SUMMARY
Behavioral performance depends on the activity of neurons in sensory cortex, but little is known about the brain's capacity to access specific neuronal signals to guide behavior. Even the individual sensory neurons that are most sensitive to a relevant stimulus are only weakly correlated with behavior [1, 2], suggesting that behavioral decisions are based on the combined activity of groups of neurons with sensitivities well matched to task demands [3, 4]. To explore how flexibly different patterns of activity can be accessed from a given cortical region, we trained animals to detect electrical microstimulation of local V1 sites. By allowing the animals to become expert at the detection of microstimulation of specific V1 sites that corresponded to particular retinotopic locations, we could measure the effects of that training on the ability of those sites to support the detection of visual stimuli. Training to detect electrical activation caused a large, reversible, retinotopically-localized impairment of thresholds for detecting visual stimuli. Retraining on visual detection restored normal thresholds, and in turn impaired thresholds for detecting microstimulation. These results suggest that there are substantial limits to the types of signals for which a local cortical region can be simultaneously optimized.
RESULTS AND DISCUSSION
In principle, the brain might have almost limitless capacity for dynamically accessing arbitrary subsets of cortical neurons. However, few experimental data speak to how efficiently and flexibly the signals of cortical neurons can be combined to guide behavior. A major obstacle to addressing this question is the fact that every sensory stimulus activates thousands of neurons that are distributed across multiple cortical areas that have overlapping neuronal response properties, making it difficult to know which neurons are likely to be most important for a given task and where they are found in cortex.
To mitigate this problem we have examined behaviors that depend on neuronal activity that is created in a predetermined cortical locus by intracortical electrical microstimulation. Although electrical stimulation is not a natural stimulus, activation of V1 by microstimulation nevertheless creates a robust sensation of a small point of light (a phosphene) in humans [5–7] and, in all likelihood, non-human primates [8, 9]. By electrically activating cortex in monkey V1 in the absence of other stimuli, we controlled which cortical site was likely to be most informative for guiding the animal's behavior in a stimulus detection task. Most importantly, by giving the animals extended training on detecting microstimulation in restricted regions of V1 subserving known visual field locations, we could study how the acquisition of expertise at detecting microstimulation of a cortical locus affects the ability of the same cortical site to support detection of visual stimuli.
We first trained two monkeys to perform a two-interval forced-choice detection task [10] using small visual stimuli. The contrast needed to detect the stimulus at different visual field locations was determined using an adaptive staircase procedure [11]. Once thresholds for detecting visual stimuli were stable over a range of eccentricities (~4 months of visual stimulus training for monkey 1, ~8 months for monkey 2), we replaced the visual stimulus with electrical microstimulation of a V1 site using a microelectrode. A V1 site was defined as a region approximately 3 by 3 mm. For each V1 site we trained the monkeys to detect the microstimulation over a course of 2 to 4 weeks, making a new electrode penetration each day, measuring a detection threshold from every block of 100 behavioral trials, and advancing the electrode between threshold measurements to traverse the thickness of V1.
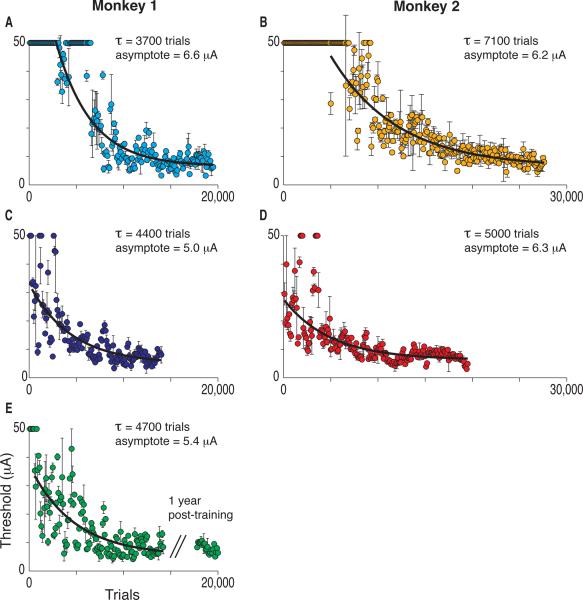
Figures 1A and 1B show the improvement in microstimulation detection thresholds at the first V1 site trained in each animal, demonstrating the brain's capacity to improve the detection of the microstimulation-induced pattern of activation with practice. We limited the stimulation current to 50 μA, and initially neither animal could reliably detect that current. However, within a few thousand trials (3–6 days of training) reliable thresholds were measured and thresholds approached a stable asymptote over 2–4 weeks of daily training. The best fitting exponential functions had decay constants of 3700 and 7100 trials. Asymptotic thresholds (6.6 and 6.2 μA) were similar to those reported previously for microstimulation of V1 [10].
Figure 1.
Improvement in Detecting Microstimulation at Different V1 Sites. Behavioral thresholds for detecting microstimulation decreased exponentially during training at each V1 site. Each color represents a different V1 site in this and all other figures. Each point is a threshold determination based on 100 trials and error bars are the 67% confidence intervals. At one site (E) we examined thresholds after a 1-year pause and found that they remained stable.
Figures 1C–E show the improvement in detection thresholds over the course of microstimulation training at other V1 sites in the same animals, with each site separated from previously trained sites by at least 5 mm (measured parallel to the cortical surface). Each new site required training to achieve asymptotic thresholds. One site in monkey 1 was re-tested after a pause of one year (Figure 1E), and a comparison with the thresholds just before the hiatus showed that thresholds remained stable without practice (before: mean 8.3 μA, 0.5 SEM, n = 23 blocks, after: mean threshold 8.1 μA, 0.3 SEM, n = 23 blocks, t-test, p = 0.71). Long lasting, spatially specific, incremental improvements in performance are hallmarks of perceptual learning [12–14]. Thus, training with microstimulation had effects similar to training with a novel sensory stimulus.
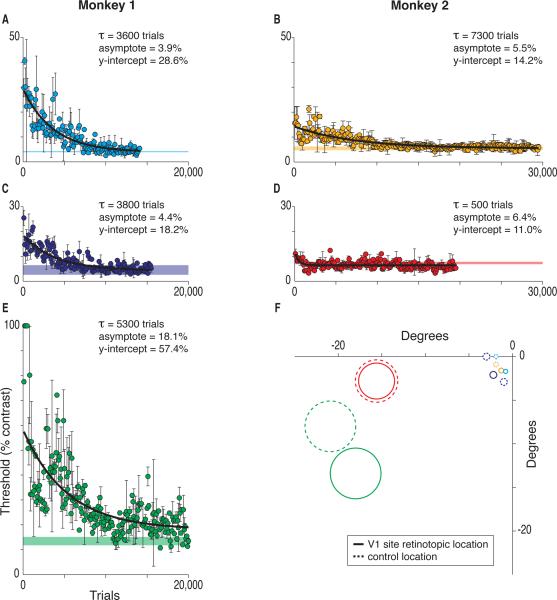
Training the animals to detect microstimulation of the V1 sites significantly impaired the detection of visual stimuli, but only at the specific retinotopic positions represented by the microstimulated V1 sites. Figure 2F illustrates the visual field locations represented at the five V1 sites (outlined in solid lines), as well as the positions of control locations (outlined in dotted lines) that were used to measure normal visual detection thresholds. Control locations were either adjacent retinotopic locations at the same eccentricity as a microstimulated representation that were tested post-microstimulation or else the microstimulated locus tested before the start of microstimulation. Colors are used consistently in all the figures to indicate different V1 sites. For each V1 site illustrated in Figures 2A–E, a horizontal band marks the 95% confidence interval for the mean threshold of the corresponding control location.
Figure 2.
Impaired Visual Thresholds after Microstimulation Training and Recovery with Visual Training. (A–E) After microstimulation training, visual detection was markedly compromised at the visual field locations represented at the microstimulated V1 sites, but recovered with visual retraining. Data are detection thresholds for visual stimuli located at the mean receptive field location mapped at the respective V1 site during microstimulation training. Format as in Figure 1. Horizontal bands mark the 95% confidence intervals for visual thresholds at the corresponding control (non-microstimulated) locations. (F) Mean receptive field locations for each V1 site are outlined in solid lines and control locations are outlined in dashed lines. Circles mark 2 SD of the Gaussian visual stimuli used at that eccentricity.
Figures 2A–E illustrate the elevated visual detection thresholds that were seen at the visual field locations corresponding to the microstimulated V1 sites after animals had been trained with microstimulation, as well as the recovery of normal thresholds with retraining with visual stimuli at those positions over 2–4 weeks. Visual thresholds were substantially elevated after training with microstimulation, although adjacent control sites had normal thresholds. Retraining restored the elevated thresholds to normal levels, and was also retinotopically specific. Retraining at one retinotopic position did not restore visual thresholds for all positions: each position had to be retrained separately.
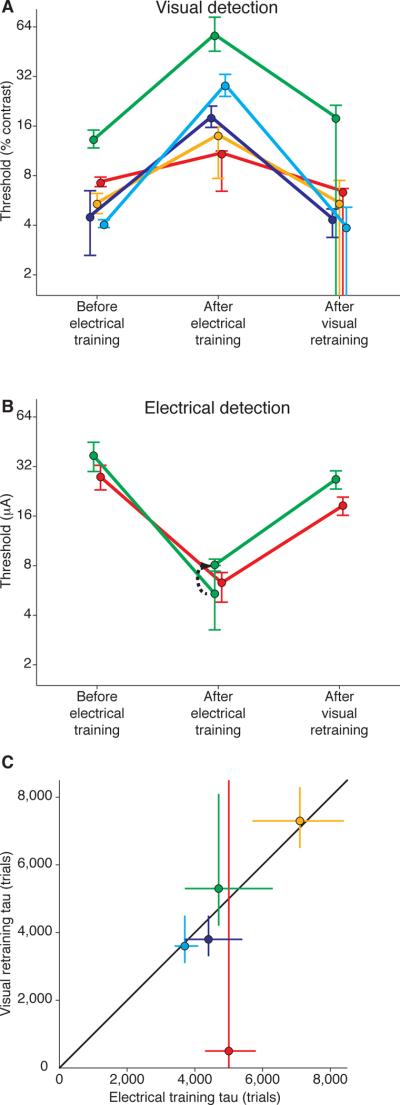
Figure 3 summarizes the reciprocal changes in visual and electrical thresholds. Figure 3A plots visual detection thresholds before microstimulation, after microstimulation, and following retraining on visual detection. Visual thresholds were elevated at the microstimulated retinotopic positions by factors that ranged from 1.5 to 7. These are large threshold elevations, similar, for example, to impairment on detecting directions of motion following ablation of the middle temporal visual area [15–16]. Visual detection thresholds varied before microstimulation because the sites spanned eccentricities from 1.9° to 22.4°, and our scaling of the visual stimulus with eccentricity did not perfectly compensate for changes in sensitivity across eccentricities.
Figure 3.
Interactions Between Visual and Microstimulation Training. (A) Thresholds for visual stimulus detection. Visual thresholds “before electrical training” correspond to the mean thresholds of the control locations described and plotted in Figure 2. Visual thresholds “after electrical training” and “after visual retraining” plot the y-intercepts and asymptotes of the exponential functions fit to the visual stimulus threshold data in Figures 2A–E. Error bars are 95% confidence intervals. (B) Thresholds for electrical stimulation detection. Before and after electrical training correspond to the y-intercepts and asymptotes of the exponential functions fit to the microstimulation threshold data in Figures 1A–E. For the green site the asymptote of the microstimulation training curve is plotted as well as (black arrow) the mean threshold tested one year later (n = 23 blocks), before visual retraining, which occurred over a period of three weeks. Microstimulation thresholds `after visual retraining' are the mean electrical thresholds based on 12 threshold determinations from each of 4 different electrode penetrations at the V1 site (n = 48 blocks). Error bars are 95% confidence intervals. (C) Exponents of the fitted functions for electrical training curves and visual retraining curves were very similar at each site. Error bars are 67% confidence intervals.
Figure 3B illustrates that just as electrical training impaired visual detection, visual retraining impaired electrical detection. At the one V1 site that was accessible for additional microstimulation in each subject (see Experimental Procedures), microstimulation detection thresholds were measured following visual retraining. Two to three weeks of visual retraining at the corresponding visual field locations caused microstimulation thresholds to rise approximately three-fold (from 8.1 to 26.8 μA for monkey 1; from 6.3 to 18.5 μA for monkey 2). Without visual retraining, these microstimulation thresholds had remained stable for many months.
Training improved visual and microstimulation thresholds at similar rates at each V1 site. Figure 3C plots the exponent for the microstimulation learning curve (Figure 1) and that for the visual learning curve (Figure 2) for each V1 site, as well as the line of equality. There was no significant difference between the learning time constants for electrical and visual stimulation within V1 sites (Friedman's test, χ2 = 0.20, p = 0.65).
Several findings argue that the visual thresholds were not elevated due to damage associated with microelectrode penetrations or the electrical stimulation. First, although microstimulation thresholds remained stable long after microstimulation training had ended (Figure 1E), retraining on visual detection, which was entirely noninvasive, greatly elevated those thresholds (Figure 3B). This suggests that it is practice detecting a different type of stimulus, not electrode penetrations and electrical stimulation, that is primarily responsible for elevating detection thresholds. Second, microstimulation thresholds improved steadily as the number of electrode penetrations and stimulus cycles increased (Figure 1), suggesting that the V1 neurons were not suffering from accumulating damage. There was no correlation between elevation in visual thresholds and either the amount of current injected at each site (r = 0.26, p = 0.62) or the number of microelectrode penetrations (r = −0.04, p = 0.95). Finally, the virtually complete recovery of visual thresholds with retraining suggests that microstimulation caused no substantial damage. The antagonistic relationship between visual and electrical thresholds is consistent with psychophysical studies showing that training on one sensory stimulus can elevate thresholds for other sensory stimuli [17−19], although those effects are much smaller than those reported here.
It is unlikely that the improvements in detection thresholds depended on the animal learning what class of percept to look for (visual versus electrical). After the monkeys became expert at detecting microstimulation at one site in V1, they still needed extensive training to achieve low thresholds at other sites. Correspondingly, visual retraining for one microstimulated V1 site did not restore thresholds for other V1 sites. Instead, each V1 site had to undergo visual stimulus relearning to restore low visual detection thresholds. Additionally, although extended practice with visual detection was needed to restore thresholds at microstimulated representations, visual detection at nearby visual field representations that had not been microstimulated was at normal levels before that visual retaining began (Figure 2F).
The inability of subjects to detect strong electrical microstimulation without practice shows that arbitrary distributions of active cortical neurons cannot be interpreted for guiding behaviors. This result is consistent with intrasurgical tests showing that human patients are insensitive to vigorous electrical stimulation over much of the cortical surface [7, 20, 21]. The spatiotemporal distribution of spiking neurons in V1 produced by microstimulation undoubtedly differs greatly from that generated by natural stimuli. Microstimulation is likely to sparsely activate a population of neurons centered around the electrode tip in a relatively synchronous way [22], while visual stimuli will activate neurons spanning multiple ocular dominance columns in layer 4, from which activity will propagate to superficial and deep layers. We speculate that because the spatiotemporal distribution of active neurons produced by the microstimulation differed markedly from that of the visual stimuli, practice was needed to alter functional connectivity to allow behavioral detection of that type of distribution.
In principle, the brain might have almost limitless capacity for dynamically accessing arbitrary subsets of cortical neurons, in which case detection of microstimulation could have been achieved with little effect on visual thresholds. Although each V1 site could support low thresholds for detecting either visual or electrical stimulation at different times, our findings suggest that at a given time the brain has relatively limited ability to access the signals from arbitrary subsets of cortical neurons.
The threshold changes described here are likely to depend on changes in functional connectivity [23, 24]. Those changes might have occurred within the stimulated cortex, in structures to which it sends its outputs, or both. The fact that visual thresholds were unaffected at retinotopic locations immediately adjacent to the loci trained with microstimulation (Figure 2) suggests that the effects of stimulating V1 did not involve changes in structures with visual receptive fields much larger than those in V1. Because thresholds for detecting electrical microstimulation have also been seen to improve for stimulation of extrastriate visual areas [10] and in frontal cortex [25], all of cortex may retain the ability to support the detection of different distributions of active neurons throughout adult life.
While it was probably critical that we assessed behavioral impairments using small targets that were represented entirely within the microstimulated part of V1, we do not yet know whether the particular features of the target are important. We predict that deficits will only be seen for tasks that depend critically on the region of cortex that is used for microstimulation training. In this regard, it will be important to learn whether training animals to detect microstimulation of extrastriate visual areas causes deficits that are selective for detection or discrimination of stimuli that are selectively represented by neurons in the microstimulated area.
We have not explored the effects of different electrical stimulus parameters. The effects of microstimulation are typically weaker with lower pulse frequencies, briefer pulses, shorter trains of pulses or when delivering the cathodal pulse first, and it is likely that we would have seen higher threshold currents with manipulations like these. However, over reasonably broad ranges, manipulations of this kind lead to quantitative, rather than qualitative differences in behavioral effects [26, 27], even when the temporal patterning of the stimulus train is changed [28]. While it is likely that the rate of learning and asymptotic thresholds will depend on electrical stimulus parameters, we suspect that our results did not depend critically on particular values. Nevertheless, it will be important in the future to explore whether there are stimulus parameters or training regimens that might permit excellent performance on detecting microstimulation without impairing visual detection.
In summary, we found that gaining expertise in detecting microstimulation of visual cortex came at the cost of impairing detection of visual stimuli. This effect on visual stimulus detection was retinotopically specific and reversible with retraining on detecting the visual stimulus. When expert performance on the detection of the visual stimulus was regained, microstimulation detection was in turn impaired. The ability of a local patch of cortex to gain the ability to support low thresholds for detecting two different types of neuronal activation points to substantial flexibility in accessing different neuronal activation patterns, yet the inability to support those low thresholds for both stimulus types at the same time indicates substantial limits in the brain's ability to use arbitrary spatiotemporal distributions of cortical activity.
EXPERIMENTAL PROCEDURES
All experiments conformed to protocols approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Behavioral Task
We trained two adult male rhesus monkeys (Macaca mulatta) on a two-interval forced choice detection task. For each trial, the animal fixated on a small white spot on a mid-gray video display background (12 cm/m2). While the animal fixated, two 250 ms intervals were presented separated by 500 ms, each interval marked by a tone. The 250 ms stimulus (visual or electrical) was delivered during one of the intervals, which was randomly selected each trial. Following a short delay after the end of the second interval (250 ms), two yellow targets appeared, 5° above and below the fixation spot. The animal indicated which interval contained the stimulus by making a saccade directly to the appropriate target, the target above the fixation spot for interval one, or the target below for interval two. The job of the animal was only to report detection of the stimulus, not the qualities of the percept itself.
Visual Stimulus
The visual stimulus was a peripheral, 2D Gaussian stimulus displayed for 250 ms, brighter than the mid-gray (12 cd/m2) video display background on which it appeared. The standard deviation (σ) of the Gaussian was determined based on its eccentricity from the fixation point so that the stimulus approximated the size of V1 receptive fields (σ=0.075·eccentricity)[29].
Electrical Stimulus
Microstimulation detection thresholds were measure by replacing the visual stimuli with microstimulation of V1. Other aspects of the task, including fixation and eye movement responses, were identical. The electrical stimulus was a 250 ms train of biphasic constant-current pulses, anodal phase first, each phase lasting 200 μs, delivered at 200 Hz. The currents delivered were limited to no more than 50 μA (all currents were the amplitude of an individual phase).
To measure microstimulation thresholds, metal microelectrodes (Pt/Ir, ~1MΩ impedance at 1 kHz) were advanced each day into a different spot in a small region of V1 (confined to about 3 by 3 mm surface area). V1 sites were either in the operculum or in the calcarine sulcus. Each threshold determination was based on 100 behavioral trials, and the electrode was advanced 100 μm every 200 trials, with the complete thickness of cortex being tested on most days. Multiunit V1 receptive fields were mapped periodically each day along each electrode track using a stimulus on the video display moved by hand.
Different V1 sites were separated by at least 5 mm (measured parallel to the cortical surface). Training at each V1 site spanned many days of microstimulation (16 median, 11–20 interquartile range). For retesting microstimulation thresholds following visual retraining, we sampled one V1 site in the calcarine sulcus in each animal. The remaining three V1 sites were in superficial cortex in the operculum, where guide tubes could not be used and transdural electrode penetrations were problematic owing to the age of the neurophysiological implants.
Data Analysis
The stimulus value (percent contrast for visual stimuli, current pulse amplitude for electrical stimuli) needed for detection of a given stimulus was determined using QUEST [30], an adaptive staircase procedure. Behavioral detection threshold was taken as the contrast needed to reach 63% of the way from chance to saturating performance (~82% correct). One block's threshold determination was based on 100 trials of the two-interval forced choice detection task.
The mean detection thresholds for the control locations (Figure 2) were determined with at least 4 blocks of 100 trials each, either post-microstimulation training (before visual retraining at the test sites) or before beginning microstimulation (site color-coded red).
Thresholds that measured ≥100% contrast or ≥50 μA are plotted in figures at those values, but were excluded from functions fits. Learning data were fit with exponential functions using unconstrained nonlinear optimizing that minimized the sum of squares error. Confidence intervals for all fit parameters of the learning curves (y-intercept, asymptote, tau) were determined using 5000-trial bootstraps of the best fitting exponential functions using sampling with replacement of the threshold determinations. P-values were computed for Pearson's linear correlation coefficients using a Student's t distribution.
HIGHLIGHTS.
-
-
Expertise in detecting visual cortex microstimulation impairs visual detection.
-
-
The effect on visual stimulus detection is retinotopically specific and reversible.
-
-
When visual performance is regained, microstimulation detection is in turn impaired.
-
-
Cortex cannot support high sensitivity for both stimulus types simultaneously.
ACKNOWLEDGMENTS
We thank Alexandra Smolyanskaya and Joe Corey for assistance in training the animals, and Aaron Seitz, Marlene Cohen, and Mark Histed for insightful comments on the manuscript. We thank Vivian Imamura and Jon Hendry for technical assistance. This work was supported by National Institutes of Health R01EY005911 and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Romo R, Salinas E. Flutter discrimination: neural codes, perception, memory and decision making. Nat. Rev. Neurosci. 2003;4:203–218. doi: 10.1038/nrn1058. [DOI] [PubMed] [Google Scholar]
- 2.Schall JD. Neural basis of deciding, choosing and acting. Nat. Rev. Neurosci. 2001;2:33–42. doi: 10.1038/35049054. [DOI] [PubMed] [Google Scholar]
- 3.Parker AJ, Newsome WT. Sense and the single neuron: probing the physiology of perception. Annu. Rev. Neurosci. 1998;21:227–277. doi: 10.1146/annurev.neuro.21.1.227. [DOI] [PubMed] [Google Scholar]
- 4.Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J. Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dobelle WH, Mladejovsky MG, Girvin JP. Artifical vision for the blind: electrical stimulation of visual cortex offers hope for a functional prosthesis. Science. 1974;183:440–444. doi: 10.1126/science.183.4123.440. [DOI] [PubMed] [Google Scholar]
- 6.Bak M, Girvin JP, Hambrecht FT, Kufta CV, Loeb GE, Schmidt EM. Visual sensations produced by intracortical microstimulation of the human occipital cortex. Med. Biol. Eng. Comput. 1990;28:257–259. doi: 10.1007/BF02442682. [DOI] [PubMed] [Google Scholar]
- 7.Lee HW, Hong SB, Seo DW, Tae WS, Hong SC. Mapping of functional organization in human visual cortex: electrical cortical stimulation. Neurology. 2000;54:849–854. doi: 10.1212/wnl.54.4.849. [DOI] [PubMed] [Google Scholar]
- 8.DeYoe EA, Lewine JD, Doty RW. Laminar variation in threshold for detection of electrical excitation of striate cortex by macaques. J. Neurophysiol. 2005;94:3443–3450. doi: 10.1152/jn.00407.2005. [DOI] [PubMed] [Google Scholar]
- 9.Tehovnik EJ, Slocum WM, Carvey CE, Schiller PH. Phosphene induction and the generation of saccadic eye movements by striate cortex. J. Neurophysiol. 2005;93:1–19. doi: 10.1152/jn.00736.2004. [DOI] [PubMed] [Google Scholar]
- 10.Murphey DK, Maunsell JH. Behavioral detection of electrical microstimulation in different cortical visual areas. Curr. Biol. 2007;17:862–867. doi: 10.1016/j.cub.2007.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept. Psychophys. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- 12.Goldstone RL. Perceptual learning. Annu. Rev. Psychol. 1998;49:585–612. doi: 10.1146/annurev.psych.49.1.585. [DOI] [PubMed] [Google Scholar]
- 13.Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- 14.Tsodyks M, Gilbert C. Neural networks and perceptual learning. Nature. 2004;431:775–781. doi: 10.1038/nature03013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) J. Neurosci. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasternak T, Merigan WH. Motion perception following lesions of the superior temporal sulcus in the monkey. Cereb. Cortex. 1994;4:247–259. doi: 10.1093/cercor/4.3.247. [DOI] [PubMed] [Google Scholar]
- 17.Sterr A, Muller MM, Elbert T, Rockstroh B, Pantev C, Taub E. Changed perceptions in Braille readers. Nature. 1998;391:134–135. doi: 10.1038/34322. [DOI] [PubMed] [Google Scholar]
- 18.Sigman M, Gilbert CD. Learning to find a shape. Nat. Neurosci. 2000;3:264–269. doi: 10.1038/72979. [DOI] [PubMed] [Google Scholar]
- 19.Seitz AR, Nanez JE, Holloway SR, Koyama S, Watanabe T. Seeing what is not there shows the costs of perceptual learning. Proc. Natl. Acad. Sci. USA. 2005;102:9080–9085. doi: 10.1073/pnas.0501026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penfield W, Rasmussen T. The Cerebral Cortex of Man. The Macmillan Company; New York: 1950. [Google Scholar]
- 21.Murphey DK, Maunsell JH, Beauchamp MS, Yoshor D. Perceiving electrical stimulation of identified human visual areas. Proc. Natl. Acad. Sci. USA. 2009;106:5389–5393. doi: 10.1073/pnas.0804998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron. 2009;63:508–522. doi: 10.1016/j.neuron.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowdhury SA, DeAngelis GC. Fine discrimination training alters the causal contribution of macaque area MT to depth perception. Neuron. 2008;60:367–377. doi: 10.1016/j.neuron.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Law CT, Gold JI. Reinforcement learning can account for associative and perceptual learning on a visual-decision task. Nat. Neurosci. 2009;12:655–663. doi: 10.1038/nn.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphey DK, Maunsell JH. Electrical microstimulation thresholds for behavioral detection and saccades in monkey frontal eye fields. Proc. Natl. Acad. Sci. USA. 2008;105:7315–7320. doi: 10.1073/pnas.0710820105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tehovnik EJ, Slocum WM. What delay fields tell us about striate cortex. J. Neurophysiol. 2007;98:559–576. doi: 10.1152/jn.00285.2007. [DOI] [PubMed] [Google Scholar]
- 27.Tehovnik EJ, Slocum WM. Microstimulation of macaque V1 disrupts target selection: effects of stimulation polarity. Exp. Brain Res. 2003;148:233–237. doi: 10.1007/s00221-002-1312-5. [DOI] [PubMed] [Google Scholar]
- 28.Kimmel DL, Moore T. Temporal patterning of saccadic eye movement signals. J. Neurosci. 2007;27:7619–7630. doi: 10.1523/JNEUROSCI.0386-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Essen DC, Newsome WT, Maunsell JH. The visual field representation in striate cortex of the macaque monkey: asymmetries, anisotropies, and individual variability. Vision Res. 1984;24:429–448. doi: 10.1016/0042-6989(84)90041-5. [DOI] [PubMed] [Google Scholar]
- 30.Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept. Psychophys. 1983;33:113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]