Abstract
What a relief! In 1955 the principle of strain release was put forward to explain the differing reactivity of axial and equitorial alcohols during oxidation. Our findings suggest that this same rationale may account for the differing rates of activation between axial and equitorial C–H bonds in C–H activation processes. In conjunction with steric and electronic considerations, strain-release can be used to qualitatively predict relative rates and site specificity of C–H activation in complex settings.
Keywords: natural products, C–H functionalization, conformational analysis
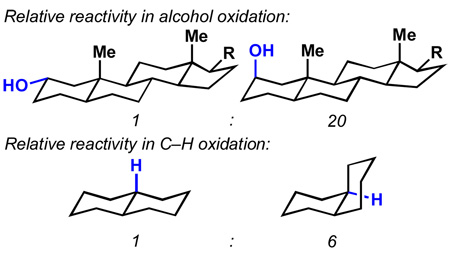
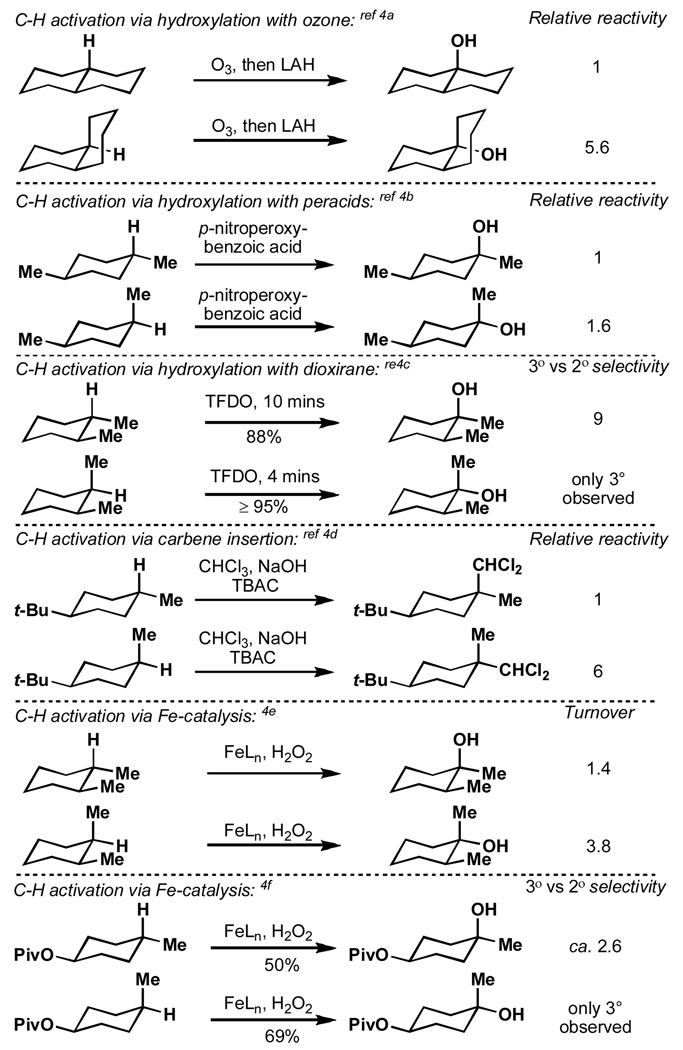
The field of C–H activation and the logic that underlies its use in complex molecule synthesis are developing at a rapid pace.[1] This is due, in part, to the great potential that such transformations could have on the various “economies” of synthesis.[2] Comprehensive observations have pointed to the importance of steric and electronic factors governing the relative rates and selectivities of such reactions. [3] Yet, in order to plan complex molecule total syntheses that utilize one or multiple C–H activation steps, a profound understanding of even the subtlest reactivity trends is needed. In particular, it has been observed on multiple occasions that equatorial C–H bonds react more rapidly than those oriented axially (Figure 1).[4] This curious axial-equatorial rate ratio appears to be independent of the reagent system employed and in some cases can be exploited to achieve site-specific C–H activation.[4c,4f,5] So far, explanations for these “orphan” observations have remained ambigouous. In this Communication, a reactivity factor that apparently has been ignored thus far in this context is proposed, one that we suspect, besides steric hindrance to reagent approach and C–H bond nucleophilicity,[6] to be co-responsible for the more rapid activation of equatorial vs. axial C–H bonds in these tertiary settings.
Figure 1.
Observations from the literature.
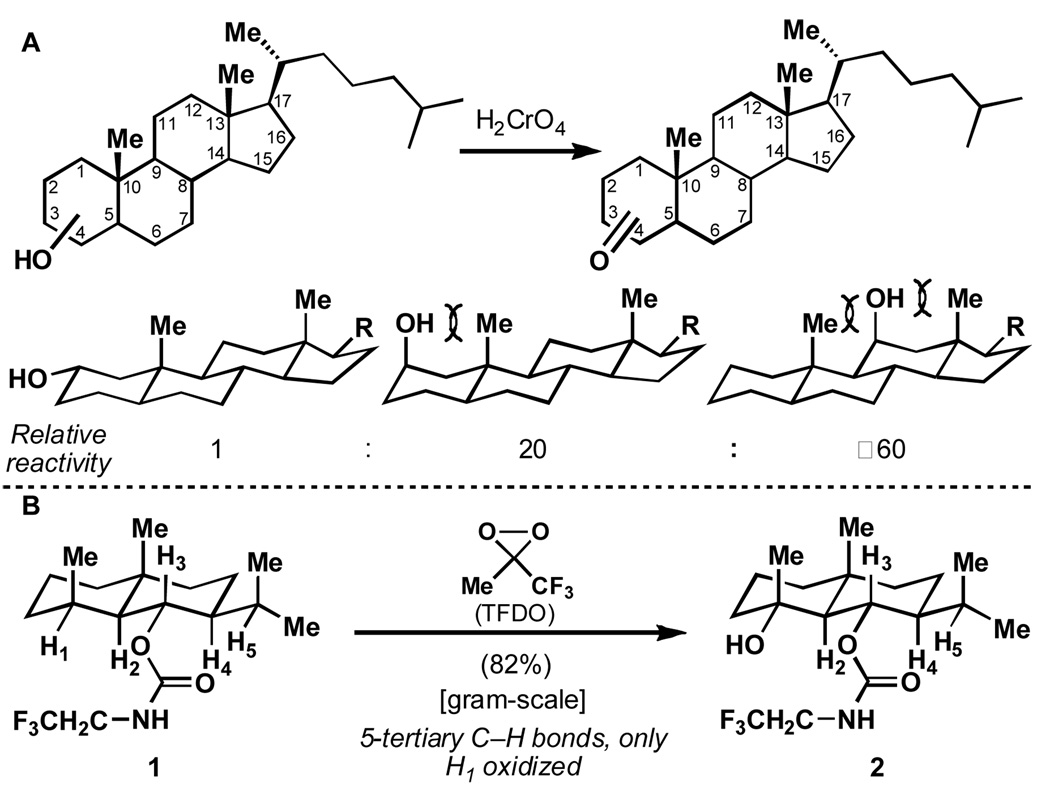
In 1955, one of us proposed strain release (Figure 2A) to explain the relative rates of reactions in which an equatorial hydrogen is also removed more rapidly than its axial counterpart, namely, in the oxidation of steroidal secondary alcohols with chromic acid (see Supporting Information for an English translation of this paper).[7] The rate acceleration in these reactions is attributed to a release of strain (1,3-diaxial interactions) in the transition state of going from an sp3 to an sp2 carbon. At the time, this work convincingly contradicted and corrected the theory[8] according to which the difference in oxidation rate of axial and equatorial alcohols was due to steric hindrance to proton abstraction by a base. In a later paper, the strain-release hypothesis was corroborated in collaboration with F. H.Westheimer and J.Rozek[9a], as well as by work by C. F. Wilcox et al[9b].
Figure 2.
(A) The principle of strain-release during alcohol oxidation; (B) Site-selective C–H oxidation: A strain-release phenomena?
A key step in the total synthesis of eudesmane terpenes[5] caused us to revisit this principle, one that has so far mainly been used to explain differences in the rates of alcohol oxidation[7,9a,9b] and solvolysis[10]. As shown in Figure 2B, the power of the Curci (TFDO) oxidation[4c] was vividly demonstrated by the conversion of 1 to 2. Among five tertiary centers present in 1, H1 was selectively activated, leading the corresponding alcohol 2 in 82% isolated yield on a gram scale. Purely electronic considerations might have led one to predict that the tertiary center of the isopropyl group (H5 in Figure 2B) would be oxidized first since its 13C NMR shift is 0.9 ppm more upfield than the carbon attached to H1.[11] Steric considerations might also support the supposition that H5 will react first. Such unusual efficiency and site-selectivity led us to consider that their origin be related to strain-release in going to the electrophilic transition state of the oxidation. In fact, numerous mechanistic studies of carbene,[12a,12b,12c] nitrene,[12d] dioxirane,[12e] and other[12f,12g] intermolecular C–H oxidations have suggested a somewhat "flattened tetrahedrality" of the tertiary carbon center in the rate-determining transitions states of such reactions (not an actual radical or cation formation but an electrophilic carbon that is bent as if to become trigonal).
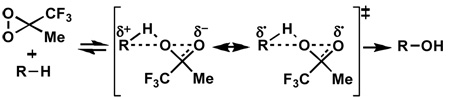
Of special interest in this context are K.N. Houk’s calculations on the mechanism of dioxirane mediated oxidations.[13] They point to "a concerted transition state with O–H abstraction much more advanced than O–C bond formation", and to a "polarized nature of the transition state (that) cause(s) the concerted oxygen insertions into tertiary CH bonds to be highly favored" (see Equation 1). These
 |
(1) |
conclusions seem perfectly compatible with a participation of strain-release as rate-influencing co-factor; they qualitatively combine concertedness with the structural prerequisite for strain-release to operate in the formation of the transition state.
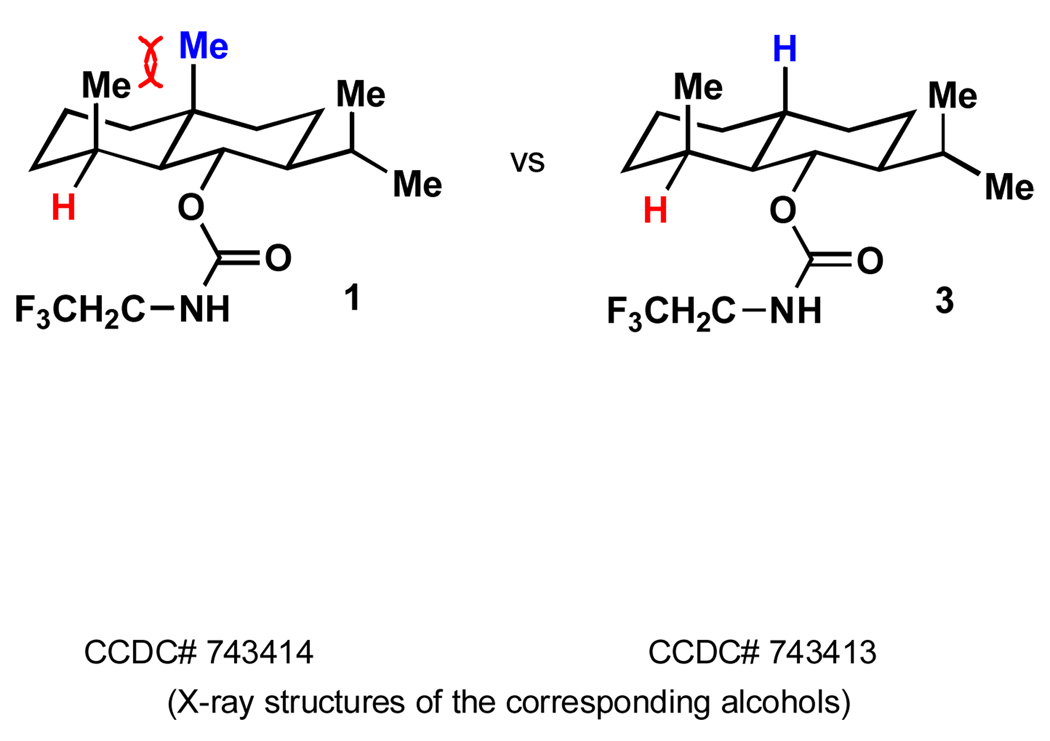
The dihydrojunenol system with its steric repulsion between two 1,3-diaxial methyl groups offers a rare opportunity to determine whether strain-release may be a contributing factor in oxidations by an electrophilic oxidant besides the factors of C–H bond nucleophilicity and steric hindrance to reagent approach.[3] It is for this very purpose that a specific eudesmane-based probe – demethyl dihydrojunenol carbamate 3 – was designed (Figure 3). In such a probe, any decrease in reactivity as compared to 1 would reflect the importance of strain-release in going to the transition state, since the electronic reactivity factors (C–H b ond nucleophilicity) in both settings are as nearly identical as they possibly can be,[14] as is the steric environment at the site of oxidation.
Figure 3.
The design of an eudesmane-based probe to account for the enhanced reactivity of equatorial C-H's.
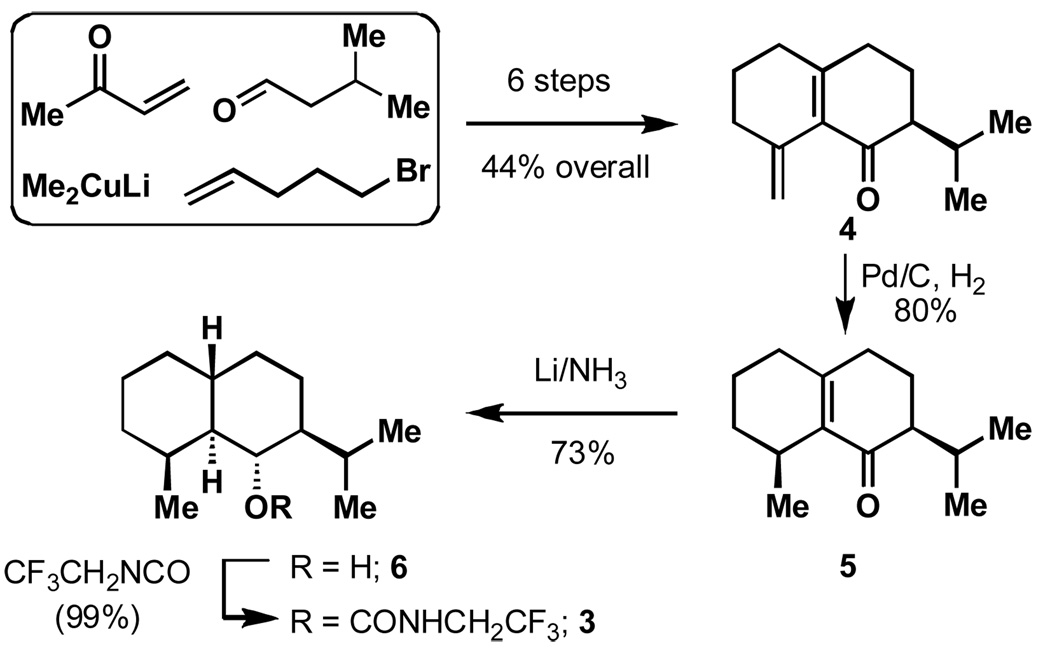
The synthesis of 3 was conducted in a similar fashion as the preparation of 1,[5] as shown in Scheme 1. Thus, the decalin framework of enone 4 was forged using methyl vinyl ketone, 3-methylbutyraldehyde, lithium dimethylcuprate and 5-bromopentene in six steps with 44% overall yield. Stereoselective hydrogenation then delivered intermediate 5 in 80% yield as the major isomer. The establishment of three requisite steric centers of de-methyl dihydrojunenol 6 was achieved in the subsequent Birch reduction in 73% yield. Lastly, carbamate formation smoothly delivered desired eudesmane probe 3 in nearly quantitative yield. The structure and stereochemical assignment were verified by X-Ray crystallography see Figure 3). Notably, probe 3 exhibits an identical conformation in the solid state and nearly identical 13C NMR chemical shifts relative to 1 (the 13C NMR shift at the key tertiary carbon atom differ by 0.1 ppm).[11a,11b,15] Not surprisingly, probe 3 reacted rapidly with TFDO in high yield to give the tertiary alcohol 7.
Scheme 1.
Synthesis of eudesmane 3.
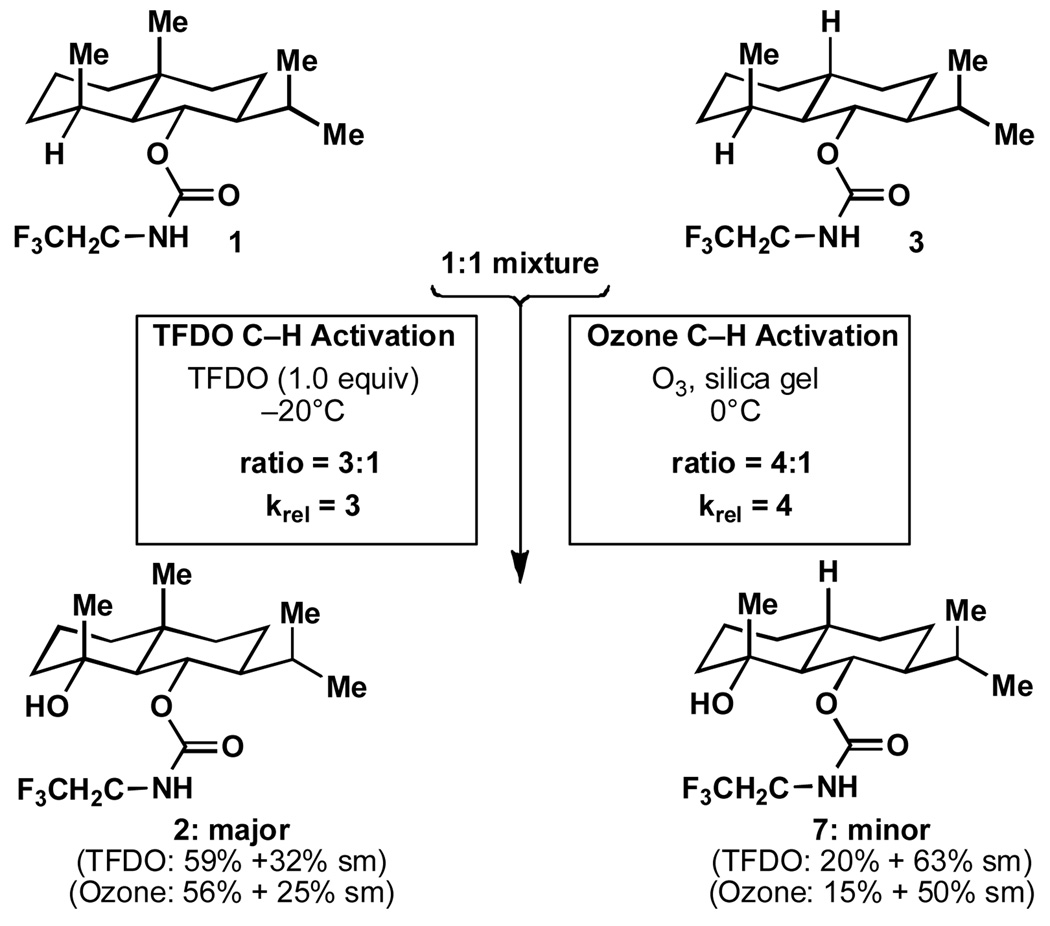
In order to establish the presence of any reactivity difference between 1 and 3 (Scheme 2), the following two experiments were conducted on a 1:1 mixture of 1 and 3 and halted before reaching full conversion (both experiments were run in triplicate and on scales ranging from 5–25 mg). Standard Curci conditions[4c] led to a 3.0–3.1:1 ratio of tertiary alcohols 2 and 7, along with recovered starting materials. To confirm that this result is not reagent-specific, the mixture was absorbed to silica gel and exposed to a stream of ozone[16] at 0 °C for 20 minutes delivering a mixture of tertiary alcohols 2 and 7 in a 3.9–4.1:1 ratio (in addition to recovered starting materials). Whereas the reaction of TFDO with both 1 and 3 was selective (only trace quantities of byproducts were observed), the reaction of 1 with ozone proceeded more efficiently (74% 2 based on recovered 1) than with 3 (numerous uncharacterized byproducts were observed and the product was formed in 30% based on recovered 3). Thus, 1 not only reacts faster with ozone than 3, it also reacts with greater selectivity.
Scheme 2.
TFDO- and Ozone-mediated oxidation of a 1:1 mixture of 1 and 3 clearly reveals the strain-release phenomenon in tertiary C–H oxidation.
The observed rate ratio of 3:1 to 4:1 in these experiments might seem modest (ΔG# ≈ 0.6 kcal/mol) for serving as evidence for the operation of a reactivity factor, particularly when compared with corresponding ratios in chromic acid oxidations of secondary alcohols. Yet there is a mechanisticically significant difference between the latter reaction and an oxidative hydroxylation of a tertiary C–H bond, in the product of which the carbon remains tetrahedral. Such rate ratios in oxidative CH activations are expected to be much smaller than in chromic acid oxidations of secondary alcohols (see Figure 2A), leading us to consider the observed ratios of Scheme 2 as significant. There is a parallel between these observations and those of classical solvolysis studies wherein similar rate differences are observed.[10] As alluded to above, these results suggest that strain release considerations can help to predict not only relative rate but also selectivity in complex settings.
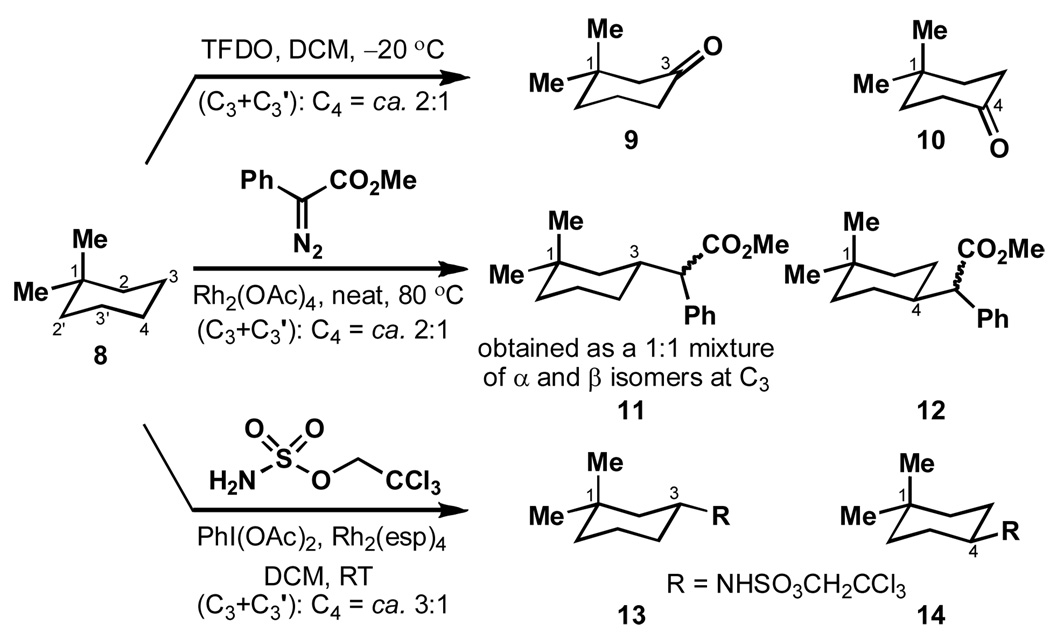
In an attempt to delineate the scope of this conclusion, a series of experiments involving dioxirane, carbene, and nitrene C–H activation were conducted on 1,1-dimethylcyclohexane (8). If the strain-release factor was operative also in methylene C–H activation, one would expect these conditions to favor activation at the C–3 carbon. As shown in Scheme 3, after correcting for statistics, TFDO and carbene activation show little to no site specificity (ca. 1:1) while nitrene activation shows a small effect (ca. 1.5:1 favoring C–3). The transition state is likely to be more tight in insertion reactions at methylene groups and, therefore, less sensitive to the strain release factor. Furthermore, the repulsion between CH3 (axial) and H (axial) in the 3-position is roughly four times smaller (0.9 vs 3.7 kcal/mol) than the correponding repulsion between two axial methyl groups (8 vs. 1) and so the effect on oxidation rates should be correpondingly smaller.3e
Scheme 3.
Strain-release is a minor factor in methylene C-H activation.
However, it is in more complex systems containing methylene groups where the strain-release factor might be operative, such as the case of sclareolide (15). Although 15 contains 26 hydrogen atoms (2 tertiary and 12 methylene), it should be possible to predict which C–H bond will react first with an electrophilic oxidant. On electronic grounds, CH2-positions at ring A may be considered most reactive since they are furthest away from the electron-withdrawing lactone ring C. Taking into account strain release, the equatorial a-CH bond at position C-2 of ring A is both the least sterically hindered and, due to the presence of two 1,3-diaxial interactions of the axial hydrogen at position C-2, expected to be most prone to the effects of strain release in the transition state of oxidation. Indeed, when commercially available sclareolide (15) was submitted to Du Bois’ nitrene insertion chemistry,[3l] product 16 (verified by NMR spectroscopy, see SI for details) was obtained in nearly quantitative yield (based on sulfonamide).
The reactivity comparison of 1 and eudesmane probe 3 brings to light the previously unrecognized importance of a reactivity factor in C–H activation that is in all likelihood attributable to strainrelease, since both the steric and electronic characteristics of these two substrates are nearly identical at the reacting site. The corroboration of these results in other settings will of course require more experiments (e.g. using substrates such as 15). For the time being, it is tempting to consider that the remarkably consistent rate differences in C–H activation of equatorial versus axial tertiary C–H bonds (see Figure 1) may result from a cooperation of reactivity factors that involve (1) C–H bond nucleophilicity, (2) steric hindrance to reagent approach, and (3) strain release. Most importantly, the work presented here may aid in the planning and execution of total syntheses that rely on the simplifying power of C–H activation logic.
Supplementary Material
Scheme 4.
Strain-release may be an important contributor in methylene activation in complex settings.
Acknowledgments
We thank Tanja Gulder for the translation of ref. 7 and Jun Shi for technical assistance. Financial support for this work was provided by Amgen, Bristol-Myers Squibb, the Skaggs Institute, and the NIH (Grant CA184785).
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
Contributor Information
Dr. Ke Chen, Department of Chemistry, The Scripps Research Institute, 10650 North Torrey Pines Road, La Jolla, CA, 92037, (USA)
Albert Eschenmoser, Laboratory of Organic Chemistry, Swiss Federal Institute of Technology, Hönggerberg HCI H309, Wolfgang-Pauli, Strasse10, CH-8093 Zürich, Switzerland.
Phil S. Baran, Department of Chemistry, The Scripps Research Institute, 10650 North Torrey Pines Road, La Jolla, CA, 92037, (USA).
References
- 1.For reviews, see Crabtree RH. J. Chem. Soc. Dalton Trans. 2001:2437–2450. Labinger JA, Bercaw JE. Nature. 2002;417:507–514. doi: 10.1038/417507a. Dick AR, Sanford MS. Tetrahedron. 2006;62:2439–2463. Godula K, Sames D. Science. 2006;312:67–72. doi: 10.1126/science.1114731. Davies HML, Manning JR. Nature. 2008;451:417–424. doi: 10.1038/nature06485. Chen X, Engle KM, Wang D-H, Yu J-Q. Angew. Chem. Int. Ed. 2009;48:5094–5115. doi: 10.1002/anie.200806273., and references cited therein.
- 2.Newhouse TR, Hoffman RW, Baran PS. Chem. Soc. Rev. 2009;39:3010–3021. doi: 10.1039/b821200g. and references cited therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.See reference 1, and the following reports: Seyferth D, Cheng YM. J. Am. Chem. Soc. 1973;95:6763–6770. Barton DHR, Basu NK, Day MJ, Hesse RH, Perchet MM, Starratt AN. J. Chem. Soc. Perkin Trans. I. 1975:2243–2251. Bovicelli P, Lupattelli P, Mincione E. J. Org. Chem. 1992;57:5052–5054. Chen H, Schlecht S, Semple TC, Hartwig JF. Science. 2000;287:1995–1997. doi: 10.1126/science.287.5460.1995. González-Núnez ME, Castellano G, Andreu C, Royo J, Báguena M, Mello R, Asensio G. J. Am. Chem. Soc. 2001;123:7487–7491. doi: 10.1021/ja003667u. Davies HML, Antoulinakis EG. J. Organomet. Chem. 2001;617:47–55. 3.20618. Yang J, Gabriele B, Belvedere S, Huang Y, Breslow R. J. Org. Chem. 2002;67:5057–5067. doi: 10.1021/jo020174u. Francisco CG, Herrera AJ, Suárez E. J. Org. Chem. 2003;68:1012–1017. doi: 10.1021/jo026314h. Díaz-Requejo MM, Belderrain TR, Nicasio MC, Pérez PJ. Dalton Trans. 2006:5559–5566. doi: 10.1039/b610183f. Daugulis O, Zaitsev VG, Shabashov D, Pham Q, Lazareva A. Synlett. 2006;20:3382–3388. Company A, Gómez L, Güell M, Ribas X, Luis JM, Que L, Jr, Costas M. J. Am. Chem. Soc. 2007;129:15766–15767. doi: 10.1021/ja077761n. Fiori KW, Du Bois J. J. Am. Chem. Soc. 2007;129:562–568. doi: 10.1021/ja0650450. Chen MS, White MC. Science. 2007;318:783–787. doi: 10.1126/science.1148597. Kurokawa T, Kim M, Du Bois J. Angew. Chem. Int. Ed. 2009;48:2777–2779. doi: 10.1002/anie.200806192., and references cited therein.
- 4.a) Varkony H, Pass S, Mazur Y. J. Chem. Soc. Chem. Comm. 1974:437–438. [Google Scholar]; b) Schneider H-J, Müller W. J. Org. Chem. 1985;50:4609–4615. [Google Scholar]; c) Mello R, Fiorentino M, Fusco C, Curci R. J. Am. Chem. Soc. 1989;111:6749–6757. [Google Scholar]; d) Likhotvorik IR, Yuan K, Brown DW, Krasutsky PA, Smyth N, Jones M., Jr Tetrahedron Lett. 1992;33:911–914. [Google Scholar]; e) Chen K, Que L., Jr J. Am. Chem. Soc. 2001;123:6327–6337. doi: 10.1021/ja010310x. [DOI] [PubMed] [Google Scholar]; f) Gómez L, Garcia-Bosch I, Company A, Benet-Buchholz J, Polo A, Sala X, Ribas X, Costas M. Angew. Chem. Int. Ed. 2009;48:5720–5723. doi: 10.1002/anie.200901865. [DOI] [PubMed] [Google Scholar]
- 5.Chen K, Baran PS. Nature. 2009;459:824–828. doi: 10.1038/nature08043. [DOI] [PubMed] [Google Scholar]
- 6.We use the term "C–H bond nucleophilicity" when referring to the "electronic reactivity factor”. It seems to us that this term pragmatically denotes the effect of what hyperconjugative and inductive electronic effects might transmit from neighboring substituents to the reaction site. Thus, this term can refer to both activation as well as deactivation of the C–H bond by neighboring substituents towards electrophilic "C–H activation" insertion reactions.
- 7.Schreiber J, Eschenmoser A. Helv. Chem. Acta. 1955;38:1529–1536. [Google Scholar]
- 8.a) Barton DHR. Experientia. 1950;6:316. doi: 10.1007/BF02170915. [DOI] [PubMed] [Google Scholar]; b) Barton DHR. J. Chem. Soc. 1953:1027. [Google Scholar]
- 9.a) Rocek J, Westheimer FH, Eschenmoser A, Moldoványl L, Schreiber J. Helv. Chem. Acta. 1962;45:2554–2567. [Google Scholar]; b) Wilcox CF, Jr, Sexton M, Wilcox MF. J. Org. Chem. 1963;28:1079–1082. [Google Scholar]
- 10.a) Brown HC, Fletcher RS. J. Am. Chem. Soc. 1949;71:1845–1854. [Google Scholar]; b) Okamoto K, Saitô S, Shingu H. Bull. Chem. Soc. Jap. 1969;42:3288–3297. [Google Scholar]; c) King JF, Coppen MJ. Can. J. Chem. 1971;49:3714–3723. [Google Scholar]; d) King JF, Lee TWS. Can. J. Chem. 1971;49:3724–3732. and references cited therein. [Google Scholar]
- 11.a) For a previous example of using (1H) nuclear magnetic resonance to predict C–H activation pattern, see: Boar RB. J. Chem. Soc. Perkin Trans. I. 1975:1275–1277.; b) For a correlation of fractional charge and carbon atom core energy to 13C chemical shifts, see: Rasmussen K. Acta Chem. Scand. A. 1980;34:155–156.
- 12.a) Davies HWL, Hansen T, Churchill MR. J. Am. Chem. Soc. 2000;122:3063–3070. [Google Scholar]; b) Brinker UH, Lin G, Xu L, Smith WB, Mieusset J-L. J. Org. Chem. 2007;72:8434–8451. doi: 10.1021/jo7013356. [DOI] [PubMed] [Google Scholar]; c) Hansen J, Autschbach J, Davies HWL. J. Org. Chem. 2009;74:6555–6563. doi: 10.1021/jo9009968. [DOI] [PubMed] [Google Scholar]; d) Breslow DS, Edwards EI, Leone R, Schleyer PVR. J. Am. Chem. Soc. 1968;90:7097–7102. [Google Scholar]; e) Curci R, D’Accolti L, Fusco C. Acc. Chem. Res. 2006;39:1–9. doi: 10.1021/ar050163y. [DOI] [PubMed] [Google Scholar]; f) Giamalva DH, Church DF, Pryor WA. J. Org. Chem. 1988;53:3429–3432. [Google Scholar]; g) Tenaglia A, Terranova E, Waegell B. J. Org. Chem. 1992;57:5523–5528. [Google Scholar]
- 13.Du X, Houk KN. J. Org. Chem. 1998;63:6480–6483. and references therein; see also. [Google Scholar]; Bach RD, Andrés JL, Su M-D, McDouall JJW. J. Am. Chem. Soc. 1993;115:5768–5775. [Google Scholar]
- 14.Due to the rigid chair-chair conformation, any hyperconjugation (electronic) effect of the bridge-headed methyl in 1 or the proton in 3 should be negligible (see ref. 4d). Strict consideration of Charton’s preferred σI parameters point to nearly identical electronic reactivity factors (C–H bond nucleophilicity) in both settings. See Charton M. Prog. Phys. Org. Chem. 1981;13:119. ;also, for an example of correlation of σI parameters and site-selectivity in TFDO CH oxidation, see ref. 3f.
- 15.The chemical shift of the reaction center in probe 3 is exactly 0.1 ppm higher than in 1, which might indicate somewhat less C–H bond nucleophilicity. However, such a small difference should be regarded as negligible. For example, the 13C shift of the tertiary carbon in cis and trans decalin differ by ca. 7 ppm yet the rate ratio of oxidation with ozone is only ca. 5.6:1 (see Figure 1 and ref. 4a). See ref. 11b for a correlation of 13C NMR shift and fractional charge.
- 16.Cohen Z, Keinan E, Mazur Y, Varkony TH. J. Org. Chem. 1975;41:2141–2142. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.