Abstract
There is growing evidence supporting a role for infections in the aetiology of childhood leukaemia. Hypotheses proposed by both Greaves and Kinlen describe childhood leukaemia to be a rare response to one or more common infections acquired through personal contacts. Previous epidemiological studies have used day-care attendance as an indicator of the increased likelihood of early and frequent exposure to infections. It is well-documented that in developed countries, exposures to common infections occur more frequently in this type of setting. Within the Northern California Childhood Leukaemia Study, the role of social contact has been assessed and a unique ‘child-hours’ summary measure incorporating information on the number of months attending a day-care, mean hours per week at this day-care and the number of children in the day-care setting was constructed. In this review, the previously reported day-care results have been described, showing that in non-Hispanic White children, children in the highest category of total child-hours of exposure had a reduced risk of acute lymphoblastic leukaemia (ALL), particularly common B-cell precursor ALL (c-ALL), compared with children without such exposures, with evidence of a dose–response effect. In addition, a literature review of relevant studies has been conducted, examining the relationship between day-care attendance and risk of childhood ALL. Overall, the 14 studies identified provided consistent support for this hypothesis, with the majority of studies reporting some evidence of a reduced risk. A meta-analysis is currently underway, which will provide a quantitative evaluation of the overall consistency and strength of the association between day-care attendance or social contact and risk of childhood ALL.
INTRODUCTION
The causes of childhood leukaemia remain largely unknown, but there is growing evidence supporting a role for infection in the aetiology of this disease, particularly for the most common sub-type, acute lymphoblastic leukaemia (ALL) (1–3). Two infection-related hypotheses of childhood leukaemia introduced in 1988 have gained popularity and are currently supported by substantial epidemiological evidence(2). Kinlen(4), in his ‘population mixing’ hypothesis proposed that childhood leukaemia may result from an abnormal immune response to specific, although unidentified, infections commonly seen with the influx of infected persons into an area previously populated with non-immune, and thus, susceptible individuals. The ‘delayed infection’ hypothesis proposed by Greaves(1,5) explains that childhood leukaemia, particularly common B-cell precursor ALL (c-ALL), may be caused by a proliferative stress-induced effect of common infections on the developing immune system of the child. Implicit in this explanation is that an adverse immune response to infections is a result of insufficient priming of the immune system usually influenced by a delay in exposure to common infectious agents during early childhood. Both the population mixing and delayed infection hypotheses are compatible with the available evidence, and in some populations, it is possible that both mechanisms may be operative.
Establishing a role for infections in childhood leukaemia aetiology has been challenging mainly due the unsuccessful attempts to identify the causal infection(s)(6) and difficulties in directly quantifying a child's exposure and/or response to infections. Evidence to date originates from a fairly large body of literature that provides support ranging from epidemiological studies of population mixing to studies using surrogate measures of exposure to infections, including birth order, child's history of infections, child's day-care and play group attendance and parental social contacts in the workplace(2). Among the surrogate measures examined, day-care attendance or activities in other similar types of settings are widely accepted as strong predictors of a child's early exposure to infections in developed countries(7,8). The transmission of infectious agents is believed to be promoted through this type of social setting due to the immaturity of children's immune systems in combination with the lack of appropriate hygienic behaviour. Previous studies have consistently shown day-care attendance to be associated with an increased risk of infectious diseases in children, particular those of the respiratory and gastrointestinal tracts (7).
This review provides an overview of the current evidence of the association between the risk of childhood leukaemia and day-care attendance, one surrogate measure of exposure to early infections, including a description of the Northern California Childhood Leukaemia Study (NCCLS) and its previously reported day-care attendance findings.
DAY-CARE ATTENDANCE AND RISK OF CHILDHOOD ALL IN THE NCCLS
Northern California Childhood Leukaemia Study
The NCCLS is an ongoing population-based case–control study designed to investigate the aetiology of pediatric leukaemias. Since 1995, incident childhood leukaemia cases have been rapidly ascertained from all major paediatric hospitals located in Northern and Central California. Newly diagnosed childhood leukaemia cases are reported to the study centre within 24–48 h of diagnosis regardless of eligibility. Comparison of case ascertainment with the California Cancer Surveillance programme data shows that the NCCLS rapid case ascertainment protocol is able to identify ∼ 90% of all newly diagnosed childhood leukaemia cases in the study region. For each eligible case, controls matched on date of birth, sex, Hispanic status (one or both biological parents are Hispanic) and maternal race are randomly selected from a list generated by the statewide birth registry maintained by the California Department of Public Health (formerly California Department of Health Services). Cases and controls are considered eligible if they are under 15 y of age (at diagnosis for cases and corresponding date for the matched controls), resided in the study region at the time of diagnosis, have a parent who speaks either English or Spanish and have no history of malignancy or cancer treatment. A detailed description of control selection is reported elsewhere(9). Owing to the unique demographic composition of the study area, ∼ 42% of the cases enrolled in the NCCLS are Hispanic.
Day-care variables and total child-hours of exposure measure
As part of the large data collection effort implemented through the NCCLS, a detailed account of the child's day-care and pre-school attendance is obtained using an in-person interview. For each day-care and/or pre-school the child attended, information on age attended, duration of time attended, hours per week and number of other children is obtained. These data are collected based on the understanding that a child's potential for exposure to infectious agents in this setting is largely influenced by the frequency and duration of attendance and the size of the facility(8). Under the assumption that exposure to infections is primarily through the child's social contacts with other children, a unique quantitative measure termed ‘total child-hours of exposure’ was calculated for each child(10). At each day-care facility, child-hours was calculated as follows: (number of months attending the day-care) × (mean hours per week at this day-care) × (number of other children at this day-care) × (4.35 weeks per month). The child-hours in each day-care setting were summed to obtain the total ‘child-hours of exposure’ for each child.
NCCLS Findings
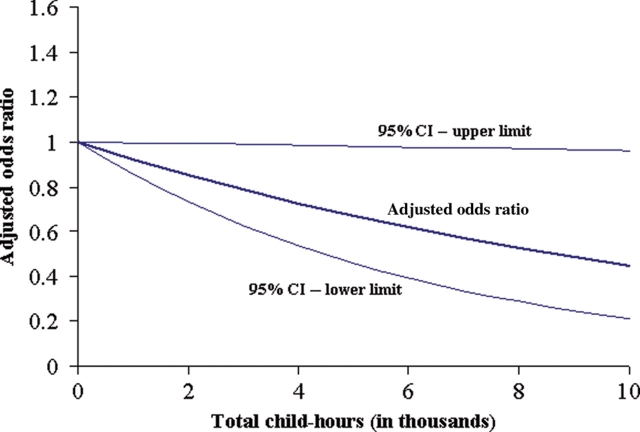
In 2002, Ma et al.(11) reported preliminary findings from the NCCLS that children who had more total child-hours of exposure had a statistically significant reduced risk of ALL. A subsequent analysis using a larger sample size from the NCCLS population resulted in the confirmation of these findings(10), but this significantly reduced risk was observed only in non-Hispanic White children and not in Hispanic children. Among non-Hispanic White children, there was evidence of a reduced risk, including a dose–response relationship, of ALL (OR 0.60, 95% CI 0.28–1.27) and c-ALL (OR 0.28, 95% CI 0.08–0.95) associated with the highest category of child-hours of exposure (≥15 000 child-hours) compared with children who did not attend day care. A statistically significant reduced risk was also observed for ALL and c-ALL when considering day-care attendance only during infancy (first year of life). Analysing the risk for each thousand child-hours as presented in Figure 1, increasing child-hours of exposure during infancy was significantly associated with a reduced risk of childhood ALL (OR 0.923, 95% CI 0.856–0.996). In contrast, day-care attendance did not appear to be associated with risk of ALL or c-ALL in Hispanic children. The descriptive analysis of day-care characteristics showed that compared with non-Hispanic White children, Hispanic children tended to start day-care at a later age, attended day-care for a shorter period of time and for fewer hours per week, have fewer child-hours of exposure at day-care and more other children living in the household. These marked differences in day-care utilisation practices and household living conditions suggest that day-care attendance may not be the major source of early exposure to infections in Hispanics. Additionally, the lower prevalence of day-care attendance among Hispanic children may have affected the statistical power to detect a significant association. As an ongoing study, the NCCLS will have the opportunity to confirm these results using a larger sample size.
Figure 1.
Total child-hours of exposure during infancy and risk of childhood acute lymphoblastic leukaemia in non-Hispanic White children enrolled in the NCCLS during 1995–2002. Odds ratios are adjusted for annual household income and maternal education and total child-hours are censored at 1 y of age.
LITERATURE REVIEW
The association between early childhood exposures to infections and childhood leukaemia risk using day-care attendance as a surrogate measure has been examined in numerous other studies. A literature search of the PubMed database was conducted to identify all original research and review articles related to childhood leukaemia and day-care attendance and/or social contacts (January 1966 through May 2008). In addition, the bibliographies of epidemiology publications on childhood leukaemia and infections were searched to identify studies that may not have been captured through the initial database search. This included the comprehensive review published in 2004 by McNally and Eden(2) on the infectious aetiology of childhood acute leukaemia. The outcome of interest was defined as clinically diagnosed leukaemia in children between the ages of 0 and 19. The exposure of interest is generally referred to as ‘day-care attendance’, which in addition to formal day-care may have included pre-school, nursery school, play groups, mother–toddler groups and other early social contact measures. A strict criterion for the meaning of ‘regular attendance’ was not defined a priori since it was assumed that this would vary between studies. Studies that created a form of social activity variable needed to have incorporated information on day-care attendance in the measure. A total of 14 studies were identified for this systematic review (10,12–24).
Table 1 presents select characteristics of 14 studies that were included in this review. The studies were all case–control in design published between 1993 and 2008 and were conducted in a number of locations throughout the world. The majority of studies implemented a population-based control selection strategy, with the exception of three studies that selected hospital-based controls (19–21). Only 1 of the 14 studies utilised a record-based day-care assessment protocol (17), while the remaining studies relied on respondent recall using standardised questionnaires administered either in-person, by telephone or by mail. All studies accounted for major confounding factors such as age, sex, race and socioeconomic status through a matched study design and/or statistical adjustment in the analysis. Of the 14 studies identified, 12 have reported either a statistically significant reduced risk associated with day-care attendance or a social contact measure or provide some evidence of a reduced risk. As expected, heterogeneity between epidemiological studies, including differences in exposure assessment and potential effects of systematic biases, is observed and is broadly discussed within the context of this review.
Table 1.
Select characteristics and results of 14 studies of day-care attendance and risk of childhood leukaemia.
| Study | Country | Outcome, agea | Exposure |
|||
|---|---|---|---|---|---|---|
| Definition | Timing | No. of cases | OR (95% CI) | |||
| Petridou et al.(20) | Greece | Leukaemia, 0–14 | Attendance at creche: yes/no | Before age 2 | 136 | 0.28 (0.09–0.88) |
| Roman et al.(22) | UK | ALL, 0–4 | Pre-school playgroup: yes/no | Year before diagnosis | 38 | 0.60 (0.20–1.80) |
| Petridou et al.(21) | Greece | Leukaemia, 0–14 | Day-care: ever/never | Birth to diagnosis | 153 | 0.83 (0.51–1.37) |
| Schuz et al.(24)b | Germany | AL, 1.5–14 | Deficit in social contacts: no/yes | Before age 2 | 921 | 0.91 (0.90–1.30) |
| Dockerty et al.(13) | New Zealand | ALL, 1.25–14 | Regular contact outside home: yes/no | First year of life | 90 | 0.65 (0.36–1.17) |
| Infante-Rivard et al.(15) | Canada | ALL, 0–9 | Day-care: entry at ≤2 y/never | At or before age 2 | 490 | 0.49 (0.31–0.77) |
| Rosenbaum et al.(23)b | USA | ALL, 0–14 | Out of home care: >36 months./none | Birth to diagnosis | 248 | 0.76 (0.70–2.52) |
| Neglia et al.(18) | USA | ALL, 1–14 | Day-care before age 2: yes/no | Before age 2 | 1744 | 0.99 (0.84–1.17) |
| Chan et al.(12) | Hong Kong | AL, 2–14 | Index and family day-care: 3-level | First year of life | 98 | 0.96 (0.70–1.32) |
| Perrillat et al.(19) | France | AL, 2–15 | Day-care attendance: yes/no | Birth to diagnosis | 246 | 0.60 (0.40–1.00) |
| Jourdan-Da Silva et al.(16) | France | ALL, 1–14 | Day-care attendance: yes/no | Birth to diagnosis | 387 | 0.70 (0.60–1.00) |
| Gilham et al.(14) | UK | ALL, 2–14 | Social activity: any/none | First year of life | 1272 | 0.66 (0.56–0.77) |
| Ma et al.(10)(White) | USA | ALL, 1–14 | Child-hours of exposure: ≥15 000/0 | Birth to diagnosis | 136 | 0.60 (0.28–1.27) |
| Ma et al., (10)(Hispanic) | USA | ALL, 1–14 | Child-hours of exposure: ≥15 000/0 | Birth to diagnosis | 120 | 1.27 (0.62–2.56) |
| Kamper-Jorgensen et al.(17) | Denmark | ALL, 0–15 | Attendance to childcare: yes/no | Before age 2 | 176 | 0.68 (0.48–0.95) |
aALL, acute lymphoblastic leukaemia; AL, acute leukaemia; age ranges are for those included in the analysis.
bSchuz et al.: Changed reference to ‘Yes-deficit in social contacts’ by calculating the inverse of the OR provided for ‘No-deficit in social contacts’; Rosenbaum et al.: Estimated the OR for ‘ > 36 months’ by calculating the inverse of the originally provided OR for ‘Stayed home’.
In one of the first studies of day-care attendance and childhood leukaemia, Petridou et al.(20) reported a reduced risk of leukaemia associated with attendance at a crèche in infancy in a Greek population (OR 0.28, 95% CI 0.09-0.88). Similarly, attendance at a crèche at any time before diagnosis appeared to be suggestive of a reduced risk. Controls were residents of the study base who attended the outpatient clinic of the hospitals where all the leukaemia patients were treated. In a subsequent Greek study comprising an independent case and hospital control series, Petridou et al.(21) were not able to confirm these results convincingly, reporting a non-significant reduced risk estimate for ever attending day-care (versus never)(21). Unlike the previous study, it is unclear whether the timing and duration of day-care attendance were assessed as part of the analysis. While there are several advantages to selecting a hospital-based control group, this strategy may introduce bias by producing a comparison population that is not representative of the study base in terms of the exposure of interest(25). Perrillat et al.(19) in a matched case–control study conducted in France, also enrolled control children who were hospitalised in the same hospital as the cases(19). The authors noted that special care was paid to selecting an appropriate control group by including many different diagnostic categories to avoid potential selection bias in the event that a particular disease was related to the exposures of interest. They found a marginally significant reduced risk of acute leukaemia associated with day-care attendance (OR 0.6, 95% CI 0.4–1.0).
In another French study conducted in an independent and slightly larger population-based group of children, Jourdan-Da Silva et al.(16) also found a reduced risk of ALL where the association was strongest when day-care was started early (<3 months of age versus never: OR 0.6, 95% CI 0.4–0.8) . They report a statistically significant trend with decreasing age of starting day-care. These results were similar when restricting the analysis to c-ALL. In a Canadian study, Infante-Rivard et al.(15) found that children who entered day-care at 2 y of age or younger had a reduced risk of ALL compared to children with no day-care attendance (OR 0.49, 95% CI 0.31–0.77) . Children who entered day-care at greater than 2 y of age also had a reduced risk, but the association was only marginally significant. In another analysis, they chose a priori to stratify children into two groups at 4 y of age because this age corresponds to the beginning of the pre-school years, a time when social contacts for children are likely to change. The risk estimates in these two groups were similarly reduced, but the effect appeared stronger among children <4 y of age (OR 0.39, 95% CI 0.15–1.04).
Recently, in the large United Kingdom Childhood Cancer Study (UKCCS), Gilham et al.(14) evaluated the effect of social activities, including day-care attendance specifically during the first year of life. The large sample of 1272 ALL cases were further classified into c-ALL and into two common cytogenetic sub-types of ALL, those with the TEL-AML1 translocation and those with hyperdiploidy. Based on their collected data, a hierarchical variable reflecting a child's overall social activity was defined based on interview data incorporating information on the frequency of regular activity with children outside of the home, frequency of attendance at a day nursery or nursery school and number of other children in attendance. These analyses indicated that social activity/day-care attendance was associated with a significantly reduced risk of all sub-types of leukaemia examined (formal day care versus no social activity in ALL: OR 0.48 95% CI 0.37–0.62).
In contrast to the compelling UKCCS results, two other large studies found little or no evidence of an association between day-care attendance/social activity and risk of childhood leukaemia. In 1999, Schuz et al.(24) published the results from a population-based German study with 921 acute leukaemia cases, including 658 c-ALL cases. This matched case–control study did not collect self-reported day-care attendance information, but instead created a ‘deficit in social contacts’ variable based on the assumption that children were likely to have attended day-care if during the first 2 y of their life both parents were in full-time work(24). The assumption made in the formulation of this social contact variable likely contributed some non-differential misclassification which tends to bias findings towards one of no effect. The case–control analysis did not show evidence of an association between deficit in social contact and risk of acute leukaemia (OR 1.1, 95% CI 0.9–1.3) or c-ALL (OR 1.0, 95% CI 0.8-1.2). The following year, there was a report from another large case–control study conducted through the Children's Cancer Group (CCG) in the USA which enrolled a series of 1744 ALL cases including 633 c-ALL cases and matched controls selected using random digit dialling (RDD)(18). In the CCG study, the investigators provided a comprehensive evaluation by leukaemia sub-type, timing of exposure and duration (months attended in day care). Their thorough evaluation of day-care attendance in total ALL, c-ALL and other ALL, provided no support for a role of infections in the risk of childhood leukaemia. There was, however, evidence of an association with a history of ear infections before the age of 2 y, with a decreasing risk associated with a greater number of reported ear infections. Despite this association, the CCG study authors were conservative in their conclusion, stating that the results provide little support for a relationship between early childhood infections and the risk of childhood ALL. Growing evidence now suggests that the use of RDD in control recruitment may result in a control group biased with respect to certain population characteristics that may be associated with exposures of interest(9,26).
In the only study of day-care attendance and childhood leukaemia conducted in Asia (Hong Kong), Chan et al.(12) constructed a 3-category index and family day-care variable which incorporated information on day-care attendance and informal social contacts, as well as the age of siblings. The authors specifically noted that the controls collected using RDD may have resulted in the over-representation of control children living in households with fewer children, and in response, all analyses in their study have been adjusted for the number of children in the household. The study specifically focused on two time periods of exposure, the first year of life and the last year before diagnosis. Overall, their results do not indicate a consistent association with day-care attendance for either ALL or c-ALL. With a total acute leukaemia series of 98 cases, statistical power may have affected their ability to detect significant associations. Furthermore, biased results from control selection cannot be ruled out as one factor that may have influenced the null findings from the CCG and Hong Kong studies.
Similar types of systematic biases resulting in socioeconomic differences between cases and controls have been implicated in other studies as well, including the large UKCCS(14) and the NCCLS(9,10). Adjustments for these differences have been implemented in the analyses, however, the possibility of residual effects cannot be ruled out. To alleviate some of this concern, results of the sub-group analysis conducted in the NCCLS among matched cases and controls who had similar annual household income showed that the pattern of association with day-care attendance persisted.
The following three studies did not find statistically significant evidence in support of the infectious hypothesis; however, in two of the three studies, small sample size may have limited their ability to detect a significant association. In an early report of a small study conducted in the UK, Roman et al.(22) reported no association in a series of 38 cases of ALL and 112 individually matched controls. The risk estimate associated with the child's attendance at a pre-school or playgroup during the year before diagnosis was reduced but lacked precision (OR 0.6, 95% CI 0.2–1.8). Dockerty et al.(13), in their population-based study of 90 cases and matched controls conducted in New Zealand, also reported a non-significant reduced risk of childhood ALL associated with children having regular contact outside the home, including attendance in day-care or other similar types of settings (OR 0.65, 95% CI 0.36–1.17). In a larger study of 255 cases of ALL and 760 frequency-matched controls conducted in the USA (New York), Rosenbaum et al.(23) evaluated the duration of out-of-home care categorised into stayed home, 1–18 months, 19–36 months and >36 months. Compared with children with >36 months of out-of-home care, children who had less out-of-home care, although elevated, were not found to be at a significantly increased risk of ALL.
Finally, in the most recent epidemiological study of day-care attendance and risk of childhood ALL published in 2008, Kamper-Jorgensen et al.(17) reported results of the first record-based day-care attendance study. Their population–based matched case-control study was nested within the cohort of all Danish children during the period 1989–2004 and included a total of 176 ALL cases and 1571 individually matched controls with complete childcare attendance data. Childcare attendance information during the first 2 y of life was obtained through the Childcare Database. A statistically significant reduced risk of childhood ALL was found for childcare attendance during the first 2 y of life compared with no childcare attendance during this time (OR 0.68, 95% CI 0.48–0.95). Several sub-type-specific analyses showed the strongest associations in B-cell precursor ALL and c-ALL.
CONCLUSIONS AND FUTURE DIRECTIONS
The majority of the studies on day-care attendance were conducted with the a priori objective of testing the biologically plausible ‘delayed infection’ hypothesis, which specifies a predicted direction of risk, timing of the exposure and the most applicable sub-type of leukaemia. Overall, the published studies have shown consistency in support of this hypothesis, with the majority of studies either reporting a statistically significant effect in the hypothesised direction or no significant association which, in many instances, can plausibly be explained by potential bias, lack of precision or both. As hypothesised, in the studies showing evidence of an association, the effects were observed for both ALL and c-ALL and in some studies were more strongly reduced in c-ALL. Furthermore, several individual studies which used detailed exposure assessment protocols demonstrated evidence of dose–response effects. For example, statistically significant trends were observed for increasing levels of child-hours of day-care attendance(10), levels of social activity(14) and age at start of day-care(16). While it is unlikely that confounding is a major source of bias in these studies, the influence of residual confounding cannot be ruled out. Some form of adjustment for confounding either through matching, stratification or adjustment during the data analysis was performed by all studies. In addition to age, sex and race/ethnicity, which were usually addressed by matching, socioeconomic status is considered to be a potential confounding factor. In terms of timing of the exposure, children that have attended day-care are assumed to have been exposed to infections at an earlier age compared with those who have not attended day-care. Thus, the observed association already reflects the critical nature of the timing of exposure. While some studies, including the NCCLS, provide evidence that the age at start of day-care may also be important in determining risk, this issue warrants further examination as most studies have not thoroughly addressed this. A meta-analysis is currently underway, which will allow for a quantitative evaluation of the overall consistency and strength of the association between day-care attendance or social contact measures and risk of childhood ALL and c-ALL. Using a series of sub-group and sensitivity analyses, the influence of differences in the study design and analytical characteristics on the combined risk estimate will be examined, namely, the impact of the larger studies, timing of exposure, potential biases in the selection of controls and the classification of leukaemia and day-care attendance.
As an indirect measure of exposure to infections, the ability of day-care attendance to act appropriately as a strong surrogate measure may vary depending on several characteristics of the facility attended and the child's pattern of attendance. This is well-documented in the epidemiological literature on childcare facilities and infections in children(7,8), indicating that the transmission and development of infectious disease are highly influenced by the age of the child, frequency and duration of attendance, structure and size of the facility. Future epidemiological studies on childhood leukaemia examining exposure to infections using this surrogate measure should attempt to obtain this type of detailed information on the facilities attended to refine the exposure classification. In addition, while day-care attendance and other social activities outside the home are generally considered a strong surrogate measure of exposure to infections early in life, they are not the only sources of exposure. Among the various possible sources, exposure to infections can commonly occur through contact with older siblings and other children living in the home(2) and the child's parents and other adults the child is frequently in contact with(27). In a nationwide survey, one study showed that among children aged 18–35 months, childcare exposure was a significant risk factor for respiratory tract illness only in children who did not have an older sibling(28). These results suggest that future studies of childhood leukaemia should account for the child's multiple possible sources of exposure when considering a full exposure history. Finally, the hypothesised causal nature of the link between infections and childhood leukaemia would be strengthened by the identification of a plausible biological mechanism for the conversion of pre-leukaemic cells following infectious exposure(1) and by the incorporation of genetic biomarkers of susceptibility and immune response into further epidemiological studies.
FUNDING
The study was supported by grants from the National Institute of Environmental Health Sciences (PS42 ES04705, R01 ES09137) and the Children with Leukaemia Foundation (UK).
REFERENCES
- 1.Greaves M. Infection, immune responses and the aetiology of childhood leukaemia. Nat. Rev. Cancer. 2006;6:193–203. doi: 10.1038/nrc1816. [DOI] [PubMed] [Google Scholar]
- 2.McNally R. J., Eden T. O. An infectious aetiology for childhood acute leukaemia: a review of the evidence. Br. J. Haematol. 2004;127:243–263. doi: 10.1111/j.1365-2141.2004.05166.x. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor S. M., Boneva R. S. Infectious etiologies of childhood leukaemia: plausibility and challenges to proof. Environ. Health Perspect. 2007;115:146–150. doi: 10.1289/ehp.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinlen L. Evidence for an infective cause of childhood leukaemia: comparison of a Scottish new town with nuclear reprocessing sites in Britain. Lancet. 1988;2:1323–1327. doi: 10.1016/s0140-6736(88)90867-7. [DOI] [PubMed] [Google Scholar]
- 5.Greaves M. Speculations on the cause of childhood acute lymphoblastic leukaemia. Leukaemia. 1988;2:120–125. [PubMed] [Google Scholar]
- 6.MacKenzie J., Greaves M. F., Eden T. O., Clayton R. A., Perry J., Wilson K. S., Jarrett R. F. The putative role of transforming viruses in childhood acute lymphoblastic leukaemia. Haematologica. 2006;91:240–243. [PubMed] [Google Scholar]
- 7.Holmes S. J., Morrow A. L., Pickering L. K. Child-care practices: effects of social change on the epidemiology of infectious diseases and antibiotic resistance. Epidemiol. Rev. 1996;18:10–28. doi: 10.1093/oxfordjournals.epirev.a017913. [DOI] [PubMed] [Google Scholar]
- 8.Osterholm M. T. Infectious disease in child day-care: an overview. Pediatrics. 1994;94:987–990. [PubMed] [Google Scholar]
- 9.Ma X., Buffler P. A., Layefsky M., Does M. B., Reynolds P. Control selection strategies in case-control studies of childhood diseases. Am. J. Epidemiol. 2004;159:915–921. doi: 10.1093/aje/kwh136. [DOI] [PubMed] [Google Scholar]
- 10.Ma X., Buffler P. A., Wiemels J. L., Selvin S., Metayer C., Loh M., Does M. B., Wiencke J. K. Ethnic difference in daycare attendance, early infections, and risk of childhood acute lymphoblastic leukaemia. Cancer Epidemiol. Biomarkers Prev. 2005;14:1928–1934. doi: 10.1158/1055-9965.EPI-05-0115. [DOI] [PubMed] [Google Scholar]
- 11.Ma X., Buffler P. A., Selvin S., Matthay K. K., Wiencke J. K., Wiemels J. L., Reynolds P. Daycare attendance and risk of childhood acute lymphoblastic leukaemia. Br. J. Cancer. 2002;86:1419–1424. doi: 10.1038/sj.bjc.6600274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan L. C., et al. Is the timing of exposure to infection a major determinant of acute lymphoblastic leukaemia in Hong Kong? Paediatr. Perinat. Epidemiol. 2002;16:154–165. doi: 10.1046/j.1365-3016.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- 13.Dockerty J. D., Skegg D. C., Elwood J. M., Herbison G. P., Becroft D. M., Lewis M. E. Infections, vaccinations, and the risk of childhood leukaemia. Br. J. Cancer. 1999;80:1483–1489. doi: 10.1038/sj.bjc.6690548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilham C., Peto J., Simpson J., Roman E., Eden T. O., Greaves M. F., Alexander F. E. Day-care in infancy and risk of childhood acute lymphoblastic leukaemia: findings from UK case-control study. BMJ. 2005;330:1294. doi: 10.1136/bmj.38428.521042.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Infante-Rivard C., Fortier I., Olson E. Markers of infection, breast-feeding and childhood acute lymphoblastic leukaemia. Br. J. Cancer. 2000;83:1559–1564. doi: 10.1054/bjoc.2000.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jourdan-Da Silva N., et al. Infectious diseases in the first year of life, perinatal characteristics and childhood acute leukaemia. Br. J. Cancer. 2004;90:139–145. doi: 10.1038/sj.bjc.6601384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamper-Jorgensen M., Woodward A., Wohlfahrt J., Benn C. S., Simonsen J., Hjalgrim H., Schmiegelow K. Childcare in the first 2 years of life reduces the risk of childhood acute lymphoblastic leukaemia. Leukaemia. 2008;22:189–193. doi: 10.1038/sj.leu.2404884. [DOI] [PubMed] [Google Scholar]
- 18.Neglia J. P., Linet M. S., Shu X. O., Severson R. K., Potter J. D., Mertens A. C., Wen W., Kersey J. H., Robison L. L. Patterns of infection and day care utilization and risk of childhood acute lymphoblastic leukaemia. Br. J Cancer. 2000;82:234–240. doi: 10.1054/bjoc.1999.0905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrillat F., et al. Day-care, early common infections and childhood acute leukaemia: a multicentre French ase-control study. Br. J. Cancer. 2002;86:1064–1069. doi: 10.1038/sj.bjc.6600091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petridou E., Kassimos D., Kalmanti M., Kosmidis H., Haidas S., Flytzani V., Tong D., Trichopoulos D. Age of exposure to infections and risk of childhood leukaemia. BMJ. 1993;307:774. doi: 10.1136/bmj.307.6907.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petridou E., et al. The risk profile of childhood leukaemia in Greece: a nationwide case-control study. Br. J. Cancer. 1997;76:1241–1247. doi: 10.1038/bjc.1997.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roman E., Watson A., Bull D., Baker K. Leukaemia risk and social contact in children aged 0-4 years in southern England. J. Epidemiol. Community Health. 1994;48:601–602. doi: 10.1136/jech.48.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenbaum P. F., Buck G. M., Brecher M. L. Early child-care and preschool experiences and the risk of childhood acute lymphoblastic leukaemia. Am. J. Epidemiol. 2000;152:1136–1144. doi: 10.1093/aje/152.12.1136. [DOI] [PubMed] [Google Scholar]
- 24.Schuz J., Kaletsch U., Meinert R., Kaatsch P., Michaelis J. Association of childhood leukaemia with factors related to the immune system. Br. J. Cancer. 1999;80:585–590. doi: 10.1038/sj.bjc.6690395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Infante-Rivard C. Hospital or population controls for case-control studies of severe childhood diseases? Am. J. Epidemiol. 2003;157:176–182. doi: 10.1093/aje/kwf174. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg E. R. Random digit dialing for control selection. A review and a caution on its use in studies of childhood cancer. Am. J. Epidemiol. 1990;131:1–5. doi: 10.1093/oxfordjournals.aje.a115462. [DOI] [PubMed] [Google Scholar]
- 27.Chang J. S., Metayer C., Fear N. T., Reinier K., Yin X., Urayama K., Russo C., Jolly K. W., Buffler P. A. Parental social contact in the work place and the risk of childhood acute lymphoblastic leukaemia. Br. J. Cancer. 2007;97:1315–1321. doi: 10.1038/sj.bjc.6604024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurwitz E. S., Gunn W. J., Pinsky P. F., Schonberger L. B. Risk of respiratory illness associated with day-care attendance: a nationwide study. Pediatrics. 1991;87:62–69. [PubMed] [Google Scholar]