Abstract
Objectives
Clinical studies have indicated the beneficial effect of an L/N-type calcium channel blocker (CCB), cilnidipine, on the progression of proteinuria in hypertensive patients compared with an L-type CCB, amlodipine. In the present study, we examined the effects of cilnidipine and amlodipine on the renal injury in spontaneously hypertensive rat/ND mcr-cp (SHR/ND) and their underlying mechanism.
Methods and results
SHR/ND were treated with vehicle (n = 10), cilnidipine [33 mg/kg per day, orally (p.o.); n = 11] or amlodipine (20 mg/kg per day, p.o.; n = 9) for 20 weeks. SHR/ND developed proteinuria in an age-dependent manner. Cilnidipine suppressed the proteinuria greater than amlodipine did. The immunohistochemical analysis showed that N-type calcium channel and Wilm’s tumor factor, a marker of podocyte, were co-expressed. SHR/ND had significantly greater desmin staining, an indicator of podocyte injury, with lower podocin and nephrin expression in the glomeruli than Wistar–Kyoto rat or SHR. Cilnidipine significantly prevented the increase in desmin staining and restored the glomerular podocin and nephrin expression compared with amlodipine. Cilnidipine also prevented the increase in renal angiotensin II content, the expression and membrane translocation of NADPH oxidase subunits and dihydroethidium staining in SHR/ND. In contrast, amlodipine failed to change these renal parameters.
Conclusion
These data suggest that cilnidipine suppressed the development of proteinuria greater than amlodipine possibly through inhibiting N-type calcium channel-dependent podocyte injury in SHR/ND.
Keywords: angiotensin II, oxidative stress, SHR/ND mcr-cp
Introduction
Metabolic syndrome is a combination of medical disorders including visceral obesity, hypertension, glucose intolerance and dyslipidemia, which are great risk factors for chronic kidney disease [1,2]. Therefore, blood pressure should be strictly controlled in patients with metabolic syndrome, especially if patients have reduced renal function. Renin–angiotensin system (RAS) inhibitors are considered to be first-line drugs due to their blood pressure-independent renoprotective effects in patients with metabolic syndrome [3,4]. However, the effects of the other antihypertensive drugs on metabolic syndrome have not been well elucidated yet, although RAS inhibitors are not always suitable for all patients; for example, in the case of pregnancy or hyperkalemia.
Several clinical studies [5–7] and studies in experimental hypertensive animals [8–11] have indicated that an L/N-type dihydropyridine calcium channel blocker (CCB), cilnidipine, displays better renal protection compared with other antihypertensive drugs, including diuretics and the other dihydropyridine CCBs. We and others have shown that the urinary protein/creatinine ratio was reduced more effectively by cilnidipine than by amlodipine, an L-type CCB, in hypertensive patients with chronic kidney disease [7,12]. However, the precise mechanisms by which cilnidipine elicits its strong anti-proteinuric effect remain unclear.
We, therefore, examined the effect of cilnidipine, compared with amlodipine, on the development of renal injury and its underlying mechanism in the spontaneously hypertensive rat/ND mcr-cp (SHR/ND), an obese SHR model.
Materials and methods
Animals
All experimental procedures were performed according to the guidelines for the care and use of animals as established by the Kagawa University and Tulane University Health Sciences Center. Male SHR/NDs were purchased from Disease Model Cooperative Research Association (Kyoto, Japan). Spontaneously hypertensive rats (SHRs) and Wistar–Kyoto rats (WKY) were purchased from SLC (Shizuoka, Japan). Animals were divided into five experimental groups as follows: group 1, WKY (n = 12); group 2, SHR (n = 12); group 3, SHR/ND + vehicle (0.5% methyl cellulose; Nacalai Tesque, Kyoto, Japan; n = 12); group 4, SHR/ND + cilnidipine [33 mg/kg per day, orally (p.o.); n = 12]; and group 5, SHR/ND + amlodipine (20 mg/kg per day, p.o.; n = 11; LKT Laboratories, St. Paul, Minnesota, USA). Preliminary experiments showed that cilnidipine and amlodipine have similar hypotensive effects in SHR/ND at these doses (data not shown).
SBP was measured in conscious rats by tail-cuff plethysmography (BP-98A; Softron, Tokyo, Japan) and 24-h urine samples were collected at 14, 18, 22, 26, 30 and 34 weeks of age. All animals underwent a 24-h acclimatization period in metabolic cages prior to urine collection. Blood and kidney samples were harvested at the end of week 34. Half of the kidney was snap-frozen in liquid nitrogen for measurement of renal angiotensin II (AngII) α content as previously described [13]. Kidney sections were either fixed in 10% formalin (pH 7.4) for histological examination or frozen in Tissue-Tek O.C.T. compound (Sakura Finetechnical, Tokyo, Japan) for dihydroethidium (DHE) staining and laser-capture microdissection. The renal cortex of the remaining kidney was snap-frozen in liquid nitrogen and stored at −80°C.
Immunohistochemistry for desmin, N-type calcium channel and Wilms’ tumor factor-1
Immunohistochemistry for desmin, N-type calcium channel (Cav2.2 subunit) and Wilms’ tumor factor 1 (WT-1) was performed by using the Histofine Simple Stain MAX-PO MULTI (Nichirei Biosciences, Tokyo, Japan) and as previously described [14–16]. Deparaffinized sections were incubated with 0.1% hydrogen peroxide for 10 min for desmin or 0.3% hydrogen peroxide in methanol for 30 min for N-type calcium channel and WT-1 to block endogenous enzymes. For antigen retrieval, sections were heated for 10 min incubation in 0.01 mol/l citrate buffer (pH 6.0) at 105°C in case of sections for WT-1. Sections for N-type calcium channel were then exposed to 0.1% Triton-X for 30 min. After blocking, sections were incubated with primary antibodies (anti-Human Desmin Mouse monoclonal antibody, D33, 1 : 500, DAKOCytomation, Glostrup, Denmark; anti-Cav2.2 antibody, 1 : 100, Alomone Labs, Jerusalem, Israel; anti-WT-1 antibody, clone 6F-H2, 1 : 100, DAKOCytomation) for 10 min (desmin) and for 1 h (N-type calcium channel) at room temperature. Antibodies were visualized by DAB substrate (DAKOCytomation); counter-staining was performed with hematoxylin (DAKOCytomation). Sections incubated without primary antibodies were used as controls. Antibody-positive areas were calculated from 20 randomly selected microscope fields (×200) in each section. The above histologic analysis was performed using a color image analyzing system (WinRooF; Mitani Co., Tokyo, Japan) in a blind manner.
Laser-capture microdissection
Laser-capture microdissection was performed as previously described [17]. Briefly, frozen tissues were subsequently cryosectioned into 8 μm sections and 30 glomeruli were microdissected from each specimen under direct visualization and catapulted into CapSure HS Laser-capture microdissection caps tubes using the laser microdissector pressure-catapulting device (LM-200; Arcturus, California, USA). Glomerular mRNA for podocin, nephrin and N-type Ca2+ channels were extracted using RNAqueous-Micro kits (Ambion Inc., Austin, Texas, USA) according to the manufacture’s protocol.
Real-time PCR
The mRNA expression of glyceraldehydes-3-phosphate dehydrogenase (GAPDH), N-type calcium channel (Cacna 1b gene), podocin, nephrin, angiotensinogen (AGT), renin, p22phox and gp91phox were analyzed by real-time PCR using a LightCycler FastStart DNA Master SYBR Green I kit or TaqMan Gene Expression Assay kits (Applied Biosystems, Foster City, California, USA). The oligonucleotide primer sequences of GAPDH, p22phox and gp91phox and PCR conditions were same as described previously [13,18]. The primer sequences of Cacna 1b, podocin, nephrin, AGT and renin were Cacna 1b forward, 5′-CATCAAGCCAGGAACCTCCTT-3′, reverse, 5′-TCATGGAATTGAGGAGGGAAAC-3′; podocin forward, 5′-CCTTTCCATGAGGTGGTAACCA-3′, reverse, 5′-GGATGGCTTTGGACACATGAG-3′; nephrin forward, 5′-CCAGAGTGGACGAACTATATTGGA-3′, reverse, 5′-GACCAGTAACTGCCCGTTATCC-3′, AGT forward, 5′-TTGTGTGAGGAGGGCTGTAT-3′, reverse, 5′-TGCTGAGAGTGTAGGTCCTG-3′; renin forward, 5′-TTGGGTGCTGAGGCAAATCT-3′, reverse, 5′-CCACATTTTGGGGGTTATCC-3′. All data were expressed as the relative differences between WKY and other groups after normalization to GAPDH expression. The PCR product for GAPDH and mouse Cacna 1b were electrophoresed in 2% agarose gels in Tris-bobate EDTA buffer and then stained with ethidium bromide.
Histological examination
Kidneys were fixed with 10% formalin (pH 7.4), embedded in paraffin, sectioned into 4 μm slices, and stained with periodic acid Schiff (PAS) reagent. PAS staining was evaluated using light microscopy according to previously described methods [14,19]. Positive glomerular sclerotic area was counted using a photoimaging system (WinRooF).
Dihydroethidium staining in kidney section
Frozen kidney segments were cut into 10 μm thick sections and placed on a glass slide. DHE (10 μmol/l; Invitrogen, Carlsbad, California, USA) was topically applied to each tissue section [20]. Slides were incubated in a light-protected humidified chamber at 37°C for 30 min. For the detection of ethidium bromide, images were assessed using a laser scanning confocal microscope system (Bio-Rad Laboratories, Hercules, California, USA) and fluorescence was detected with a 590 nm long-pass filter. The average DHE fluorescence intensity was calculated from 30–40 glomeruli from each group.
Immunoprecipitation and western blotting
Complex formation of NADPH oxidase subunits in the renal cortex was determined by co-immunoprecipitation and western blotting as previously described [21]. Briefly, approximately 1 mg protein was incubated for at least 2 h with p22phox antibody (Santa Cruz, California, USA) and immunoprecipitated with protein G plus agarose beads (Santa Cruz) overnight at 4°C. Immunocomplex-bound beads were washed four times with immunoprecipitation buffer and re-suspended in 25 μl of 2× Laemmli buffer. Samples were boiled for 3 min and proteins were separated by 10 or 12% SDS-PAGE for immunoblotting. The resolved proteins were transferred to a nitrocellulose membrane, blocked and exposed to rabbit polyclonal IgG anti p47phox or Rac-1 antibody (1 : 800; Santa Cruz) at 4°C overnight, followed by incubation with goat antirabbit IgG (1 : 1000; Cell Signaling, Danvers, Massachusetts, USA). All values were normalized by arbitrarily setting the integrated densitometric values of WKY to 1.0.
Cell culture condition, small interfering RNA transfection and dihydroethidium staining
Conditionally immortalized mouse podocyte cell lines were used for culture study [22]. Transfection of small interfering RNA (siRNA) for murine N-type Ca2+ channel (mouse Cacna1b, silencer select Predesigned siRNA; Ambion) was performed with Lipofectamine 2000 (Invitrogen). Thirty to fifty percent subconfluent podocyts in growth medium without antibiotics were transfected by Royal Park Memorial Institute 1640 free containing 4 μl of Lipofectamine 2000 reagent with 100 pmol of siRNA per well (six-well plate) for 10 h and replaced growth medium. DHE was performed in a 35 mm dish (Nalge nunc, Tokyo, Japan). Cells, which were transfected with scramble vector or siRNA for N-type calcium channel, were exposed to vehicle or angiotensin II (100 nmol/l) for 30 min. DHE (10 μmol/l) was added to the medium and the incubation was continued for 15 min.
Other analytical procedures
Urinary protein excretion was determined using a protein assay kit (microTP-test; Wako, Osaka, Japan). The degree of lipid peroxidation was determined using biochemical assays of thiobarbituric acid-reactive substances (TBARS) in renal cortical tissues [13]. Creatinine concentrations were measured using colorimetric Jaffé assay kits (creatinine-test; Wako). Serum triglyceride level was measured by the GPO DAOS glycerol method (triglyceride E test; Wako).
Statistical analysis
Values are presented as mean ± SE. Two-way analysis of variance (ANOVA) and following Bonferroni posthoc test was employed to analyze SBP and proteinuria. Statistical comparisons of the differences between treatments for other parameters were performed using one-way ANOVA combined with the Newman–Keuls posthoc test. A P value less than 0.05 was considered statistically significant.
Results
SBP, postprandial blood glucose, plasma total triglycerides and body weight
The SBP of SHR/ND was similar to that of SHR at 34 weeks of age (Fig. 1a); both animals showed significant hypertension compared with WKY throughout the experimental period. Treatment with cilnidipine or amlodipine led to comparable decreases in SBP in SHR/ND. SHR/ND showed higher postprandial blood glucose levels compared with WKY and SHR at 34 weeks of age (Table 1). Administration of cilnidipine or amlodipine did not significantly affect plasma glucose level in SHR/ND. SHR/ND exhibited higher levels of serum triglycerides than WKY and SHR did, which were significantly suppressed by cilnidipine, but not amlodipine, possibly a secondary effect of antiproteinuric effect [23] of cilnidipine (data on proteinuria is described below). At the end of the study, SHR/ND had higher body weight than WKY and SHR (Table 1). Treatment with cilnidipine or amlodipine did not affect the body weight in SHR/ND.
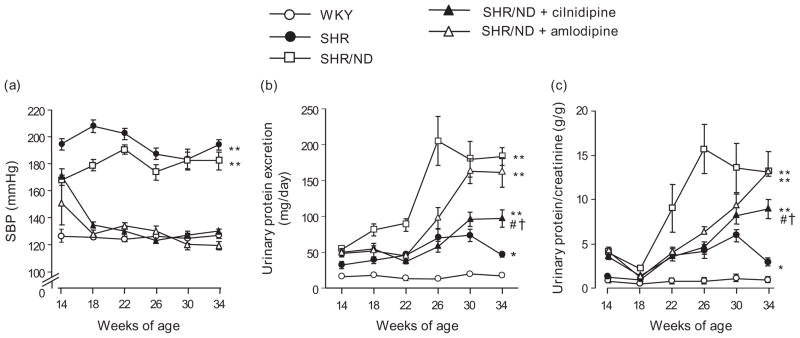
Fig. 1.
SBP (a), urinary protein excretion (b) and urinary protein excretion/creatinine ratio (c). All treatments decreased SBP to levels comparable with that of WKY. SHR/ND showed substantial proteinuria compared with WKY or SHR. Compared with amlodipine, cilnidipine greatly suppressed the progression of proteinuria in SHR/ND. *P < 0.05, **P < 0.01 vs. WKY, #P < 0.05 vs. SHR/ND, †P < 0.05 vs. SHR/ND + amlodipine at 34 weeks old (n = 11–12). SHR, spontaneously hypertensive rat; WKY, Wistar–Kyoto rats.
Table 1.
Characteristics of each group at 34 weeks of age
| WKY (n = 12) | SHR (n = 12) | SHR/ND (n = 12) | SHR/ND + cilnidipine (n = 12) | SHR/ND + amlodipine (n = 11) | |
|---|---|---|---|---|---|
| BW (g) | 451 ± 4 | 422 ± 6* | 722 ± 13* | 702 ± 10* | 698 ± 16* |
| BG (mmol/l) | 5.2 ± 0.2 | 5.4 ± 0.3 | 9.8 ± 1.0* | 9.1 ± 0.9* | 11.5 ± 0.8* |
| PTG (mg/dl) | 87 ± 5 | 78 ± 9 | 341 ± 41* | 174 ± 20*,# | 350 ± 101* |
| Pcre (mg/dl) | 0.56 ± 0.02 | 0.55 ± 0.02 | 0.55 ± 0.02 | 0.55 ± 0.02 | 0.55 ± 0.02 |
| PAngII (fmol/ml) | 14 ± 2 | 15 ± 2 | 9 ± 3* | 6 ± 1* | 15 ± 3 |
| TBARS (nmol/mg protein) | 0.21 ± 0.02 | 0.28 ± 0.04 | 0.47 ± 0.03* | 0.27 ± 0.02# | 0.35 ± 0.08 |
Values are mean ± SE. BW, body weight; BG, postprandial blood glucose; Pcre, plasma creatinine; PAngII, plasma AngII; PTG, plasma triglyceride; SHR, spontaneously hypertensive rat; TBARS, thiobarbituric acid-reactive substances; WKY, Wistar–Kyoto rats.
P < 0.05 vs. WKY.
P < 0.05 vs. SHR/ND.
Plasma creatinine, urinary protein excretion and urinary protein/creatinine ratio
At 34 weeks of age, no significant difference in plasma creatinine level was observed between the groups. SHR/ND showed marked age-dependent increases in urinary protein excretion and protein/creatinine ratio (Fig. 1b and c) in which the value at 34 weeks of age (183 ± 12 mg/day and 13.1 ± 0.5 g/g, respectively) was substantially higher than that of WKY (17 ± 2 mg/day and 0.9 ± 0.1 g/g, respectively) or SHR (46 ± 3 mg/day and 3.0 ± 0.2 g/g, respectively). Treatment with cilnidipine significantly suppressed the urinary protein excretion and protein/creatinine ratio through 22–34 weeks of age in SHR/ND (97 ± 12 mg/day and 8.9 ± 1.1 g/g at 34 weeks of age, respectively; P < 0.01). On the contrary, treatment with amlodipine initially attenuated the development of urinary protein excretion and protein/creatinine ratio (SHR/ND vs. SHR/ND + amlodipine: 90 ± 8 vs. 45 ± 6 mg/day and 9.5 ± 3.0 vs. 4.0 ± 0.7 g/g at 22 weeks of age and 205 ± 34 vs. 97 ± 15 mg/day and 16.2 ± 2.7 vs. 7.1 ± 0.7 g/g at 26 weeks of age, respectively; P < 0.01), but there was no difference in the value between untreated animals and amlodipine-treated animals at 34 weeks of age (161 ± 21 mg/day and 12.5 ± 0.9 g/g).
N-type calcium channel expression in podocyte
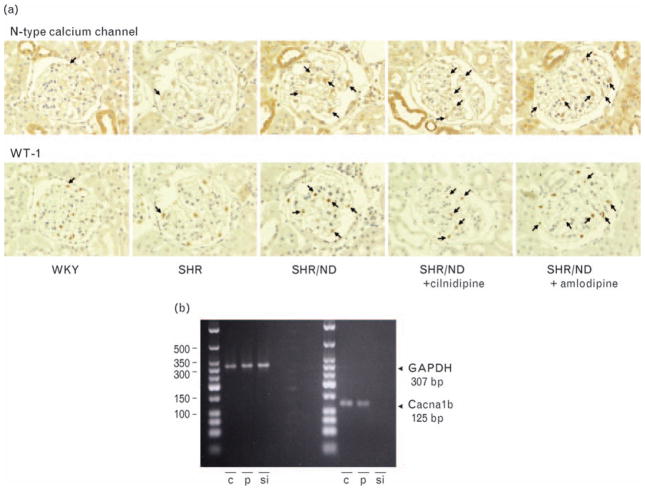
As cilnidipine attenuated the proteinuria greater than amlodipine, we next evaluated the location of N-type calcium channel by immunohistochemistry in the kidney cross-section. The immunoreactivity for N-type calcium channel was found in vascular walls, possibly in the nerves in adventitia, distal tubules and glomerular podocyte. Because we observed that treatment with cilnidipine suppressed the development of proteinuria, we focused on the N-type calcium channel in podocyte. We confirmed the expression of N-type calcium channel in podocyte by using three different approaches. First, we detected the mRNA expression of N-type calcium channel in the laser-captured isolated glomeruli. The expression level tended to be higher in the glomeruli of SHR/ND (the ratio of N-type calcium channel mRNA and GAPDH mRNA; 1.2 ± 0.2-fold) and amlodipine-treated SHR/ND (1.5 ± 0.3-fold) compared with that of the other three groups (WKY, 0.8 ± 0.1-fold; SHR, 0.9 ± 0.2-fold: SHR + cilnidipine, 0.9 ± 0.3-fold), but the difference was not statistically significant. However, we could not eliminate the possibility that laser-captured samples were contaminated with nerve endings that exist in the walls of afferent and efferent arteries. Therefore, we also examined the co-expression of N-type calcium channel with podocyte-specific protein, WT-1 (Fig. 2a). In the consecutive kidney sections, we detected the immunoreactivity for both N-type calcium channel and WT-1 in the same cells of glomeruli in SHR/ND; however, the coexpression was hard to detect in the glomeruli of WKY and SHR because immunostaining for N-type calcium channel was weak in these two strains. Finally, we analyzed the N-type calcium channel mRNA expression in the cultured podocytes. The mRNA level we detected in the cultured podocytes was abolished by siRNA for Ntype calcium channel (Fig. 2b).
Fig. 2.
The co-expression of N-type calcium channel with podocyte-specific nuclear protein, WT-1 (a) and the expression of N-type calcium channel in cultured podocyte (b). The representative glomerulus pictures from WKY and SHR/ND were shown. The immunoreactivities for both N-type calcium channel and WT-1 were found in the same cells (arrows) analyzed in the consecutive kidney sections (1.5 μm thick). Reverse-transcription PCR analysis revealed significant expression of the N-type calcium channel product (125 bp) in podocyte (p) and cerebral cortex (c) as a positive control, and the band was abolished by siRNA transfection of N-type calcium channel (si) with the same proportion of GAPDH expression (307 bp) (n = 3). SHR, spontaneously hypertensive rat; WKY, Wistar–Kyoto rats; WT-1, Wilms’ tumor factor 1.
Glomerular histological analysis
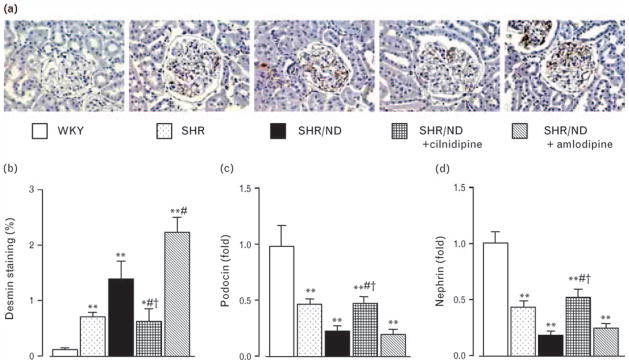
In order to examine the protective effect of cilnidipine in podocytes, we evaluated the glomerular podocyte damage by analyzing antidesmin antibody-positive areas [14] and compared the area between cilnidipine-treated and amlodipine-treated groups. At age 34 weeks, antibody- positive areas were markedly greater in SHR and all SHR/ND groups compared with WKY (Fig. 3a and b). However, positively stained areas in cilnidipine-treated rats were significantly smaller compared with those in untreated and amlodipine-treated SHR/ND.
Fig. 3.
Representative immunohistochemistry images (a) and the antibody-positive area (b) for desmin, and glomerular expression of podocin (c) and nephrin (d) mRNA. Podocyte injury revealed by desmin and mRNA expression of glomerular podocin and nephrin was increased in SHR/ND, which was significantly improved by cilnidipine but not by amlodipine. *P < 0.05, **P < 0.01 vs. WKY, #P < 0.05 vs. SHR/ND, †P < 0.05 vs. SHR/ND + amlodipine (n = 11–12). SHR, spontaneously hypertensive rat; WKY, Wistar–Kyoto rats.
To further analyze the podocyte damage, slit diaphragm proteins, podocin and nephrin, expression in the laser-captured glomeruli were measured (Fig. 3c and d). The mRNA levels of podocin and nephrin were downregulated in SHR and were further decreased in SHR/ND compared with those in WKY. Cilnidipine treatment restored the expression levels in SHR/ND to the levels similar to those in SHR. Amlodipine treatment did not affect the expression levels of these mRNA.
The PAS-positive areas to estimate the glomerular sclerotic changes in the renal cortex of WKY, SHR and SHR/ND were similar at 34 weeks of age (15 ± 1, 15 ± 1 and 16 ± 1%, respectively). Treatment did not affect the PAS-positive area in SHR/ND (cilnidipine 16 ± 1%; amlodipine 17 ± 1%; hydralazine 16 ± 1%).
AngII, angiotensinogen and renin in the kidney
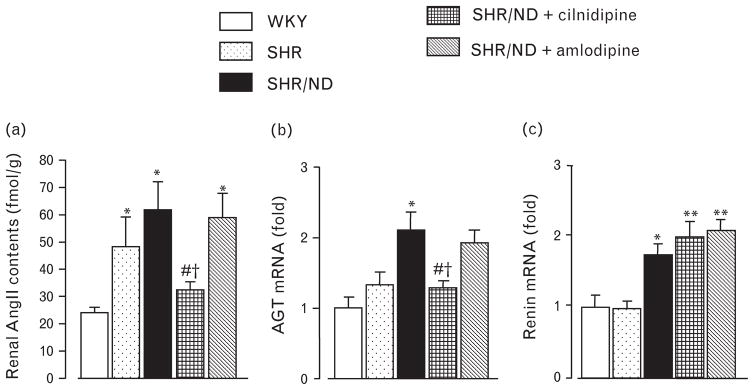
As renoprotective effect of cilnidipine was implicated in the reduction of the activity of the RAS [11], we analyzed the AngII content in the kidney. SHR and SHR/ND showed higher AngII content in renal tissue at 34 weeks of age (Fig. 4a) compared with those in WKY. AngII levels in renal tissue of SHR/ND were significantly reduced by cilnidipine treatment, but were not significantly affected by amlodipine treatment. SHR/ND showed higher AGT mRNA expression in renal cortical tissues (Fig. 4b). Administration of cilnidipine significantly suppressed AGT gene expression in renal cortical tissues, whereas amlodipine treatment had no effect. Renin mRNA expression was higher in renal cortical tissue of SHR/ND than in WKY and was not affected by any treatment (Fig. 4c). Plasma AngII levels tended to be lowered by cilnidipine treatment and increased by amlodipine, but these changes were not statistically significant (Table 1).
Fig. 4.
Renal AngII content (a) and mRNA level of AGT (b) and renin (c) in renal cortical tissues. SHR/ND had higher renal AngII levels and mRNA levels of AGT and renin compared with WKY. Cilnidipine treatment decreased renal AngII and AGT levels, but amlodipine had no effect. *P < 0.05, **P < 0.01 vs. WKY, #P < 0.05 vs. SHR/ND, †P < 0.05 vs. SHR/ND + amlodipine (n = 11–12). SHR, spontaneously hypertensive rat; WKY, Wistar-Kyoto rats.
Thiobarbituric acid-reactive substances content, dihydroethidium staining and NADPH oxidase subunits expression and complex formation in the kidney
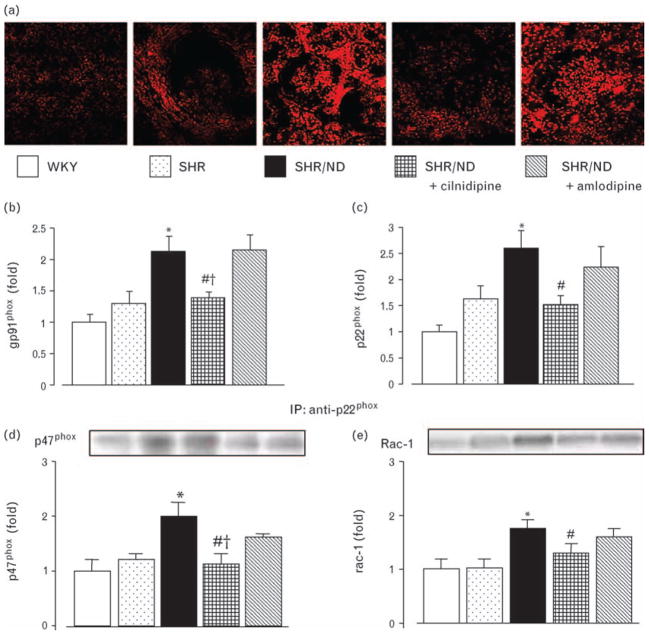
TBARS content [13] and DHE staining [20] were analyzed as oxidative stress markers. At 34 weeks of age, SHR/ND showed higher renal cortical TBARS content than WKY or SHR (Table 1). Cilnidipine, but not amlodipine, significantly inhibited the increase in TBARS content. DHE fluorescence (Fig. 5a) was greater in SHR/ND (2.55 ± 0.12-fold) than in WKY (1.00 ± 0.10-fold) or SHR (1.44 ± 0.06-fold). Cilnidipine significantly suppressed the increase in DHE fluorescence (1.49 ± 0.07-fold), but amlodipine had no effect (2.05 ± 0.08-fold).
Fig. 5.
Representative pictures of DHE (a), gene expression of gp91phox (b) and p22phox (c) and complex formation of p22phox with p47phox (d) or Rac-1 (e) in renal cortical tissues. Increased renal cortical mRNA expression of gp91phox and p22phox in SHR/ND was significantly suppressed by cilnidipine but not by amlodipine. Similarly, increased complex formation of p22phox with p47phox or Rac-1 was suppressed by cilnidipine but not by amlodipine. *P < 0.05 vs. WKY, #P < 0.05 vs. SHR/ND, †P < 0.05 vs. SHR/ND + amlodipine. DHE, dihydroethidium; SHR, spontaneously hypertensive rat; WKY, Wistar–Kyoto rats.
The levels of gp91phox and p22phox mRNA were significantly greater in SHR/ND than in WKY or SHR (Fig. 5b and c). Administration of cilnidipine suppressed the increase in mRNA levels of both gp91phox and p22phox, whereas amlodipine had no effect on expression levels. Protein complex formation of p47phox or Rac-1 with p22phox of NADPH oxidase subunits, which are necessary for NADPH oxidase to produce superoxide [24], were significantly increased in SHR/ND (Fig. 5d and e). Cilnidipine, but not amlodipine, significantly suppressed the increases in complex formation of p47phox or Rac-1 with p22phox of NADPH oxidase.
Dihydroethidium staining in podocyte
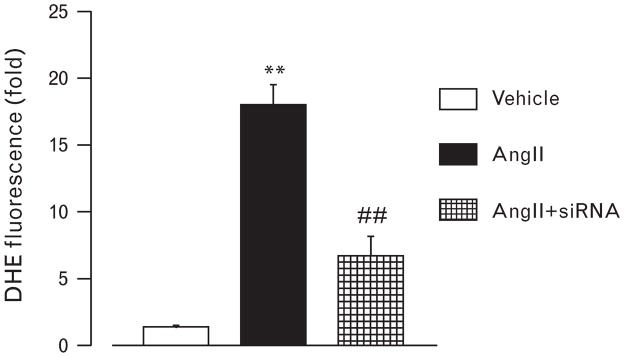
To support the results of in-vivo study, we next evaluated the effect of AngII on superoxide production in podocyte. Treatment with AngII remarkably increased the DHE fluorescence in the cultured murine podocyte compared with vehicle treatment (Fig. 6). The increase in DHE fluorescence was significantly inhibited by siRNA for N-type calcium channel.
Fig. 6.
DHE staining in cultured murine podocytes. AngII prominently increased dihydroethidium fluorescence in the podocytes. Gene silence of N-type calcium channel prevented the increase in DHE fluorescence in response to AngII. **P < 0.01 vs. control, ##P < 0.01 vs. AngII. DHE, dihydroethidium.
Discussion
Calcium channels are expressed not only in vascular smooth muscle cells but also in other cells in the kidney; for example, T-type calcium channels are expressed in collecting ducts [25] and L-type calcium channels are mainly expressed in vessels but are also expressed in tubular cells [26]. N-type calcium channel are known to be expressed in the nerve endings and to be implicated in the regulation of nerve activity by maintaining the intra-cellular calcium level [27]. However, few studies have explored the role of N-type calcium channel expressing in the other cells. Here, we showed the first evidence for the expression of N-type calcium channels in podocyte, a cell that plays an important role in glomerular filtration barrier [28]. As N-type calcium channel blockade in addition to L-type calcium channel blockade by cilnidipine elicited the better suppression of the podocyte damage (the increase in desmin staining and the reduction of slit-diaphragm proteins) and the proteinuria than the inhibition of L-type calcium channel by amlodipine, it can be considered that the inhibition of N-type calcium channel by cilnidipine in podocyte may prevent the podocyte injury and lead to the antiproteinuric activity of cilnidipine in SHR/ND.
AngII induces superoxide production by activating NADPH oxidase in many tissues including kidney and is implicated in the development of proteinuria and renal injury in experimental hypertensive [29] or diabetic rats [30]. Furthermore, AngII increased NADPH oxidase activity, subunits expression and superoxide production [31] and altered the phenotype of podocyte cytoskeleton by reactive oxygen species [32] in cultured murine podocytes, indicating that AngII could stimulate oxidative stress and induce podocyte injury, thereby accelerating proteinuria. In fact, in the present study, both increased renal AngII levels and oxidative stress were observed in SHR/ND, which were accompanied by podocyte injury and proteinuria. Furthermore, treatment with cilnidipine, but not with amlodipine, significantly suppressed these changes. These findings suggest that cilnidipine, independent of its hypotensive effect, elicits podocyte protection and antiproteinuric effect in SHR/ND through the reduction of AngII and a subsequent reduction in oxidative stress. A limitation of the current study is that we could not directly measure the changes in the AngII levels and oxidative stress in podocytes of SHR/ND because of the technical limitations on quantitative analysis in vivo. However, the in-vitro results that showed N-type calcium channel-dependent superoxide production by AngII could partially support our hypothesis. In addition, we recently reported that cilnidipine had stronger antioxidant activity than amlodipine in vitro [33]. Thus, cilnidipine may participate in the further reduction of AngII-induced oxidative stress through the inhibitory effect of N-type calcium channel and its direct antioxidative effect in podocytes, although the mechanism by which cilnidipine suppressed AngII level in vivo still remains unclear in the present study.
An L-type calcium channel blocker, amlodipine, initially suppressed proteinuria in SHR/ND; however, it reached levels similar to those at week 34. Moreover, amlodipine did not restore the reduction of podocin and nephrin expression and, rather, increased the desmin staining, suggesting that amlodipine has no protective effect on podocytes. It is also possible that the initial antiproteinuric effect of amlodipine results from the blood pressure-lowering effect. Indeed, several studies have reported that the changes in intracellular calcium concentration, an important physiological role of calcium channel, in response to AngII [34], catecholamine [35] and acetylcholine [36], were not inhibited by L-type CCBs in podocytes, suggesting that L-type calcium channel may not play important roles in podocytes.
The mechanisms by which cilnidipine suppressed renal AGT expression and AngII levels are not clear in the present results. One possibility is that cilnidipine inhibits the vicious cycle of RAS and oxidative stress in the kidney. Hsieh et al. [37] reported that exposure of rat proximal tubular cells to high glucose or AngII-induced oxidative stress and AGT expression that were suppressed by radical scavengers or NADPH oxidase inhibitor. They have also demonstrated that selective over-expression of renal catalase prevents the glucose-induced or AngII-induced oxidative stress and AGT upregulation [38] and that overexpression of AGT elicits RAS inhibitor-inhibitable [39] or antioxidant-inhibitable [40] renal injury. Taken together, oxidative stress, which could be triggered by hyperglycemia, seems to increase AGT gene expression, AngII production and further oxidative stress in the kidney. Cilnidipine may reduce AGT expression in the kidney of SHR/ND through inhibition of this vicious cycle. However, our animal studies were not designed to address these issues and further in-vitro studies are needed to clarify the precise mechanisms by which the observed effects are elicited.
It is widely known that renal sympathetic nerve activity plays an important role in renin secretion through β-adrenergic receptor-dependent pathway in the juxtaglomerular apparatus [41]. As N-type calcium channel is originally known to express in the nerves, several reports have examined the effect of cilnidipine on norepinephrine secretion and circulating RAS [11,42]. Konda et al. [42] recently reported that cilnidipine did not change plasma norepinephrine and AngII levels and plasma renin activity (PRA) despite having a blood pressure-lowering effect in SHR and that amlodipine increased PRA and plasma AngII. They concluded that cilnidipine, but not amlodipine, suppressed reflex sympathetic hyperactivity and RAS activation, caused by blood pressure reduction, through N-type calcium channel blockade. A similar tendency was observed in our current study; cilnidipine tended to suppress and amlodipine tended to increase plasma AngII level in SHR/ND. Therefore, it can be considered that cilnidipine functions as a regulator of RAS through inhibition of N-type calcium channel on the renal nerves and that this effect partially explains the renoprotective effects of cilnidipine.
CCBs are reported to regulate glomerular pressure by changing the afferent and efferent arteriole tone. Zhou et al. [9] demonstrated that cilnidipine decreased single nephron filtration fraction, glomerular capillary pressure, afferent and efferent arteriole resistance and proteinuria in nitric oxide synthase-inhibited SHR, indicating that cilnidipine attenuated the glomerular hypertension and prevented the proteinuria. Similar arteriole responses were observed in the kidney of dogs [43] and hydronephrotic SHR [44]. Importantly, Konno and Kimura [44] revealed that nifedipine, another L-type CCB, dilated afferent, but not efferent, artery, whereas cilnidipine dilated both afferent and efferent artery, suggesting that N-type calcium channel inhibition by cilnidipine might elicit efferent vasodilation. Therefore, it can be expected that the effect of cilnidipine on glomerular hemodynamics explains part of the renoprotective effect of cilnidipine in SHR/ND in the present study. However, Hayashi et al. [43] reported that the effects of cilnidipine and amlodipine on the efferent/afferent ratio arteriole dilation were similar in hydronephrotic kidney. Taken together, it seems likely that cilnidipine elicits renoprotective effect by regulating glomerular hemodynamics; however, whether this effect causes the difference between cilnidipine and amlodipine in the present study remains unclear.
In conclusion, cilnidipine suppressed the development of proteinuria in SHR/ND greater than amlodipine did, possibly, through the inhibition of N-type calcium channel in the podocyte. The inhibiting effects of cilnidipine on renal RAS and oxidative stress may also be involved in its beneficial effect in metabolic syndrome patients. The present findings suggest that cilnidipine treatment could be a candidate for therapeutic strategies in hypertensive metabolic syndrome patients with renal disease.
Acknowledgments
This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (20590253) and by a grant from Kagawa University Project Research 2008 (to A.N.) and from the National Institute of Diabetes and Digestive and Kidney Diseases (RO1DK072408 to H.K.). We are grateful to Ajinomoto Co. for supplying cilnidipine.
Abbreviations
- AGT
angiotensinogen
- AngII
angiotensin II
- CCB
calcium channel blocker
- DHE
dihydroethidium
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
- PRA
plasma renin acivity
- RAS
renin–angiotensin system
- SHR
spontaneously hypertensive rats
- SHR/ND
spontaneously hypertensive rat/ND mcr-cp
- TBARS
thiobarbituric acid-reactive substances
- WKY
Wistar–Kyoto rats
- WT-1
Wilms’ tumor factor 1
Footnotes
There are no conflicts of interest.
References
- 1.Muntner P, He J, Chen J, Fonseca V, Whelton PK. Prevalence of nontraditional cardiovascular disease risk factors among persons with impaired fasting glucose, impaired glucose tolerance, diabetes, and the metabolic syndrome: analysis of the Third National Health and Nutrition Examination Survey (NHANES III) Ann Epidemiol. 2004;14:686–695. doi: 10.1016/j.annepidem.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Kurella M, Lo JC, Chertow GM. Metabolic syndrome and the risk for chronic kidney disease among nondiabetic adults. J Am Soc Nephrol. 2005;16:2134–2140. doi: 10.1681/ASN.2005010106. [DOI] [PubMed] [Google Scholar]
- 3.de Vinuesa SG, Goicoechea M, Kanter J, Puerta M, Cachofeiro V, Lahera V, et al. Insulin resistance, inflammatory biomarkers, and adipokines in patients with chronic kidney disease: effects of angiotensin II blockade. J Am Soc Nephrol. 2006;17:S206–S212. doi: 10.1681/ASN.2006080916. [DOI] [PubMed] [Google Scholar]
- 4.Yano Y, Hoshide S, Ishikawa J, Noguchi C, Tukui D, Takanori H, et al. The differential effects of angiotensin II type 1 receptor blockers on microalbuminuria in relation to low-grade inflammation in metabolic hypertensive patients. Am J Hypertens. 2007;20:565–572. doi: 10.1016/j.amjhyper.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Rose GW, Kanno Y, Ikebukuro H, Kaneko M, Kaneko K, Kanno T, et al. Cilnidipine is as effective as benazepril for control of blood pressure and proteinuria in hypertensive patients with benign nephrosclerosis. Hypertens Res. 2001;24:377–383. doi: 10.1291/hypres.24.377. [DOI] [PubMed] [Google Scholar]
- 6.Kojima S, Shida M, Yokoyama H. Comparison between cilnidipine and amlodipine besilate with respect to proteinuria in hypertensive patients with renal diseases. Hypertens Res. 2004;27:379–385. doi: 10.1291/hypres.27.379. [DOI] [PubMed] [Google Scholar]
- 7.Fujita T, Ando K, Nishimura H, Ideura T, Yasuda G, Isshiki M, et al. Antiproteinuric effect of the calcium channel blocker cilnidipine added to renin–angiotensin inhibition in hypertensive patients with chronic renal disease. Kidney Int. 2007;72:1543–1549. doi: 10.1038/sj.ki.5002623. [DOI] [PubMed] [Google Scholar]
- 8.Varagic J, Susic D, Frohlich ED. Cilnidipine improves spontaneously hypertensive rat coronary hemodynamics without altering cardiovascular mass and collagen. J Hypertens. 2002;20:317–322. doi: 10.1097/00004872-200202000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X, Ono H, Ono Y, Frohlich ED. N- and L-type calcium channel antagonist improves glomerular dynamics, reverses severe nephrosclerosis, and inhibits apoptosis and proliferation in an l-NAME/SHR model. J Hypertens. 2002;20:993–1000. doi: 10.1097/00004872-200205000-00035. [DOI] [PubMed] [Google Scholar]
- 10.Konda T, Enomoto A, Matsushita J, Takahara A, Moriyama T. The N- and L-type calcium channel blocker cilnidipine suppresses renal injury in Dahl rats fed a high-sucrose diet, an experimental model of metabolic syndrome. Nephron Physiol. 2005;101:1–13. doi: 10.1159/000085713. [DOI] [PubMed] [Google Scholar]
- 11.Konda T, Enomoto A, Takahara A, Yamamoto H. Effects of L/N-type calcium channel antagonist, cilnidipine on progressive renal injuries in Dahl salt-sensitive rats. Biol Pharm Bull. 2006;29:933–937. doi: 10.1248/bpb.29.933. [DOI] [PubMed] [Google Scholar]
- 12.Morimoto S, Yano Y, Maki K, Iwasaka T. Renal and vascular protective effects of cilnidipine in patients with essential hypertension. J Hypertens. 2007;25:2178–2183. doi: 10.1097/HJH.0b013e3282c2fa62. [DOI] [PubMed] [Google Scholar]
- 13.Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, et al. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16:703–711. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishiyama A, Nakagawa T, Kobori H, Nagai Y, Okada N, Konishi Y, et al. Strict angiotensin blockade prevents the augmentation of intrarenal angiotensin II and podocyte abnormalities in type 2 diabetic rats with microalbuminuria. J Hypertens. 2008;26:1849–1859. doi: 10.1097/HJH.0b013e3283060efa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pardo NE, Hajela RK, Atchison WD. Acetylcholine release at neuromuscular junctions of adult tottering mice is controlled by N-(cav2.2) and R-type (cav2.3) but not L-type (cav1.2) Ca2+ channels. J Pharmacol Exp Ther. 2006;319:1009–1020. doi: 10.1124/jpet.106.108670. [DOI] [PubMed] [Google Scholar]
- 16.Yokoi H, Mukoyama M, Mori K, Kasahara M, Suganami T, Sawai K, et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int. 2008;73:446–455. doi: 10.1038/sj.ki.5002722. [DOI] [PubMed] [Google Scholar]
- 17.Ekuni D, Firth JD, Putnins EE. Regulation of epithelial cell growth factor receptor protein and gene expression using a rat periodontitis model. J Periodontal Res. 2006;41:340–349. doi: 10.1111/j.1600-0765.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- 18.Rahman M, Nishiyama A, Guo P, Nagai Y, Zhang GX, Fujisawa Y, et al. Effects of adrenomedullin on cardiac oxidative stress and collagen accumulation in aldosterone-dependent malignant hypertensive rats. J Pharmacol Exp Ther. 2006;318:1323–1329. doi: 10.1124/jpet.106.105106. [DOI] [PubMed] [Google Scholar]
- 19.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimura S, Zhang GX, Nishiyama A, Nagai Y, Nakagawa T, Miyanaka H, et al. D-allose, an all-cis aldo-hexose, suppresses development of salt-induced hypertension in Dahl rats. J Hypertens. 2005;23:1887–1894. doi: 10.1097/01.hjh.0000182523.29193.e3. [DOI] [PubMed] [Google Scholar]
- 21.Li JM, Shah AM. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J Biol Chem. 2002;277:19952–19960. doi: 10.1074/jbc.M110073200. [DOI] [PubMed] [Google Scholar]
- 22.Mundel P, Reiser J, Zuniga Mejia Borja A, Pavenstadt H, Davidson GR, Kriz W, et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 23.Shearer GC, Stevenson FT, Atkinson DN, Jones H, Staprans I, Kaysen GA. Hypoalbuminemia and proteinuria contribute separately to reduced lipoprotein catabolism in the nephrotic syndrome. Kidney Int. 2001;59:179–189. doi: 10.1046/j.1523-1755.2001.00478.x. [DOI] [PubMed] [Google Scholar]
- 24.Groemping Y, Lapouge K, Smerdon SJ, Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 25.Andreasen D, Jensen BL, Hansen PB, Kwon TH, Nielsen S, Skott O. The alpha(1G)-subunit of a voltage-dependent Ca(2+) channel is localized in rat distal nephron and collecting duct. Am J Physiol Renal Physiol. 2000;279:F997–F1005. doi: 10.1152/ajprenal.2000.279.6.F997. [DOI] [PubMed] [Google Scholar]
- 26.Zhao PL, Wang XT, Zhang XM, Cebotaru V, Cebotaru L, Guo G, et al. Tubular and cellular localization of the cardiac L-type calcium channel in rat kidney. Kidney Int. 2002;61:1393–1406. doi: 10.1046/j.1523-1755.2002.00267.x. [DOI] [PubMed] [Google Scholar]
- 27.Murakami M, Ohba T, Xu F, Satoh E, Miyoshi I, Suzuki T, et al. Modified sympathetic nerve system activity with overexpression of the voltage-dependent calcium channel beta3 subunit. J Biol Chem. 2008;283:24554–24560. doi: 10.1074/jbc.M802319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comper WD, Hilliard LM, Nikolic-Paterson DJ, Russo LM. Disease-dependent mechanisms of albuminuria. Am J Physiol Renal Physiol. 2008;295:F1589–F1600. doi: 10.1152/ajprenal.00142.2008. [DOI] [PubMed] [Google Scholar]
- 29.Vaziri ND, Bai Y, Ni Z, Quiroz Y, Pandian R, Rodriguez-Iturbe B. Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. J Pharmacol Exp Ther. 2007;323:85–93. doi: 10.1124/jpet.107.123638. [DOI] [PubMed] [Google Scholar]
- 30.Onozato ML, Tojo A, Goto A, Fujita T, Wilcox CS. Oxidative stress and nitric oxide synthase in rat diabetic nephropathy: effects of ACEI and ARB. Kidney Int. 2002;61:186–194. doi: 10.1046/j.1523-1755.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 31.Whaley-Connell A, Habibi J, Nistala R, Cooper SA, Karuparthi PR, Hayden MR, et al. Attenuation of NADPH oxidase activation and glomerular filtration barrier remodeling with statin treatment. Hypertension. 2008;51:474–480. doi: 10.1161/HYPERTENSIONAHA.107.102467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu HH, Hoffmann S, Endlich N, Velic A, Schwab A, Weide T, et al. Mechanisms of angiotensin II signaling on cytoskeleton of podocytes. J Mol Med. 2008;86:1379–1394. doi: 10.1007/s00109-008-0399-y. [DOI] [PubMed] [Google Scholar]
- 33.Hishikawa K, Takase O, Idei M, Fujito T. Comparison of antioxidant activity of cilnidipine and amlodipine. Kidney Int. 2009;76:230–231. doi: 10.1038/ki.2009.146. [DOI] [PubMed] [Google Scholar]
- 34.Nitschke R, Henger A, Ricken S, Gloy J, Muller V, Greger R, et al. Angiotensin II increases the intracellular calcium activity in podocytes of the intact glomerulus. Kidney Int. 2000;57:41–49. doi: 10.1046/j.1523-1755.2000.00810.x. [DOI] [PubMed] [Google Scholar]
- 35.Huber TB, Gloy J, Henger A, Schollmeyer P, Greger R, Mundel P, et al. Catecholamines modulate podocyte function. J Am Soc Nephrol. 1998;9:335–345. doi: 10.1681/ASN.V93335. [DOI] [PubMed] [Google Scholar]
- 36.Nitschke R, Henger A, Ricken S, Muller V, Kottgen M, Bek M, et al. Acetylcholine increases the free intracellular calcium concentration in podocytes in intact rat glomeruli via muscarinic M(5) receptors. J Am Soc Nephrol. 2001;12:678–687. doi: 10.1681/ASN.V124678. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh TJ, Fustier P, Wei CC, Zhang SL, Filep JG, Tang SS, et al. Reactive oxygen species blockade and action of insulin on expression of angiotensinogen gene in proximal tubular cells. J Endocrinol. 2004;183:535–550. doi: 10.1677/joe.1.05871. [DOI] [PubMed] [Google Scholar]
- 38.Brezniceanu ML, Liu F, Wei CC, Tran S, Sachetelli S, Zhang SL, et al. Catalase overexpression attenuates angiotensinogen expression and apoptosis in diabetic mice. Kidney Int. 2007;71:912–923. doi: 10.1038/sj.ki.5002188. [DOI] [PubMed] [Google Scholar]
- 39.Liu F, Brezniceanu ML, Wei CC, Chenier I, Sachetelli S, Zhang SL, et al. Overexpression of angiotensinogen increases tubular apoptosis in diabetes. J Am Soc Nephrol. 2008;19:269–280. doi: 10.1681/ASN.2007010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu F, Wei CC, Wu SJ, Chenier I, Zhang SL, Filep JG, et al. Apocynin attenuates tubular apoptosis and tubulointerstitial fibrosis in transgenic mice independent of hypertension. Kidney Int. 2009;75:156–166. doi: 10.1038/ki.2008.509. [DOI] [PubMed] [Google Scholar]
- 41.Bie P, Damkjaer M. Renin secretion and total body sodium: pathways of integrative control. Clin Exp Pharmacol Physiol. 2009 doi: 10.1111/j.1440-1681.2009.05316.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Konda T, Enomoto A, Aritomi S, Niinuma K, Koganei H, Ogawa T, et al. Different effects of L/N-type and L-type calcium channel blockers on the renin-angiotensin-aldosterone system in SHR/Izm. Am J Nephrol. 2009;30:155–161. doi: 10.1159/000210396. [DOI] [PubMed] [Google Scholar]
- 43.Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, Saruta T. Ca2+ channel subtypes and pharmacology in the kidney. Circ Res. 2007;100:342–353. doi: 10.1161/01.RES.0000256155.31133.49. [DOI] [PubMed] [Google Scholar]
- 44.Konno Y, Kimura K. Vasodilatory effect of cilnidipine, an L-type and N-type calcium channel blocker, on rat kidney glomerular arterioles. Int Heart J. 2008;49:723–732. doi: 10.1536/ihj.49.723. [DOI] [PubMed] [Google Scholar]