Abstract
Purpose
To interrogate grade II, III, and IV gliomas and characterize the critical effectors within the PI3-kinase pathway upstream and downstream of mTOR.
Experimental design
Tissues from 87 patients who were treated at UCSF between 1990 and 2004 were analyzed. Twenty-eight grade II, 17 grade III glioma, 26 grade IV gliomas, and 16 non-tumor brain specimens were analyzed. Protein levels were assessed by immunoblots; RNA levels were determined by polymerase chain reaction amplification. To address the multiple comparisons, first an overall analysis was done comparing the four groups using Spearman’s Correlation Coefficient. Only if this analysis was statistically significant were individual pairwise comparisons done.
Results
Multiple comparison analyses revealed a significant correlation with grade for all variables examined, except phosphorylated-S6. Expression of phosphorylated-4E-BP1, phosphorylated-PKB/Akt, PTEN, TSC1, and TSC2 correlated with grade (P < 0.01 for all). We extended our analyses to ask whether decreases in TSC proteins levels were due to changes in mRNA levels, or due to changes in post-transcriptional alterations. We found significantly lower levels of TSC1 and TSC2 mRNA in GBMs than in grade II gliomas or non-tumor brain (P < 0.01).
Conclusions
Expression levels of critical signaling molecules upstream and downstream of mTOR differ between non-tumor brain and gliomas of any grade. The single variable whose expression did not differ between non-tumor brain and gliomas was phosphorylated-S6, suggesting that other protein kinases, in addition to mTOR, contribute significantly to S6 phosphorylation. mTOR provides a rational therapeutic target in gliomas of all grades, and clinical benefit may emerge as mTOR inhibitors are combined with additional agents.
Keywords: Phosphatidylinositol 3-kinase, Gliomas, Signal transduction, mTOR
Introduction
For glioma patients, tumor grade is a longstanding key determinant of clinical outcome. More recently, molecular characteristics of individual tumors have provided insight into the clinical course and prognosis of patients. Such molecular features can help predict not only the natural history of a glioma but also the likelihood that it will respond to conventional chemotherapeutic alkylating agents [1], and novel molecularly targeted therapies [2, 3]. Key among such molecular features is the status of the phosphoinositide 3-kinase (PI3-kinase) signaling pathway, whose deregulation in gliomas plays critical roles influencing prognosis and determining response.
The PI3-kinase pathway is frequently aberrantly activated in gliomas. PTEN is the lipid phosphatase that antagonizes PI3-kinase activity by dephosphorylating the 3′ phosphate of the product of PI3-kinase activity–phosphatidylinositol (3,4,5) trisphosphate (PtdIns (3,4,5) P3). PTEN thus functions as a tumor suppressor protein in gliomas, among many other human malignancies. We previously reported that glioblastoma (GBM), the highest-grade glioma, has decreased PTEN expression that correlates with poor patient survival [4]. Whereas the role of PTEN in the behavior and prognosis of GBMs has been well established, its role in the development and progression of lower-grade gliomas remains unclear.
Elements within the PI3-kinase cascade, in addition to PTEN, likely contribute to the development, clinical behavior, and therapeutic response of gliomas. The best-characterized downstream effector of PI3-kinase is protein kinase B (PKB/Akt). As expected, activity of PKB/Akt, reflected in its state of phosphorylation, inversely correlates with PTEN expression in GBM cell lines [5] and primary tumors [4, 6]. A critical effector of PI3-kinase and PKB/Akt activities is mammalian target of Rapamycin (mTOR). mTOR phosphorylates at least two substrates, p70S6 kinase and the translational repressor eukaryotic initiation factor 4E binding protein 1 (4E-BP1) [7]. Although S6 ribosomal protein (S6) is phosphorylated by p70S6 kinase and therefore lies downstream of mTOR, previous studies have not found a correlation between S6 phosphorylation and either PTEN levels or PKB/Akt phosphorylation in primary GBM specimens [6], likely reflecting the importance of other pathways in controlling mTOR activity in these tumors. Improved understanding and greater insight into these regulatory pathways are critical for determining patient response in ongoing and future clinical trials of mTOR inhibitors.
Key regulators of mTOR activity are the tumor suppressor genes TSC1 and TSC2. Germline mutations in these genes cause tuberous sclerosis (TSC), a disorder characterized by various tumors, predominantly benign in nature, including hamartomas and lymphangioleiomyomas (LAM) [8]. TSC1 and TSC2 encode the proteins hamartin and tuberin, respectively, that form a heterodimeric protein complex and inhibit mTOR activity. Tuberin possesses a GTPase activating domain that acts on the small GTPase Rheb [9]. Increased levels of GTP-bound Rheb are thought to increase mTOR activity through as-of-yet unclear mechanisms. Although mutations in TSC1 and TSC2 are found in tuberous sclerosis and LAM, mutations in these genes are extremely rare in malignant tumors, reported in only bladder carcinomas, at a frequency of less than 10% [10]. However, mechanisms other than gene mutation or loss of heterozygosity (LOH) may play a role in reducing expression of hamartin and/or tuberin in a larger spectrum of human tumors.
mTOR has recently emerged as an attractive target for therapeutic intervention in human tumors [11]. While there are no tumor-associated activating somatic mutations documented in mTOR, its biochemical location downstream of activated growth factor receptors and PI3-kinase leads to elevated mTOR activity in many tumors. Documentation of increased mTOR activity in many human malignancies has propelled the introduction of several mTOR inhibitors into clinical practice. Among these, rapamycin is a bacterially derived macrocyclic compound that in complex with a cellular protein FKBP12 binds mTOR and displaces its substrate-targeting subunit raptor. Rapamycin thus leads to dephosphorylation of S6 and 4E-BP1, and a consequent decrease in cellular protein translation. Rapamycin treatment decreases growth and proliferation of many cancer cells, and can increase cellular susceptibility to apoptosis, lending enthusiasm to its use as an anti-cancer drug.
Gliomas constitute an attractive malignancy for the incorporation of rapamycin therapy. PTEN mutation, a genetic aberration frequently found in gliomas, appears to render tumors particularly susceptible to mTOR inhibition [12]. In addition, rapamycin and its analogues may shift GBM differentiation from astrocytic to oligodendroglial lineages in some tumor cells [13]. Rapamycin and its derivatives are currently in clinical trials for multiple indications, including malignant gliomas, and although they have demonstrated clinical promise, their potential has not been fully characterized. A more comprehensive understanding of the signaling elements affected by mTOR inhibitors will likely allow more successful exploitation of these anti-neoplastic agents. We have therefore undertaken an analysis of the levels and phosphorylation status of proteins upstream and downstream of mTOR in a panel of glioma specimens. Evaluation of these proteins in gliomas of various grades may help shed light on their roles in glioma initiation and progression. Elucidation of the biochemical relationships among these signaling molecules and their associations with patient survival may help identify appropriate pathways and substrates for novel therapeutic agents.
Materials and methods
Patients and tissue samples
Tissues from 87 patients who were treated at the University of California, San Francisco (UCSF) between 1990 and 2004 were analyzed. Forty-six of these patients had been analyzed in a previous study. We acquired 28 grade II gliomas, 17 grade III gliomas, 26 GBMs and 16 non-tumor brain samples, all obtained at initial diagnosis. All non-tumor brain specimens were acquired from surgical resections for seizure disorders. All specimens were frozen immediately after surgery and stored at −80°C at the UCSF Tumor Research Center Tissue Bank. Institutional board approval was obtained prior to study initiation. Formalin-fixed, paraffin-embedded sections were prepared for all tissues. All tumor sections were examined by a neuropathologist to verify the samples consisted of >70% tumor. Non-tumor brain specimens were acquired from 16 patients undergoing surgery for epilepsy and were reviewed to verify the absence of tumor.
Tissue homogenization
Frozen tissue specimens were thawed and homogenized. Portions measuring 100–300 mg of each tissue sample were manually homogenized in a Dounce homogenizer in 0.2–1 ml of 1% NP40 lysis buffer [17.7 ml sterile H2O, 25 mM NAF, 150 mM NaCl, 20 mM Tris-pH 8.0, 1 mM NaVO4, 1% Nonidet P40, 1 mM EGTA-pH 8.0, 1 mM EDTA, 1 mM DTT, 1 pill protease inhibitors (complete mini, Roche)], transferred to microfuge tubes, and incubated for at least 10 min on ice. The lysates were further homogenized by sonication for 15 s using a Sonic Dismembrator (Fisher Scientific) set to 4.5. Lysates were cooled down on ice for 15 s, sonicated again for 15 s, and then placed on ice for at least 15 min. Microfuge tubes were spun for 15 min at 13,000 × g at 4°C and supernatants were transferred into new microfuge tubes.
Immunoblot analysis
Protein concentrations for all lysates were quantified using the Lowry assay (Bio-Rad). Expression levels of PTEN, phosphorylated-S6, phosphorylated-PKB/Akt and phosphorylated-4E-BP1 were determined for all grade II, grade III, GBM and non-tumor brain samples by immunoblot analyses. In addition, TSC1 and TSC2 levels were determined for non-tumor brain, grade II and GBM samples. Expression of these proteins was normalized to β-actin levels. Cell lysates were diluted 1:1 in SDS-PAGE loading buffer. About 10–20 μg of protein per tissue lysate were electrophoresed on a 4–20% Tris/glycine SDS-polyacrylamide gel and transferred to Immobilon P PVDF membrane. Membranes were blocked with 5% non-fat dry milk in TBST (Tris-buffered saline containing 0.1% Tween 20), followed by incubation with primary antibody in either 5% bovine serum albumin or 5% non-fat dry milk in TBST, depending on the primary antibody used. Membranes were incubated overnight and after two short washes and three 10 min washes with 0.5% non-fat dry milk in TBST, were incubated with secondary antibody diluted in 5% nonfat dry milk in TBST. Membranes were then washed with TBST for two short washes and three 10 min washes. The ECL Detection System for Western blot Analysis (Amersham) was followed according to the manufacturer’s instructions for antibody detection. Films were developed and scanned into the computer hard drive. Scion image computer software from Meyer Instruments, Inc. was used to quantify bands of appropriate sizes. The following primary antibodies were used: anti-β-actin (Sigma Chemical Co.), anti-phosphorylated-S6 (Cell Signaling), anti-phosphorylated-PKB/Akt (from our labs), anti-phosphorylated-4E-BP1 (Cell Signaling), anti-PTEN (Cell Signaling), anti-TSC1 (a generous gift from Jeffrey DeClue), and anti-TSC2 (Santa Cruz Biotechnology).
Measurement of TSC1 and TSC2 RNA levels
Total RNA was extracted from non-tumor brain and tumor specimens using TRIzol reagent (Invitrogen Life Technology, Carlsbad, CA) and purified on RNeasy columns (Qiagen). One μg of total RNA was used for reverse transcription followed by PCR amplification using gene specific primers in the presence of a fluorogenic Taqman™ probe (Applied Biosystems Inc.). Values were normalized relative to the expression of β-glucuronidase amplified in the same RT-PCR reaction.
Statistical analysis
Expression of the individual protein bands of PTEN, TSC1, TSC2, phosphorylated-S6, phosphorylated-PKB/Akt, and phosphorylated-4E-BP1 were normalized to β-actin levels. All the individual protein bands on various immunoblots were subsequently normalized to one another by quantifying and then normalizing the data to control tumor samples. These tumor samples and controls were used as the pivot points for normalization of respective protein bands across different immunoblots, because no single blot could accommodate all samples in this study.
SPSS for Windows, Version 10.0.07 (SPSS, Inc., Chicago, IL) was used for statistical analyses and preparation of figures. To address the multiple comparisons issue, first an overall analysis was done comparing the four groups using Spearman’s Correlation Coefficient. Only if this analysis was statistically significant were individual pairwise comparisons done using the Kruskal–Wallis test. To assess correlation between molecular markers, the Spearman’s Correlation Coefficient was used including all samples. We then tested for correlations using the Cochran–Mantel–Haenszel test to adjust for grade.
All P-values reported are from two-tailed hypothesis tests. The actual value for expression levels was used as a continuous variable for these analyses. Survival was measured from date of surgery. For the assessment of expression/activity as predictors among GBM cases, Cox Regression analyses were done. Deaths were verified by examination of medical records and the Social Security Death Index. In cases where no death could be confirmed, patients were censored at the date last known to be alive.
Results
Correlations between signaling molecules and glioma grade
Current clinical trials are evaluating the efficacy of agents that inhibit the activity of mTOR in gliomas. We characterized the status of upstream regulators and downstream effectors of mTOR in gliomas of all grades as well as in non-tumor brain specimens. Although key elements downstream of PI3-kinase are deregulated in GBMs, the status of such molecules in lower grade gliomas remains largely unknown. We characterized phosphorylated-4E-BP1 and phosphorylated-S6 as well as β-actin as a loading control, in 28 GBMs, 17 grade III gliomas, 26 grade II gliomas, and 16 non-tumor brain specimens.
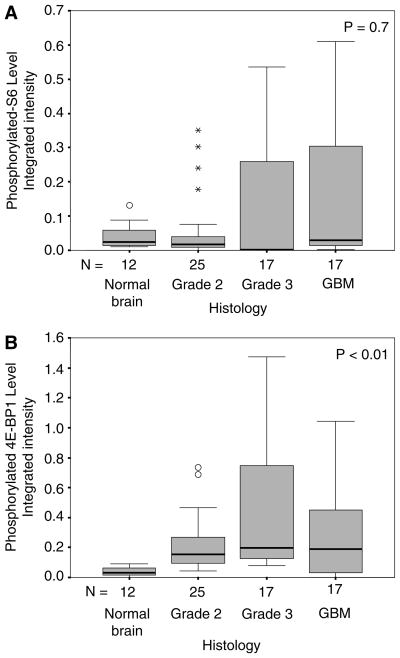
We asked whether expression levels of these phosphorylated proteins, which reflects their activation, correlate with histologic grade. Expression levels of phosphorylated-4E-BP1 and phosphorylated-S6 are displayed as boxplots (Fig. 1a, b) according to histologic grade (non-tumor brain, grade II glioma, grade III glioma, and GBM) and analyzed by the Spearman’s Correlation Coefficient test. Within a boxplot, the black horizontal line represents the median value, the gray box represents the middle 50% of values lying between 25 and 75%, and the horizontal gray lines represent minimum and maximum values. Outliers (defined as data points more than two standard deviations from the median) are shown as open circles (◦), and extreme values (defined as data points more than three standard deviations from the median) are shown as stars (*). Immunoblots from which expression levels of phosphorylated-4E-BP1 and phosphorylated-S6 were quantitated, and then normalized as described in materials and methods above, are shown in Supplemental Fig. 1a, b.
Fig. 1.
Boxplots depicting, (a) phosphorylated-S6 levels and (b) phosphorylated-4E-BP1 levels, in non-tumor brain, grade II gliomas, grade III gliomas, and glioblastoma (GBM). Black horizontal line represents the median value for each group; gray box represents the middle 50% of the values, lying between 25 and 75%; horizontal gray lines represent the minimum and maximum values observed in each group; (o) represents outlier greater than two standard deviations from the median; (*) represents extreme value greater than three standard deviations from the median. Statistical analysis was performed using the Spearman’s Correlation Coefficient
Multiple comparison analyses, including all four groups (non-tumor brain, grade II, III, and IV gliomas) revealed a significant correlation with grade for phosphorylated-4E-BP1 (P < 0.01), but not for phosphorylated-S6 (P = 0.7; Table 1). This correlation of phosphorylated-4E-BP1 levels with grade was driven chiefly by differences between non-tumor brain and grade II, III, and IV gliomas (P < 0.01, P < 0.01, P = 0.02, respectively); there were no differences in expression of phosphorylated-4E-BP1 between the various grade gliomas (P = 0.16 for grade II vs. III; P = 0.91 for grade II vs. IV; P = 0.22 for grade III vs. IV).
Table 1.
Correlations between signaling molecules and glioma grade
| Groups | Normal vs. tumor P-value | Spearman’s Correlation Coefficient |
P values for pairwise comparisons |
||||||
|---|---|---|---|---|---|---|---|---|---|
| SCC | P-value | Normal vs Gr. 2 | Normal vs Gr. 3 | Normal vs Gr. 4 | Gr. 2 vs Gr. 3 | Gr. 3 vs Gr. 4 | Gr. 2 vs Gr. 4 | ||
| pS6 | 0.433 | 0.049 | 0.687 | 0.230 | 0.182 | 0.478 | 0.212 | 0.028 | 0.121 |
| p4E-BP1 | 0.000 | 0.334 | 0.004 | 0.000 | 0.000 | 0.020 | 0.155 | 0.221 | 0.908 |
| pPKB | 0.005 | 0.396 | 0.000 | 0.123 | 0.006 | 0.002 | 0.011 | 0.502 | 0.014 |
| PTEN | 0.093 | −0.517 | 0.000 | 0.845 | 0.451 | 0.000 | 0.233 | 0.005 | 0.000 |
| TSC1 mRNA | 0.000 | −0.736 | 0.000 | 0.483 | N/A* | 0.000 | N/A* | N/A* | 0.001 |
| TSC1 protein | 0.000 | −0.694 | 0.000 | 0.224 | N/A* | 0.000 | N/A* | N/A* | 0.000 |
| TSC2 mRNA | 0.002 | −0.578 | 0.000 | 0.586 | N/A* | 0.000 | N/A* | N/A* | 0.008 |
| TSC2 protein | 0.001 | −0.798 | 0.000 | 0.870 | N/A* | 0.000 | N/A* | N/A* | 0.000 |
No Grade 3 in TSC1 and TSC2 analyses
As signaling upstream of mTOR is known to be deregulated in gliomas, we examined the association between tumor grade and two upstream molecules, PTEN and PKB/Akt. Phosphorylated-PKB/Akt expression was associated with glioma grade (P < 0.01; Table 1). For phosphorylated-PKB/Akt, correlation with grade was driven by marked differences between grade II tumors or non-tumor brain compared to either grade III, or grade IV gliomas (P = 0.01 for grade II versus either grade III or IV; P < 0.01 for non-tumor brain versus grade III or IV). No difference was observed between grade III and IV tumors (P = 0.5) or between non-tumor brain and grade II gliomas (P = 0.12). PTEN expression showed a significant inverse correlation with glioma grade (P < 0.01; Table 1). This correlation was driven by significantly decreased expression levels in grade IV tumors compared to non-tumor brain, grade II, or III gliomas (P < 0.01 for all).
Characterization of TSC1 and TSC2 in gliomas
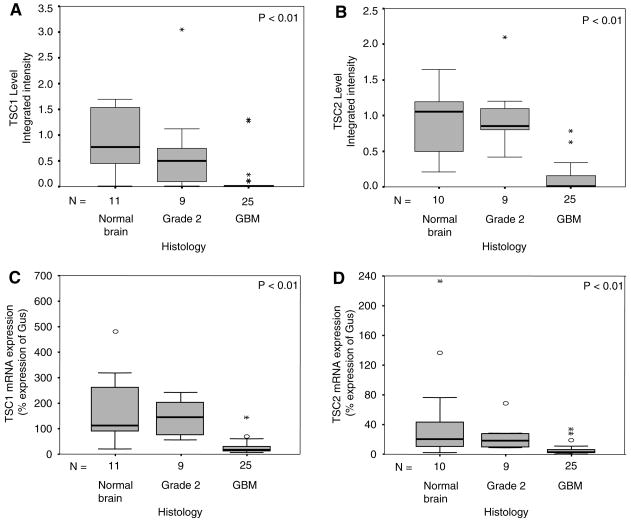
Observed elevations of phosphorylated-4E-BP1 in gliomas likely reflect increased mTOR activity. Hamartin and tuberin, encoded by the tumor suppressor genes TSC1 and TSC2, form a heterodimeric protein complex that inhibits mTOR activity. In evaluating the mechanism of increased mTOR activity documented in gliomas, we asked whether decreased expression levels of hamartin and tuberin may relieve the inhibitory control of mTOR and lead to elevated mTOR activity. Sufficient tissues were available from 25 GBMs, nine grade II gliomas, and 11 non-tumor brain specimens to measure TSC1 and TSC2 protein levels in conjunction with β-actin as a loading control. In addition, sufficient tissues were available from 25 GBMs, six grade II gliomas, and 11 non-tumor brain specimens to measure TSC1 and TSC2 mRNA levels.
TSC1 and TSC2 protein levels inversely correlated with tumor grade (P < 0.01; Fig. 2a; Table 1). Pairwise analyses showed significantly lower levels of TSC1 expression in GBMs than in grade II gliomas or non-tumor brain tissue (P < 0.01 for both pairwise comparisons). Similarly, TSC2 expression levels were lower in GBMs compared to grade II gliomas or non-tumor brain specimens (P < 0.01 for both pairwise comparisons; Fig. 2b). Neither TSC1 nor TSC2 proteins differed between non-tumor brain and grade II tumors (P = 0.22 and P = 0.87, respectively). We next analyzed whether decreases in TSC proteins levels were due to changes in mRNA levels, or due to changes in post-transcriptional alterations such as differences in translation or stability. Figure 2c, d, respectively, show that TSC1 and TSC2 mRNA levels are also decreased in GBM samples compared to grade II gliomas and non-tumor brain tissues (P < 0.01; Table 1).
Fig. 2.
Boxplots depicting, (a) TSC1 expression levels, (b) TSC2 expression levels, (c) TSC1 mRNA levels, and (d) TSC2 mRNA levels in non-tumor brain, grade II gliomas, and GBMs. Depiction is explained in detail in Fig. 1 legend
Correlations among molecules upstream and downstream of mTOR parallel known biochemical functions
In vitro studies are elucidating the relationships among elements within the PI3-kinase cascade. We explored whether biochemical interactions documented in vitro would be reflected in primary tumors. Expression levels of TSC1 and TSC2, both upstream regulators of mTOR, correlated directly with each other (P < 0.01 with and without adjustment for grade). We did not find a similar correlation between phosphorylation of the two downstream effectors of mTOR, S6 and 4E-BP1 (P = 0.06 without adjustment for grade and 0.14 with adjustment for grade).
Next we examined whether there were significant correlations between upstream regulators and downstream effectors of mTOR. The key signaling molecule PKB/Akt positively regulates mTOR by phosphorylating and inactivating TSC2 and by phosphorylating and activating mTOR directly. We therefore conjectured a positive association between expression of phosphorylated-PKB/Akt and the phosphorylation states of downstream targets of mTOR, 4E-BP1 and S6. Indeed, we found a significant direct correlation between levels of phosphorylated-PKB/Akt and phosphorylated-4E-BP1 when all tumors were included in the analysis (P = 0.046), although when adjusted for grade the correlation lost statistical significance (P = 0.10). In contrast, there was no significant association between expression of phosphorylated-PKB/Akt and phosphorylated-S6 (P > 0.2 with and without adjustment for grade).
No correlation between mTOR pathway components and survival
PI3-kinase is a multifactorial signaling molecule with a number of downstream targets, including the mTOR pathway that participates in diverse cellular functions. PTEN expression has previously been shown to correlate with survival in gliomas. We asked whether expression and activity of components of the mTOR pathway would predict patient survival among the GBM patients. There were insufficient numbers of events in the grade II and III cohorts to assess correlation with survival; all 26 GBM patients had died. Using Cox proportional hazards regression analyses we found no significant correlations between TSC1, TSC2, phosphorylated-4E-BP1, or phosphorylated-S6 levels and patient survival (P > 0.2 for all molecular markers).
Discussion
The rapid introduction of mTOR inhibitors into clinical practice and its incorporation into multimodality therapy with additional targeted inhibitors necessitate increased insight into the roles of mTOR, its upstream regulators and downstream effectors, in tumor pathogenesis and progression. In this study we have further demonstrated that increased mTOR activity likely plays an important role in the pathogenesis of adult gliomas. By directly examining surgical specimens of adult gliomas, we have shown that 4E-BP1, a direct downstream substrate of mTOR, exhibits increased phosphorylation in gliomas of different grades, whereas phosphorylation of ribosomal protein S6, itself phosphorylated by the mTOR substrate p70S6K, did not correlate with glioma grade. In addition, TSC1 and TSC2, which form a complex that negatively regulates mTOR activity, demonstrate decreased mRNA and protein levels in GBMs compared to grade II tumors and non-tumor brain.
Our previous study [4], as well as, those of others [6, 14] have shown that PKB/Akt phosphorylation and activity are increased in GBMs relative to non-tumor brain specimens. PKB/Akt activity has been linked to increased mTOR activation through several mechanisms, including direct phosphorylation of mTOR by PKB/Akt [15, 16], PKB/Akt-mediated phosphorylation of TSC2 (reviewed in [17]), and inhibition of AMP kinase, which prevents the phosphorylation and inhibition of TSC2 [18]. We therefore expected a correlation between phosphorylated-PKB/Akt and mTOR activity, as reflected by phosphorylated-4E-BP1. Our results confirm this anticipated correlation, although they do not identify which of the above candidate mechanisms of regulation occur in gliomas.
Interestingly, our analysis of gliomas of various grades failed to reveal a significant correlation between phosphorylated-PKB/Akt and phosphorylated-S6, an observation previously made in studies of GBM [6]. This discrepancy between associations of phosphorylated-PKB/Akt with phosphorylated-4E-BP1 versus phosphorylated S6 may be due to additional protein kinases that phosphorylate S6 in the absence of mTOR activity. For example, the p90rsk family of protein kinases that are regulated through the coordinated activities of ERK1/2 and PDK1 [19] plays an important role in S6 phosphorylation under some circumstances. While S6 is predominantly phosphorylated by p70S6K in most mammalian adult tissues, phosphorylation of S6 by p90rsk is important during meiosis in mammalian and amphibian oocytes [20, 21], and to a lesser extent in growth factor stimulated hepatocytes [22]. Greater insight into the role of p90rsk in phosphorylating S6, and other targets of p70S6K in human tumors, will help design successful treatment strategies utilizing rapamycin analogues as single agents, or in combination with MEK inhibitors.
Although germline mutations in TSC1 and TSC2 are seen in the hamartoma syndrome tuberous sclerosis, mutations in these genes are extremely uncommon in sporadic tumors. None of a myriad of tumors studied to date have demonstrated mutations in TSC1 or TSC2 [10], except for bladder cancer in which only five of 51 tumors showed loss of one copy of TSC1 combined with mutation of the remaining allele [23]. Benign lesions observed in TSC patients rarely progress to malignant tumors, an observation that has been extended to animal models of TSC. The low rate of malignant transformation in these lesions may be due, at least in part, to negative feedback inhibition of PI3-kinase signaling that occurs as a result of increased mTOR activity [24]. Consistent with this hypothesis, TSC2+/− PTEN+/− mice, in which PI3-kinase attenuation is associated with TSC loss, display dramatically enhanced malignant potential compared to TSC2+/− or PTEN+/− mice [25]. It follows that decreased levels of TSC1 and TSC2 may be limited to tumors that have a high frequency of PTEN mutations, such as GBMs. Interestingly, low levels of TSC1 or TSC2 were not seen in low-grade gliomas, consistent with the absence of PTEN mutations seen in these tumors.
Another subtlety in the interaction between TSC1/TSC2 and PTEN is highlighted by recent publications. Such studies suggest that the loss of PTEN in fact is not always sufficient to activate mTOR. In such cases loss of TSC1 and TSC2 would be required to activate mTOR. This point is exemplified by reports of breast tumor cell lines in which PTEN is mutated and PKB/Akt phosphorylation can be decreased using p110β PI3K inhibitors, but these inhibitors do not affect the phosphorylation of mTOR substrates 4EBP1 and S6 (Edgar et al. 2008, AACR abstract LB-26). Finally, Decreased protein levels of TSC1 and TSC2 in GBMs were associated with decreased mRNA levels. Promoter regions of both TSC1 and TSC2 genes contain CpG islands, as defined by the UCSC genome browser (www.genome.ucsc.edu). The analysis of these promoters in GBM samples for methylation therefore seems warranted. Mechanistic issues notwithstanding, our data reveal complex interactions among regulators and effectors of mTOR that contribute to the development of gliomas of all grades.
Supplementary Material
Acknowledgments
This research was supported in part by NIH-PO1 NS-42927-27A2 (D.A.H-K and K.R.L), NIH Brain Tumor SPORE grant P50 CA097257 (D.A.H-K, K.R.L), The Nancy and Stephen Grand Philanthropic Fund and NIH/NCRR UCSF-CTSI UL1 RR024131 (D.A.H-K), CA 82103 (K.R.L. and M.S.B.), RO1CA79548 (D.S.) and DOD TS030017 (D.S.).
Contributor Information
Ralph P. Ermoian, Department of Radiation Oncology, The University of California, San Francisco, 1600 Divisadero St. Suite H1031, San Francisco, CA 94143-1708, USA
Tania Kaprealian, Department of Radiation Oncology, The University of California, San Francisco, 1600 Divisadero St. Suite H1031, San Francisco, CA 94143-1708, USA.
Kathleen R. Lamborn, Department of Neurosurgery, Brain Tumor Research Center, The University of California, San Francisco, 505 Parnassus Ave, Box 0112, San Francisco, CA 94143, USA
Xiaodong Yang, Department of Radiation Oncology, The University of California, San Francisco, 1600 Divisadero St. Suite H1031, San Francisco, CA 94143-1708, USA.
Nannette Jelluma, Department of Radiation Oncology, The University of California, San Francisco, 1600 Divisadero St. Suite H1031, San Francisco, CA 94143-1708, USA.
Nils D. Arvold, Department of Radiation Oncology, The University of California, San Francisco, 1600 Divisadero St. Suite H1031, San Francisco, CA 94143-1708, USA
Ruth Zeidman, Cancer Research Institute, The University of California, San Francisco, 2340 Sutter St, San Francisco, CA 94143, USA.
Mitchel S. Berger, Department of Neurosurgery, Brain Tumor Research Center, The University of California, San Francisco, 505 Parnassus Ave, Box 0112, San Francisco, CA 94143, USA
David Stokoe, Department of Neurosurgery, Brain Tumor Research Center, The University of California, San Francisco, 505 Parnassus Ave, Box 0112, San Francisco, CA 94143, USA. Cancer Research Institute, The University of California, San Francisco, 2340 Sutter St, San Francisco, CA 94143, USA.
Daphne A. Haas-Kogan, Email: dhaaskogan@radonc.ucsf.edu, Department of Radiation Oncology, The University of California, San Francisco, 1600 Divisadero St. Suite H1031, San Francisco, CA 94143-1708, USA. Department of Neurosurgery, Brain Tumor Research Center, The University of California, San Francisco, 505 Parnassus Ave, Box 0112, San Francisco, CA 94143, USA
References
- 1.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J ed. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 2.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 3.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJ Moa051918. [DOI] [PubMed] [Google Scholar]
- 4.Ermoian RP, Furniss CS, Lamborn KR, et al. Dysregulation of PTEN and protein kinase B is associated with glioma histology and patient survival. Clin Cancer Res. 2002;8:1100–1106. [PubMed] [Google Scholar]
- 5.Haas-Kogan DA, Shalev N, Wong M, et al. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr Biol. 1998;8:1195–1198. doi: 10.1016/S0960-9822(07)00493-9. [DOI] [PubMed] [Google Scholar]
- 6.Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- 7.Richardson CJ, Schalm SS, Blenis J. PI3-kinase and TOR: PIKTORing cell growth. Semin Cell Dev Biol. 2004;15:147–159. doi: 10.1016/j.semcdb.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Kwiatkowski DJ. Tuberous sclerosis: from tubers to mTOR. Ann Hum Genet. 2003;67:87–96. doi: 10.1046/j.1469-1809.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 9.Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573–576. doi: 10.1016/j.tibs. 2003.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Knowles MA, Hornigold N, Pitt E. Tuberous sclerosis complex (TSC) gene involvement in sporadic tumours. Biochem Soc Trans. 2003;31:597–602. doi: 10.1042/BST0310597. [DOI] [PubMed] [Google Scholar]
- 11.Houghton PJ, Huang S. mTOR as a target for cancer therapy. Curr Top Microbiol Immunol. 2004;279:339–359. doi: 10.1007/978-3-642-18930-2_20. [DOI] [PubMed] [Google Scholar]
- 12.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA. 2001;98:10314–10319. doi: 10.1073/pnas.1710 76798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu X, Pandolfi PP, Li Y, et al. mTOR promotes survival and astrocytic characteristics induced by PTEN/Akt signaling in glioblastoma. Neoplasia. 2005;7:356–368. doi: 10.1593/neo.04595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakravarti A, Zhai G, Suzuki Y, et al. The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004;22:1926–1933. doi: 10.1200/JCO.2004.07.193. [DOI] [PubMed] [Google Scholar]
- 15.Nave BT, Ouwens M, Withers DJ, et al. Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and aminoacid deficiency on protein translation. Biochem J. 1999;344:427–431. doi: 10.1042/0264-6021:3440427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekulic A, Hudson CC, Homme JL, et al. A direct linkage between the phosphoinositide 3-kinase-Akt signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–3513. [PubMed] [Google Scholar]
- 17.McManus EJ, Alessi DR. TSC1-TSC2: a complex tale of PKB-mediated S6K regulation. Nat Cell Biol. 2002;4:E214–E216. doi: 10.1038/ncb0902-e214. [DOI] [PubMed] [Google Scholar]
- 18.Hahn-Windgassen A, Nogueira V, Chen CC, et al. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 19.Frodin M, Jensen CJ, Merienne K, et al. A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J. 2000;19:2924–2934. doi: 10.1093/emboj/19.12.2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gavin AC, Schorderet-Slatkine S. Ribosomal S6 kinase p90rsk and mRNA cap-binding protein eIF4E phosphorylations correlate with MAP kinase activation during meiotic reinitiation of mouse oocytes. Mol Reprod Dev. 1997;46:383–391. doi: 10.1002/(SICI)1098-2795(199703). 46:3<383::AID-MRD18>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Schwab MS, Kim SH, Terada N, et al. p70(S6 K) controls selective mRNA translation during oocyte maturation and early embryogenesis in Xenopus laevis. Mol Cell Biol. 1999;19:2485–2494. doi: 10.1128/mcb.19.4.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pende M, Um SH, Mieulet V, et al. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hornigold N, Devlin J, Davies AM, et al. Mutation of the 9q34 gene TSC1 in sporadic bladder cancer. Oncogene. 1999;18:2657–2661. doi: 10.1038/sj.onc.1202854. [DOI] [PubMed] [Google Scholar]
- 24.Manning BD. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manning BD, Logsdon MN, Lipovsky AI, et al. Feedback inhibition of Akt signaling limits the growth of tumors lacking TSC2. Genes Dev. 2005;19:1773–1778. doi: 10.1101/gad.1314605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.