Abstract
α-defensins are released from granules of leukocytes and are implicated in inflammatory and fibrotic lung diseases. In the present study, the effects of α-defensins on the proliferation and collagen synthesis of lung fibroblasts were examined. We found that α-defensin-1 and α-defensin-2 induced dose-dependent increases in the incorporation of 5-bromo-2′-deoxy-uridine into newly synthesized DNA in two lines of human lung fibroblasts (HFL-1 and LL-86), suggesting that α-defensin-1 and α-defensin-2 stimulate the proliferation of lung fibroblasts. α-defensin-1 and α-defensin-2 also increased collagen-I mRNA (COL1A1) levels and protein contents of collagen-I and active/dephosphorylated β-catenin without changes in total β-catenin protein content in lung fibroblasts (HFL-1 and LL-86). Inhibition of the β-catenin signaling pathway using quercetin prevented increases in cell proliferation and the protein content of collagen-I and active/dephosphorylated β-catenin in lung fibroblasts, and in COL1A1 mRNA levels and collagen release into culture medium induced by α-defensin-1 and α-defensin-2. Knocking-down β-catenin using small interfering RNA technology also prevented α-defensin-induced increases in cell proliferation and the protein content of collagen-I and active/dephosphorylated β-catenin in lung fibroblasts, and in COL1A1 mRNA levels. Moreover, increases in the phosphorylation of glycogen synthase kinase 3β, accumulation/activation of β-catenin, and collagen synthesis induced by α-defensin-1 and α-defensin-2 were prevented by p38 mitogen-activated protein kinase inhibitor SB203580 and phosphoinositide 3-kinase inhibitor LY294002. These results indicate that α-defensin-1 and α-defensin-2 stimulate proliferation and collagen synthesis of lung fibroblasts. The β-catenin signaling pathway mediates α-defensin-induced increases in cell proliferation and collagen synthesis of lung fibroblasts. α-defensin-induced activation of β-catenin in lung fibroblasts might be caused by phosphorylation/inactivation of glycogen synthase kinase 3β as a result of the activation of the p38 mitogen-activated protein kinase and phosphoinositide 3-kinase/Akt pathways.
Keywords: β-catenin, collagen, defensins, fibroblasts, proliferation
Introduction
Defensins are small cationic peptides with approximately 30–40 amino acids. There are two isoforms of defensins: α-defensin and β-defensin. α-defensin-1 to -4, also called human neutrophil peptide (HNP-1 to -4), are mainly presented in granules of neutrophils [1,2]. They account for 50% of the protein content of neutrophil granules. α-defensins are released from granules of leukocytes at inflammatory sites [3,4]. Increased α-defensin levels in bronchial alveolar lavage and/or plasma have been observed in a number of inflammatory lung diseases, such as diffuse panbronchiolitis, acute respiratory distress syndrome and α1-antitrypsin deficiency [1,4–6]. The role of α-defensins in these diseases is not clear. Interestingly, it has been found that α-defensin levels in bronchial alveolar lavage and/or plasma are increased in fibrotic lung diseases, such as cystic fibrosis and idiopathic pulmonary fibrosis (IPF) [7,8]. A significant amount of α-defensins can be found outside neutrophils in fibrotic foci in the lungs of patients with IPF [8]. Moreover, inflammatory lung diseases with neutrophil infiltration are complicated with fibroproliferative lesions [9]. Thus, α-defensins might play an important role in the formation of fibroproliferative lesions in inflammatory lung diseases. It has been reported that α-defensins stimulate the proliferation of airway epithelial cells, NIH 3T3 fibroblasts and dermal fibroblasts [10,11]. In the present study, we also found that α-defensin-1 and α-defensin-2 stimulated the proliferation and collagen synthesis of two other cell lines of lung fibroblasts (HFL-1 and LL86). However, the mechanism responsible for the α-defensin-induced proliferation of lung fibroblasts is not understood.
The Wnt pathway has been identified as one of the numerous signaling pathways critical for the precise temporal and spatial control of lung morphogenesis [12]. β-catenin is a key regulatory protein in the Wnt cascade. In nonstimulated cells, β-catenin is largely associated with cadherin. There is very little β-catenin in the cytoplasm or nucleus because β-catenin in the cytoplasm is phosphorylated by glycogen synthase kinase 3β (GSK3β) and is targeted for ubiquitination and subsequent degradation by the proteasome [13]. When cells are stimulated, proteasome-mediated degradation of β-catenin is prevented. β-catenin accumulates and translocates into the nucleus, forms a complex with T cell factor/lymphocyte enhancer factor-1 (TCF/LEF-1) family transcription factors, and regulates Wnt target genes and proliferation [14]. β-catenin activation has been implicated in fibroproliferative pulmonary disorders, including IPF and hyperoxic lung injuries [15,16]. It is not clear whether β-catenin activation plays any role in α-defensin-induced proliferation and collagen synthesis of lung fibroblasts. In the present study, we found that α-defensin-1 and α-defensin-2 induced increases in the phosphorylation of GSK3β and β-catenin activation and that inhibition of β-catenin activation prevented α-defensin-induced proliferation and collagen synthesis of lung fibroblasts, suggesting that the β-catenin signaling pathway plays a mediating role in these processes.
Results
α-defensin-1 and α-defensin-2 increased proliferation and collagen synthesis in HFL-1 lung fibroblasts
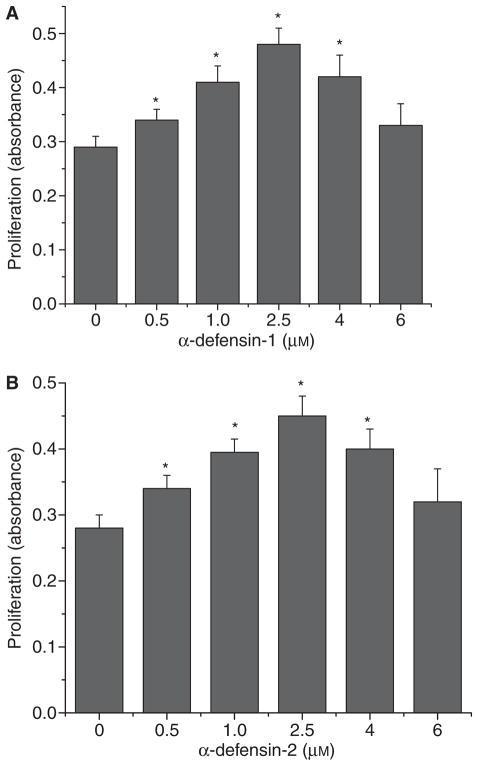
To study the effects of α-defensin-1 and α-defensin-2 on lung fibroblast proliferation, HFL-1 lung fibroblasts were incubated with α-defensin-1 and α-defensin-2 (0.5–6 μM) for 24 h and then the incorporation of 5-bromo-2′-deoxy-uridine (BrdU) into the cells was assayed. As shown in Fig. 1A, incubation of HFL-1 lung fibroblasts with α-defensin-1 induced dose-dependent increases in BrdU incorporation, suggesting that α-defensin-1 induces an increase in the proliferation of lung fibroblasts. The maximum effect of α-defensin-1 was observed with concentrations of 2.5 μM. Similar results were obtained with HFL-1 lung fibroblasts incubated with α-defensin-2 (Fig. 1B).
Fig. 1.
Effect of α-defensin-1 and α-defensin-2 on cell proliferation of lung fibroblasts HFL-1. Lung fibroblasts HFL1 were incubated with or without α-defensin-1 (A: 0.5–6 μM) and α-defensin-2 (B: 0.5–6 μM) for 24 h, after which cell proliferation was assayed as described in the Experimental procedures; n = 4, *P < 0.05 versus control (concentration 0).
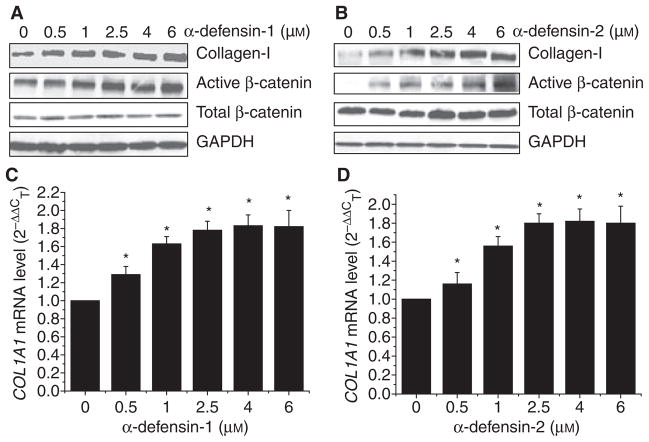
To study whether α-defensin-1 and α-defensin-2 increases collagen synthesis, HFL-1 lung fibroblasts were incubated with α-defensin-1 and α-defensin-2 (0.5–6 μM) for 24 h and then collagen protein content and the collagen-I mRNA (COL1A1) level were assayed. We found that incubation of HFL-1 lung fibroblasts with α-defensin-1 induced a dose-dependent increase in collagen-I protein content and the COL1A1 mRNA level (Fig. 2A,C). Similar results were obtained with HFL-1 lung fibroblasts incubated with α-defensin-2 (Fig. 2B,D).
Fig. 2.
Effect of α-defensin-1 and α-defensin-2 on protein contents of active/dephosphrylated β-catenin, total β-catenin and collagen-I and COL1A1 mRNA levels of in lung fibroblasts HFL-1. Lung fibroblasts HFL1 were incubated with α-defensin-1 (A, C: 0.5–6 μM) and α-defensin-2 (B, D: 0.5–6 μM) for 24 h, after which protein contents of active/dephosphrylated β-catenin, total β-catenin and collagen-I were measured using western blot analysis and COL1A1 mRNA levels were assayed using quantitative real-time RT-PCR as described in the Experimental procedures. (A, B) Showing representative blots of three separate experiments. (C, D) Bar graphs depicting changes in COL1A1 mRNA levels; n = 3, *P < 0.05 versus control (concentration 0). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
α-defensin-1 and α-defensin-2 also enhanced proliferation and collagen synthesis in LL-86 lung fibroblasts (Figs S1 and S2), suggesting that α-defensin-induced increases in proliferation and collagen synthesis are a generalized phenomenon among lung fibroblasts.
α-defensin-1 and α-defensin-2 increased active/dephosphorylated β-catenin in lung fibroblasts
As shown in Fig. 2A,B, incubation of HFL-1 lung fibroblasts with α-defensin-1 and α-defensin-2 induced dose-dependent increases in active/dephosphorylated β-catenin in HFL-1 lung fibroblasts. However, total β-catenin protein content was not affected. These results suggest that α-defensins induce the dephosphorylation of β-catenin and cause β-catenin accumulation in the nuclei of HFL-1 lung fibroblasts. Incubation of LL-86 lung fibroblasts with α-defensin-1 and α-defensin-2 also caused increases in active/dephosphorylated β-catenin without a significant alteration in total β-catenin protein content (Figs S1 and S2), suggesting that α-defensin-induced activation of the β-catenin signaling pathway is a generalized phenomenon among lung fibroblasts.
Inhibition of the β-catenin signaling pathway using quercetin blocked the α-defensin-induced increase in lung fibroblast proliferation and collagen synthesis
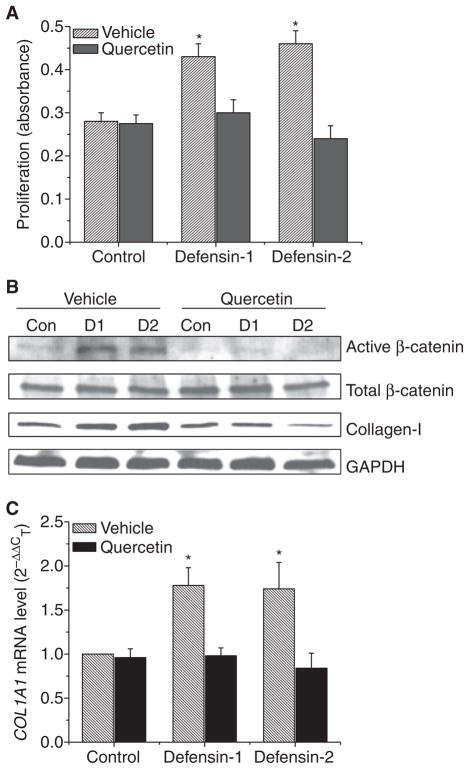
To investigate the role of β-catenin activation in the defensin-induced increase in lung fibroblast proliferation, lung fibroblasts (HFL-1) were incubated with or without α-defensin-1 and α-defensin-2 (2.5 μM) in the presence and absence of quercetin (10 μM) for 24 h, after which the protein contents of active/dephosphorylated β-catenin, total β-catenin and collagen-I, cell proliferation, and the COL1A1 mRNA level were assayed. We found that quercetin prevented an increase in the protein content of active/dephosphorylated β-catenin without changes in total β-catenin (Fig. 3B). Correspondingly, quercetin prevented an increase in cell proliferation and in collagen-I protein content and the COL1A1 mRNA level induced by α-defensin-1 and α-defensin-2 (Fig. 3A–C). These results indicate that α-defensin-induced increases in lung fibroblast proliferation and collagen synthesis involve the β-catenin signaling pathway.
Fig. 3.
Effect of quercetin on α-defensin-induced alterations in cell proliferation, intracellular protein contents of active/dephosphorylated β-catenin, total β-catenin and collagen-I in lung fibroblasts. Lung fibroblasts HFL1 were incubated with or without α-defensin-1 and α-defensin-2 (2.5 μM) in the presence and absence of quercetin (10 μM) for 24 h, after which cell proliferation (A), protein contents of active/dephosphrylated β-catenin, total β-catenin and collagen-I (B) and COL1A1 mRNA levels (C) were measured as described in the Experimental procedures. The images in (B) are representative blots of three separate experiments. Con, control; D1, α-defensin-1; D2, α-defensin-2. (A, C) n = 3, *P < 0.05 versus control vehicle group without defensins and quercetin.
Inhibition of β-catenin signaling pathway using quercetin blocked the α-defensin-induced increase in collagen release from lung fibroblasts
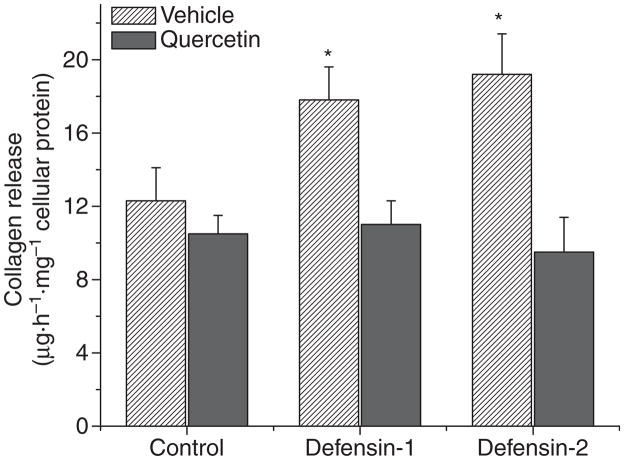
To study whether the α-defensin-induced alteration in intracellular collagen-I protein content in lung fibroblasts results in corresponding changes in collagen release from the cells, the collagen content in the culture medium of lung fibroblasts (HFL-1) treated without or with α-defensin-1 and α-defensin-2 (2.5 μM) in the presence and absence of quercetin (10 μM) was determined. As shown in Fig. 4, incubation of lung fibroblasts with α-defensin-1 and α-defensin-2 resulted in an increase in collagen content in the culture medium, which corresponds to changes in intracellular collagen-I protein content. In addition, quercetin prevented an α-defensin-induced increase in collagen content in the culture medium (Fig. 4).
Fig. 4.
Effect of quercetin on α-defensin-induced alterations in collagen release from lung fibroblasts. Lung fibroblasts HFL1 were incubated with or without α-defensin-1 and α-defensin-2 (2.5 μM) in the presence and absence of quercetin (10 μM) for 24 h, after which collagen release was measured by determining collagen contents in the culture medium as described in the Experimental procedures; n = 3, *P < 0.05 versus control (Vehicle).
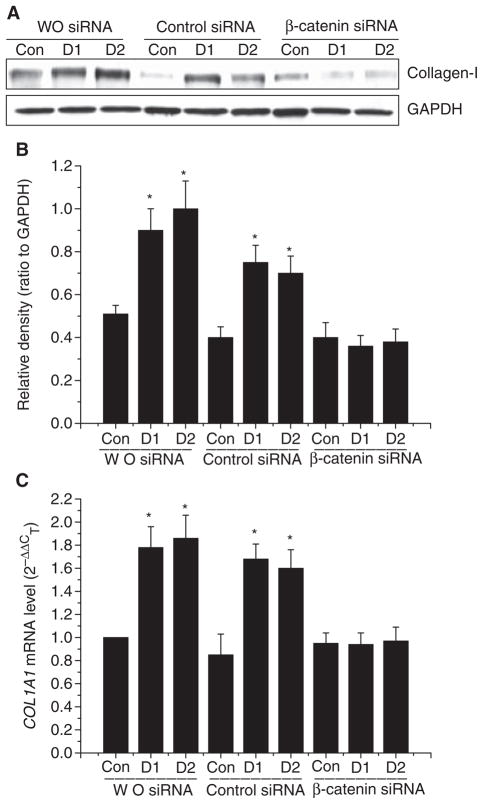
Knocking-down β-catenin prevented the α-defensin-induced increase in lung fibroblast proliferation and collagen synthesis
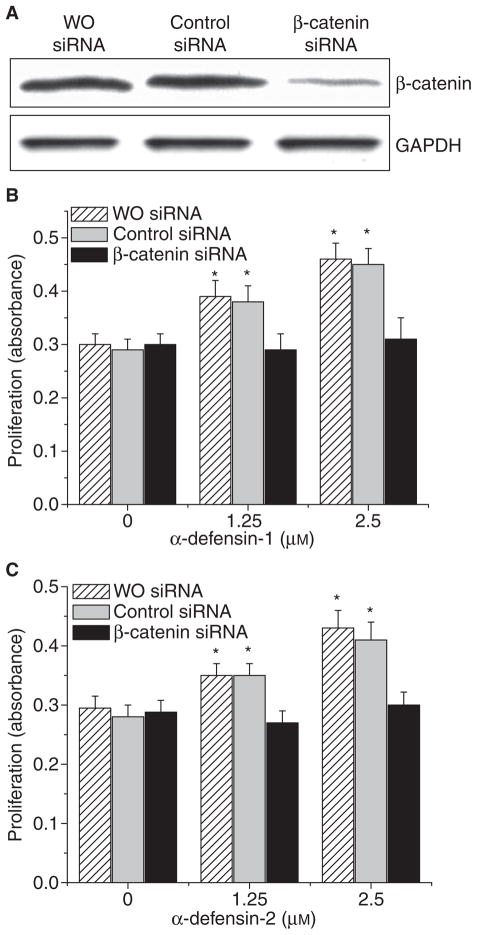
To clarify whether β-catenin is responsible for defensin-induced increases in lung fibroblast proliferation and in collagen synthesis, the protein expression of β-catenin in lung fibroblasts (HFL-1) was knocked down by using its small interfering RNA (siRNA). As shown in Fig. 5A, transfection of HFL-1 lung fibroblasts with siRNA targeting the mRNA of β-catenin significantly knocked down the protein expression of β-catenin. Knocking-down the protein expression of β-catenin prevented increases in lung fibroblast proliferation (Fig. 5B,C) and collagen-I protein content (Fig. 6A,B) as well as the COL1A1 mRNA level (Fig. 6C) caused by α-defensin-1 and α-defensin-2.
Fig. 5.
Effect of knocking down β-catenin on cell proliferation of lung fibroblasts. Lung fibroblasts HFL1 were transfected with siRNAs targeted to β-catenin and luciferase (control sequence). After 72 h, the cells were incubated with or without α-defensin-1 (B: 1.25–2.5 μM) and α-defensin-2 (C: 1.25–2.5 μM) for 24 h, after which cell proliferation was assayed as described in the Experimental procedures; n = 4, *P < 0.05 versus control (concentration 0). (A) Showing a representative image of immunoblot against active/dephosphorylated β-catenin from three experiments.
Fig. 6.
Effect of knocking down β-catenin on collagen synthesis of lung fibroblasts. Lung fibroblasts HFL1 were transfected with siRNAs targeted to β-catenin and luciferase (control sequence). After 72 h, the cells were incubated with or without α-defensin-1 (2.5 μM) and α-defensin-2 (2.5 μM) for 24 h after which collagen-I protein contents (A, B), and COL1A1 mRNA levels (C) were measured as described in the Experimental procedures. The images in (A) are representative blots of three separate experiments. (B) Bar graph depicting changes in densities of the blots in (A); n = 3, *P < 0.05 versus control group. Con, control; D1, α-defensin-1; D2, α-defensin-2.
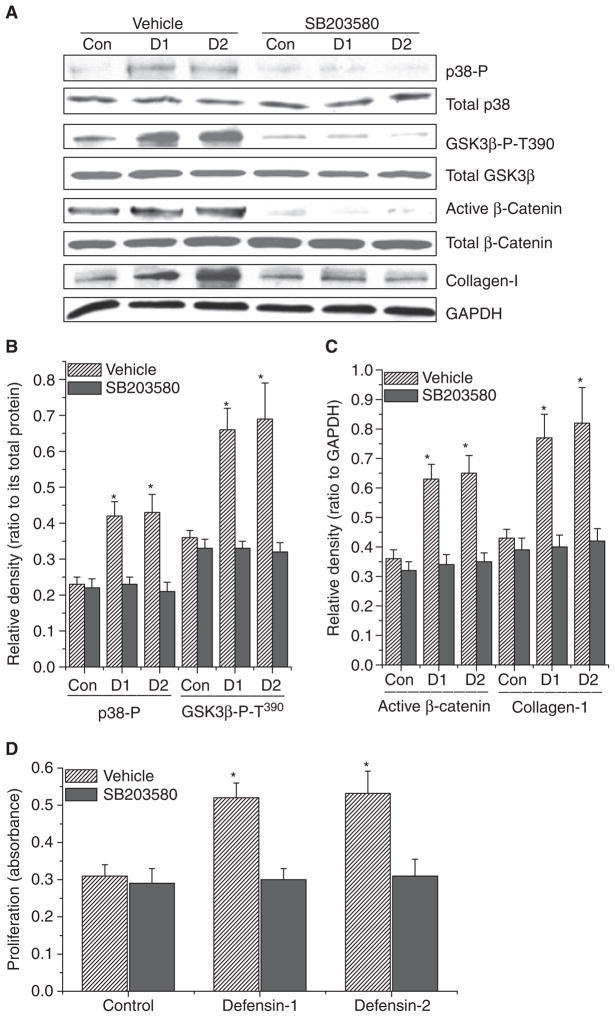
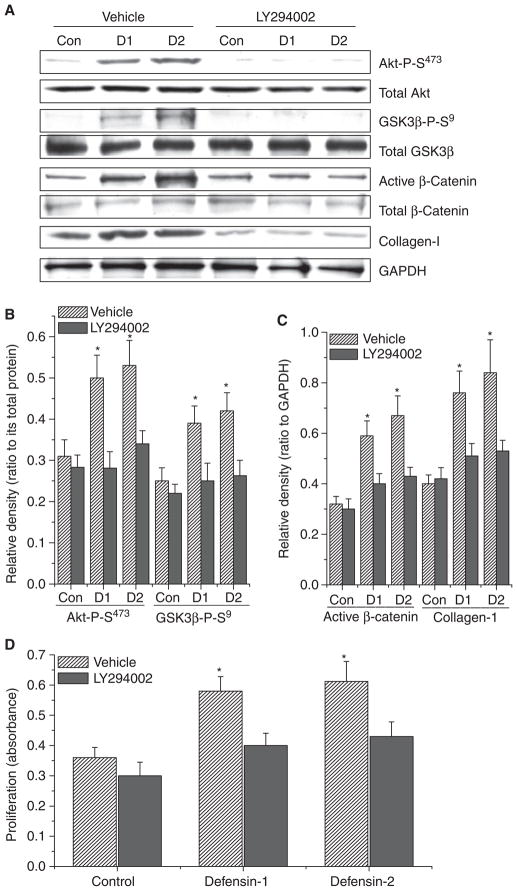
p38 mitogen-activated protein (MAP) kinase inhibitor SB203580 and phosphoinositide 3-kinase (PI3K) inhibitor LY294002 prevented increases in the phosphorylation of GSK3β, dephosphorylation/activation of β-catenin, and collagen synthesis induced by α-defensin-1 and α-defensin-2
The signaling molecule upstream of β-catenin is GSK3β. It has been previously reported that p38 MAP kinase and PI3K/Akt directly phosphorylate GSK3β on Thr390 and Ser9, respectively, leading to inactivation of GSK3β and accumulation/activation of β-catenin [17,18]. To determine whether α-defensin-induced collagen synthesis and the accumulation/activation of β-catenin in lung fibroblasts are caused by activation of p38 MAP kinase and PI3K/Akt, lung fibroblasts (HFL-1) were incubated with or without α-defensin-1 and α-defensin-2 (2.5 μM) in the presence and absence of SB203580 (10 μM), a specific selective inhibitor of p38 MAP kinase, or LY294002 (20 μM), a specific inhibitor of PI3K, for 1–24 h, after which the protein contents of phosphorylated p38 MAP kinase, total p38 MAP kinase, phosphorylated Akt, total Akt, phosphorylated GSK3β, total GSK3β, active/dephosphorylated β-catenin, total β-catenin and collagen-I, and cell proliferation were determined. As shown in Figs 7A,B and 8A,B, the incubation of lung fibroblasts with α-defensin-1 and α-defensin-2 induced increases in the protein contents of phosphorylated p38 MAP kinase, Ser473-phosphorylated Akt, Thr390-phosphorylated and Ser9-phosphorylated GSK3β without an alteration in the protein contents of total p38 MAP kinase, total Akt, and total GSK3β, suggesting that α-defensin-1 and α-defensin-2 cause the activation of p38 MAP kinase and PI3K/Akt and increase phosphorylation of GSK3β on Thr390 and Ser9. SB203580 and LY294002 prevented α-defensin-induced increases in the protein contents of Thr390-phosphorylated and Ser9-phosphorylated GSK3β, active/dephosphorylated β-catenin, and collagen-I (Figs 7A–C and 8A–C). Moreover, α-defensin-induced increases in cell proliferation were prevented by SB203580 and LY294002 (Figs 7D and 8D).
Fig. 7.
Effect of MAP kinase inhibitor SB203580 on α-defensin-induced alterations in intracellular protein contents of phosphorylated p38 MAP kinase, total p38 MAP kinase, Thr390-phosphorylated GSK3β (GSK3β-P-T390), total GSK3β, active/dephosphorylated β-catenin, total β-catenin and collagen-I and cell proliferation in lung fibroblasts. Lung fibroblasts HFL1 were incubated with or without α-defensin-1 and α-defensin-2 (2.5 μM) in the presence and absence of SB203580 (10 μM) for 1–24 h, after which protein contents of phosphorylated p38 MAP kinase, total p38 MAP kinase, GSK3β-P-T390, total GSK3β, active/dephosphorylated β-catenin, total β-catenin and collagen-I (A–C) and cell proliferation (D) were measured as described in the Experimental procedures. The images in (A) are representative blots of three separate experiments. (B, C) Bar graphs depicting changes in densities of the blots in (A); n = 3, *P < 0.05 versus control vehicle group without defensins and SB203580. Con, control; D1, α-defensin-1; D2, α-defensin-2.
Fig. 8.
Effect of PI3K inhibitor LY294002 on α-defensin-induced alterations in intracellular protein contents of phosphorylated Akt, total Akt, Ser9-phosphorylated GSK3β (GSK3β-P-S9), total GSK3β, active/dephosphorylated β-catenin, total β-catenin and collagen-I and cell proliferation in lung fibroblasts. Lung fibroblasts HFL1 were incubated with or without α-defensin-1 and α-defensin-2 (2.5 μM) in the presence and absence of LY294002 (20 μM) for 1–24 h, after which protein contents of phosphorylated Akt, total Akt, GSK3β-P-S9, total GSK3β, active/dephosphorylated β-catenin, total β-catenin and collagen-I (A–C) and cell proliferation (D) were measured as described in the Experimental procedures. The images in (A) are representative blots of three separate experiments. (B, C) Bar graphs depicting changes in densities of the blots in (A); n = 3, *P < 0.05 versus control vehicle group without defensins and LY294002. Con, control; D1, α-defensin-1; D2, α-defensin-2.
Discussion
The plasma concentrations of α-defensins are approximately 0.03 μM in normal volunteers, but rise to 1.5–2.0 μM in patients with IPF and acute respiratory distress syndrome [6,8]. The concentrations of α-defensins in the lower respiratory tract epithelial lining fluid in patients with α1-antitrypsin deficiency could reach as high as 2.0 μM [19]. Significant amounts of α-defensins can also be found outside neutrophils in the fibrotic foci in the lungs of IPF patients [5,8]. In addition to potential antimicrobial effects, α-defensins in the lung tissue may be responsible for the formation of fibroproliferative lesions or remodeling in inflammatory lung diseases. Proliferation and collagen synthesis of lung fibroblasts plays a pivotal role in these processes. In the present study, we have demonstrated that α-defensin-1 and α-defensin-2 stimulate lung fibroblast proliferation and collagen synthesis in two lines of lung fibroblasts, suggesting that α-defensins may be implicated in the formation of fibroproliferative lesions or remodeling in neutrophil-infiltrated lung tissue. The effects of α-defensin-1 and α-defensin-2 on lung fibroblast proliferation are concentration-dependent. α-defensins in concentrations similar to those in inflammatory lung diseases promote proliferation and collagen synthesis of lung fibroblasts. The stimulatory effects of α-defensins on collagen synthesis reach a plateau at higher concentrations, whereas the proliferative effects are reduced. The results obtained in the present study are consistent with the mitogenic effects of α-defensins previously reported for airway epithelial cells, NIH 3T3 fibroblasts and dermal fibroblasts [10,11].
The mechanism for α-defensin-induced increase in the proliferation and collagen synthesis of lung fibroblasts has not been clarified. Yoshioka et al. [20] indicated that α-defensin activated the MAP kinase signaling pathway. Aarbiou et al. [21] also reported that MAP kinase kinase inhibitor U0126 inhibited α-defensin-induced proliferation of A549 lung epithelial cells, suggesting that α-defensins mediate cell proliferation through the MAP kinase signaling pathway. In the present study, we found that α-defensin-1 and α-defensin-2 caused increases in the protein content of active/dephosphorylated β-catenin without changes in total β-catenin, suggesting that α-defensins activate the β-catenin signaling pathway. We then studied the role of the β-catenin signaling pathway in α-defensin-induced increases in the proliferation and collagen synthesis of lung fibroblasts using quercetin. Quercetin, which inhibits the Wnt/β-catenin signaling pathway through its upstream kinases, blocks β-catenin/Tcf transcriptional activity by decreasing active/dephosphorylated β-catenin and Tcf-4 proteins [22–24]. The results obtained in the present study show that inhibition of the β-catenin signaling pathway by quercetin prevented increases in lung fibroblast proliferation and collagen synthesis induced by α-defensin-1 and α-defensin-2. Furthermore, by using a different method (i.e. siRNA technology), we have demonstrated that knocking-down the protein expression of β-catenin inhibited increases in lung fibroblast proliferation and collagen synthesis caused by α-defensin-1 and α-defensin-2. Taken together, these results indicate that the β-catenin signaling pathway mediates α-defensin-induced increases in cell proliferation and collagen synthesis of lung fibroblasts.
The canonical Wnt/β-catenin signaling pathway is initiated when Wnts bind to frizzed receptor and lipoprotein receptor-related protein 5/6, which leads to dephosphorylation/accumulation of β-catenin and subsequent transcription of its target genes [25]. It is unlikely that α-defensin-induced activation of the β-catenin signaling pathway and the increase in collagen synthesis of lung fibroblasts are Wnt-dependent because the soluble Wnt-antagonist secreted frizzled-related-protein-1 (sFRP-1) did not affect the α-defensin-induced increase in collagen synthesis (Fig. S3). It has been well defined that β-catenin is phosphorylated by GSK-3β and subsequently ubiquitinized and degraded by proteasome in the Wnt/β-catenin signaling pathway [26]. Thornton et al. [17] reported that p38 MAP kinase directly phosphorylated GSK3β on Thr390, leading to inactivation of GSK3β and accumulation/activation of β-catenin. The inactivation of GSK3β can also be caused by phophorylation of its N-terminus at Ser9 by Akt [18]. Syeda et al. [27] reported that a mixture of HNP, predominantly consisting of α-defensins-1 and -2, induced activation of the p38 MAP kinase and PI3K/Akt pathways in human monocytes (U937). The mixture of HNP activated only the PI3K/Akt pathway in human lung epithelial cells (A549) [27]. Our data indicate that inhibition of p38 MAP kinase and PI3K prevents the phosphorylation of GSK3β on Thr390 and Ser9 and an increase in active/dephosphorylated β-catenin induced by α-defensins in lung fibroblasts, suggesting that α-defensin-induced activation of β-catenin in lung fibroblasts is caused by phosphorylation/inactivation of GSK3β as a result of the activation of p38 MAP kinase and PI3K/Akt.
The Wnt/β-catenin signaling pathway is required for proper lung mesenchymal growth and vascular development [12,28]. Up-regulation of the Wnt/β-catenin pathway was observed in the lungs of neonatal rats with hyperoxic lung remodeling [29]. Extensive nuclear accumulation of β-catenin was found in bronchiolar proliferative lesions, damaged alveolar structures and fibrotic foci in the lungs of IPF patients [30]. Collagen-I expression and cell proliferation in human skin fibroblasts in response to irradiation both depend on activation of the Wnt/β-catenin pathway [31]. The results obtained in the present study indicate that activation of the β-catenin signaling pathway mediates α-defensin-induced increases in cell proliferation and collagen-I synthesis of lung fibroblasts. Cell proliferation mediated by the β-catenin signaling pathway is attributed to the direct target genes of β-catenin-TCF/LEF-1, such as the genes for cyclin D, fibroblast growth factor, fibronectin, endothelin, surviving, etc. We have obtained data indicating that α-defensin-1 and α-defensins-2 induce an increase in the protein content of cyclin D and that quercetin inhibits the α-defensin-induced increase in cyclin D protein content in HFL-1 lung fibroblasts (Fig. S4), suggesting that cyclin D might be related to the α-defensin-induced increase in lung fibroblast proliferation. Collagen-I is the major component of the extracellular matrix in the fibrotic lesions of lung fibrosis. Although there is no direct evidence showing that collagen-I is a direct transcriptional target of TCF/LEF-1, there is compelling evidence to suggest that the β-catenin signaling pathway leads to up-regulation of collagen-I synthesis in fibroblasts [30–32]. Another important feature of fibroblasts in the pathogenesis of fibrotic lesion concerns cell motility. Our data (W. Han and Y. Su, unpublished data) show that α-defensin-1 and α-defensin-2 enhance the cell migration of HFL-1 lung fibroblasts in a Boyden chamber assay, providing evidence to support the role of α-defensins in the formation of inflammatory fibroproliferative lesions.
In summary, we have demonstrated that α-defensin-1 and α-defensin-2 stimulate the proliferation and collagen synthesis of lung fibroblasts. Our novel findings on the role of the β-catenin signaling pathway in α-defensin-induced increases in proliferation and collagen synthesis of lung fibroblasts open the door to the possibility that manipulation of the α-defensins/β-catenin signaling pathway will provide a new avenue for preventing or reversing the formation of fibroproliferative lesions in inflammation-related pulmonary diseases, such as diffuse panbronchiolitis, acute respiratory distress syndrome, chronic obstructive pulmonary disease and hyperoxic lung injuries, as well as other inflammatory diseases associated with fibroproliferative lesions.
Experimental procedures
Materials
α-defensin-1 and α-defensin-2 were obtained from Bachem (Torrance, CA, USA). Anti-collagen-I antibody was obtained from Novus Biologicals (Littleton, CO, USA). Antibodies against active/dephosphorylated β-catenin, total β-catenin and cyclin D were obtained from Millipore (Billerica, MA, USA). Antibodies against Thr180/Tyr182-phosphorylated p38 MAP kinase and total p38 MAP kinase were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against Thr390-phosphorylated GSK3β, Ser9-phosphorylated GSK3β, total GSK3β, Ser473-phosphorylated Akt, total Akt and GAPDH were obtained from Cell Signaling Technology (Danvers, MA, USA). sFRP-1 was obtained from R&D Systems (Minneapolis, MN, USA). SB203580 and LY294002 were obtained from Calbiochem (San Diego, CA, USA).
Cell culture
Two lines of human lung fibroblasts (HFL-1 and LL-86) were obtained from the American Type Culture Collection (Rockville, MD, USA). Cells were cultured in accordance with the manufacturer’s instructions. Third-to-tenth passage cells that were equilibrated in serum-free medium for 24 h were used for all experiments.
siRNA knock-down of β-catenin
The expression of β-catenin was silenced using siRNA technology. The target sequences for the mRNA of β-catenin were 5′-AAAGCTGATATTGATGGACAG-3′. The siRNA against luciferase mRNA was used as a control siRNA. The target sequence for luciferase mRNA was 5′-AACGTACGCGGAATACTTCGA-3′. The siRNAs were custom-synthesized by Qiagen (Valencia, CA, USA) and were transfected into lung fibroblasts using Qiagen RNAiFest transfection reagent in accordance with the manufacturer’s instructions. Two days after transfection, the medium was changed to serum free medium. After 24 h, the protein content of β-catenin, cell proliferation, and collagen protein and mRNA were evaluated.
Cell proliferation assay
Proliferation of lung fibroblasts was assayed with a kit from Roche (Indianapolis, IN, USA) that monitors the incorporation of BrdU into newly synthesized DNA. The BrdU was detected using anti-BrdU-peroxidase conjugate in accordance with the manufacturer’s instructions. After reactions were stopped, A450 was measured with a BioTek F600 microplate reader (BioTek Inc., Winooski, VT, USA).
Immunoblot analysis
Three days after siRNA transfection, cells were harvested. Samples (20–30 μg of protein) were denatured and electrophoresed on 7.5% SDS-PAGE. Separated proteins were electrotransferred to nitrocellulose membranes, incubated with 5% fat-free milk for 2 h, and then incubated with monoclonal antibodies against collagen-I, active/dephosphorylated β-catenin, total β-catenin, Thr180/Tyr182-phosphorylated p38 MAP kinase, total p38 MAP kinase, Ser473-phosphorylated Akt, total Akt, Thr390-phosphorylated GSK3β, Ser9-phosphorylated GSK3β, total GSK3β and GAPDH overnight at 4 °C and then washed with 50 mL of 0.1% Tween-20, 20 mM Tris-HCl (pH 7.5) and 150 mM NaCl (TTBS) three times for 10 min. Secondary goat anti-mouse IgG conjugated to alkaline phosphatase (Bio-Rad, Hercules, CA, USA) was diluted in TTBS plus 5% nonfat milk and incubated with the membranes at room temperature for 1–2 h. After the membranes were washed with TTBS, enhanced chemiluminescence (ImmunStar; Bio-Rad) was used to visualize the reactive proteins followed by densitometric quantification using IMAGE J (NIH, Bethesda, MD, USA).
Determination of COL1A1 mRNA
After treatment, total RNA of lung fibroblasts was extracted by using an RNeasy Mini kit from Qiagen. COL1A1 mRNA content was determined by quantitative real time RT-PCR. RNAs in 200 ng of each sample were reverse-transcripted. Real-time PCR was performed using ABI 7500 Sequence Detector (Perkin-Elmer Applied Biosystem, Foster City, CA, USA) with the conditions: 95 °C for 10 min, 40 cycles of 95 °C for 15 s and 60 °C for 1 min. All the primers and probes were purchased from Applied Biosystems. The results were expressed as 2−ΔΔCT using 18s rRNA as a reference.
Quantitation of collagen release
Collagen release from lung fibroblasts was quantitated by measuring collagen content in the culture medium using a Sircol collagen assay kit from Biocolor Ltd (Carrickfergus, UK), expressed as μg·h−1·mg−1 cellular protein.
Statistical analysis
In each experiment, experimental and control lung fibroblasts were matched for age, seeding density and number of passages to avoid variation in tissue culture factors that can influence measurements of cell proliferation, collagen and β-catenin. Results are shown as the mean ± SE for n experiments. Student’s paired t-test was used to determine the significance of differences between the means of experimental and control cells. P < 0.05 was considered statistically significant.
Supplementary Material
Fig. S1. Effect of α-defensin-1 and α-defensin-2 on cell proliferation of lung fibroblasts LL-86.
Fig. S2. Effect of α-defensin-1 and α-defensin-2 on protein contents of active/dephosphrylated β-catenin, total β-catenin and collagen-I and COL1A1 mRNA levels in lung fibroblasts LL-86.
Fig. S3. Effect of sFRP-1 on α-defensin-induced alterations in collagen-I protein contents.
Fig. S4. Effect of α-defensin-1 and α-defensin-2 on cyclin D protein contents in lung fibroblasts HFL-1.
Doc. S1. Results of supplemental experiments.
Acknowledgments
This work was supported by NIH grant R01HL088261, Flight Attendant Medical Research Institute grants 032040 and 072104, and American Heart Association Greater Southeast Affiliate grants 0555322B and 0855338E.
Abbreviations
- BrdU
5-bromo-2′-deoxy-uridine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GSK3β
glycogen synthase kinase 3β
- HNP
human neutrophil peptide
- IPF
idiopathic pulmonary fibrosis
- MAP
mitogen-activated protein
- PI3K
phosphoinositide 3-kinase
- sFRP-1
secreted frizzled-related-protein-1
- siRNA
small interfering RNA
- TCF/LEF-1
T cell factor/lymphocyte enhancer factor-1
References
- 1.Aarbiou J, Rabe KF, Hiemstra PS. Role of defensins in inflammatory lung disease. Ann Med. 2002;34:96–101. doi: 10.1080/07853890252953482. [DOI] [PubMed] [Google Scholar]
- 2.Cole AM, Waring AJ. The role of defensins in lung biology and therapy. Am J Respir Med. 2002;1:249–259. doi: 10.1007/BF03256616. [DOI] [PubMed] [Google Scholar]
- 3.Ross DJ, Cole AM, Yoshioka D, Park AK, Belperio JA, Laks H, Strieter RM, Lynch JP, III, Kubak B, Ardehali A, et al. Increased bronchoalveolar lavage human beta-defensin type 2 in bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2004;78:1222–1224. doi: 10.1097/01.tp.0000137265.18491.75. [DOI] [PubMed] [Google Scholar]
- 4.Hiratsuka T, Mukae H, Iiboshi H, Ashitani J, Nabeshima K, Minematsu T, Chino N, Ihi T, Kohno S, Nakazato M. Increased concentrations of human beta-defensins in plasma and bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis. Thorax. 2003;58:425–430. doi: 10.1136/thorax.58.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wencker M, Brantly ML. Cytotoxic concentrations of alpha-defensins in the lungs of individuals with alpha(1)-antitrypsin deficiency and moderate to severe lung disease. Cytokine. 2005;32:1–6. doi: 10.1016/j.cyto.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Ashitani J, Mukae H, Arimura Y, Sano A, Tokojima M, Nakazato M. High concentrations of alpha-defensins in plasma and bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. Life Sci. 2004;75:1123–1134. doi: 10.1016/j.lfs.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Chen CI, Schaller-Bals S, Paul KP, Wahn U, Bals R. Beta-defensins and LL-37 in bronchoalveolar lavage fluid of patients with cystic fibrosis. J Cyst Fibros. 2004;3:45–50. doi: 10.1016/j.jcf.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Mukae H, Iiboshi H, Nakazato M, Hiratsuka T, Tokojima M, Abe K, Ashitani J, Kadota J, Matsukura S, Kohno S. Raised plasma concentrations of alpha-defensins in patients with idiopathic pulmonary fibrosis. Thorax. 2002;57:623–628. doi: 10.1136/thorax.57.7.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keane MP, Strieter RM, Lynch JP, III, Belperio JA. Inflammation and angiogenesis in fibrotic lung disease. Semin Respir Crit Care Med. 2006;27:589–599. doi: 10.1055/s-2006-957331. [DOI] [PubMed] [Google Scholar]
- 10.Oono T, Shirafuji Y, Huh WK, Akiyama H, Iwatsuki K. Effects of human neutrophil peptide-1 on the expression of interstitial collagenase and type I collagen in human dermal fibroblasts. Arch Dermatol Res. 2002;294:185–189. doi: 10.1007/s00403-002-0310-6. [DOI] [PubMed] [Google Scholar]
- 11.Van Wetering S, Tjabringa GS, Hiemstra PS. Interactions between neutrophil-derived antimicrobial peptides and airway epithelial cells. J Leukoc Biol. 2005;77:444–450. doi: 10.1189/jlb.0604367. [DOI] [PubMed] [Google Scholar]
- 12.Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- 13.Eberhart CG, Argani P. Wnt signaling in human development: beta-catenin nuclear translocation in fetal lung, kidney, placenta, capillaries, adrenal, and cartilage. Pediatr Dev Pathol. 2001;4:351–357. doi: 10.1007/s10024001-0037-y. [DOI] [PubMed] [Google Scholar]
- 14.Biswas P, Canosa S, Schoenfeld J, Schoenfeld D, Tucker A, Madri JA. PECAM-1 promotes beta-catenin accumulation and stimulates endothelial cell proliferation. Biochem Biophys Res Commun. 2003;303:212–218. doi: 10.1016/s0006-291x(03)00313-9. [DOI] [PubMed] [Google Scholar]
- 15.Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, Rehan VK. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-β and Wnt signaling, protection by rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1031–L1041. doi: 10.1152/ajplung.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS ONE. 2008;3:e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thornton TM, Pedraza-Alva G, Deng B, Wood CD, Aronshtam A, Clements JL, Sabio G, Davis RJ, Matthews DE, Doble B, et al. Phosphorylation by p38 MAPK as an alternative pathway for GSK3beta inactivation. Science. 2008;320:667–670. doi: 10.1126/science.1156037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 19.Spencer LT, Paone G, Krein PM, Rouhani FN, Rivera-Nieves J, Brantly ML. Role of human neutrophil peptides in lung inflammation associated with alpha1-antitrypsin deficiency. Am J Physiol Lung Cell Mol Physiol. 2004;286:L514–L520. doi: 10.1152/ajplung.00099.2003. [DOI] [PubMed] [Google Scholar]
- 20.Yoshioka S, Mukae H, Ishii H, Kakugawa T, Ishimoto H, Sakamoto N, Fujii T, Urata Y, Kondo T, Kubota H, et al. Alpha-defensin enhances expression of HSP47 and collagen-1 in human lung fibroblasts. Life Sci. 2007;80:1839–1845. doi: 10.1016/j.lfs.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Aarbiou J, Ertmann M, Van Wetering S, van Noort P, Rook D, Rabe KF, Litvinov SV, Van Krieken JH, De Boer WI, Hiemstra PS. Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J Leukoc Biol. 2002;72:167–174. [PubMed] [Google Scholar]
- 22.Park CH, Chang JY, Hahm ER, Park S, Kim HK, Yang CH. Quercetin, a potent inhibitor against beta-catenin/Tcf signaling in SW480 colon cancer cells. Biochem Biophys Res Commun. 2005;328:227–234. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- 23.Roman-Gomez J, Cordeu L, Agirre X, Jimenez-Velasco A, San Jose-Eneriz E, Garate L, Calasanz MJ, Heiniger A, Torres A, Prosper F. Epigenetic regulation of Wnt-signaling pathway in acute lymphoblastic leukemia. Blood. 2007;109:3462–3469. doi: 10.1182/blood-2006-09-047043. [DOI] [PubMed] [Google Scholar]
- 24.Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, Jeong WI, Ryu SY, Do SH, Lee CS, et al. Quercetin suppresses proinflammatory cytokines production through MAP kinases and NF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol Cell Biochem. 2003;243:153–160. doi: 10.1023/a:1021624520740. [DOI] [PubMed] [Google Scholar]
- 25.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 26.Moon RT. Wnt/beta-catenin pathway. Sci STKE. 2005;2005 cm1. [Google Scholar]
- 27.Syeda F, Liu HY, Tullis E, Liu M, Slutsky AS, Zhang H. Differential signaling mechanisms of HNP-induced IL-8 production in human lung epithelial cells and monocytes. J Cell Physiol. 2008;214:820–827. doi: 10.1002/jcp.21279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shu W, Jiang YQ, Lu MM, Morrisey EE. Wnt7b regulates mesenchymal proliferation and vascular development in the lung. Development. 2002;129:4831–4842. doi: 10.1242/dev.129.20.4831. [DOI] [PubMed] [Google Scholar]
- 29.Dasgupta C, Sakurai R, Wang Y, Guo P, Ambalavanan N, Torday JS, Rehan VK. Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling, protection by rosiglitazone. Am J Physiol Lung Cell Mol Physiol. 2009;296:L1031–L1041. doi: 10.1152/ajplung.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, et al. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gurung A, Uddin F, Hill RP, Ferguson PC, Alman BA. Beta-catenin is a mediator of the response of fibroblasts to irradiation. Am J Pathol. 2009;174:248–255. doi: 10.2353/ajpath.2009.080576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effect of α-defensin-1 and α-defensin-2 on cell proliferation of lung fibroblasts LL-86.
Fig. S2. Effect of α-defensin-1 and α-defensin-2 on protein contents of active/dephosphrylated β-catenin, total β-catenin and collagen-I and COL1A1 mRNA levels in lung fibroblasts LL-86.
Fig. S3. Effect of sFRP-1 on α-defensin-induced alterations in collagen-I protein contents.
Fig. S4. Effect of α-defensin-1 and α-defensin-2 on cyclin D protein contents in lung fibroblasts HFL-1.
Doc. S1. Results of supplemental experiments.