Abstract
Background
Corin is a transmembrane protease that processes natriuretic peptides in the heart. Like many membrane proteins, corin is shed from the cell surface.
Methods and Results
In this study, we obtained plasma samples from healthy controls and patients with heart failure (HF) and acute myocardial infarction (AMI). Soluble corin levels in plasma were measured by an ELISA method. In healthy adults (n=198), plasma corin levels were 690 (SD 260) pg/mL. The corin levels did not differ significantly among different age groups. In patients with HF (n=291), plasma corin levels were significantly lower compared to that of healthy controls (365 (SD 259) pg/mL, p<0.001). The reduction in plasma corin levels appeared to correlate with the severity of HF. In patients of New York Heart Association (NYHA) classes II, III and IV, plasma corin levels were 450 (SD 281) (n=69), 377 (SD 270) (n=132), and 282 (SD 194) (n=90) pg/mL, respectively (p<0.001 class II vs. IV; p<0.05 class III vs. IV). In contrast, plasma corin levels in patients with AMI (n=73) were similar to that of healthy controls (678 (SD 285) pg/mL, p>0.05).
Conclusions
Soluble corin was detected in human plasma. Plasma corin levels were reduced significantly in patients with HF but not AMI. Our results indicate that corin deficiency may contribute to the pathogenesis of HF and that plasma corin may be used as a biomarker in the diagnosis of HF.
Keywords: corin, natriuretic peptides, heart failure, biomarker
Heart failure (HF) is a major disease that affects ∼5.7 million Americans1. The disease has a high mortality and its annual costs exceed $37 billion in the United States1. The mechanisms underlying HF are complex, involving a variety of structural and biological alterations that directly or indirectly impair cardiac function2-4. When HF progresses to an end-stage, medical options are limited. Thus, timely diagnosis and early intervention are important for managing this life-threatening disease.
Corin is a transmembrane protease that regulates blood pressure and cardiac function5, 6. The enzyme is expressed primarily in cardiomyocytes, where it converts inactive pro-atrial natriuretic peptide (pro-ANP) and pro-brain natriuretic peptide (pro-BNP) to active peptides. Under high blood pressure or volume overload, the production of the natriuretic peptides in the heart is increased to promote natriuresis, diuresis, and vasodialation7-9. In HF, these natriuretic peptide-mediated actions serve as an important compensatory mechanism to lower blood volume/pressure and improve cardiac function. In mice, corin deficiency leads to hypertension, cardiac hypertrophy, and impaired cardiac function10. In humans, corin gene variants have been associated with an increased risk for hypertension and cardiac hypertrophy in African Americans11, 12, a population known for their high prevalence of cardiovascular disease13, 14.
Many membrane proteins are shed from the cell surface in a regulated manner and can be detected in plasma15, 16. Topologically, corin belongs to the type II transmembrane serine protease family17, 18. Corin has a cytoplasmic tail and a single-span transmembrane domain near the N-terminus. In its extracellular region, there are two frizzled-like domains, eight LDL receptor repeats, one scavenger receptor-like domain and a trypsin-like protease domain6, 19. The transmembrane domain anchors corin to the cell surface but is not required for its catalytic activity20, 21. Within the family of type II transmembrane serine proteases, several members such as enteropeptidase22, 23, hepsin24 and matriptases25, 26 were shown to be shed from the cell surface. We also detected soluble corin in cell culture medium, indicating that corin is shed from the cells (unpublished data). We hypothesized, therefore, that shed corin may enter into blood circulation and that plasma corin levels may reflect cellular homeostasis within the heart. To test this hypothesis, we measured plasma corin in healthy controls and patients with heart disease.
Methods
Study Population
This study was approved by the local Ethics Committees, and participants gave informed consent. A total of 291 patients with HF from three hospitals in Jiangsu Province, China were included. These patients were hospitalized for symptoms of HF such as fatigue, shortness of breath and edema at rest or with exercise (New York Heart Association (NYHA) functional classes II, III or IV). Some patients were previously diagnosed with HF and re-hospitalized for acute decompensation. The average value of ejection fraction in these HF patients was 51.9 (SD 16.9) %. Patients with chronic obstructive lung disease, congenital heart disease and cancer were excluded. In addition, 73 patients with acute myocardial infarction (AMI) and 198 healthy subjects, who underwent routine medical check-ups at the hospitals and had no medical history of cardiovascular disease, were also included. All participants were ethnic Han Chinese, which is the predominant population in that region of China.
Clinical Diagnosis
To confirm the diagnosis of HF, paper or electronic medical records were reviewed to obtain information on medical history, clinical examination, ECG, echocardiography, chest X-ray, and other laboratory tests of the patients. Cardiac arrhythmia was confirmed by ECG or 24-hour Holter monitoring. Valvular heart disease was confirmed by echocardiography. As part of routine practice, all patients underwent evaluation for HF diagnosis and determination of disease severity by clinical history and laboratory tests including echocardiogram. On the basis of available data, the diagnosis of HF and the underlying pathogenesis were determined by experienced cardiologists who cared for the patients but were blinded to the study. The HF functional class for each patient was assessed by HF specialists based on the NYHA classification standards. Most HF patients were treated with diuretics, ACE inhibitors, angiotensin receptor blockers and β-blockers, according to clinical management guidelines.
For AMI patients, the diagnosis was based on at least two of the following three criteria: 1) a clinical history of characteristic chest pain; 2) serial electrocardiographic tracings indicative of myocardial infarction, such as ST segment elevation, new left bundle-branch block, ST segment depression and T wave inversion; and 3) characteristic elevation of serum cardiac enzymes. A troponin test also was done in some patients for the diagnosis of AMI. Blood samples were collected within 12 hours of the ischemic onset to measure soluble corin.
Measurement of Plasma Soluble Corin
Blood samples were collected into tubes containing EDTA as an anticoagulant. Plasma samples were obtained by centrifugation at 3,000 g for 10 min. Aliquots of samples were prepared, stored at -80°C, and used within 12 weeks. Samples from HF, AMI and healthy control groups were stored in similar lengths of time. In a pilot study, soluble corin appeared to be stable in plasma samples frozen at -80°C after several cycles of freezing and thawing. The results were consistent with the findings from a recent report27. We used an ELISA kit (R&D Systems, Minneapolis, MN) to measure soluble corin levels in plasma. In brief, microtiter plates were coated with an anti-corin antibody. Plasma samples or recombinant human corin protein standards were added and incubated at room temperature for 2 hours. The plates were washed with a buffer and a biotinylated anti-human corin antibody was added and incubated for 2 hours. After washing, peroxidase-conjugated streptavidin was added and incubated at room temperature for 20 min. The reaction was visualized by adding a horseradish peroxidase substrate (3,3′,5,5′-tetramethylbenzidine, TMB) and the optical density was monitored with a spectrometer at wavelength of 450 nm.
Statistical Analysis
The analysis was done using the MedCalc software (version 10.4.0.0; Mariakerke, Belgium) and the Statistical Analysis Software (version 9.0; SAS Institute, Cary, NC). Data are presented as mean value and standard deviation (SD) in parenthesis. Comparisons of plasma corin levels in healthy controls and patient groups were performed by ANOVA followed by Tukey's post test. Multiple linear regression analysis was performed to identify independent predictors for plasma corin levels in HF patients. Variables in the analysis included sex, age, hypertension, coronary artery disease, left ventricular ejection fraction, cardiomyopathy, cardiac arrhythmia, diabetes, and NYHA classification. A residual analysis of the regression model indicated a non-normal distribution of corin levels in HF patients. A square root transformation resulted in normally distributed data. The standard errors as percentages of the coefficients, however, remained essentially the same with or without the transformation. The final results presented were from the analysis of the transformed data. All probabilities were two-tailed and p values less than 0.05 were considered as statistically significant.
Results
By ELISA, we detected corin protein in human plasma. In 198 plasma samples from healthy volunteers, soluble corin levels were 690 (SD 260) pg/mL (Fig. 1). The corin level did not appear to change in different age groups. When this control cohort was divided into three age groups: 16-25 (n=40), 26-50 (n=100), and >50 (n=58) years, the levels of soluble corin in these three groups were 619 (SD 251), 676 (SD 286), and 730 (SD 302) pg/mL, respectively. No statistical significant difference was found among these groups (p=0.17). Plasma corin levels in males (n=104) appeared to be higher than that in females (n=94) (798 (SD 285) vs. 551 (SD 224) pg/mL, p<0.001).
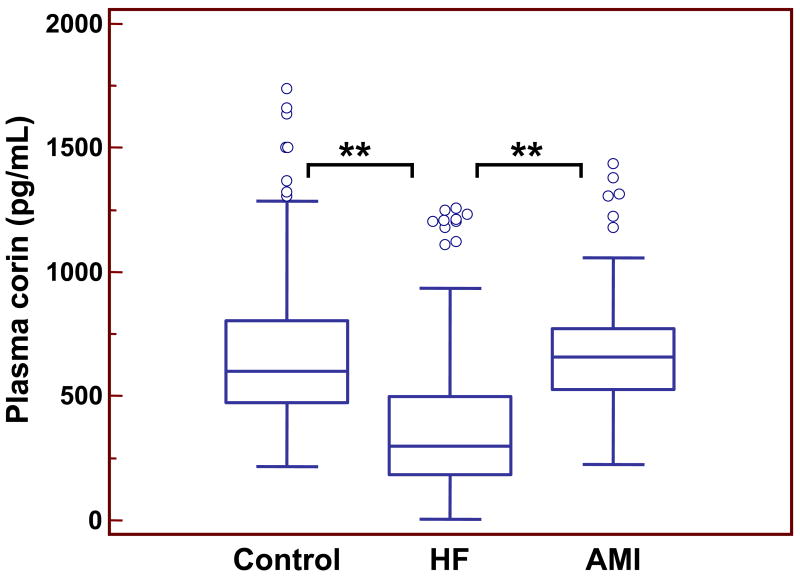
Figure 1.
Plasma corin levels in controls and patients with HF and AMI. Results were from 198 healthy controls, 291 HF and 73 AMI patients. Each box represents the median and interquartile range (IQR) values. The outliers that are >1.5 times of IQR are indicated by open circles. The vertical I bars indicate the non-outlier minimum and maximum. **p<0.001 vs. control or AMI.
Next we measured plasma soluble corin in HF patients. The base-line characteristics of these patients and healthy controls are shown in Table 1. The mean age of the HF patients was 67.5 (SD 13.3) years. There were 170 men (58.4%) and 121 women (41.6%). The results showed that plasma corin levels were significantly lower in this cohort of HF patients (365 (SD 259) pg/mL, p<0.001) compared to that of healthy controls (Fig. 1). In HF patients, corin levels in males (n=170) appeared to be higher than that of females (n=121) (387 (SD 272) vs. 335 (SD 225) pg/mL), but the difference was not statistically significant (p=0.092).
Table 1.
Characteristics of Controls and Patients with HF and AMI
| Characteristics | Control | HF | AMI |
|---|---|---|---|
| Age | 41.2 (SD 18.8)* (n=198) |
67.5 (SD 13.3)† (n=291) |
64.5 (SD 11.6) (n=73) |
| Sex (%)‡ | |||
| Male | 104 (52.5) | 170 (58.4) | 43 (58.9) |
| Female | 94 (47.5) | 121 (41.6) | 30 (41.1) |
| Medical History (%) | |||
| Hypertension | 0 (0) | 175 (60.1) | 36 (49.3) |
| Diabetes | 0 (0) | 65 (22.3) | 10 (13.7) |
| Valvular heart disease | 0 (0) | 42 (14.4) | NA |
| Cardiomyopathy | 0 (0) | 46 (15.8) | 0 (0) |
| Coronary artery disease | 0 (0) | 95 (32.6) | 73 (100) |
| Cardiac arrhythmia | 0 (0) | 37 (12.7) | NA |
| Others | 0 (0) | 58 (19.9) | 32 (43.8) |
HF, heart failure; AMI, acute myocardial infarction;
p<0.001 control vs. HF or AMI;
p>0.05 HF vs. AMI;
Sex distributions were not statistically different among three groups; NA, not available.
We also measured plasma soluble corin in AMI patients. The mean age of this group of patients was 64.5 (SD 11.6) years. There were 58.9% men and 41.1% women (Table 1). Interestingly, plasma corin levels in AMI patients did not differ significantly from that of the control group. The values were 678 (SD 285) pg/mL (n=73, p>0.05 vs. control; p<0.001 vs. HF) (Fig. 1). Like in the healthy control group, corin levels in males (n=43) were higher than that in females (n=30) with AMI (799 (SD 272) vs. 534 (SD 135) pg/mL, p<0.001). These results suggest that the reduction of plasma corin levels, as measured under our current experimental conditions, may be related more closely to the pathological changes associated with HF than that of AMI.
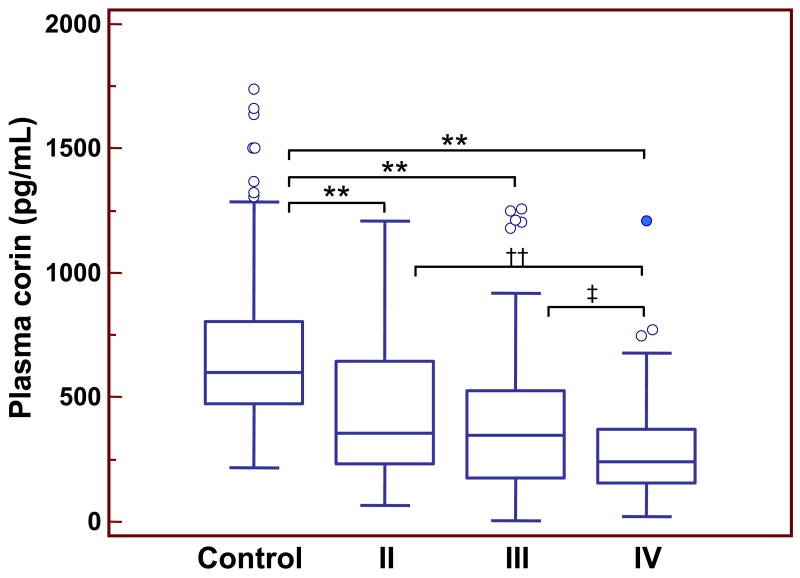
In addition, the reduction in plasma corin levels in HF patients appeared to correlate with the severity of the disease. The levels of plasma corin were lower progressively in patients with more severe HF. In patients of NYHA classes II (n=69; 56.5% male), III (n=132; 59.1% male) and IV (n=90; 58.9% male), the plasma soluble corin levels were 450 (SD 281), 377 (SD 270), and 282 (SD 194) pg/mL, respectively (p<0.001 class II vs. class IV; p<0.05 class III vs. class IV) (Fig. 2).
Figure 2.
Plasma corin levels in controls and HF patients of NYHA classes II-IV. Results were from 198 normal controls, 69 HF patients of class II, 132 HF patients of class III, and 90 HF patients of class IV. Each box represents the median and IQR values. The outliers that are >1.5 and >3 times of IQR are indicated by open and filled circles, respectively. The vertical I bars indicate the non-outlier minimum and maximum. **p<0.001 vs. control; ††p<0.001 vs. IV; ‡p<0.05 vs. IV.
We did multiple linear regression analysis to identify variables that may independently predict plasma corin levels (Table 2). The results showed that hypertension (p=0.0012) and NYHA classes (p=0.0016 for II; p<0.0001 for III and IV) were strong independent predictors for low soluble corin levels in HF patients. Sex also was an independent predictor (p<0.0001). In contrast, age, coronary artery disease, left ventricular ejection fraction and diabetes did not appear to be independent predictors for plasma corin levels in HF patients.
Table 2.
Multiple Linear Regression to Predict Plasma Corin Levels in HF*
| Variable | Coefficient | Standard Error | t | p |
|---|---|---|---|---|
| Sex† | -2.62 | 0.56 | -4.72 | <0.0001 |
| Age | -0.01 | 0.02 | 0.46 | 0.6471 |
| Hypertension | -2.59 | 0.79 | -3.27 | 0.0012 |
| Coronary artery disease | 0.21 | 0.85 | 0.25 | 0.8021 |
| LV ejection fraction | 0.00 | 0.02 | 0.16 | 0.8744 |
| Diabetes mellitus | 0.31 | 0.94 | 0.33 | 0.7394 |
| NYHA class II | -4.00 | 1.26 | -3.17 | 0.0016 |
| NYHA class III | -6.64 | 1.27 | -5.22 | <0.0001 |
| NYHA class IV | -8.65 | 1.39 | -6.22 | <0.0001 |
HF, heart failure; LV, left ventricular; NYHA, New York Heart Association. The response variables were analyzed using square root transformed data. The R-square = 0.32.
The coding for dummy variables: sex (male=0, female=1), hypertension, coronary artery disease, and diabetes (yes=1, no=0), NYHA class II (DN1=1, DN2=0, DN3=0), III (DN1=0, DN2=1, DN3=0), and IV (DN1=0, DN2=0, DN3=1) with the normal controls being the reference.
Discussion
Corin is a cardiac membrane protease that activates pro-natriuretic peptides28. To date, the role of corin in pathological conditions such as HF remains unclear. In this study, we showed that corin was detectable in human plasma, indicating that corin was shed from cells and later entered the circulation. The results suggested that plasma corin may serve as a biomarker to indicate the status of cardiomyocytes and, possibly, pathological conditions in the heart.
To test this hypothesis, we measured plasma corin in HF and AMI patients. Our results showed that corin levels were significantly lower in HF patients compared to that of healthy controls. Moreover, the reduction closely correlated with the severity of the disease. In our study, the mean age of the healthy group was younger than that of the HF group (41.2 (SD18.8) vs. 67.5 (SD 13.2)). It appears, however, plasma corin levels did not change significantly over age. In healthy subjects, interestingly, plasma corin levels appeared to be higher in males than females but the reason for such an apparent gender difference is unknown. In HF patients, however, this difference between males and females did not reach statistical significance.
In contrast to the reduced levels in HF patients, plasma corin levels did not differ significantly in AMI patients compared to that of healthy controls (Fig. 1). The data suggest that low plasma corin levels were associated more closely with pathological changes in HF than that in acute ischemic cardiac injury. In our study, the samples from AMI patients were collected within 12 hours of disease onset. Further studies are needed with samples from more time points to determine if plasma corin levels vary over a longer course after AMI. Previously, other shed membrane proteins, such as tumor necrosis factor α (TNF-α) and interleukin-1 receptors, also were identified in HF patient plasma29, 30. Unlike plasma corin, however, these soluble proteins were increased in both HF and AMI29-32, suggesting that the shedding of these cytokine receptors may be a part of the inflammatory response to heart damage or stress.
Corin is most abundantly expressed in the heart19, 33. In Northern analysis with multiple human tissues, corin mRNA was detected only in the heart19. By other more sensitive methods, low levels of mouse or rat corin mRNA were detected in other tissues, including scar myofibroblasts, developing kidneys, chondrocytes, lung cancer cells, and certain regions of the brain19, 34-36. Recently, corin mRNA and protein also were detected in mouse skin hair follicles37. The function of corin in these extra-cardiac tissues remains to be determined. The low levels of plasma corin observed in HF patients are likely to reflect either the chronic loss of cardiomyocytes, reduced corin production either in the heart or other tissues, accelerated clearance of plasma corin, and/or down-regulation of corin shedding that was associated with failing hearts.
In multiple linear regression analysis, hypertension and NYHA class were two strong independent predictors for low plasma corin levels. The primary corin function is to regulate blood pressure by activating natriuretic peptides, which in turn promote natriuresis, diuresis and vasodilation. The ANP-mediated pathway also has a local anti-hypertrophic function in the heart, which is independent of its systemic blood pressure lowering function38-40. Consistently, knockout mice lacking corin developed hypertension and cardiac hypertrophy10. A similar cardiac hypertrophy phenotype was reported in a naturally occurring mutant mouse strain, in which the corin gene was disrupted by genetic inversion41. Corin knockout mice also had reduced ejection fractions10. These data indicate that corin is important in maintaining normal blood pressure and cardiac function in vivo.
The human corin gene is on chromosome 4p12-13, which has 22 exons and spans >200 kb in length42. Population genetic studies identified single nucleotide polymorphisms (SNPs) in the corin gene, which were present in patients with hypertension and cardiac hypertrophy11, 12. In cell-based studies, these SNPs were found to alter corin protein structure and impair its biological activity43. The results suggest that corin defects may contribute to hypertension and heart disease in humans. Plasma levels of unprocessed pro-ANP and pro-BNP are highly elevated in patients with severe HF44-50, indicating that processing these natriuretic peptides becomes rate-limiting as the disease progresses. It appears, therefore, that low plasma corin levels in HF patients may reflect the underlying disease mechanism in the heart.
Corin is a newly-identified protease that is essential for processing natriuretic peptides in the heart6, 10. We have much to learn about the biology of this new enzyme and its role of in cardiovascular disease. At this time, we do not know which enzyme(s) shed corin from cells. It remains to be determined if and how corin shedding is regulated under physiological and pathological conditions. Our finding of low plasma corin levels in HF patients suggests that corin may play an important role in the development and/or progression of HF in patients and that plasma corin may be used as a biomarker for HF diagnosis. At this time, our study has its limitations because of its retrospective nature and a relatively small set of patient samples from a single ethnic group. Our data, however, should encourage designing future prospective studies with larger cohorts of patients with acute and chronic HF from different ethnic populations. Additional studies are important to determine if plasma corin levels are altered in HF patients over a longer period after acute decompensation and/or following medical treatment. Such studies shall help to understand the role of corin in heart disease and to define the diagnostic and prognostic values of plasma corin for HF.
Acknowledgments
We thank Robert S. Butler for helping statistical analysis.
Funding Sources: This work was supported in part by grants from the Ohio Third Frontier Program, the Ralph Wilson Medical Research Foundation, and the NIH (R01HL089298, R01HL089298-S1 to Q.W.).
Footnotes
Disclosure
All authors claim no potential conflict of interest relevant to this article.
References
- 1.American Heart Association. Heart disease and stroke statistics--2009 update. Dallas, Texas: American Heart Association; [Google Scholar]
- 2.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–18. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 3.McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365:1877–89. doi: 10.1016/S0140-6736(05)66621-4. [DOI] [PubMed] [Google Scholar]
- 4.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–51. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 5.Wu Q. The serine protease corin in cardiovascular biology and disease. Front Biosci. 2007;12:4179–90. doi: 10.2741/2379. [DOI] [PubMed] [Google Scholar]
- 6.Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–6. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–68. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Lee CY, Burnett JC., Jr Natriuretic peptides and therapeutic applications. Heart Fail Rev. 2007;12:131–42. doi: 10.1007/s10741-007-9016-3. [DOI] [PubMed] [Google Scholar]
- 9.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–8. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 10.Chan JC, Knudson O, Wu F, Morser J, Dole WP, Wu Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785–90. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dries DL, Victor RG, Rame JE, Cooper RS, Wu X, Zhu X, Leonard D, Ho SI, Wu Q, Post W, Drazner MH. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–10. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 12.Rame JE, Drazner MH, Post W, Peshock R, Lima J, Cooper RS, Dries DL. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–64. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- 13.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 14.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. Jama. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 15.Garton KJ, Gough PJ, Raines EW. Emerging roles for ectodomain shedding in the regulation of inflammatory responses. J Leukoc Biol. 2006;79:1105–16. doi: 10.1189/jlb.0106038. [DOI] [PubMed] [Google Scholar]
- 16.Murphy G. Regulation of the proteolytic disintegrin metalloproteinases, the ‘Sheddases’. Semin Cell Dev Biol. 2009;20:138–45. doi: 10.1016/j.semcdb.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem. 2009;284:23177–81. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu Q. Type II transmembrane serine proteases. Curr Top Dev Biol. 2003;54:167–206. doi: 10.1016/s0070-2153(03)54009-1. [DOI] [PubMed] [Google Scholar]
- 19.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–35. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- 20.Knappe S, Wu F, Madlansacay MR, Wu Q. Identification of domain structures in the propeptide of corin essential for the processing of proatrial natriuretic peptide. J Biol Chem. 2004;279:34464–71. doi: 10.1074/jbc.M405041200. [DOI] [PubMed] [Google Scholar]
- 21.Knappe S, Wu F, Masikat MR, Morser J, Wu Q. Functional analysis of the transmembrane domain and activation cleavage of human corin: design and characterization of a soluble corin. J Biol Chem. 2003;278:52363–70. doi: 10.1074/jbc.M309991200. [DOI] [PubMed] [Google Scholar]
- 22.Hadorn B, Steiner N, Sumida C, Peters TJ. Intestinal enterokinase. Mechanisms of tts “secretion” into the lumen of the small intestine. Lancet. 1971;1:165–6. doi: 10.1016/s0140-6736(71)91936-2. [DOI] [PubMed] [Google Scholar]
- 23.Kitamoto Y, Yuan X, Wu Q, McCourt DW, Sadler JE. Enterokinase, the initiator of intestinal digestion, is a mosaic protease composed of a distinctive assortment of domains. Proc Natl Acad Sci U S A. 1994;91:7588–92. doi: 10.1073/pnas.91.16.7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Q. Gene targeting in hemostasis. Hepsin Front Biosci. 2001;6:D192–200. doi: 10.2741/a604. [DOI] [PubMed] [Google Scholar]
- 25.Lin CY, Anders J, Johnson M, Dickson RB. Purification and characterization of a complex containing matriptase and a Kunitz-type serine protease inhibitor from human milk. J Biol Chem. 1999;274:18237–42. doi: 10.1074/jbc.274.26.18237. [DOI] [PubMed] [Google Scholar]
- 26.Velasco G, Cal S, Quesada V, Sanchez LM, Lopez-Otin C. Matriptase-2, a membrane-bound mosaic serine proteinase predominantly expressed in human liver and showing degrading activity against extracellular matrix proteins. J Biol Chem. 2002;277:37637–46. doi: 10.1074/jbc.M203007200. [DOI] [PubMed] [Google Scholar]
- 27.Peleg A, Jaffe AS, Hasin Y. Enzyme-linked immunoabsorbent assay for detection of human serine protease corin in blood. Clin Chim Acta. 2009;409:85–9. doi: 10.1016/j.cca.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci USA. 2000;97:8525–9. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okuyama M, Yamaguchi S, Nozaki N, Yamaoka M, Shirakabe M, Tomoike H. Serum levels of soluble form of Fas molecule in patients with congestive heart failure. Am J Cardiol. 1997;79:1698–701. doi: 10.1016/s0002-9149(97)00228-2. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–6. doi: 10.1161/01.cir.0000047274.66749.fe. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu M, Fukuo K, Nagata S, Suhara T, Okuro M, Fujii K, Higashino Y, Mogi M, Hatanaka Y, Ogihara T. Increased plasma levels of the soluble form of Fas ligand in patients with acute myocardial infarction and unstable angina pectoris. J Am Coll Cardiol. 2002;39:585–90. doi: 10.1016/s0735-1097(01)01800-9. [DOI] [PubMed] [Google Scholar]
- 32.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–6. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hooper JD, Scarman AL, Clarke BE, Normyle JF, Antalis TM. Localization of the mosaic transmembrane serine protease corin to heart myocytes. Eur J Biochem. 2000;267:6931–7. doi: 10.1046/j.1432-1033.2000.01806.x. [DOI] [PubMed] [Google Scholar]
- 34.Calderone A, Bel-Hadj S, Drapeau J, El-Helou V, Gosselin H, Clement R, Villeneuve L. Scar myofibroblasts of the infarcted rat heart express natriuretic peptides. J Cell Physiol. 2006;207:165–73. doi: 10.1002/jcp.20548. [DOI] [PubMed] [Google Scholar]
- 35.Ono Y, Nakatani T, Sakamoto Y, Mizuhara E, Minaki Y, Kumai M, Hamaguchi A, Nishimura M, Inoue Y, Hayashi H, Takahashi J, Imai T. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134:3213–25. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- 36.Wu F, Wu Q. Corin-mediated processing of pro-atrial natriuretic peptide in human small cell lung cancer cells. Cancer Res. 2003;63:8318–22. [PubMed] [Google Scholar]
- 37.Enshell-Seijffers D, Lindon C, Morgan BA. The serine protease Corin is a novel modifier of the Agouti pathway. Development. 2008;135:217–25. doi: 10.1242/dev.011031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holtwick R, van Eickels M, Skryabin BV, Baba HA, Bubikat A, Begrow F, Schneider MD, Garbers DL, Kuhn M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J Clin Invest. 2003;111:1399–407. doi: 10.1172/JCI17061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knowles JW, Esposito G, Mao L, Hagaman JR, Fox JE, Smithies O, Rockman HA, Maeda N. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J Clin Invest. 2001;107:975–84. doi: 10.1172/JCI11273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molkentin JD. A friend within the heart: natriuretic peptide receptor signaling. J Clin Invest. 2003;111:1275–7. doi: 10.1172/JCI18389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nigrovic PA, Gray DH, Jones T, Hallgren J, Kuo FC, Chaletzky B, Gurish M, Mathis D, Benoist C, Lee DM. Genetic inversion in mast cell-deficient (W(sh)) mice interrupts corin and manifests as hematopoietic and cardiac aberrancy. Am J Pathol. 2008;173:1693–701. doi: 10.2353/ajpath.2008.080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan J, Hinzmann B, Yan W, Wu F, Morser J, Wu Q. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem. 2002;277:38390–8. doi: 10.1074/jbc.M205686200. [DOI] [PubMed] [Google Scholar]
- 43.Wang W, Liao X, Fukuda K, Knappe S, Wu F, Dries DL, Qin J, Wu Q. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–8. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ando K, Hirata Y, Emori T, Shichiri M, Kurosawa T, Sato K, Marumo F. Circulating forms of human atrial natriuretic peptide in patients with congestive heart failure. J Clin Endocrinol Metab. 1990;70:1603–7. doi: 10.1210/jcem-70-6-1603. [DOI] [PubMed] [Google Scholar]
- 45.Azizi C, Maistre G, Kalotka H, Isnard R, Barthelemy C, Masson F, Pham P, Pousset F, Eurin J, Lechat P, Komajda M, Carayon A. Plasma levels and molecular forms of proatrial natriuretic peptides in healthy subjects and in patients with congestive heart failure. J Endocrinol. 1996;148:51–7. doi: 10.1677/joe.0.1480051. [DOI] [PubMed] [Google Scholar]
- 46.Chen HH. Heart failure: a state of brain natriuretic peptide deficiency or resistance or both! J Am Coll Cardiol. 2007;49:1089–91. doi: 10.1016/j.jacc.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 47.Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr, Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (BNP-32) in severe human heart failure. Proc Natl Acad Sci U S A. 2005;102:17442–7. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang F, O'Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, Apple FS, Maisel AS, Pollitt NS, Protter AA. Evidence for functional heterogeneity of circulating B-type natriuretic peptide. J Am Coll Cardiol. 2007;49:1071–8. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu H, Masuta K, Aono K, Asada H, Sasakura K, Tamaki M, Sugita K, Yamada K. Molecular forms of human brain natriuretic peptide in plasma. Clin Chim Acta. 2002;316:129–35. doi: 10.1016/s0009-8981(01)00745-8. [DOI] [PubMed] [Google Scholar]
- 50.Yandle TG, Richards AM, Gilbert A, Fisher S, Holmes S, Espiner EA. Assay of brain natriuretic peptide (BNP) in human plasma: evidence for high molecular weight BNP as a major plasma component in heart failure. J Clin Endocrinol Metab. 1993;76:832–8. doi: 10.1210/jcem.76.4.8473392. [DOI] [PubMed] [Google Scholar]