM199411033311804. [DOI] [PubMed] [Google Scholar]
- 23.Donadio JV, Grande JP. The role of fish oil/omega-3 fatty acids in the treatment of IgA nephropathy. Semin Nephrol. 2004;24:225–243. doi: 10.1016/j.semnephrol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 25.Engler MB, Engler MM. Docosahexaenoic acid--induced vasorelaxation in hypertensive rats: mechanisms of action. Biol Res Nurs. 2000;2:85–95. doi: 10.1177/109980040000200202. [DOI] [PubMed] [Google Scholar]
- 26.Engler MB, Engler MM, Browne A, Sun YP, Sievers R. Mechanisms of vasorelaxation induced by eicosapentaenoic acid (20:5n-3) in WKY rat aorta. Br J Pharmacol. 2000;131:1793–1799. doi: 10.1038/sj.bjp.0703754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engler MB, Engler MM, Ursell PC. Vasorelaxant properties of n-3 polyunsaturated fatty acids in aortas from spontaneously hypertensive and normotensive rats. J Cardiovasc Risk. 1994;1:75–80. [PubMed] [Google Scholar]
- 28.Forde P, Scribner AW, Dial R, Loscalzo J, Trolliet MR. Prevention of hypertension and renal dysfunction in Dahl rats by alpha-tocopherol. J Cardiovasc Pharmacol. 2003;42:82–88. doi: 10.1097/00005344-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Frenoux JM, Prost ED, Belleville JL, Prost JL. A polyunsaturated fatty acid diet lowers blood pressure and improves antioxidant status in spontaneously hypertensive rats. J Nutr. 2001;131:39–45. doi: 10.1093/jn/131.1.39. [DOI] [PubMed] [Google Scholar]
- 30.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–1499. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Goumenos DS, Tsamandas AC, Oldroyd S, Sotsiou F, Tsakas S, Petropoulou C, Bonikos D, El Nahas AM, Vlachojannis JG. Transforming growth factor-β1 and myofibroblasts: a potential pathway towards renal scarring in human glomerular disease. Nephron. 2001;87:240–248. doi: 10.1159/000045921. [DOI] [PubMed] [Google Scholar]
- 32.Grande J, Walker H, Holub B, Warner G, Keller D, Haugen J, Donadio J, Dousa T. Suppressive effects of fish oil on mesangial cell proliferation in vitro and in vivo. Kidney Int. 2000;57:1027–1040. doi: 10.1046/j.1523-1755.2000.00930.x. [DOI] [PubMed] [Google Scholar]
- 33.Grande JP, Donadio JV. Dietary fish oil supplementation in IgA nephropathy: A therapy in search of a mechanism? Nutrition. 1998;14:240–242. doi: 10.1016/s0899-9007(97)00437-1. [DOI] [PubMed] [Google Scholar]
- 34.Grande JP, Donadio JV. Role of dietary fish oil supplementation in IgA nephropathy. Mechanistic implications. Minerva Urol Nefrol. 2001;53:201–209. [PubMed] [Google Scholar]
- 35.Gu JW, Tian N, Shparago M, Tan W, Bailey AP, Manning RD., Jr Renal NF{kappa}B Activation and TNF{alpha} Up-regulation Correlate with Salt-sensitive Hypertension in Dahl Salt-sensitive (SS) Rats. Am J Physiol Regul Integr Comp Physiol. 2006 doi: 10.1152/ajpregu.00153.2006. [DOI] [PubMed] [Google Scholar]
- 36.Hagiwara S, Makita Y, Gu L, Tanimoto M, Zhang M, Nakamura S, Kaneko S, Itoh T, Gohda T, Horikoshi S, Tomino Y. Eicosapentaenoic acid ameliorates diabetic nephropathy of type 2 diabetic KKAy/Ta mice: involvement of MCP-1 suppression and decreased ERK1/2 and p38 phosphorylation. Nephrol Dial Transplant. 2006;21:605–615. doi: 10.1093/ndt/gfi208. [DOI] [PubMed] [Google Scholar]
- 37.Hamaguchi A, Kim S, Izumi Y, Iwao H. Chronic activation of glomerular mitogen-activated protein kinases in Dahl salt-sensitive rats. J Am Soc Nephrol. 2000;11:39–46. doi: 10.1681/ASN.V11139. [DOI] [PubMed] [Google Scholar]
- 38.Harper CR, Jacobson TA. The fats of life: the role of omega-3 fatty acids in the prevention of coronary heart disease. Arch Intern Med. 2001;161:2185–2192. doi: 10.1001/archinte.161.18.2185. [DOI] [PubMed] [Google Scholar]
- 39.Harris WS. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65:1645S–1654S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 40.Heitzer T, Wenzel U, Hink U, Krollner D, Skatchkov M, Stahl RA, MacHarzina R, Brasen JH, Meinertz T, Munzel T. Increased NAD(P)H oxidase-mediated superoxide production in renovascular hypertension: evidence for an involvement of protein kinase C. Kidney Int. 1999;55:252–260. doi: 10.1046/j.1523-1755.1999.00229.x. [DOI] [PubMed] [Google Scholar]
- 41.Helton WS, Espat NJ. Defining mechanisms of ω-3 fatty-acid activity. Nutrition. 2001;17:674. doi: 10.1016/s0899-9007(01)00548-2. [DOI] [PubMed] [Google Scholar]
- 42.Hermann M, Shaw S, Kiss E, Camici G, Buhler N, Chenevard R, Luscher TF, Grone HJ, Ruschitzka F. Selective COX-2 inhibitors and renal injury in salt-sensitive hypertension. Hypertension. 2005;45:193–197. doi: 10.1161/01.HYP.0000153053.82032.bf. [DOI] [PubMed] [Google Scholar]
- 43.Hisaki R, Fujita H, Saito F, Kushiro T. Tempol attenuates the development of hypertensive renal injury in Dahl salt-sensitive rats. Am J Hypertens. 2005;18:707–713. doi: 10.1016/j.amjhyper.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 44.Hobbs LM, Rayner TE, Howe PR. Dietary fish oil prevents the development of renal damage in salt-loaded stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1996;23:508–513. doi: 10.1111/j.1440-1681.1996.tb02770.x. [DOI] [PubMed] [Google Scholar]
- 45.Iwai J. Dahl salt-sensitive and salt-resistant rats. Hypertension. 1987;1987:I18–I20. [Google Scholar]
- 46.Jia Q, Zhou HR, Shi Y, Pestka JJ. Docosahexaenoic acid consumption inhibits deoxynivalenol-induced CREB/ATF1 activation and IL-6 gene transcription in mouse macrophages. J Nutr. 2006;136:366–372. doi: 10.1093/jn/136.2.366. [DOI] [PubMed] [Google Scholar]
- 47.Jinde K, Nikolic-Paterson DJ, Huang XR, Sakai H, Kurokawa K, Atkins RC, Lan HY. Tubular phenotypic change in progressive tubulointerstitial fibrosis in human glomerulonephritis. Am J Kidney Dis. 2001;38:761–769. doi: 10.1053/ajkd.2001.27693. [DOI] [PubMed] [Google Scholar]
- 48.Khalfoun B, Thibault F, Watier H, Bardos P, Lebranchu Y. Docosahexaenoic and eicosapentaenoic acids inhibit in vitro human endothelial cell production of interleukin-6. Adv Exp Med Biol. 1997;400B:589–597. [PubMed] [Google Scholar]
- 49.Kielar ML, Jeyarajah DR, Penfield JG, Lu CY. Docosahexaenoic acid decreases IRF-1 mRNA and thus inhibits activation of both the IRF-E and NFkappa d response elements of the iNOS promoter. Transplantation. 2000;69:2131–2137. doi: 10.1097/00007890-200005270-00030. [DOI] [PubMed] [Google Scholar]
- 50.Kimura S, Minami M, Saito H, Kobayashi T, Okuyama H. Dietary docosahexaenoic acid (22:6n-3) prevents the development of hypertension in SHRSP. Clin Exp Pharmacol Physiol. 1995;22 1:S308–309. doi: 10.1111/j.1440-1681.1995.tb02931.x. [DOI] [PubMed] [Google Scholar]
- 51.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106:2747–2757. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 52.Kushiro T, Fujita H, Hisaki R, Asai T, Ichiyama I, Kitahara Y, Koike M, Sugiura H, Saito F, Otsuka Y, Kanmatsuse K. Oxidative stress in the Dahl salt-sensitive hypertensive rat. Clin Exp Hypertens. 2005;27:9–15. doi: 10.1081/ceh-200044244. [DOI] [PubMed] [Google Scholar]
- 53.Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, Moorhead JF, Varghese Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005;67:867–874. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol. 2004;15:1–12. doi: 10.1097/01.asn.0000106015.29070.e7. [DOI] [PubMed] [Google Scholar]
- 56.Lo CJ, Chiu KC, Fu M, Chu A, Helton S. Fish oil modulates macrophage P44/P42 mitogen-activated protein kinase activity induced by lipopolysaccharide. JPEN J Parenter Enteral Nutr. 2000;24:159–163. doi: 10.1177/0148607100024003159. [DOI] [PubMed] [Google Scholar]
- 57.Lopez-Franco O, Suzuki Y, Sanjuan G, Blanco J, Hernandez-Vargas P, Yo Y, Kopp J, Egido J, Gomez-Guerrero C. Nuclear factor-κ B inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am J Pathol. 2002;161:1497–1505. doi: 10.1016/s0002-9440(10)64425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lowry O, Rosebrough A, Farr A, Randall R. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 59.Lungershausen YK, Abbey M, Nestel PJ, Howe PR. Reduction of blood pressure and plasma triglycerides by omega-3 fatty acids in treated hypertensives. J Hypertens. 1994;12:1041–1045. [PubMed] [Google Scholar]
- 60.Massaro M, Habib A, Lubrano L, Turco SD, Lazzerini G, Bourcier T, Weksler BB, De Caterina R. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci U S A. 2006;103:15184–15189. doi: 10.1073/pnas.0510086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 62.Medeiros FJ, Aguila MB, Mandarim-de-Lacerda CA. Renal cortex remodeling in streptozotocin-induced diabetic spontaneously hypertensive rats treated with olive oil, palm oil and fish oil from Menhaden. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2006;75:357–365. doi: 10.1016/j.plefa.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 63.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD., Jr Oxidative Stress in Dahl Salt-Sensitive Hypertension. Hypertension. 2003;41:1346–1352. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 64.Meng S, Cason GW, Gannon AW, Racusen LC, Manning RD., Jr Oxidative stress in Dahl salt-sensitive hypertension. Hypertension. 2003;41:1346–1352. doi: 10.1161/01.HYP.0000070028.99408.E8. [DOI] [PubMed] [Google Scholar]
- 65.Meng S, Roberts LJ, 2nd, Cason GW, Curry TS, Manning RD., Jr Superoxide dismutase and oxidative stress in Dahl salt-sensitive and -resistant rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R732–738. doi: 10.1152/ajpregu.00346.2001. [DOI] [PubMed] [Google Scholar]
- 66.Mori TA. Omega-3 fatty acids and hypertension in humans. Clin Exp Pharmacol Physiol. 2006;33:842–846. doi: 10.1111/j.1440-1681.2006.04451.x. [DOI] [PubMed] [Google Scholar]
- 67.Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000;102:1264–1269. doi: 10.1161/01.cir.102.11.1264. [DOI] [PubMed] [Google Scholar]
- 68.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104. doi: 10.1097/01.mco.0000214566.67439.58. [DOI] [PubMed] [Google Scholar]
- 69.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–1737. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 70.Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523–533. doi: 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- 71.Nath K, Grande J, Croatt A, Likely S, Hebbel R, Enright H. Intracellular targets in heme protein induced renal injury. Kidney Int. 1998;53:100–111. doi: 10.1046/j.1523-1755.1998.00731.x. [DOI] [PubMed] [Google Scholar]
- 72.Nath K, Haggard J, Croatt A, Grande J, Poss K, Alam J. The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am J Pathol. 2000;156:1527–1535. doi: 10.1016/S0002-9440(10)65024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nath K, Vercellotti G, Grande J, Miyoshi H, Paya C, Manivel J, Haggard J, Croatt A, Payne W, Alam J. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int. 2001;59:106–117. doi: 10.1046/j.1523-1755.2001.00471.x. [DOI] [PubMed] [Google Scholar]
- 74.Nath KA. The functional significance of induction of heme oxygenase by oxidant stress [editorial; comment] J Lab Clin Med. 1994;123:461–463. [PubMed] [Google Scholar]
- 75.Nielsen GL, Faarvang KL, Thomsen BS, Teglbjaerg KL, Jensen LT, Hansen TM, Lervang HH, Schmidt EB, Dyerberg J, Ernst E. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. 1992;22:687–691. doi: 10.1111/j.1365-2362.1992.tb01431.x. [DOI] [PubMed] [Google Scholar]
- 76.Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M, Shokoji T, Kimura S, Abe Y. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J Am Soc Nephrol. 2004;15:306–315. doi: 10.1097/01.asn.0000108523.02100.e0. [DOI] [PubMed] [Google Scholar]
- 77.Nishiyama A, Yoshizumi M, Rahman M, Kobori H, Seth DM, Miyatake A, Zhang GX, Yao L, Hitomi H, Shokoji T, Kiyomoto H, Kimura S, Tamaki T, Kohno M, Abe Y. Effects of AT1 receptor blockade on renal injury and mitogen-activated protein activity in Dahl salt-sensitive rats. Kidney Int. 2004;65:972–981. doi: 10.1111/j.1523-1755.2004.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nordoy A, Marchioli R, Arnesen H, Videbaek J. n-3 polyunsaturated fatty acids and cardiovascular diseases. Lipids. 2001;36:S127–129. doi: 10.1007/s11745-001-0695-7. [DOI] [PubMed] [Google Scholar]
- 79.Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-κB inhibition by omega -3 fatty acids modulates LPS-stimulated macrophage TNF-α transcription. Am J Physiol Lung Cell Mol Physiol. 2003;284:L84–89. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- 80.O'Donnell MP, Kasiske BL, Katz SA, Schmitz PG, Keane WF. Lovastatin but not enalapril reduces glomerular injury in Dahl salt-sensitive rats. Hypertension. 1992;20:651–658. doi: 10.1161/01.hyp.20.5.651. [DOI] [PubMed] [Google Scholar]
- 81.Pairet M, Engelhardt G. Distinct isoforms (COX-1 and COX-2) of cyclooxygenase: possible physiological and therapeutic implications. Fundam Clin Pharmacol. 1996;10:1–17. doi: 10.1111/j.1472-8206.1996.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 82.Pownall HJ, Brauchi D, Kilinc C, Osmundsen K, Pao Q, Payton-Ross C, Gotto AM, Jr, Ballantyne CM. Correlation of serum triglyceride and its reduction by omega-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high-density and low-density lipoproteins. Atherosclerosis. 1999;143:285–297. doi: 10.1016/s0021-9150(98)00301-3. [DOI] [PubMed] [Google Scholar]
- 83.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984;26:137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 84.Rangan GK, Wang Y, Tay YC, Harris DC. Inhibition of nuclear factor-kappaB activation reduces cortical tubulointerstitial injury in proteinuric rats. Kidney Int. 1999;56:118–134. doi: 10.1046/j.1523-1755.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- 85.Rapp J. Dahl salt-susceptible and salt-resistant rats. A review. Hypertension. 1982;4:753–763. doi: 10.1161/01.hyp.4.6.753. [DOI] [PubMed] [Google Scholar]
- 86.Rapp JP, Dene H. Development and characteristics of inbred strains of Dahl salt-sensitive and salt-resistant rats. Hypertension. 1985;7:340–349. [PubMed] [Google Scholar]
- 87.Rayner TE, Howe PR. Purified omega-3 fatty acids retard the development of proteinuria in salt-loaded hypertensive rats. J Hypertens. 1995;13:771–780. [PubMed] [Google Scholar]
- 88.Reddi AS, Nimmagadda VR, Arora R. Effect of antihypertensive therapy on renal artery structure in type 2 diabetic rats with hypertension. Hypertension. 2001;37:1273–1278. doi: 10.1161/01.hyp.37.5.1273. [DOI] [PubMed] [Google Scholar]
- 89.Sanders PW. Salt intake, endothelial cell signaling, and progression of kidney disease. Hypertension. 2004;43:142–146. doi: 10.1161/01.HYP.0000114022.20424.22. [DOI] [PubMed] [Google Scholar]
- 90.Schneider SM, Fung VS, Palmblad J, Babior BM. Activity of the leukocyte NADPH oxidase in whole neutrophils and cell- free neutrophil preparations stimulated with long-chain polyunsaturated fatty acids. Inflammation. 2001;25:17–23. doi: 10.1023/a:1007019510569. [DOI] [PubMed] [Google Scholar]
- 91.Shimamura T, Wilson AC. Influence of dietary fish oil on the aortic, myocardial, and renal lesions of SHR. J Nutr Sci Vitaminol (Tokyo) 1991;37:581–590. doi: 10.3177/jnsv.37.581. [DOI] [PubMed] [Google Scholar]
- 92.Stone NJ. Fish consumption, fish oil, lipids, and coronary heart disease. Circulation. 1996;94:2337–2340. doi: 10.1161/01.cir.94.9.2337. [DOI] [PubMed] [Google Scholar]
- 93.Sullivan JM. Salt sensitivity. Definition, conception, methodology, and long-term issues. Hypertension. 1991;17:I61–68. doi: 10.1161/01.hyp.17.1_suppl.i61. [DOI] [PubMed] [Google Scholar]
- 94.Takizawa T, Takasaki I, Shionoiri H, Ishii M. Progression of glomerulosclerosis, renal hypertrophy, and an increased expression of fibronectin in the renal cortex associated with aging and salt-induced hypertension in Dahl salt-sensitive rats. Life Sci. 1997;61:1553–1558. doi: 10.1016/s0024-3205(97)00734-0. [DOI] [PubMed] [Google Scholar]
- 95.Terano T, Tanaka T, Tamura Y, Kitagawa M, Higashi H, Saito Y, Hirai A. Eicosapentaenoic acid and docosahexaenoic acid inhibit vascular smooth muscle cell proliferation by inhibiting phosphorylation of Cdk2-cyclinE complex. Biochem Biophys Res Commun. 1999;254:502–506. doi: 10.1006/bbrc.1998.9976. [DOI] [PubMed] [Google Scholar]
- 96.Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD., Jr Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension. 2005;45:934–939. doi: 10.1161/01.HYP.0000160404.08866.5a. [DOI] [PubMed] [Google Scholar]
- 97.Tomoda F, Takata M, Kinuno H, Tomita S, Yasumoto K, Inoue H. Renal structural properties in prehypertensive Dahl salt-sensitive rats. Hypertension. 2000;36:68–72. doi: 10.1161/01.hyp.36.1.68. [DOI] [PubMed] [Google Scholar]
- 98.USRDS USRDS. 2001 Annual Data Report. Bethesda, MD: National Institutes of Health - National Institute of Diabetes and Digestive and Kidney Disease; 2001. Atlas of End-Stage Renal Disease in the United States. [Google Scholar]
- 99.Vaskonen T, Laakso J, Mervaala E, Sievi E, Karppanen H. Interrelationships between salt and fish oil in stroke-prone spontaneously hypertensive rat. Blood Press. 1996;5:178–189. doi: 10.3109/08037059609062127. [DOI] [PubMed] [Google Scholar]
- 100.Wang HH, Hung TM, Wei J, Chiang AN. Fish oil increases antioxidant enzyme activities in macrophages and reduces atherosclerotic lesions in apoE-knockout mice. Cardiovasc Res. 2004;61:169–176. doi: 10.1016/j.cardiores.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 101.Wang PX, Sanders PW. Mechanism of hypertensive nephropathy in the Dahl/Rapp rat: a primary disorder of vascular smooth muscle. Am J Physiol Renal Physiol. 2005;288:F236–242. doi: 10.1152/ajprenal.00213.2004. [DOI] [PubMed] [Google Scholar]
- 102.Watanabe S, Katagiri K, Onozaki K, Hata N, Misawa Y, Hamazaki T, Okuyama H. Dietary docosahexaenoic acid but not eicosapentaenoic acid suppresses lipopolysaccharide-induced interleukin-1 beta mRNA induction in mouse spleen leukocytes. Prostaglandins Leukot Essent Fatty Acids. 2000;62:147–152. doi: 10.1054/plef.2000.0134. [DOI] [PubMed] [Google Scholar]
- 103.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 104.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yin K, Chu ZM, Beilin LJ. Effect of fish oil feeding on blood pressure and vascular reactivity in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 1990;17:235–239. doi: 10.1111/j.1440-1681.1990.tb01313.x. [DOI] [PubMed] [Google Scholar]
- 106.Ying WZ, Sanders PW. Enhanced expression of EGF receptor in a model of salt-sensitive hypertension. Am J Physiol Renal Physiol. 2005;289:F314–321. doi: 10.1152/ajprenal.00003.2005. [DOI] [PubMed] [Google Scholar]
- 107.Yusufi A, Cheng J, Thompson M, Walker H, Gray C, Warner G, Grande J. Differential effects of low-dose docosahexaenoic acid and eicosapentaenoic acid on the regulation of mitogenic signaling pathways in mesangial cells. J Lab Clin Med. 2003;141:318–329. doi: 10.1016/S0022-2143(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 108.Zhao Y, Joshi-Barve S, Barve S, Chen LH. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23:71–78. doi: 10.1080/07315724.2004.10719345. [DOI] [PubMed] [Google Scholar]


