Abstract
The development of the hypothalamic paraventricular nucleus (PVN) involves several factors that work together to establish a cell group that regulates neuroendocrine functions and behaviors. A number of molecular markers were noted within the developing PVN, including estrogen receptors (ER), neuronal nitric oxide synthase (nNOS) and brain derived neurotrophic factor (BDNF). By contrast, immunoreactive gamma-aminobutyric acid (GABA) was found in cells and fibers surrounding the PVN. Two animal models were used to test the hypothesis that GABA works through GABAA and GABAB receptors to influence the development of the PVN. Treatment with bicuculline to decrease GABAA receptor signaling from embryonic day (E)10 to 17 resulted in fewer cells containing immunoreactive (ir)-ERα in the region of the PVN versus control. GABABR1 receptor subunit knockout mice were used to examine the PVN at P0 without GABAB signaling. In female but not male GABABR1 subunit knockout mice, the positions of cells containing ir-ERα shifted from medial to lateral compared to wildtype controls, while the total number of ir-ERα containing cells was unchanged. In E17 knockout mice, ir-nNOS cells and fibers were spread over a greater area. There was also a significant decrease in ir-BDNF in the knockout mice in a region dependent manner. Changes in cell position and protein expression subsequent to disruption of GABA signaling may be due, in part, to changes in nNOS and BDNF signaling. Based on the current study, the PVN can be added as another site where GABA exerts morphogenetic actions in development.
Keywords: cell migration, cell differentiation, development, estrogen receptor α, GABAB receptor, GABAA receptor, BDNF, nitric oxide
Introduction
The paraventricular nucleus of the hypothalamus (PVN) lies at the dorsal limit of the classical hypothalamus at the base of the diencephalon. It has been implicated in a broad array of homeostatic and behavioral functions ranging from neuroendocrine and cardiovascular control to affective, ingestive, and defensive behaviors (Herman et al., 2005; Swanson and Sawchenko, 1983). Numerous peptides, neurotransmitters (e.g., corticotropin releasing hormone (CRH), arginine vasopressin (AVP), oxytocin (OT)); (Armstrong et al., 1980; Ford-Holevinski et al., 1991; Swanson and Sawchenko, 1983), and other proteins including calbindin (Brager et al., 2000) and neuronal nitric oxide synthase (nNOS) (Bernstein et al., 1998) characterize the chemoarchitecture of the PVN. Steroid hormone receptors are also among the markers located in cells within and around the PVN. Thus, populations of cells that contain immunoreactive estrogen receptors-α (ir-ERα), ERβ, glucocorticoid (GR) and androgen (AR) receptors characterize select regions inside and surrounding the PVN (Mitra et al., 2003; Simerly et al., 1990; Suzuki and Handa, 2004).
The development of the PVN has been studied using Nissl stains, neuronal birthdating, and the identification of cell phenotypes at early ages. Based on Nissl staining, the PVN is first visible as a cell group between embryonic day (E)15 and E17 in mice (Karim and Sloper, 1980; Shimada and Nakamura, 1973). A number of different cell phenotypes that delineate regions within the PVN are expressed early in development. Estrogen and glucocorticoid receptors are among those found at embryonic ages (Owen and Matthews, 2003; Tobe et al., 2005). Neuroactive peptides such as OT and AVP (Okamura et al., 1983), transcription factors, neurotrophic factors (e.g., BDNF; (Fujioka et al., 2003), and neurotransmitters also are expressed by PVN neurons early in development (Michaud et al., 1998; Xu and Fan, 2007). These cells are derived from precursors along the third ventricle with distinct timelines; more lateral (magnocellular) cells are the first to undergo their final mitotic division and move away from the third ventricle, between E10.5 and E12.5, in the developing mouse (Karim and Sloper, 1980; Okamura et al., 1983). Cells that occupy the more medial (parvocellular) region of the PVN are generated later.
Differentiation of the PVN requires the expression of particular transcription factors, including Sim1 (Michaud et al., 1998), Arnt2 (Michaud et al., 2000), Otp (Acampora et al., 1999; Wang and Lufkin, 2000), Brn2 and Nkx2.2 (Caqueret et al., 2006). In the absence of Sim1, there was a decrease in OT and AVP expression in the PVN and the supraoptic nucleus suggesting that Sim1 is required for terminal cell differentiation of these cell types in both the PVN and SON (Michaud et al., 1998). More recently it has been suggested that Sim1 expression may also be important for cell migration within the region of the PVN (Xu and Fan, 2007). Cell proliferation as indicated by bromodeoxyuridine incorporation and cell death as indicated by TUNEL positive cells were similar in Sim1 mutants compared to wildtype mice, however, the distribution of Sim1 presumptive cells in the mutant showed an altered migratory phenotype that may be mediated through the direct regulation of PlexinC1 (a receptor known to be involved in migration and axon guidance) by Sim1 (Xu and Fan, 2007).
Migration at the cellular level is mediated by molecular communication between cells often from small molecules or polypeptides released to diffuse through extracellular space. Several neurotransmitters/neuropeptides including, GABA, serotonin, dopamine, and endogenous opiates have been suggested to act as neurotrophic factors or morphogens in various brain regions (Lauder, 1993; Nguyen et al., 2001). GABA has been shown to influence cell movements within one hypothalamic nucleus, the ventromedial nucleus (VMN), through GABAA (Dellovade et al., 2001) and GABAB (Davis et al., 2002; McClellan et al., 2008) receptors. The distribution pattern of GABAergic cells and fibers surrounding the region of the developing VMN provides potential boundary information for influencing cells moving into the ventrolateral region of the nucleus (McClellan et al., 2006). The current study examined the relationship of GABAergic cells and fibers to the developing PVN, which they surround to determine if similar roles might be in play.
To test the hypothesis that GABA plays a role in PVN development, experiments were conducted to address potential roles for both GABAA and GABAB receptors. To determine the potential role of GABA acting through GABAA receptors, pregnant mice were administered bicuculline injections to examine cell positions and immunoreactive ERα in the PVN region of fetal brains. To determine the potential role of GABA acting through GABAB receptors, the distribution of specific cell types was examined in the PVN of GABABR1 receptor knockout mice that we had previously analyzed for VMN development (McClellan et al., 2008). Immunoreactive ERα, nNOS, and BDNF were three proteins of interest examined for location within and outside of the developing PVN (Tobet et al., 2009). ERα was found in cells of the dorsal and medial region of the PVN and also in a population of cells lateral to the PVN (lateral hypothalamus/ perifornical region). The location of immunoreactive ERα cells has previously been shown to be a useful marker for identifying alterations in cell position in other regions of the developing hypothalamus (Tobet et al., 2002). GABAB receptor knockout mice showed a change in the location of ERα cells within the VMN, and the population of immunoreactive ERα cells found just lateral to the PVN represents a group of cells that likely migrate through the PVN into a dense region of GABAergic fibers.
Interest in immunoreactive nNOS derives from its role in catalyzing the synthesis of nitric oxide (NO) and because it is located in specific regions within the embryonic and adult PVN. Nitric oxide released in the PVN plays a role in the neuroendocrine stress response as well as cardiovascular responses (Orlando et al., 2008b). It also has roles influencing migration, proliferation, and differentiation within the developing brain (Bicker, 2005; Peunova and Enikolopov, 1995; Peunova et al., 2001). We examined the position of nNOS immunoreactive cells and fibers in the PVN of GABAB receptor knockout and wildtype animals to determine if GABAB receptors may play a role in the positioning of these cells during development.
BDNF is found early in development within the PVN and is a key neurotrophic factor controlling diverse brain functions in development and in adulthood. BDNF is a member of the neurotrophin family of proteins with activity mediated by binding to a specific receptor tyrosine kinase (TrkB) as well as the non-selective receptor p75LNTR (Tapia-Arancibia et al., 2004). BDNF has been shown to be an important molecule in the development and differentiation of the central and peripheral nervous systems, including an influence on neuronal migration (Borghesani et al., 2002; Zhou et al., 2007). The PVN contains high levels of BDNF and TrkB mRNA and these overlap substantially with the distribution of AVP and CRH (Givalois et al., 2004). In prenatal hypothalamic neurons, BDNF influences the release of GABA, while in turn, GABA stimulates BDNF expression (Obrietan et al., 2002). In the current study, we show that GABAergic elements surround the developing PVN and that GABAB receptors may play a role in determining the positions of cells containing immunoreactive ERα and nNOS. We also show that GABABR1 subunit knockouts exhibit a selective decrease in immunoreactive BDNF in the PVN leading to the hypothesis that GABA and BDNF may act synergistically to determine aspects of PVN development.
Materials and Methods
Animals
Mice used in this study were all from lines generated on a C57BL/6J background. The majority of the experiments used a transgenic line of mice lacking functional GABAB receptors. Mice with disruption of GABAB receptor signaling were generated on a C57BL/6 background through the insertion of a gene encoding β-galactosidase in the coding region of the R1 subunit of the GABAB receptor (Prosser et al., 2001). Heterozygous breeding pairs were used to generate homozygous null, heterozygous, and wild type animals to be used for Nissl staining and immunohistochemistry. Animals were mated overnight and checked for vaginal plugs the following morning. The day of plug was designated as E0. Pups were transcardially perfused on either day E15, E17 or postnatal day (P)0. Pregnant mice were anesthetized with a combination of ketamine (80mg/kg) and xylazine (8mg/kg) and embryos were removed individually before being perfused with 2mL (E15 and E17) or 5mL (P0) 4% paraformaldehyde in 0.1M phosphate buffer using a hand held 10ml syringe. Body weight and/ or crown rump length measurements were taken to verify ages (E15, CRL= 14-15mm; E17, BW= 0.7-0.95g, CRL= 17-18mm). Sex determination was made through direct inspection of the gonads or PCR analysis for the Y-chromosome sry gene. Brains were post-fixed in 4% paraformaldehyde overnight and were placed in 0.1M phosphate buffer and stored at 4°C until tissue sectioning. To generate embryos with decreased GABAA receptor activation during the time of PVN development, pregnant dams were injected (s.c.) with 1mg/kg (Bless et al., 2000; Nguyen-Ba-Charvet et al., 2004) bicuculline methobromide (B-4013, Sigma, St. Louis, MO) in 100μl water twice per day from the evening of E10 thru the morning of E17 (only one injection on E10 and E17). Vehicle treatment was with 100μl water alone. Embryos were transcardially perfusion fixed as described above on the afternoon of E17. All experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Colorado State University Animal Care and Use Committee.
Genotyping
Genotyping of tail DNA was done as described previously (McClellan et al., 2008). Mice were genotyped for the GABABR1 knockout allele and the sry gene using a standard Taq polymerase PCR kit (Qiagen, Valencia, CA).
Nissl staining
Brain tissue collected from pups at ages E15, E17, and P0 were embedded in 5% agarose and cut into 50μm thick coronal sections using a vibrating microtome (VT1000S; Leica Microsystems, Wetzlar, Germany). Tissue sections were mounted onto glass slides pre-coated with gelatin and left to dry at room temperature overnight. Sections were rinsed in 50% ethanol, rehydrated in distilled water, and stained with a 0.1% thionin solution. Sections were rinsed in 70% ethanol with glacial acetic acid for color differentiation. Sections were then dehydrated in a graded series of ethanol washes, placed in xylene, and then coverslipped using Permount (Fisher Scientific, Waltham, MA). Selected sections were processed for immunohistochemistry and counterstained following the same Nissl procedure described here but were first rehydrated in a graded series of ethanol washes and distilled water after soaking off coverslips in xylene.
Immunohistochemistry
Brain tissue collected from pups at ages E15, E17, and P0 were embedded in 5% agarose and cut into 50μm or 60μm thick coronal sections using a vibrating microtome (Leica VT1000S). Alternating sections were collected in 0.05M phosphate buffered saline (PBS), pH 7.5. Excess unreacted aldehyde was neutralized using a 30-minute incubation in 0.1M glycine and 15 minutes in 0.5% sodium borohydride in PBS. After washing the tissue sections in PBS the sections were incubated in a PBS blocking solution for at least 30 minutes at 4°C containing 5% normal goat serum, 0.3% Triton-X 100 (Tx) and 1% hydrogen peroxide. Following the blocking step, the tissue was incubated in primary antisera containing 1%BSA and 0.3%Tx. Primary antibodies used (Table 1) were: ERα (1:5000; C1355, Upstate Biotechnology, Charlottesville, VA), BDNF (1:200; sc-546, N-20, Santa Cruz Biotechnology, Santa Cruz, CA), CRH (1:25,000; generously provided by Dr. W. Vale), vasopressin (1:10,000; Immunostar, Hudson, WI), ERβ (1:500; early lot number 10967002 of Z8P, Zymed Laboratories, San Francisco, CA), nNOS (1:10,000; Immunostar), calbindin (1:5000; AB1778, Millipore, Billerica, MA and 1:40,000; D-28K, Sigma-Aldrich, St. Louis, MO), galanin (1:8000; Millipore (Brown et al., 1999), GABA (1:500; Immunostar), GAD67 (1:5000; Chemicon), NPY (1:8000; Immunostar), GABAA receptor subunits γ2 (1μg/ml; generously provided by Dr. W. Sieghart) and α1 (1:1000; Phosphosolutions, Inc.). Tissue sections were incubated over 2 nights at 4°C with primary antisera. Sections were washed at room temperature in PBS containing 1% normal goat serum and 0.02% Tx. Sections were incubated at room temperature in secondary antisera buffer containing 1% normal goat serum and 0.32% Tx with a biotin conjugated anti-rabbit secondary diluted to 1:2500 (Rabbit IgG-fab fragment; Jackson Immunoresearch, West Grove, PA). Sections were developed using a Vectastain ABC Elite kit (Vector Laboratories; Burlingame, CA) for 1 hour at room temperature and visualized with a 5 minute incubation in a solution containing 0.025% diaminobenzidine with 0.02% nickel and 0.02%H2O2 diluted in tris-buffered saline (pH 7.5). Pre-incubation with peptide served as an antibody control for BDNF (5μg peptide: 1μg antiserum for 1 hour at room temperature, data not shown), while similarity of immunoreactivity among multiple antisera served for others (e.g., calbindin, nNOS, and comparison of GABA to GAD; see below and Table 1), similarity to literature reports for others (e.g., AVP, OT, galanin), as well as omission of primary antisera that resulted in non-detectable reaction product.
Table 1.
List of antibodies used in experiments. This list includes antibodies used for tissue analysis as well as those used to verify specificity.
| Primary Antibody | Catalog Number | Lot Number | Species | Immunogen | Company |
|---|---|---|---|---|---|
| ERα (C1355) | 06-935 | JBC1371375 | Rabbit | TYYIPPEAE GFPNTI |
Millipore |
| BDNF (N-20) | sc546 | A0207 | Rabbit | human BDNF aa128-147 RHSDPARRGELSV CDSISEW GI - P23560 |
Santa Cruz Biotechnology |
| ERβ (Z8P) | 51-7900 | 10967002 | Rabbit | mouse ERβ aa 468-485 CSTEDSKSKEGS QNLQSQ |
Zymed Laboratories |
| CRH | N/A | N/A | Rabbit | rat hypothalamic CRH | Provided by Dr. Wylie Vale |
| vasopressin | AB1565 | 17090354 | Rabbit | whole arginine vasopressin conjugated to thyroglobulin | Millipore |
| nNOS | 24287 | 436002 | Rabbit | human C-terminal peptide aa 1419-1433 |
Immunostar |
| calbindin | AB1778 | LV1378360 | Rabbit | recombinant mouse calbindin | Millipore |
| calbindin | C9848 | 117K4757 | Mouse monoclonal | bovine kidney calbindin-D | Sigma-Aldrich |
| GABA | 20094 | 517211 | Rabbit | GABA coupled to BSA | Immunostar |
| GAD 67 | AB5992 | unknown | Rabbit | Recombinant feline GAD67 | Chemicon |
| GABABR1 (GP311) | N/A | N/A | Guinea Pig | RQQLRSRRHPPT PPDPSGGLPRGP SE |
Provided by Dr. Margeta-Mitrovic |
| NPY | 22940 | 550212 | Rabbit | synthetic porcine NPY conjugated to BSA | Immunostar |
| galanin | AB1985 | 18010442 | Rabbit | human galanin | Millipore |
Antibody Characterization
Specific information on company, lot number and immunogen can be found in table 1. Below is additional evidence of antigen specificity for antisera and antibodies used in this paper produced in our lab or taken from the literature.
ERα (C1355)
This antiserum recognizes a 66kDa band on Western blot using in vitro translated ERα, rat pituitary and uterine lysates as well as lysates from COS-1 cells transfected with rat ERα but not in untransfected COS-1 cells and does not cross react with ERβ (data provided by supplier; Millipore; Friend et al. 1997). In addition, others have shown that preadsorption with ERα peptide eliminated immunoreactivity while preadsorption with ERβ did not change immunoreactivity in rat CNS (Papka et al., 2001).
BDNF (N-20)
The pattern of immunoreactivity seen with this antiserum closely matches the pattern of reactivity seen by in situ hybridization studies with a riboprobe recognizing BDNF mRNA. In addition, when preadsorbed overnight at 4°C with 5 μg of blocking peptide provided by Santa Cruz (sc-546 P) in 1 ml primary antibody buffer with 1 μg BDNF (N-20) antiserum, immunoreactivity was eliminated.
ERβ (Z8P; lot number 10967002)
Others have shown that this antiserum recognizes in vitro translated rat ERβ but not ERα and an ERβ-like 60-kDa protein from rat granulosa cells and ovary extracts on Western blots, and that the immunoreactivity pattern of this antiserum overlaps the mRNA pattern of ERβ by in situ hybridization (multiple regions in rat CNS) (Shughrue and Merchenthaler, 2001). We have further determined that immunoreactivity was absent in formaldehyde fixed brain tissue from ERβ knockout mice (generously provided by P. Bonthuis and Dr. E.F. Rissman, University of Virginia School of Medicine).
CRH
On dot blot there was no cross-reactivity with the peptides, melanin concentrating hormone or αMSH while CRH peptide was strongly recognized (Van Bockstaele et al., 1996). In addition others have shown that immunolabeling in rat was eliminated by preadsorption with synthetic CRH (Sigma C-3042) (Tagliaferro et al., 2008). The pattern of CRH immunoreactivity in the PVN for the current experiments is consistent with prior results in mice (e.g., Keegan et al., 1994; Alon et al., 2009).
Vasopressin
The manufacturer has shown that this antiserum does not cross react with oxytocin peptide in western blots (data provided by supplier; Millipore). Previous studies have also shown that preadsorption with synthetic vasopressin peptide (10μM) resulted in a complete loss of immunolabeling (Das et al., 2007). The pattern of vasopressin immunoreactivity in the PVN for the current experiments is consistent with prior results in mice and rats (e.g., Vacher et al., 2002; Samponpum and Sladek, 2003).
nNOS
We have found no specific immunoreactivity using a C-terminal directed antiserum (Immunostar 24287) in nNOS knockout mice (exon 6 deletion generously provided by P. Huang, Massachusetts General Hospital and Harvard Medical School; Gyurko et al., 2002). Use of an N-terminal specific nNOS antiserum (Zymed, cat 61-7000), produced a similar pattern of immunoreactivity in wild type mice and also failed to detect immunoreactive protein in the knockout (as also reported in Gyurko et al., 2002).
Calbindin
Two calbindin immunoreagents have been used, one rabbit polyclonal antiserum (AB1778; Millipore) and one mouse monoclonal antibody (C9848; Sigma-Aldrich). The pattern of immunoreactivity was similar between the two immunoreagents and matched that seen in our previous studies of embryonic mouse hypothalamus (Edelmann et al., 2007). AB1778 has a specific reaction product of 28kD on Western blots as stated by the manufacturer and seen in our own studies (adult mouse hypothalamic homogenate). Others have shown a lack of immunoreactivity in mouse (C9848) following preadsorption with 100μM calbindin peptide (Huynh et al., 2000).
GABA
The specificity of the antiserum was evaluated using competitive inhibition enzyme-linked immunosorbent assay. Preadsorption of this antiserum with conjugates of GABA completely eliminate labeling, while preadsoprtion with other conjugates (glutamate, aspartate, beta alanine, tyrosine, taurine, glycine, alanine) did not inhibit the antiserum’s ability to bind GABA (data provided by supplier; Immunostar). There is notable similarity of immunoreactivity for GABA and its synthetic enzyme GAD67 in fetal mouse hypothalamus (Tobet et al., 1999).
GAD67
This antiserum preferentially recognized GAD67 over GAD65 on Western blots of recombinant proteins and rat brain homogenate illustrated by a band at 67kDa (data provided by supplier; Chemicon). The immunoreactivity pattern was similar to mRNA by in situ hybridization (multiple regions in rat CNS) and preadsorption with bacterially produced rat GAD65 did not change the immunoreactivity pattern in rat brain while preadsorption with bacterially produced rat GAD67 blocked all specific immunoreactivity (Esclapez et al., 1994).
GABABR1
Others have verified specific immunoreactivity by preabsorption with an excess of immunogen peptide (Table 1) that led to a complete loss of immunoreactivity (Margeta-Mitrovic et al., 1999; Belenky et al., 2008).
NPY
Immunoreactivity was blocked with preabsorption of excess NPY but not with peptide YY, avian pancreatic polypeptide, b-endorphin, vasoactive intestinal peptide, cholecystokinin, or somatostatin (data provided by supplier; Immunostar). The pattern of NPY immunoreactivity for the current experiments was consistent with prior results in the arcuate and PVN in mice and rats (e.g., Tobet et al., 1999; Dellovade et al., 2000; Legradi and Lechan, 1998).
Galanin
Preadsorption with excess porcine-galanin (H-6580, Bachem) resulted in loss of specific immunoreactivity in the region of the PVN in chick brains (Klein et al., 2006). The pattern of galanin immunoreactivity for the current experiments was consistent with prior results in the dorsal hypothalamic area in mice and rats (e.g., Brown et al., 1999; Wittman et al., 2004).
Analysis
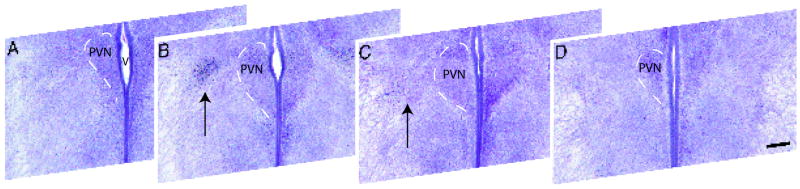
Images of the rostral PVN were taken at 10x magnification on an Olympus BH2 microscope with an Insight QE digital camera using Spot Advanced Software. Images were normalized for optimal contrast using Adobe Photoshop software (version CS for Macintosh). For the analysis of cells containing ir-ERα, images were opened in IP Lab Imaging software (Scanalytics Inc. part of BD Biosciences, Rockville, MD) and grids, which include columns (100μm wide) and rows (100μm tall) were placed over the images with the boundaries being the edge of the third ventricle and the dorsal boundary of the PVN. Four tissue sections from each embryonic brain contained a part of the PVN that was subdivided from rostral to caudal (Fig. 1; A-D). The sections were categorized based on the rostral / caudal location and angle of cut. Only those sections containing ERα immunoreactivity in a cell grouping lateral to the PVN and within the PVN were included in the analysis (Fig. 1; B). The total area of immunoreactivity was measured for each column using IP Lab Imaging software. Totals were also calculated for overall immunoreactivity summing all the columns. In addition, to validate the area analysis and for the analysis of Bicuculline treated animals, cell counts were taken for sections of the PVN that contained ERα immunoreactivity in the region just lateral to the PVN. Cells containing ir-ERα were counted and designated as being within the region of the PVN or part of the cell group located just lateral to the PVN. The number of cells inside and outside the PVN was totaled for the left and right sides of the brain and averaged for each section. To account for possible over estimation of cell numbers, Abercrombie’s formula was used to correct all cell counts, T/(T+h) where T=section thickness and h=mean object diameter (ir-ERα cell nucleus) (Guillery 2002). For P0 sections (GABAB KO vs WT), the formula was 60μm/(60μm+6.4μm) and for E17 sections (Bicuculline vs Vehicle), the formula was 50μm/(50μm+5.6μm) resulting in a correction factor of 0.9 in both instances.
Figure 1.
Digital images show 50μm thick coronal sections taken in the region of the PVN at E17. Sections contain immunoreactive ERα and were counterstained for Nissl substance to delineate the boundaries of the paraventricular nucleus (dotted line). This series of sections shows the rostral (A) to caudal (D) extent of the PVN. All sections used for analysis came from the central region of the PVN (B, C). PVN= paraventricular nucleus, V= third ventricle, scale bars= 100μm.
Immunoreactive nNOS was quantified using the grid method as described above with the exception of using smaller 50μm squares in the grid. The central section of the PVN at E17 was taken for analysis and tissue sections were matched for angle of cut. The total area of immunoreactivity and the immunoreactivity for each 50μm × 50μm square was taken for comparison. The immunoreactive nNOS was found in both cell bodies and fibers, which are combined in the data analysis. Males and females were combined for data analysis as the results were similar between the sexes.
For BDNF analysis, images were normalized to ensure that the pixel intensities were spread across the dynamic range, and then the darkest one third of the pixels were segmented for quantification of the area occupied by immunopositive cells (Davis et al., 2004). Tissue sections chosen for BDNF analysis were in the central region of the PVN, similar to the region found in Fig. 1 B,C. The area of immunoreactive cells was measured in each column. Totals were calculated only for boxes that were within the region of the PVN and those boxes that were outside of the PVN were not included in the analysis. Column analysis was used to determine changes in location within the PVN. Males and females were combined for data analysis as the results were similar between the sexes.
For ir-ERα, statistical significance was determined by ANOVA for genotype × location (column as a repeated measure) or in the cases where cell counts were used by ANOVA for genotype (KO vs WT) or treatment (Bicuculline vs vehicle) × location (inside or outside the PVN as a repeated measure) using SPSS software (SPSS Inc., Chicago, IL). For ir-BDNF, statistical significance was determined by ANOVA for genotype × location (column as a repeated measure). For ir-nNOS, statistical significance was determined by ANOVA for genotype.
Digital images for figures were acquired as noted above for analysis. Image adjustments were made using Adobe Photoshop software (version CS for Macintosh). Images in figure 1 were converted to a composite figure using Adobe Illustrator (version CS for Macintosh). All other images were converted to grayscale and sizes were adjusted for the appropriate resolution to create the final composite figures. Images were enhanced for contrast by adjusting levels and using the unsharp mask tool to improve clarity.
Results
PVN cell phenotypes at E15
Several immunohistochemical characteristics selectively delineated the developing PVN based on cytoarchitecture, prior to its emergence and lateral extension. Thus, many different cell phenotypes have already established positions within the developing nucleus. Figure 2 shows the distribution of a subset of immunoreactive markers that have differentiated by E15 in the PVN. These include cells containing CRH (Fig. 2A), vasopressin (2B), calbindin (2C), nNOS (2D), ERβ (2E), Erα (2F), galanin (2G), and NPY (2H). Based on the distribution of these various cell types it is clear that aspects of the cellular architecture are established at young ages, however, the size of the nucleus and the number of cells occupying the PVN increases with further development. Subnuclear compartments at the early ages would be difficult to assign. Of the molecular markers examined, immunoreactive NPY was the only one not intrinsic to cells of the PVN. Unfortunately, cell bodies of origin were not discernible. Of the remaining molecular markers for neurons intrinsic to the PVN, none had immunoreactive processes extending much beyond the nucleus through P0.
Figure 2.
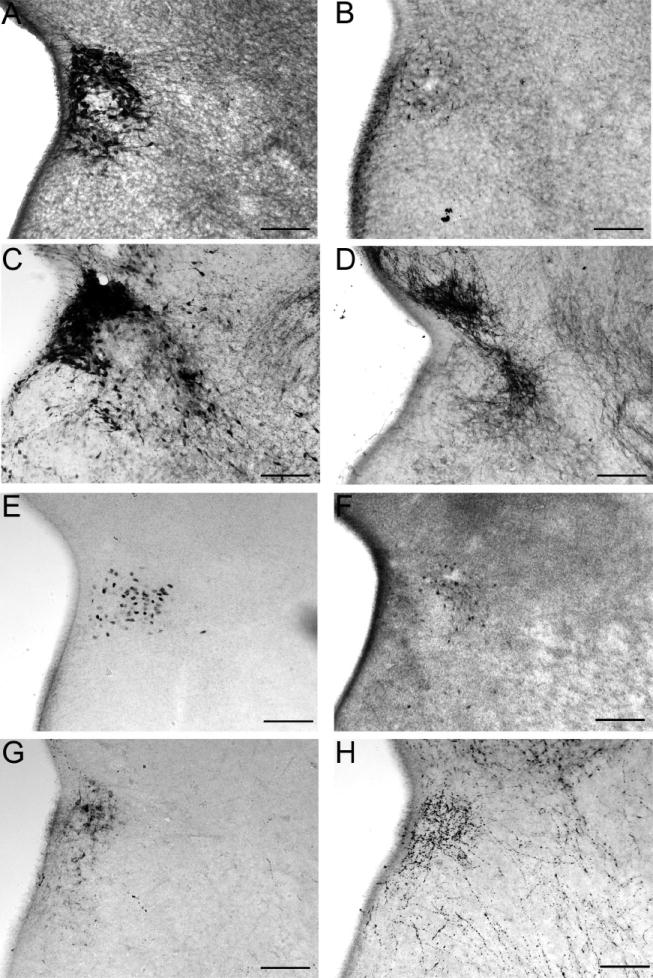
Digital images show several immunoreactive peptides, receptors, and signaling molecules that populate the paraventricular nucleus (PVN) by embryonic day 15. Corticotropin releasing hormone (A), vasopressin (B), calbindin (C), neuronal nitric oxide synthase (D), estrogen receptor (ER)β (E), ERα (F), galanin (G), and neuropeptide Y (H) delineated distinct populations of cells in the PVN at early ages (scale bars = 50μm). The third ventricle is the white region on the left of each image. As the PVN developed, becoming larger in volume, the numbers of cells expressing these markers increased but with similar patterns of distribution.
The relationship of GABA to the PVN
Cell bodies and fibers containing immunoreactive GABA or GAD67 surrounded the developing PVN as early as E13. This is relatively unique to two regions within the developing hypothalamus - the PVN and the VMN. The progression from E13 (Fig. 3A), to E15 (Fig. 3B), to E17 (Fig. 3C), shows the dense populations of GABAergic cells and fibers that surround the PVN. By adulthood, GABAergic fibers completely fill the nucleus (data not shown).
Figure 3.
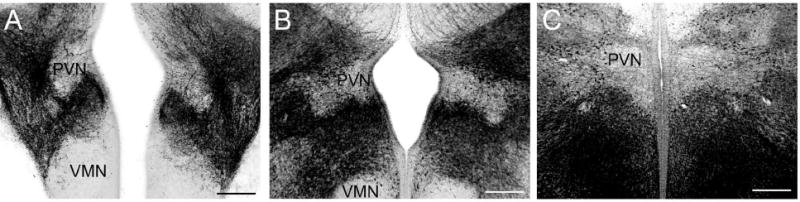
Digital images show immunoreactive GAD 67 (A) or GABA (B-C) surrounded the region of the developing paraventricular nucleus of the hypothalamus (PVN) at embryonic ages. At E13 (A), E15 (B), and E17 (C) in the mouse, the PVN region is one of two hypothalamic regions where GABA is found surrounding the nucleus in development. The ventromedial nucleus of the hypothalamus (VMN), the other nuclear group where this has been found, is shown within the images of panels A and B. By adulthood GABAergic fibers have moved into the region of the PVN and VMN (data not shown). At the center of each image is the third ventricle (scale bars = 100μm).
GABA signaling through the GABAA receptor influences the number of ir-ERα cells
Pregnant mice were given daily bicuculline injections starting at E10 and embryos were perfused on E17. Sections taken for analysis correspond to those in Fig. 1B and 1C and are the only two sections that contain the lateral population of ir-ERα cells. Cell counts were taken for ir-ERα within the PVN and outside of the PVN on each side of the brain across two sections. Cell counts were combined from both sides of the brain and sections were analyzed as a two-way ANOVA for treatment by location as a repeated measure. There was a significant interaction between subjects in location by treatment (F(1,4) = 50.1, p < 0.01). Bicuculline treated pups had a 30% decrease in the total number of ir-ERα cells compared to the control (Fig. 4). While the decrease in ir-ERα cells was evident in cells just lateral to the PVN, there was a greater effect (more than a 54% decrease with bicuculline treatment) seen within the PVN.
Figure 4.
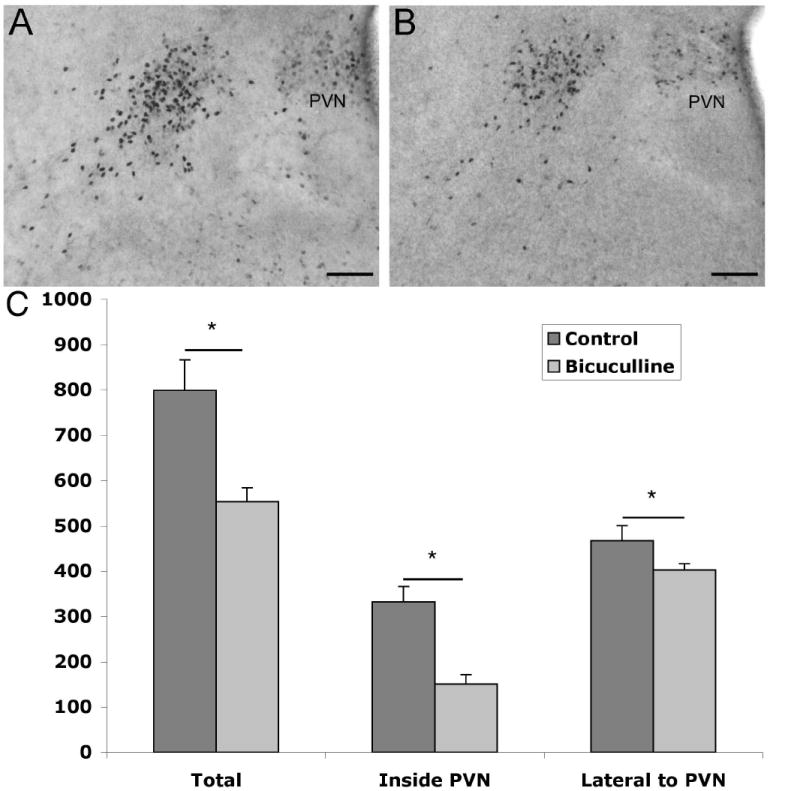
Digital images show ERα immunoreactivity at E17 in female mice exposed to bicuculline between days E10 and E17. Bicuculline treatment (panel B, n=3) as compared to saline controls (panel A, n=3) caused about a 30% decrease in total ERα immunoreactivity (C). Immunoreactive ERα levels were lower both within the paraventricular nucleus (PVN; third ventricle is to the right in each image) and outside following 1mg/kg bicuculline treatment. There was a significant interaction between subjects in location by treatment (p < 0.01; scale bars =100μm).
To determine if the lateral cell group containing ir-ERα was truly inside or outside of the boundaries of the PVN, sections processed for ir-ERα at E15 and E17 (Fig. 5A,B) were counterstained for Nissl substance to highlight the cytoarchitecture. This cell group appears to be distant from the ventricle as early in development as E15 (Fig. 5A), more lateral still at E17 (Fig. 5B), and into adulthood (Fig. 5C). In adulthood, the columns of the fornix are clearly visible and this population of cells containing ir-ERα, outside the PVN, is located just dorsal to the descending columns of the fornix.
Figure 5.
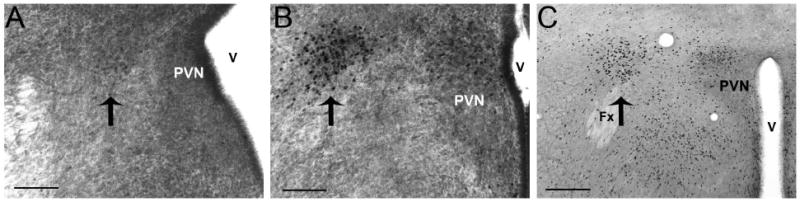
Digital images of sections containing immunoreactive ERα that were counterstained with thionin to show the boundaries of the paraventricular nucleus (PVN) and the lateral group of cells containing immunoreactive ERα outside of these boundaries (E15 in A, E17 in B, scale bars = 50μm). In the adult (C), this lateral group of ERα cells is still visible outside of the PVN and appears to be just dorsal to the descending columns of the fornix (Fx). The third ventricle is the white area to the right in each image (scale bars =250μm).
GABA influences the location of ERα cells through the GABAB receptor in a sex dependent manner
Brains from mice lacking functional GABAB receptors were analyzed for ir-ERα at P0. A spatial analysis from tissue sections corresponding in angle and location to those in Figure 1B revealed a difference in the medial to lateral distribution of cells containing ir-ERα. Tissue sections were obtained and the section through the central region of the PVN (containing a large density of ERα immunoreactive cells lateral to the PVN) was used for analysis (corresponding to Fig. 1B). To analyze this distribution of cells, 100μm wide columns were graphically placed over images of the tissue and pixel density was determined for each column. The columns were numbered 1-7 with column 1 being closest to the ventricle. GABABR1 knockouts were compared to wildtype animals and separated by sex. The data were analyzed as immunoreactive areas in a three-way ANOVA, sex by genotype by location considered as a repeated measure. The analysis showed a significant three-way interaction (F(6,120) = 2.43, p < 0.05) that clearly reflected the fact that a knockout phenotype was only evident in females, and was dependent upon location. In female knockout mice there was an approximate 80% decrease in ir-ERα within the fist 100μm from the ventricle (p < 0.05) and an approximate 70% increase in immunoreactive area between 500 and 600μm from the ventricle as compared to sections from wildtype mice (p<0.05; Fig. 6A-C). There were no differences in the distribution of ir-ERα in the same regions in males (Fig. 6D-F). Furthermore, there was no difference in the overall amount of ir-ERα, which included the PVN and the lateral population. Given the thickness of sections (60um) and the high density of cells in the PVN, we cannot conclude that the total area of immunoreactivity is representative of the total ERα cell number. Analyzing immunoreactivity levels by column aims at understanding the changing distribution of cells containing ir-ERα protein and does not account for total ERα protein levels or the number of cells expressing ERα.
Figure 6.
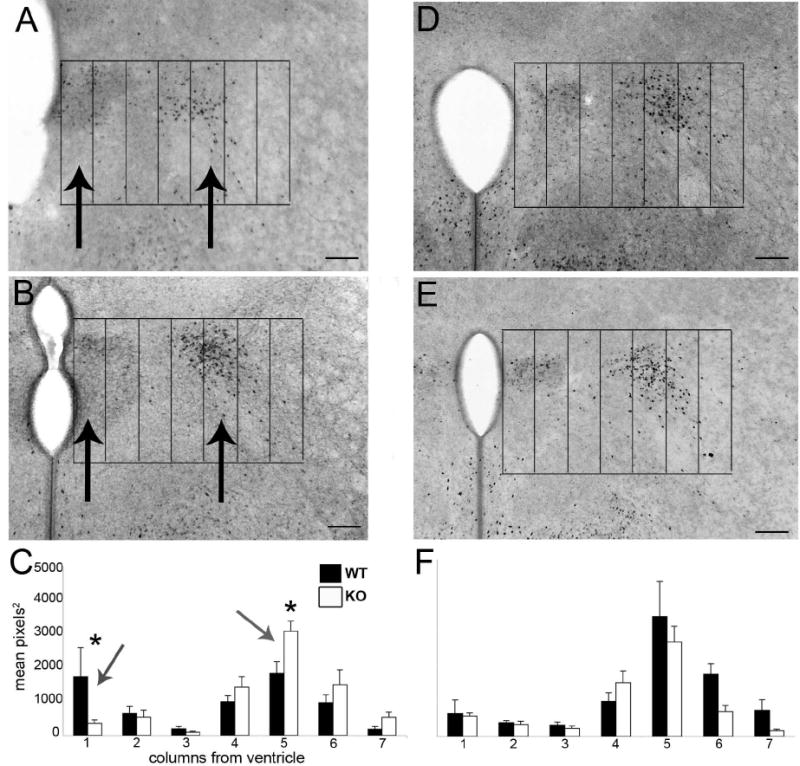
Digital mages show ERα immunoreactivity at P0 in GABABR1 subunit wildtype (A,D) and knockout (B,E) mice in the plane of section corresponding to figure 4B. Seven 100μm wide columns were placed over images with the edge of the third ventricle and the top of the paraventricular nucleus as the boundaries for the grid. Female GABABR1 subunit knockouts (n=6) had an approximately 80% decrease in immunoreactivity in column 1, as measured by mean pixels2, the first 100μm from the ventricle (Nguyen-Ba-Charvet et al., 2004), and an approximately 70% increase in immunoreactive area in column 5 (400-500μm from the ventricle), versus wildtype littermates (n=6). There was no difference in levels of immunoreactivity in male GABABR1 subunit knockout mice (D-F; n=6) as compared to wildtype (n=6) (scale bars =100μm).
To verify the results seen with our column area analysis, individual cell counts were taken from tissue sections corresponding to those used for analysis of immunoreactive area. Overall cell counts were taken for the region within the PVN, and the region outside of the PVN from one half of the brain. The cell count data was highly correlated to the immunoreactive area measurements (e.g., for total area correlated with total cell number, r = 0.80, p < 0.05) and the results were similar to those described above). Female GABABR1 wildtype mice had greater cell numbers in locations within the PVN (Mean ± SEM: WT, 34.5 ± 9.9; KO, 25.6 ± 2.7; n = 6/7 group) while female knockout mice had greater cell numbers in locations lateral to the PVN (WT, 83.6 ± 11.8; KO, 125.1 ± 6.2; n=6 group). There was a statistically significant difference of location by genotype between the GABABR1 knockout and wildtype female mice (F(1,11) = 5.86, p<0.05) as analyzed by repeated measures ANOVA. There were no differences found in males.
GABAB and GABAA receptor subunits are found within the PVN at embryonic ages
Adjacent sections were processed for ir-ERα and ir-GABABR1 subunit to determine if those cells containing ir-ERα might have GABAB receptors. Although the cellular resolution is hampered by the distribution of a membrane protein, the R1 subunit of the GABAB receptor was found in cells within the boundaries of the PVN and was found laterally in the region where the ERα cells are positioned just outside the PVN (Fig. 7A, B). GABAA receptor subunits are also located in cells within or around the developing PVN. Immunoreactive GABAA receptor subunit γ2 is notably found within the PVN (Fig. 7C) and immunoreactive α1 subunits (Fig. 7D) are found toward the periphery of the nucleus and in lateral locations.
Figure 7.
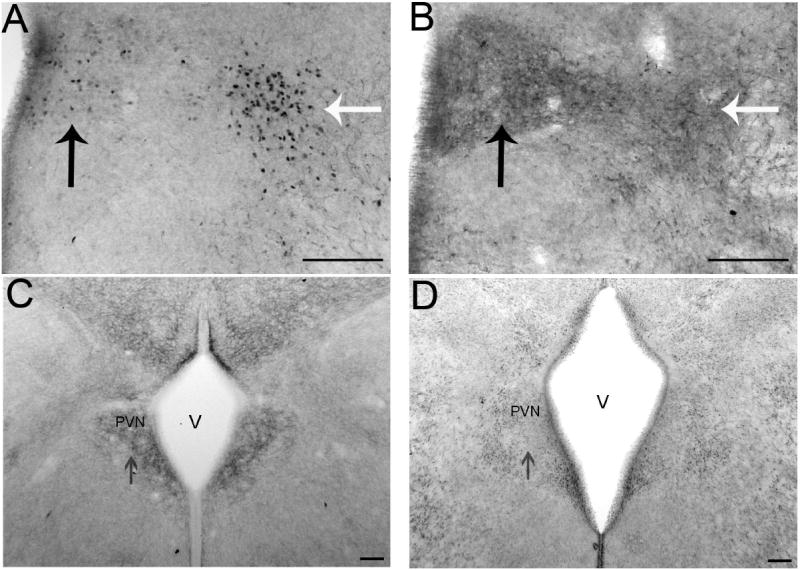
Digital images show immunoreactive GABA receptor subunits and their relationship to ERα in the paraventricular nucleus (PVN). Adjacent sections with ERα (A) and GABABR1 subunit (B) immunoreactivity at E17. Black arrows point to immunoreactivity within the region of the paraventricular nucleus (PVN) and white arrows point to immunoreactivity lateral to the classical boundaries of the PVN. Based on the locations of the immunoreactive elements in adjacent sections, these proteins may be expressed in some of the same cells. γ2 (C) and α1 (D) are two GABAA receptor subunits expressed in the PVN at E17 (V= third ventricle; scale bars =100μm).
GABABR1 subunit knockouts exhibited a change in the location of cells expressing nNOS
The expression pattern of immunoreactive nNOS changed across the rostral-caudal expanse of the nucleus with the more rostral sections containing more nNOS immunoreactivity within the nucleus and more caudal sections containing more nNOS positive cells along the lateral boundaries of the PVN (data not shown). GABABR1 subunit knockouts were analyzed to determine if GABAB signaling plays a role in the distribution of immunoreactive nNOS within the region of the PVN. In the central PVN, the area covered by nNOS immunoreactive cells and fibers was greater in PVN containing sections from knockout mice compared to wild type littermates. A grid was used to quantify the spread of immunoreactivity in the PVN of E17 GABABR1 subunit knockout and wildtype mice. There was a significant difference in the spread of immunoreactivity between genotypes (F(1,8) = 8.4, p < 0.03). The number of 50μm × 50μm squares containing ir-nNOS in the wildtype mice was 30.1 ± 1.8, while the number of squares containing ir-nNOS in the knockout was 38.4 ± 1.9 (Fig. 8 A-E). The total area of actual immunoreactivity did not differ between the groups (Fig. 8F).
Figure 8.
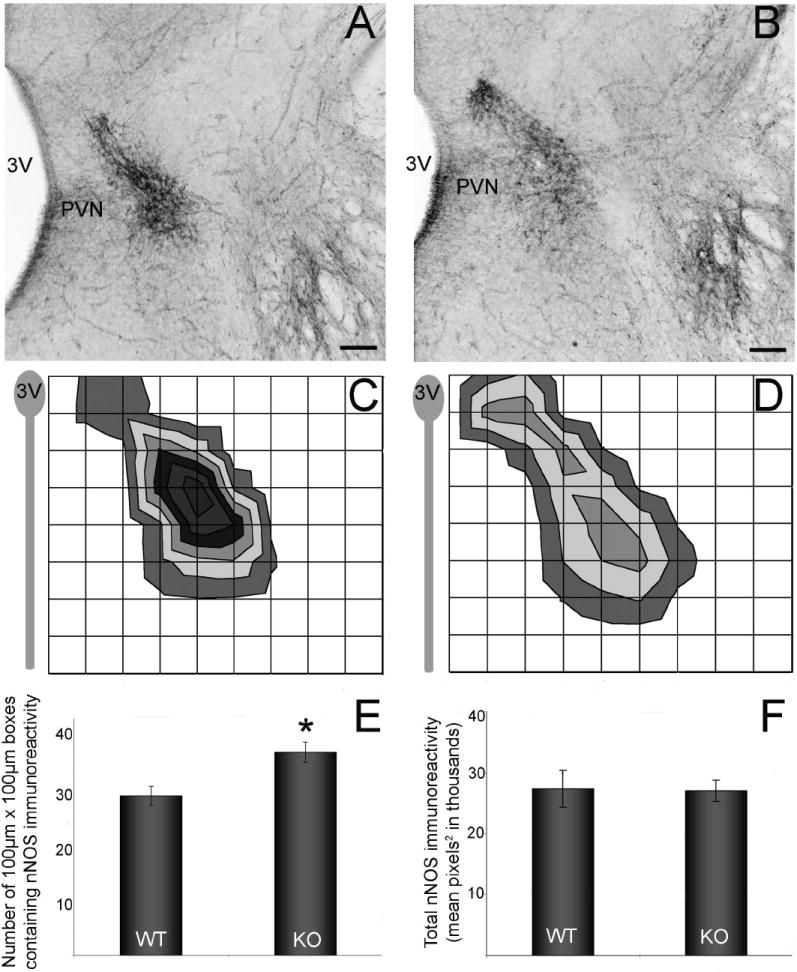
Digital images show nNOS immunoreactive cells and fibers in the paraventricular nucleus (PVN) at E17 (A, B). Immunoreactive elements are more dispersed in the GABABR1 knockout (B,D n=5) in comparison to wildtype littermates (A,C n=5). NNOS immunoreactivity in the central PVN is found near the lateral boundaries of the PVN. To visualize the spread of immunoreactivity, a grid was placed over the image of the PVN (using the top of the PVN as the dorsal boundary and the third ventricle as the medial boundary). In the knockout, there was an approximate 20% increase in the number of boxes containing immunoreactive elements (E). The overall amount of immunoreactivity did not change (F) (scale bars =100μm). *Asterisk represents p<0.03.
GABABR1 subunit knockouts exhibited a decrease in BDNF immunoreactive elements
BDNF protein is expressed throughout the PVN as early as E15 and through adulthood (data not shown). At E17, GABABR1 subunit knockout mice had decreased BDNF immunoreactivity in the PVN in a region dependent manner (Fig. 9). Tissue processed for ir-BDNF in GABABR1 subunit wildtype and knockout animals was normalized for variability in background levels and was analyzed using a spatial grid analysis. All boxes were determined as being a part of the PVN or outside of the region of the PVN. The data were analyzed as immunoreactive area in a two-way ANOVA, genotype × column location (considered as a repeated measure). Calculated by total immunoreactive area over all columns, the knockouts exhibited a 32% decrease in immunoreactive area. The analysis showed a significant effect between genotypes (F(1,9) = 5.93, p < 0.05). While the overall genotype by column location interaction was not statistically significant, those columns in the central region of the PVN (300-500μm from the edge of the third ventricle) had mean values outside the 95% confidence intervals for genotype comparisons (Fig 9C). There was not an obvious difference between the females and males and the sexes were combined for the analysis. The VMN is another site rich in BDNF (Connor et al., 1997) and where GABAB receptors play a role in nuclear development (Davis et al., 2002; McClellan et al., 2008). A similar analysis of ir-BDNF in the VMN of GABABR1 subunit knockout mice showed no discernible change in immunoreactivity (data not shown).
Figure 9.
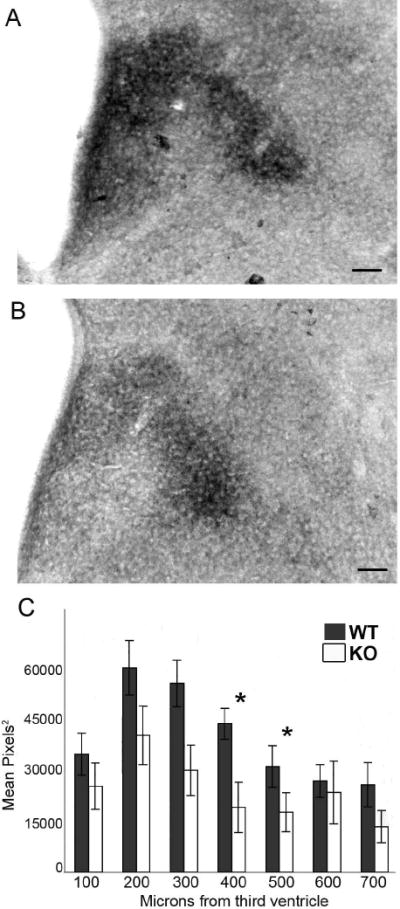
GABABR1 subunit knockout mice (B, n=5) had a 32% decrease in immunoreactive BDNF in the PVN compared to wildtype mice (A, n=7) with a significant difference between genotypes (p<0.05). Analyzing the immunoreactivity by location revealed that the largest decrease in protein levels was between 300 and 500μm from the edge of the third ventricle (C, scale bars =100μm). *Asterisks represent p<0.05.
Discussion
Development of the hypothalamus involves the interactions of many factors, including transcription factors, secreted factors, cell signaling molecules and extracellular matrix proteins to accomplish the goal of forming and connecting key components of specific nuclear groups. We have identified several molecules expressed early in PVN development; specifically BDNF, calbindin, nNOS, ERα and β, galanin and NPY. These markers and others (Caqueret et al., 2006), illustrate that a partially organized and heterogeneous nucleus is apparent before the PVN is discernible by Nissl stain. They provide tools to track the development of the PVN and identify molecules (GABA, NO, and BDNF) that could regulate the development of the PVN.
The current study delineated a ring like pattern of GABA and GAD expression in elements surrounding the PVN during embryonic development while GABAA and GABAB receptor subunits are enriched in cells of the developing PVN. This pattern is fundamental to the hypothesis that GABA acts to help form the boundary of the developing PVN and could provide gradient information to cells in the region. This GABA pattern of immunoreactivity is similar to one seen in the developing VMN where GABA effects on cell positioning were previously described (Dellovade et al., 2001; Davis et al., 2002; McClellan et al., 2006; McClellan et al., 2008). Before the establishment of axonal connections, GABA is not likely acting in its traditional role as a neurotransmitter at the level of the synapse (Taylor et al., 1990; van den Pol, 1997). The functional significance of GABA in early development may be to influence several developmental processes including proliferation, migration and differentiation (Lauder, 1993; Nguyen et al., 2001).
The focus of this study was to identify GABA influences on cell position within the region of the PVN during a developmental time period when the PVN is being established as a nucleus. We hypothesize that when there is a difference in the distribution of immunoreactive elements across columns without a change in total immunoreactive area, this may indicate a change in the position of cells. Measurements of immunoreactive area do not represent total levels of protein. Additional studies would be needed to determine whether there are changes in the levels of protein content. The goal of this study was to determine where the cells containing specific proteins were distributed across the region. However, in our analysis we found evidence for potential differences in protein expression levels based on cell counts (ERα) and area measurements of immunoreactivity (BDNF).
Cells immunoreactive for ERα were affected differently by the disruption of GABAA versus GABAB receptor signaling. In female, but not male, GABABR1 knockout mice there was less ir-ERα located closer to the third ventricle and more ir-ERα grouped lateral to the PVN. In the absence of a significant alteration in the total area of ERα immunoreactive cells in the region as a whole, this difference may reflect an influence on cell positions and potentially migration. In contrast, when GABAA signaling was disrupted, female mice exhibited an overall decrease in the number of cells containing ir-ERα, with a greater effect being found within the boundaries of the PVN. The overall decrease in the number of cells containing ir-ERα upon the disruption of GABAA signaling may be indicative of a decrease in the number of cells containing ir-ERα or the amount of protein being made in some of these cells rendering them undetectable. The difference in immunoreactive cell numbers between bicuculline and control treated mice could be mediated by GABA at several levels, ranging from gene expression to cell death. As the focus of the current study was to examine influences on cell position, we did not conduct additional studies on the nature of the influence of GABAA signaling on cells containing ir-ERα. To examine influences on cell position we analyzed area of immunoreactivity within specified regions of the tissue (100μm wide columns). This measurement allows us to visualize medial to lateral placement of cells within the PVN and outside of the PVN that are expressing various proteins of interest including ERα. The measures of immunoreactive area represent distribution of protein vs. total amount of protein throughout the region.
GABAA and GABAB receptors work through two very different signaling pathways (ionotropic versus metabotropic) and this may explain the different roles they play in the development of the PVN. Both receptor types have been shown to play a role in VMN (Davis et al., 2002; Dellovade et al., 2000; McClellan et al., 2008) as well as cortical development (Behar et al., 1996; Behar et al., 1998). With regards to VMN development, both receptor types seem to play similar roles in migration, influencing the position and spread of ir-ERα cells as well as influencing the speed of migrating neurons. In the cortex, however, GABAA and GABAB receptors play different roles, influencing the likelihood of motion and the movement through individual layers (Behar et al., 1998). This study implicates a role for GABA in the differentiation (through GABAA) and cell positioning (through GABAB) of neurons immunoreactive for ERα in the PVN of female mice.
The lateral population of ERα cells that was altered in female mice with disrupted GABA signaling may be part of the lateral hypothalamic/perifornical region of the hypothalamus. The perifornical region is part of the hypothalamic area controlling emotional responses (HACER) in the primate (Smith et al., 1990) and is involved in the regulation of cardiovascular responses to emotions (Risold et al., 1994). The perifornical population of cells within the hypothalamus has been implicated in aggressive behaviors and autonomic cardiovascular responses and contains a large number of orexin positive cell bodies (Peyron et al., 1998; Steininger et al., 2004). Estradiol has been shown to enhance the sensitivity of PVN CRH neurons involved in the HPA response (Lund et al., 2006). Studies involving rats that were implanted with capsules releasing estradiol benzoate directly in the region of the PVN exhibited changes in their stress response (Lund et al., 2006). Although this response is largely thought to act through ERβ positive cells within the rat PVN, this response could be mediated through the lateral population of cells containing ERα of which about 70% are GABAergic neurons with projections to the PVN in the adult rat. In fact it has been shown that estradiol can block glucocorticoid dependent negative feedback on the PVN via ERα (Weiser and Handa, 2009). This lateral population of ERα containing cells may be part of the limbic inputs that exert an inhibitory tone on the PVN (Herman et al., 2005). Thus, altering the position or levels of ERα could change the ability of hormones to modulate the HPA axis. Hormone modulation of the HPA axis is not limited to estrogens as there is also evidence for androgens modulating HPA axis function at the level of the PVN (Williamson et al., 2005).
In the context of the current results, we reexamined the pattern of ERα immunoreactivity in the archived slides of the PVN region of GABAA receptor β3 subunit knockout mice (Dellovade et al., 2001) and found no changes. However, the GABAA receptor β3 subunit is expressed at extremely low levels if at all in the embryonic PVN (data not shown). Therefore it is unlikely that the β3 subunit could be involved in the GABAA receptor response found in this study. With a number of possible subunits in the developing PVN (e.g., Fig. 7) it is likely that other subunits are making up the GABAA receptors within the PVN (Fenelon et al., 1995).
There are a number of indications of sex differences in PVN regulation and function (Handa et al., 1994; Rhodes and Rubin, 1999), even though there are relatively few findings of sexual dimorphism (differences based on cytoarchitecture and anatomy) in the adult or neonatal PVN. One recent finding is a difference in the number of CRH neurons in the brains of human subjects. Men had more CRH neurons than women in the PVN and men, but not women, had a significant increase in the number of CRH neurons with age (Bao and Swaab, 2007). Our data suggests a sex difference in the positioning of cells containing ir-ERα that was only revealed when GABAB receptor signaling was impaired. Many sex differences in the PVN may only become apparent under specific physiological states or in circumstances of altered development.
Nitric oxide, similar to GABA, has established and necessary functions in the adult PVN. NO is involved in the autonomic regulation of the heart and upon heart failure, nNOS levels decrease in the brain (Kang et al., 2009). It also regulates the release of vasopressin from PVN neurons and plays a role in regulating the neuroendocrine stress response (Orlando et al., 2008a; Orlando et al., 2008b). At early embryonic ages (E15-E17) ir-nNOS is expressed in the PVN (Edelmann et al., 2007), therefore it is possible that NO is involved in processes related to PVN development. In the GABABR1 knockout, the total area of nNOS immunoreactivity did not change but the spread of cells and fibers was significantly increased. This could be a further indication that GABA is acting as a boundary cue for cells moving into the lateral region of the PVN. Without normal GABAB signaling, cells moving in to this region do not end up in the correct location. Immunoreactive nNOS is found in cells and fibers, therefore, this result could also indicate that fibers within this region of the PVN are extending out in a disrupted pattern presaging altered connectivity at older ages. As NO is a bioactive signaling agent in its own right, the change in the distribution of nNOS may further influence the development of other elements within or connected to the PVN region.
BDNF is a neurotrophic factor that has characterized roles in neuronal growth, migration, survival and differentiation (Chiaramello et al., 2007; Fukumitsu et al., 2006; Gorski et al., 2003; Turner et al., 2006). BDNF is synthesized in specified locations within the embryonic hypothalamus, including a concentrated group of cells synthesizing BDNF in the PVN (Tapia-Arancibia et al., 2004). In the current study, BDNF was found in the PVN by E15, in time to play a role in PVN development and differentiation or to report on the influence of other factors. By E17, in the GABABR1 subunit knockout mice, there was a region-specific decrease in ir-BDNF within the PVN. Immunocytochemical methods were used to examine location of ir-BDNF in the region of the PVN and are not a valid method to evaluate BDNF protein levels per se. A grid placed over individual tissue sections revealed that the central part of the PVN is where the most obvious decrease in ir-BDNF was seen. There was a decrease in the area of ir-BDNF in the columns occupying the central region of the nucleus that could indicate that cells in this region are no longer synthesizing sufficient levels of BDNF to reach the level of detection. Although additional indicators of the phenotype of these cells remain to be determined, the decrease in the central PVN indicates that only selected cells within the PVN may be affected by a loss in GABAB receptor signaling. GABAB signaling could contribute to either the terminal differentiation or cell positioning of cells that are dependent on GABA. More globally it is clear that not all cells expressing BDNF in the brain are affected by GABAB receptor signaling. In the VMN, there were no differences in BDNF mRNA (McClellan et al., 2008) or immunoreactive protein (current study) in GABABR1 knockout mice. GABAB agonist in hippocampal cells increased BDNF expression (Ghorbel et al., 2005), and in embryonic hypothalamic cultures GABA increased the expression of BDNF through the MAPK and CREB pathway (Obrietan et al., 2002). Thus, GABA signaling may be involved in the differentiation of neurons synthesizing BDNF and the disruption of GABA signaling through the deletion of the GABAB receptor may influence the differentiation of BDNF cells within the PVN.
The PVN is the ultimate regulator in the hypothalamo-pituitary-adrenal (HPA) axis and the hormonal response to stress. Anxiety-like and depressive-like behaviors in animal models result from manipulating the HPA axis and increasing circulating levels of CRH (Kasckow et al., 2001). The importance of the PVN and the HPA axis as it relates to anxiety-related disorders has also been shown in human studies (Bao et al., 2008; Bao and Swaab, 2007; Wang et al., 2008b). Understanding normal development of the PVN and which factors contribute to its development can help us further understand this nuclear group and how abnormal development could ultimately lead to altered function and dysregulation of the HPA axis. The results of the current study suggest that GABA impacts neurons developing in or moving through the region of the PVN. In the absence of a functional GABABR1 subunit, cell positions (ERα and nNOS) and locations of protein expression (BDNF) were altered in and around the PVN. A second GABABR1 subunit knockout mouse, generated by a separate group, exhibit increased anxiety-like behaviors as compared to their littermate counterparts (Mombereau et al., 2004; Schuler et al., 2001). There is also evidence in the literature for changes in depression and anxiety with altered GABAA receptor signaling (Kalueff and Nutt, 2007). BDNF function in adulthood has been linked to depression and other mood disorders (Angelucci et al., 2005; Hashimoto et al., 2004), although less has been done developmentally. However, the recent findings of depression in forebrain selective murine knockouts of BDNF (Chan et al., 2006; Monteggia et al., 2007) suggest that there may be significant developmental contributions of BDNF to adult depression. Interestingly, disruption of the transcription factor SF-1 alters the differentiation of the VMN (McClellan et al., 2006) including causing a VMN-specific decrease in BDNF expression and an increase in anxiety-like behavior (Zhao et al., 2008). Altered Nitric Oxide signaling has also been implicated in depression. Specifically, individuals with depression have increased nNOS expression in the brain and altered levels of NO metabolites in circulation which are normalized with antidepressant therapies (Herken et al., 2007; Oliveira et al., 2008; Yanik et al., 2004). Also in animal models L-Arginine (precursor of NO) increased depressive like behaviors while NOS inhibitors augmented the effects of other antidepressants (Wang et al., 2008a).
GABA may potentially mediate aspects of PVN development to include BDNF expression, nNOS location and ERα location and expression. All of these molecules are important for regulating adult PVN and therefore HPA axis function. In addition there is a clear case to be made for nNOS and BDNF in PVN development based on their functions in the development of other brain regions. Although most evidence for anxiety or depression related effects of NO, BDNF and GABA have been in adult models and much work remains to be done, we suggest that some of the predisposition to these behaviors seen in humans and animal models is due to altered formation of the PVN, a master regulator of the HPA axis.
Acknowledgments
This work was supported by NIH grant PO1-MH082679 (SAT), NIH training grant HD07031-29 (KM), and NSF GK-12 training grant DGE-0841259 (SAT, MS). We thank Cory Wolfe, Stephanie Chase, Michelle Edelmann, Brian Searcy, and Megan Walker for technical assistance along the way. We thank Paul Bonthuis and Dr. Emilie Rissman for providing brains from ERβ knockout mice. We thank Drs. Robert Handa and Jill Goldstein for comments on the manuscript.
References
- Acampora D, Postiglione MP, Avantaggiato V, Di Bonito M, Vaccarino FM, Michaud J, Simeone A. Progressive impairment of developing neuroendocrine cell lineages in the hypothalamus of mice lacking the Orthopedia gene. Genes Dev. 1999;13(21):2787–2800. doi: 10.1101/gad.13.21.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon T, Zhou L, Pérez CA, Garfield AS, Friedman JM, Heisler LK. Transgenic mice expressing green fluorescent protein under the control of the corticotropin-releasing hormone promoter. Endocrinology. 2009;150(12):5626–5632. doi: 10.1210/en.2009-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelucci F, Brene S, Mathe AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10(4):345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- Armstrong WE, Warach S, Hatton GI, McNeill TH. Subnuclei in the rat hypothalamic paraventricular nucleus: a cytoarchitectural, horseradish peroxidase and immunocytochemical analysis. Neuroscience. 1980;5(11):1931–1958. doi: 10.1016/0306-4522(80)90040-8. [DOI] [PubMed] [Google Scholar]
- Azumaya Y, Tsutsui K. Localization of galanin and its binding sites in the quail brain. Brain Res. 1996;727(1-2):187–95. doi: 10.1016/0006-8993(96)00379-4. [DOI] [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57(2):531–553. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Bao AM, Swaab DF. Gender difference in age-related number of corticotropin-releasing hormone-expressing neurons in the human hypothalamic paraventricular nucleus and the role of sex hormones. Neuroendocrinology. 2007;85(1):27–36. doi: 10.1159/000099832. [DOI] [PubMed] [Google Scholar]
- Behar TN, Li YX, Tran HT, Ma W, Dunlap V, Scott C, Barker JL. GABA stimulates chemotaxis and chemokinesis of embryonic cortical neurons via calcium-dependent mechanisms. J Neurosci. 1996;16(5):1808–1818. doi: 10.1523/JNEUROSCI.16-05-01808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, O’Connell C, Barker JL. Differential response of cortical plate and ventricular zone cells to GABA as a migration stimulus. J Neurosci. 1998;18(16):6378–6387. doi: 10.1523/JNEUROSCI.18-16-06378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky MA, Yarom Y, Pickard GE. Heterogeneous expression of gamma-aminobutyric acid and gamma-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. J Comp Neurol. 2008;506(4):708–32. doi: 10.1002/cne.21553. [DOI] [PubMed] [Google Scholar]
- Bernstein HG, Stanarius A, Baumann B, Henning H, Krell D, Danos P, Falkai P, Bogerts B. Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience. 1998;83(3):867–875. doi: 10.1016/s0306-4522(97)00461-2. [DOI] [PubMed] [Google Scholar]
- Bicker G. STOP and GO with NO: nitric oxide as a regulator of cell motility in simple brains. Bioessays. 2005;27:495–505. doi: 10.1002/bies.20221. [DOI] [PubMed] [Google Scholar]
- Bless EP, Westaway WA, Schwarting GA, Tobet SA. Effects of gamma-aminobutyric acid(A) receptor manipulation on migrating gonadotropin-releasing hormone neurons through the entire migratory route in vivo and in vitro. Endocrinology. 2000;141(3):1254–1262. doi: 10.1210/endo.141.3.7348. [DOI] [PubMed] [Google Scholar]
- Borghesani PR, Peyrin JM, Klein R, Rubin J, Carter AR, Schwartz PM, Luster A, Corfas G, Segal RA. BDNF stimulates migration of cerebellar granule cells. Development. 2002;129(6):1435–1442. doi: 10.1242/dev.129.6.1435. [DOI] [PubMed] [Google Scholar]
- Brager DH, Sickel MJ, McCarthy MM. Developmental sex differences in calbindin-D(28K) and calretinin immunoreactivity in the neonatal rat hypothalamus. J Neurobiol. 2000;42(3):315–322. [PubMed] [Google Scholar]
- Brown AE, Mani S, Tobet SA. The preoptic area/anterior hypothalamus of different strains of mice: sex differences and development. Brain Res Dev Brain Res. 1999;115(2):171–182. doi: 10.1016/s0165-3806(99)00061-9. [DOI] [PubMed] [Google Scholar]
- Caqueret A, Boucher F, Michaud JL. Laminar organization of the early developing anterior hypothalamus. Dev Biol. 2006;298(1):95–106. doi: 10.1016/j.ydbio.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Chan JP, Unger TJ, Byrnes J, Rios M. Examination of behavioral deficits triggered by targeting Bdnf in fetal or postnatal brains of mice. Neuroscience. 2006;142(1):49–58. doi: 10.1016/j.neuroscience.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Chiaramello S, Dalmasso G, Bezin L, Marcel D, Jourdan F, Peretto P, Fasolo A, De Marchis S. BDNF/ TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signalling pathways. Eur J Neurosci. 2007;26(7):1780–1790. doi: 10.1111/j.1460-9568.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- Connor B, Young D, Yan Q, Faull RL, Synek B, Dragunow M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Brain Res Mol Brain Res. 1997;4(1-2):71–81. doi: 10.1016/s0169-328x(97)00125-3. [DOI] [PubMed] [Google Scholar]
- Das M, Vihlen CS, Legradi G. Hypothalamic and brainstem sources of pituitary adenylate cyclase-activating polypeptide nerve fibers innervating the hypothalamic paraventricular nucleus in the rat. J Comp Neurol. 2007;500(4):761–76. doi: 10.1002/cne.21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AM, Henion TR, Tobet SA. Gamma-aminobutyric acidB receptors and the development of the ventromedial nucleus of the hypothalamus. J Comp Neurol. 2002;449(3):270–280. doi: 10.1002/cne.10293. [DOI] [PubMed] [Google Scholar]
- Davis AM, Seney ML, Walker HJ, Tobet SA. Differential colocalization of Islet-1 and estrogen receptor alpha in the murine preoptic area and hypothalamus during development. Endocrinology. 2004;145(1):360–366. doi: 10.1210/en.2003-0996. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Davis AM, Ferguson C, Sieghart W, Homanics GE, Tobet SA. GABA influences the development of the ventromedial nucleus of the hypothalamus. J Neurobiol. 2001;49(4):264–276. doi: 10.1002/neu.10011. [DOI] [PubMed] [Google Scholar]
- Dellovade TL, Young M, Ross EP, Henderson R, Caron K, Parker K, Tobet SA. Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J Comp Neurol. 2000;423(4):579–589. doi: 10.1002/1096-9861(20000807)423:4<579::aid-cne4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Edelmann M, Wolfe C, Scordalakes EM, Rissman EF, Tobet S. Neuronal nitric oxide synthase and calbindin delineate sex differences in the developing hypothalamus and preoptic area. Dev Neurobiol. 2007;67(10):1371–1381. doi: 10.1002/dneu.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenelon VS, Sieghart W, Herbison AE. Cellular localization and differential distribution of GABAA receptor subunit proteins and messenger RNAs within hypothalamic magnocellular neurons. Neuroscience. 1995;64(4):1129–1143. doi: 10.1016/0306-4522(94)00402-q. [DOI] [PubMed] [Google Scholar]
- Ford-Holevinski TS, Castle MR, Herman JP, Watson SJ. Microcomputer-based three-dimensional reconstruction of in situ hybridization autoradiographs. J Chem Neuroanat. 1991;4(5):373–385. doi: 10.1016/0891-0618(91)90044-d. [DOI] [PubMed] [Google Scholar]
- Friend KE, Resnick EM, Ang LW, Shupnik MA. Specific modulation of estrogen receptor mRNA isoforms in rat pituitary throughout the estrous cycle and in response to steroid hormones. Mol Cell Endocrinol. 1997;131(2):147–155. doi: 10.1016/s0303-7207(97)00098-1. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Fujioka A, Endoh H, Sakata Y, Furukawa S, Nakamura S. Materno-fetal coordination of stress-induced Fos expression in the hypothalamic paraventricular nucleus during pregnancy. Neuroscience. 2003;118(2):409–415. doi: 10.1016/s0306-4522(02)00781-9. [DOI] [PubMed] [Google Scholar]
- Fukumitsu H, Ohtsuka M, Murai R, Nakamura H, Itoh K, Furukawa S. Brain-derived neurotrophic factor participates in determination of neuronal laminar fate in the developing mouse cerebral cortex. J Neurosci. 2006;26(51):13218–13230. doi: 10.1523/JNEUROSCI.4251-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbel MT, Becker KG, Henley JM. Profile of changes in gene expression in cultured hippocampal neurones evoked by the GABAB receptor agonist baclofen. Physiol Genomics. 2005;22(1):93–98. doi: 10.1152/physiolgenomics.00202.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givalois L, Naert G, Rage F, Ixart G, Arancibia S, Tapia-Arancibia L. A single brain-derived neurotrophic factor injection modifies hypothalamo-pituitary-adrenocortical axis activity in adult male rats. Mol Cell Neurosci. 2004;27(3):280–295. doi: 10.1016/j.mcn.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brain-derived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci. 2003;23(17):6856–6865. doi: 10.1523/JNEUROSCI.23-17-06856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW. On counting and counting errors. J Comp Neurol. 2002;447(1):1–7. doi: 10.1002/cne.10221. [DOI] [PubMed] [Google Scholar]
- Gyurko R, Leupen S, Huang PL. Deletion of exon 6 of the neuronal nitric oxide synthase gene in mice results in hypogonadism and infertility. Endocrinology. 2002;143(7):2767–2774. doi: 10.1210/endo.143.7.8921. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O’Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28(4):464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res Brain Res Rev. 2004;45(2):104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, Kap O, Yumru M, Savas HA, Akyol O. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res. 2007;38(2):247–252. doi: 10.1016/j.arcmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Huynh DP, Figueroa K, Hoang N, Pulst SM. Nuclear localization or inclusion body formation of ataxin-2 are not necessary for SCA2 pathogenesis in mouse or human. Nat Genet. 2000;26(1):44–50. doi: 10.1038/79162. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. 2007;24(7):495–517. doi: 10.1002/da.20262. [DOI] [PubMed] [Google Scholar]
- Kang YM, He RL, Yang LM, Qin DN, Guggilam A, Elks C, Yan N, Guo Z, Francis J. Brain tumour necrosis factor-{alpha} modulates neurotransmitters in hypothalamic paraventricular nucleus in heart failure. Cardiovasc Res. 2009;83(4):737–46. doi: 10.1093/cvr/cvp160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim MA, Sloper JC. Histogenesis of the supraoptic and paraventricular neurosecretory cells of the mouse hypothalamus. J Anat. 1980;130(Pt 2):341–347. [PMC free article] [PubMed] [Google Scholar]
- Kasckow JW, Baker D, Geracioti TD., Jr Corticotropin-releasing hormone in depression and post-traumatic stress disorder. Peptides. 2001;22(5):845–851. doi: 10.1016/s0196-9781(01)00399-0. [DOI] [PubMed] [Google Scholar]
- Keegan CE, Karolyi IJ, Knapp LT, Bourbonais FJ, Camper SA, Seasholtz AF. Expression of corticotropin-releasing hormone transgenes in neurons of adult and developing mice. Mol Cell Neurosci. 1994;5(6):505–514. doi: 10.1006/mcne.1994.1062. [DOI] [PubMed] [Google Scholar]
- Klein S, Jurkevich A, Grossmann R. Sexually dimorphic immunoreactivity of galanin and colocalization with arginine vasotocin in the chicken brain (Gallus gallus domesticus) J Comp Neurol. 2006;499(5):828–839. doi: 10.1002/cne.21132. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16(6):233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Légrádi G, Lechan RM. The arcuate nucleus is the major source for neuropeptide Y-innervation of thyrotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology. 1998;139(7):3262–3270. doi: 10.1210/endo.139.7.6113. [DOI] [PubMed] [Google Scholar]
- Lund I, Lundeberg T, Carleson J, Sonnerfors H, Uhrlin B, Svensson E. Corticotropin releasing factor in urine--a possible biochemical marker of fibromyalgia. Responses to massage and guided relaxation. Neurosci Lett. 2006;403(1-2):166–171. doi: 10.1016/j.neulet.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI. Immunohistochemical localization of GABA(B) receptors in the rat central nervous system. J Comp Neurol. 1999;405(3):299–321. doi: 10.1002/(sici)1096-9861(19990315)405:3<299::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- McClellan KM, Calver AR, Tobet SA. GABAB receptors role in cell migration and positioning within the ventromedial nucleus of the hypothalamus. Neuroscience. 2008;151(4):1119–1131. doi: 10.1016/j.neuroscience.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan KM, Parker KL, Tobet S. Development of the ventromedial nucleus of the hypothalamus. Front Neuroendocrinol. 2006 doi: 10.1016/j.yfrne.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Michaud JL, DeRossi C, May NR, Holdener BC, Fan CM. ARNT2 acts as the dimerization partner of SIM1 for the development of the hypothalamus. Mech Dev. 2000;90(2):253–261. doi: 10.1016/s0925-4773(99)00328-7. [DOI] [PubMed] [Google Scholar]
- Michaud JL, Rosenquist T, May NR, Fan CM. Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev. 1998;12(20):3264–3275. doi: 10.1101/gad.12.20.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144(5):2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mombereau C, Kaupmann K, Froestl W, Sansig G, van der Putten H, Cryan JF. Genetic and pharmacological evidence of a role for GABA(B) receptors in the modulation of anxiety- and antidepressant-like behavior. Neuropsychopharmacology. 2004;29(6):1050–1062. doi: 10.1038/sj.npp.1300413. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parada LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol Psychiatry. 2007;61(2):187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Nguyen L, Rigo JM, Rocher V, Belachew S, Malgrange B, Rogister B, Leprince P, Moonen G. Neurotransmitters as early signals for central nervous system development. Cell Tissue Res. 2001;305(2):187–202. doi: 10.1007/s004410000343. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet KT, Picard-Riera N, Tessier-Lavigne M, Baron-Van Evercooren A, Sotelo C, Chedotal A. Multiple roles for slits in the control of cell migration in the rostral migratory stream. J Neurosci. 2004;24(6):1497–1506. doi: 10.1523/JNEUROSCI.4729-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, Gao XB, Van Den Pol AN. Excitatory actions of GABA increase BDNF expression via a MAPK-CREB-dependent mechanism--a positive feedback circuit in developing neurons. J Neurophysiol. 2002;88(2):1005–1015. doi: 10.1152/jn.2002.88.2.1005. [DOI] [PubMed] [Google Scholar]
- Okamura H, Fukui K, Koyama E, Tsutou HL, Tsutou T, Terubayashi H, Fujisawa H, Ibata Y. Time of vasopressin neuron origin in the mouse hypothalamus: examination by combined technique of immunocytochemistry and [3H]thymidine autoradiography. Brain Res. 1983;285(2):223–226. doi: 10.1016/0165-3806(83)90055-x. [DOI] [PubMed] [Google Scholar]
- Oliveira RM, Guimaraes FS, Deakin JF. Expression of neuronal nitric oxide synthase in the hippocampal formation in affective disorders. Braz J Med Biol Res. 2008;41(4):333–341. doi: 10.1590/s0100-879x2008000400012. [DOI] [PubMed] [Google Scholar]
- Orlando GF, Langnaese K, Schulz C, Wolf G, Engelmann M. Neuronal nitric oxide synthase gene inactivation reduces the expression of vasopressin in the hypothalamic paraventricular nucleus and of catecholamine biosynthetic enzymes in the adrenal gland of the mouse. Stress. 2008a;11(1):42–51. doi: 10.1080/10253890701449867. [DOI] [PubMed] [Google Scholar]
- Orlando GF, Wolf G, Engelmann M. Role of neuronal nitric oxide synthase in the regulation of the neuroendocrine stress response in rodents: insights from mutant mice. Amino Acids. 2008b;35(1):17–27. doi: 10.1007/s00726-007-0630-0. [DOI] [PubMed] [Google Scholar]
- Owen D, Matthews SG. Glucocorticoids and sex-dependent development of brain glucocorticoid and mineralocorticoid receptors. Endocrinology. 2003;144(7):2775–2784. doi: 10.1210/en.2002-0145. [DOI] [PubMed] [Google Scholar]
- Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, Saunders PT, Shupnik M. Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001;304(2):193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- Peunova N, Enikolopov G. Nitric oxide triggers a switch to growth arrest during differentiation of neuronal cells. Nature. 1995;375:68–73. doi: 10.1038/375068a0. [DOI] [PubMed] [Google Scholar]
- Peunova N, Scheinker V, Cline H, Enikolopov G. Nitric oxide is an essential regulator of cell proliferation in Xenopus brain. J Neurosci. 2001;21:8809–8818. doi: 10.1523/JNEUROSCI.21-22-08809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, Schenck E, Warmerdam BS, Clapham C, Reavill C, Rogers DC, Stean T, Upton N, Humphreys K, Randall A, Geppert M, Davies CH, Pangalos MN. Epileptogenesis and enhanced prepulse inhibition in GABA(B1)-deficient mice. Mol Cell Neurosci. 2001;17(6):1059–1070. doi: 10.1006/mcne.2001.0995. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Rubin RT. Functional sex differences (‘sexual diergism’) of central nervous system cholinergic systems, vasopressin, and hypothalamic-pituitary-adrenal axis activity in mammals: a selective review. Brain Res Brain Res Rev. 1999;30(2):135–152. doi: 10.1016/s0165-0173(99)00011-9. [DOI] [PubMed] [Google Scholar]
- Risold PY, Canteras NS, Swanson LW. Organization of projections from the anterior hypothalamic nucleus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348(1):1–40. doi: 10.1002/cne.903480102. [DOI] [PubMed] [Google Scholar]
- Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31(1):47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- Shimada M, Nakamura T. Time of neuron origin in mouse hypothalamic nuclei. Exp Neurol. 1973;41(1):163–173. doi: 10.1016/0014-4886(73)90187-8. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J Comp Neurol. 2001;436(1):64–81. [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Smith OA, DeVito JL, Astley CA. Neurons controlling cardiovascular responses to emotion are located in lateral hypothalamus-perifornical region. Am J Physiol. 1990;259(5 Pt 2):R943–954. doi: 10.1152/ajpregu.1990.259.5.R943. [DOI] [PubMed] [Google Scholar]
- Somponpun SJ, Sladek CD. Osmotic regulation of estrogen receptor-beta in rat vasopressin and oxytocin neurons. J Neurosci. 2003;23(10):4261–4269. doi: 10.1523/JNEUROSCI.23-10-04261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steininger TL, Kilduff TS, Behan M, Benca RM, Landry CF. Comparison of hypocretin/orexin and melanin-concentrating hormone neurons and axonal projections in the embryonic and postnatal rat brain. J Chem Neuroanat. 2004;27(3):165–181. doi: 10.1016/j.jchemneu.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Handa RJ. Regulation of estrogen receptor-beta expression in the female rat hypothalamus: differential effects of dexamethasone and estradiol. Endocrinology. 2004;145(8):3658–3670. doi: 10.1210/en.2003-1688. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Rage F, Givalois L, Arancibia S. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004;25(2):77–107. doi: 10.1016/j.yfrne.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Taylor J, Docherty M, Gordon-Weeks PR. GABAergic growth cones: release of endogenous gamma-aminobutyric acid precedes the expression of synaptic vesicle antigens. J Neurochem. 1990;54(5):1689–1699. doi: 10.1111/j.1471-4159.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol. 2008;506(4):616–26. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe I, Ishida Y, Tanaka M, Endoh H, Fujioka T, Nakamura S. Effects of repeated maternal stress on FOS expression in the hypothalamic paraventricular nucleus of fetal rats. Neuroscience. 2005;134(2):387–395. doi: 10.1016/j.neuroscience.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Henderson RG, Whiting PJ, Sieghart W. Special relationship of gamma-aminobutyric acid to the ventromedial nucleus of the hypothalamus during embryonic development. J Comp Neurol. 1999;405(1):88–98. doi: 10.1002/(sici)1096-9861(19990301)405:1<88::aid-cne7>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Tobet S, Dellovade TL, Parker K, Homanics GE. Positioning estrogen receptor alpha-containing cells during hypothalamic development. In: Handa R, editor. Neuroplasticity, Development, and Steroid Hormone Action. 2002. pp. 59–72. [Google Scholar]
- Tobet S, Knoll JG, Hartshorn C, Aurand E, Stratton M, Kumar P, Searcy B, McClellan K. Brain sex differences and hormone influences: a moving experience? J Neuroendocrinol. 2009;21(4):387–392. doi: 10.1111/j.1365-2826.2009.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BA, Sparrow J, Cai B, Monroe J, Mikawa T, Hempstead BL. TrkB/BDNF signaling regulates photoreceptor progenitor cell fate decisions. Dev Biol. 2006;299(2):455–465. doi: 10.1016/j.ydbio.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher CM, Frétier P, Créminon C, Calas A, Hardin-Pouzet H. Activation by serotonin and noradrenaline of vasopressin and oxytocin expression in the mouse paraventricular and supraoptic nuclei. J Neurosci. 2002;22(5):1513–1522. doi: 10.1523/JNEUROSCI.22-05-01513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996;364(3):523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- van den Pol AN. GABA immunoreactivity in hypothalamic neurons and growth cones in early development in vitro before synapse formation. J Comp Neurol. 1997;383(2):178–188. doi: 10.1002/(sici)1096-9861(19970630)383:2<178::aid-cne5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Wang D, An SC, Zhang X. Prevention of chronic stress-induced depression-like behavior by inducible nitric oxide inhibitor. Neurosci Lett. 2008a;433(1):59–64. doi: 10.1016/j.neulet.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Wang SS, Kamphuis W, Huitinga I, Zhou JN, Swaab DF. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: the presence of multiple receptor imbalances. Mol Psychiatry. 2008b;13(8):786–799. 741. doi: 10.1038/mp.2008.38. [DOI] [PubMed] [Google Scholar]
- Wang W, Lufkin T. The murine Otp homeobox gene plays an essential role in the specification of neuronal cell lineages in the developing hypothalamus. Dev Biol. 2000;227(2):432–449. doi: 10.1006/dbio.2000.9902. [DOI] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159(2):883–895. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M, Bingham B, Viau V. Central organization of androgen-sensitive pathways to the hypothalamic-pituitary-adrenal axis: implications for individual differences in responses to homeostatic threat and predisposition to disease. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1239–1248. doi: 10.1016/j.pnpbp.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Wittmann G, Sarkar S, Hrabovszky E, Liposits Z, Lechan RM, Fekete C. Galanin- but not galanin-like peptide-containing axon terminals innervate hypophysiotropic TRH-synthesizing neurons in the hypothalamic paraventricular nucleus. Brain Res. 2004;1002(1-2):43–50. doi: 10.1016/j.brainres.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Xu C, Fan CM. Allocation of paraventricular and supraoptic neurons requires Sim1 function: a role for a Sim1 downstream gene PlexinC1. Mol Endocrinol. 2007;21(5):1234–1245. doi: 10.1210/me.2007-0034. [DOI] [PubMed] [Google Scholar]
- Yanik M, Vural H, Tutkun H, Zoroglu SS, Savas HA, Herken H, Kocyigit A, Keles H, Akyol O. The role of the arginine-nitric oxide pathway in the pathogenesis of bipolar affective disorder. Eur Arch Psychiatry Clin Neurosci. 2004;254(1):43–47. doi: 10.1007/s00406-004-0453-x. [DOI] [PubMed] [Google Scholar]
- Zhao L, Kim KW, Ikeda Y, Anderson KK, Beck L, Chase S, Tobet SA, Parker KL. CNS-Specific Knockout of Steroidogenic Factor 1 Results in Increased Anxiety-Like Behavior. Mol Endocrinol. 2008;22(6):1403–15. doi: 10.1210/me.2008-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Porcionatto M, Pilapil M, Chen Y, Choi Y, Tolias KF, Bikoff JB, Hong EJ, Greenberg ME, Segal RA. Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron. 2007;55(1):53–68. doi: 10.1016/j.neuron.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]