Summary
Background
c-kit is a receptor tyrosine kinase family member expressed in hematopoietic stem cells. c-kit is also transiently expressed in cardiomyocyte precursors during development, and in a rare cell population in the normal adult heart. Here, the cardiomyogenic potential of c-kit+ cells isolated from normal neonatal, normal adult and infarcted adult mouse hearts was evaluated.
Methods and Results
Magnetic activated cell sorting (MACS) was used to prepare c-kit+ cells from the hearts of ACT-EGFP/MHC-nLAC double transgenic mice. These animals exhibit widespread enhanced green fluorescent protein (EGFP) expression and cardiomyocyte-restricted nuclear β-galactosidase activity, thus permitting simultaneous tracking of cell survival and differentiation. A subset of the c-kit+ cells from double transgenic neonatal hearts acquired a cardiomyogenic phenotype when co-cultured with fetal cardiomyocytes (2.4% of all EGFP+ cells screened), but not when cultured alone or when co-cultured with mouse fibroblasts (0.03% and 0.05% of the EGFP+ cells screened, respectively). In contrast, c-kit+ cells from normal adult double transgenic hearts failed to undergo cardiomyogenic differentiation when co-cultured with non-transgenic fetal cardiomyocytes (>18,000 EGFP+ cells screened) or when transplanted into normal or infarcted adult mouse hearts (14 EGFP+ grafts examined). A single c-kit+ cell from an infarcted double transgenic adult heart was observed to acquire a cardiomyogenic phenotype in co-culture (>37,000 EGFP+ cells screened).
Conclusions
These data suggest that the ability of cardiac-resident c-kit+ cells to acquire a cardiomyogenic phenotype is subject to temporal limitations, or alternatively that the cardiomyogenic population is lost. Elucidation of the underlying molecular basis may permit robust cardiomyogenic induction in adult-derived cardiac c-kit+ cells.
Keywords: c-kit, cardiac progenitor cells, transgenic mice, cardiomyogenesis, cardiac regeneration
Introduction
Transplantation of donor myocytes or myogenic stem cells is emerging as a potential therapeutic intervention for the treatment of heart failure.1 Consequently, considerable effort is being invested to identify markers for stem cells with cardiomyogenic activity. c-kit (also known as CD117) is a member of the receptor tyrosine kinase family which is expressed at high levels in a number of hematopoietic progenitors, including hematopoietic stem cells, multipotent progenitors and common myeloid progenitors.2–4 c-kit is also expressed in early thymocyte progenitors, mast cells, melanocytes, interstitial cells of Cajal, and prostate stem cells.
c-kit expression has recently been observed in cardiovascular progenitors. For example, Wu and colleagues used a reporter transgene to isolate cells expressing the cardiomyogenic transcription factor Nkx-2.5 in differentiating embryonic stem cell (ESC) cultures. A subset of these cells expressed modest levels of c-kit, and gave rise to both cardiomyocytes and smooth muscle cells in vitro, suggesting that c-kit marked bi-potent cardiovascular progenitors.5 A similar approach was used by Christifirou and colleagues, who further demonstrated that cardiomyocytes, smooth muscle cells and endothelial cells could be derived from the sub-population of cells expressing c-kit, Nkx2-5 and Flk-1.6 Flk-1 was previously reported to be expressed in cardiovascular progenitors derived from ESCs.7
c-kit expression has also been reported in cardiovascular precursors during in vivo development. Using a BAC reporter transgene expressing EGFP under the regulation of the c-kit promoter, Tallini and colleagues demonstrated that neonatal hearts contain cells co-expressing c-kit and Flk-1.8 c-kit reporter transgene expression was also observed in neonatal cardiomyocytes with α-actinin immune reactivity, and expression levels appeared to be inversely related to the level of differentiation. These data are consistent with the notion that c-kit expression marks cardiomyogenic precursors, and that expression is extinguished with terminal differentiation. In support of this, Tallini and colleagues further demonstrated that clonally-amplified EGFP-expressing cells from neonatal hearts carrying the c-kit reporter transgene gave rise to cardiomyocytes, smooth muscle cells and endothelial cells, similar to what was observed for ESC-derived c-kit+ cells.8 Transient c-kit expression in neonatal cardiomyocytes was also observed by Li and colleagues via immune cytologic analyses,9 and was thought to be critical for the termination of cardiomyocyte cell cycle activity.
The role of c-kit expressing cells in the adult heart is less clear. Experiments with adult mice with diminished levels of c-kit activity10 or with mice carrying reporter transgenes8 suggested that c-kit expressing cells are predominantly involved with post-injury revascularization and beneficial myofibroblast-mediated remodeling. Other studies suggest that c-kit immune reactivity in the adult heart is limited to mast cells.11 In contrast, transplantation of in vitro amplified c-kit+ cells from adult rat12 or human13 hearts was thought to result in overt myocardial regeneration, with the transplanted c-kit+ cells giving rise to endothelial cells, smooth muscle cells and cardiomyocytes. In support of this, Kubo and colleagues demonstrated that adenovirus-transduced c-kit+ cells from failing human hearts could give rise to cardiomyocytes in vitro.14 Unfortunately, differences in the experimental approaches and read-outs employed in these various studies make it difficult to retrospectively evaluate the relative cardiomyogenic potential of cardiac-resident c-kit+ cells at different stages of development.
Co-culture with cardiomyocytes has been used previously to characterize the cardiomyogenic potential of a number of progenitor cell populations.14–16 Here, co-culture with fetal mouse cardiomyocytes was employed to directly compare the cardiomyogenic potential of cardiac-resident c-kit+ cells isolated from normal neonatal, normal adult and infarcted adult mouse hearts. The experiments utilized a combination of reporter transgenes and immune cytology to monitor cell survival and cardiomyogenic potential. A subset of the c-kit+ cells isolated from neonatal hearts acquired a cardiomyogenic phenotype in co-culture, independent of cell fusion events. However, this activity was markedly diminished in c-kit+ cells isolated from normal or infarcted adult hearts. Moreover, c-kit+ cells from adult hearts failed to undergo cardiomyogenic differentiation when transplanted into either normal or infarcted adult hearts. These data suggest that the ability of cardiac-resident c-kit+ cells to acquire a cardiomyogenic phenotype is subject to temporal limitations, or alternatively that the cardiomyogenic population is lost.
Methods (see On-Line Methods section for a complete description)
Mice
ACT-EGFP (C57BL/6-Tg(ACTB-EGFP)1Osb/J) and [DBA/2J × C57Bl/6J]f1 mice were from the Jackson Laboratory (Bar Harbor, ME). MHC-nLAC mice17 utilize the mouse alpha-cardiac Myosin Heavy Chain promoter to target expression of a nuclear β-galactosidase reporter. NOD/SCID-IL2-gamma null mice were from the Indiana University Simon Cancer Center.
Flow cytometry of c-kit+ cells
Cardiac cells were isolated by mincing whole neonatal or adult hearts in 0.1% Collagenase IV, incubating for 45 minutes at 37°C18 and then filtering through a 40-μm mesh. Alternatively, whole hearts were minced in Liberase/Blendzyme for 45 minutes at 37°C. Cells were stained with PerCP-conjugated antibody against CD45 or anti-c-kit-PE antibody for 45 minutes. Cells were analyzed using a FACSCalibur flow cytometer.
MACS-isolation of c-kit+ cells from the heart
Cells from enzymatically-dispersed whole hearts (normal or infarcted) were reacted with magnetic beads conjugated with mouse anti-c-kit antibody for 20 min. and subsequently separated by magnet-activated cell sorting into c-kit+ and c-kit− fractions.
Co-culture of c-kit+ cells with fetal mouse cardiomyocytes
Embryonic day 15 whole hearts were dissociated with collagenase I for 60 minutes at 37°C, plated on gelatin-coated dishes at a density of 25,000 to 50,000 cells per cm2 and cultured for 24 hours in DMEM supplemented with 10% FBS and antibiotics. The medium was replaced with DMEM supplemented with 10% FBS, 10 ug/mL insulin, 10 ug/mL transferrin and antibiotics and seeded with freshly isolated c-kit+ cells at a density of 2,500 to 5,000 cells per cm2.
Myocardial infarction and intra-cardiac grafting
Myocardial infarction was performed in 12 week old mice as described previously.19 For intra-cardiac grafting, donor cells suspended in 3 μl phosphate-buffered saline were injected into the anterior and posterior infarct border zones of the ischemic myocardium after coronary ligation, or in normal hearts.
Results
Characterization and isolation of myocardial c-kit+ cells
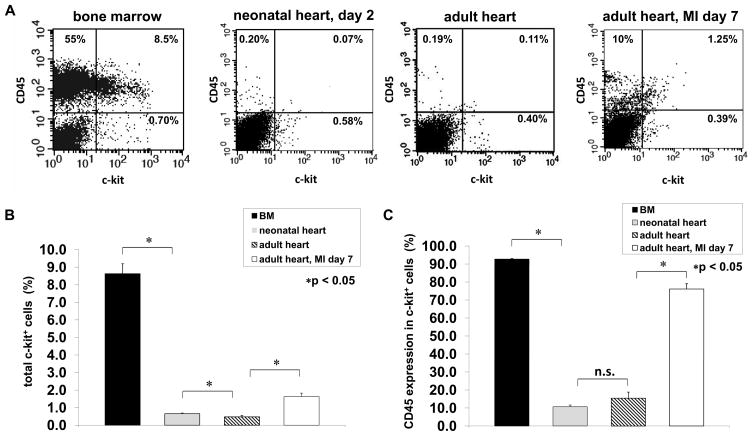
Fluorescence-activated cell sorting (FACS) was used to quantitate and characterize the c-kit+ cells in normal neonatal and adult hearts, as well as in infarcted adult hearts. c-kit+ cells from adult bone marrow were used as a reference. Isotype-matched antibodies were used to set threshold levels for detecting c-kit and CD45 immune reactivity. c-kit+/CD45− and c-kit+/CD45+ populations were readily observed in representative dot plots from each source (Figure 1A). Quantitative analyses (Figure 1B and C) revealed that approximately 9% of the mono-nucleated cells from the bone marrow expressed c-kit, the majority of which (>90%) co-expressed the hematopoietic lineage marker CD45. In the neonatal heart, 0.65% of the cells were c-kit+, and of these approximately 10% were CD45+. In the adult heart, approximately 0.5% of the cells expressed c-kit, of which approximately 15% co-expressed CD45. Myocardial infarction via permanent coronary artery occlusion resulted in an approximately 3-fold increase in the number of cells expressing c-kit in adult hearts, of which the majority (76%) co-expressed CD45 when analyzed at 7 days post-infarction, in agreement with published observations.10
Figure 1. Characterization c-kit+ cells.
(A) Representative FACS dot plots showing the distribution of CD45+/c-kit−, CD45−/c-kit+ and CD45+/c-kit+ cells from bone marrow, neonatal heart, and normal and infarcted adult heart. (B) Quantitative analysis of c-kit+ cells from bone marrow, neonatal heart and normal and infarcted adult heart. (C) Quantitative analysis of the percentage of c-kit+ cells co-expressing the hematopoietic marker CD45. Data represent mean ± SEM (n=6). *p < 0.05; n.s.: not significant.
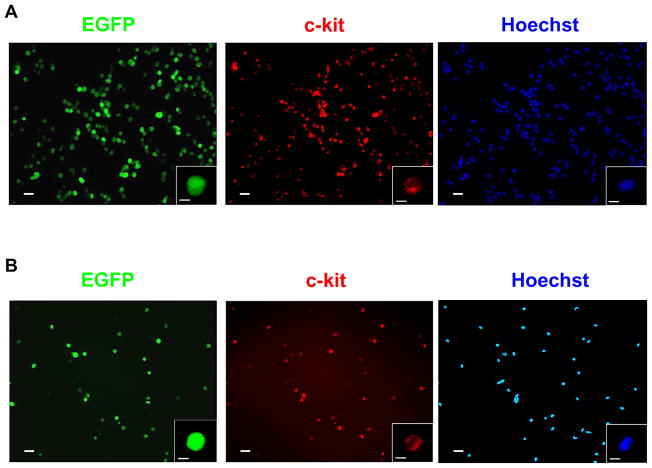
Magnetic-activated cell sorting (MACS) was employed to isolate c-kit+ cells from mice carrying the ACT-EGFP reporter transgene (which targets EGFP expression under the β-actin promoter).20 The vast majority of cells obtained from neonatal or normal adult hearts following two cycles of MACS purification exhibited EGFP epi-fluorescence (Figure 2A and B, respectively). Immune cytology analyses revealed that >90% of cells with EGFP epi-fluorescence also exhibited c-kit immune reactivity. Similar results were obtained from infarcted adult hearts (not shown).
Figure 2. Isolation of c-kit+ cells.
c-kit+ cells were isolated using 2 consecutive isolation procedures with a MACS column. Representative cell preparations from neonatal (A) and normal adult (B) hearts are shown. The left panel shows EGFP epi-fluorescence, the middle panel shows c-kit+ immune reactivity (Rhodamine-conjugated secondary antibody) and the right panel shows Hoechst nuclear staining (blue). The insets depict high magnification images of a single cell from the field. Bar = 20 microns in low power images, 5 microns in insets.
c-kit+ cells derived from neonatal hearts acquire a cardiac phenotype following co-culture with fetal cardiomyocytes
Co-culture with cardiomyocytes was used to quantitate the cardiomyogenic potential of cardiac-resident c-kit+ from various stages of development. Cells were prepared from double transgenic mice carrying the ACT-EGFP and MHC-nLAC reporter transgenes. As shown above, the ACT-EGFP reporter targets EGFP expression to most cardiac-resident c-kit+ cells. The MHC-nLAC reporter targets cardiomyocyte-restricted nuclear β-galactosidase activity which is detected by reacting with the chromogenic β-galactosidase substrate X-GAL.17 Thus, the presence of c-kit+ cells in co-cultures could be readily quantitated via the presence of EGFP epi-fluorescence or anti-EGFP immune reactivity, and their cardiomyogenic differentiation could be quantitated by co-localization of anti-EGFP and anti-α-actinin immune reactivity or by induction of nuclear β-galactosidase activity. Control experiments using fetal cardiomyocytes confirmed the fidelity of the reporter transgenes for these analyses (see Supplemental Figure 1).
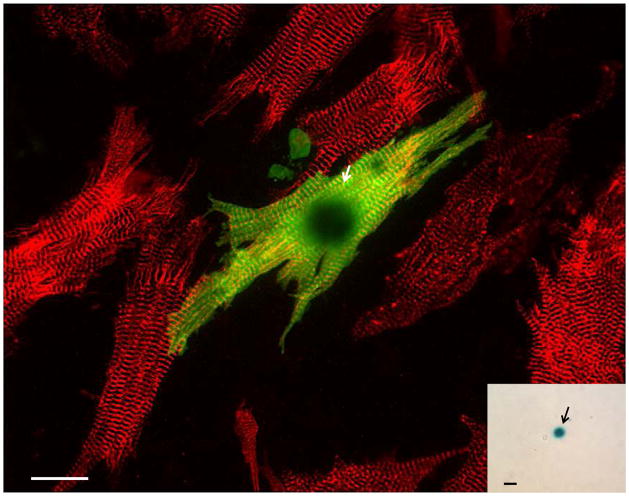
Fetal cardiomyocytes from non-transgenic mice were plated, and 24 hours later the cultures were seeded with MACS-isolated c-kit+ cells prepared from the hearts of neonatal ACT-EGFP/MHC-nLAC double transgenic mice. After 7 days of co-culture, a subset of the cells with EGFP epi-fluorescence exhibited robust contractile activity (Supplemental Video 1). The samples were fixed, reacted with X-GAL, and processed for EGFP (Alexa488-conjugated antibody) and α-actinin (rhodamine-conjugated secondary antibody) immune reactivity. A subset of the EGFP-expressing cells exhibited highly mature sarcomeric structure as evidenced by α-actinin immune reactivity (Figure 3); moreover, all of the EGFP-positive cells with α-actinin immune reactivity also exhibited nuclear β-galactosidase activity (Figure 3, inset). Two to 3% of the neonatal heart-derived c-kit+/EGFP+ cells exhibited a cardiomyogenic phenotype when co-cultured with non-transgenic fetal cardiomyocytes (Table 1). In contrast, EGFP-expressing, β-galactosidase positive cardiomyocytes were only rarely detected when the neonatal heart-derived c-kit+ cells were cultured alone, or when co-cultured with NIH-3T3 cells (Table 1).
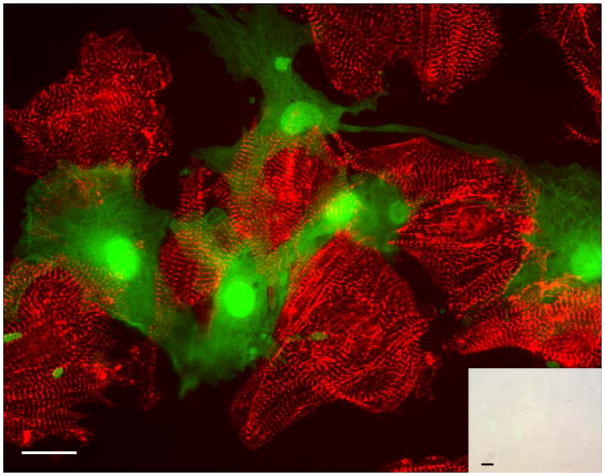
Figure 3. Cardiac-resident c-kit+ cells from neonatal hearts exhibit robust cardiomyogenic differentiation when co-cultured with fetal cardiomyocytes.
Non-transgenic fetal cardiomyocyte cultures were seeded with neonatal c-kit+ cells from ACT-EGFP/MHC-nLAC double transgenic hearts and cultured for 7 days. The cultures were then fixed and processed for β-galactosidase activity (blue), EGFP (green) and α-actinin (red) immune reactivity. EGFP-expressing cardiomyocytes exhibiting mature sarcomeric structure (yellow due to the overlay of green EGFP and red α-actinin signals) were readily detected by fluorescence microscopy; these cells also exhibited β-galactosidase activity when visualized under bright field illumination (inset). Bar = 20 microns in the fluorescent image, 20 microns in the bright field inset. Arrows mark β-galactosidase+ nuclei. Bright field, single color fluorescence and merged images for these cells are shown in the Supplemental Data section.
Table 1.
Quantification of co-culture experiments with cardiac-resident c-kit+ cells:
| A. Cardiomyogenic Assays | |||||
|---|---|---|---|---|---|
| c-kit+ Cell | Co-culture | # EGFP+ | # act.+ | # nLAC+ | % CM-genic |
| ACT-EGFP/MHC-nLAC (normal adult) | NON-TXG fetal CM | 18,866 | 0 | 0 | 0 |
| ACT-EGFP/MHC-nLAC (MI adult) | NON-TXG fetal CM | 37,372 | 1 | 1 | 0.003 |
| ACT-EGFP/MHC-nLAC (neonatal) | NON-TXG fetal CM | 6,995 | 165 | 165 | 2.4 |
| ACT-EGFP/MHC-nLAC(neonatal) | None | 5,946 | 2 | 2 | 0.03 |
| ACT-EGFP/MHC-nLAC(neonatal) | NIH-3T3 | 6,556 | 3 | 3 | 0.05 |
| B. Cell Fusion Assay | |||||
| c-kit+ Cell | Co-culture | # EGFP+ | # EGFP+/act.+ | # EGFP+/act.+/nLAC+ | % Fusion |
| Act-EGFP(neonatal) | MHC-nLAC fetal CM | 3,766 | 144 (3%) | 2 | 0.05 |
Abbreviations: NON-TXG, non-transgenic; CM, cardiomyocytes; act., α-actinin
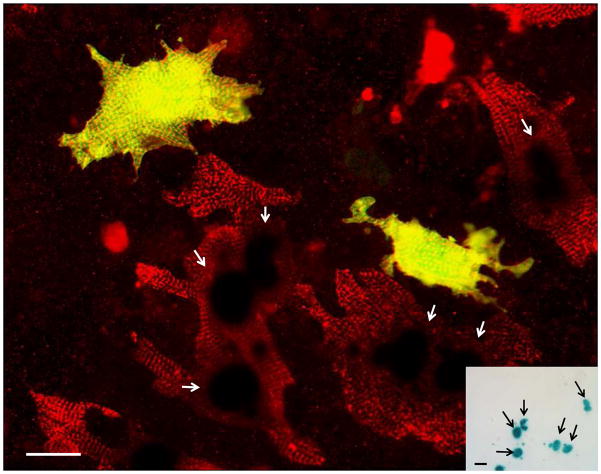
To determine if cell fusion contributed to the apparent cardiomyogenic events, fetal cardiomyocyte cultures were prepared from single transgenic MHC-nLAC mice. Twenty-four hours later, the cultures were seeded with MACS-purified c-kit+ cells prepared from the hearts of single transgenic neonatal ACT-EGFP mice. The cultures were fixed 7 days later and processed as described above. Two to 3% of the cells with EGFP immune reactivity also exhibited α-actinin immune reactivity (Figure 4). However, the preponderance of these cells did not exhibit MHC-nLAC reporter transgene activity (which was readily detected in cardiomyocytes lacking EGFP epi-fluorescence; see Figure 4, inset). Indeed, only two cells with MHC-nLAC reporter transgene activity were observed when more than 3,500 EGFP-expressing cells were screened (Table 1), suggesting a cardiomyocyte/c-kit+ cell fusion rate of 0.05% under these assay conditions.
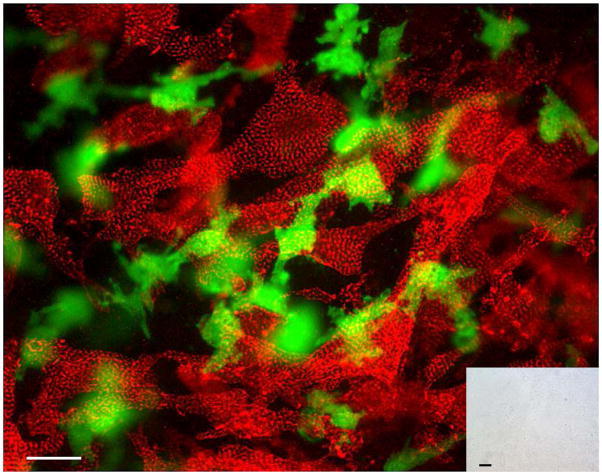
Figure 4. Cardiac-resident c-kit+ cells from neonatal hearts exhibit robust cardiomyogenic differentiation in the absence of cell fusion.
Fetal cardiomyocyte cultures from single transgenic MHC-nLAC mice were seeded with c-kit+ cells from neonatal ACT-EGFP hearts and cultured for 7 days. The cultures were then fixed and processed for β-galactosidase activity (blue), EGFP (green) and α-actinin (red) immune reactivity. EGFP-expressing cardiomyocytes exhibiting mature sarcomeric structure (yellow due to the overlay of green EGFP and red α-actinin signals) were readily detected by fluorescence microscopy; however these cells lacked β-galactosidase activity when visualized under bright field illumination (inset). Bar = 20 microns in the fluorescent image, 20 microns in the bright field inset. Arrows mark β-galatosidase positive nuclei. Bright field, single color fluorescence and merged images for these cells are shown in the Supplemental Data section.
c-kit+ cells derived from adult hearts have very limited cardiomyogenic potential following co-culture with fetal cardiomyocytes or intra-cardiac engraftment
Next, c-kit+ cells prepared from normal adult mice were tested for cardiomyogenic activity. Fetal cardiomyocytes from non-transgenic mice were plated, and 24 hours later the cultures were seeded with MACS-isolated c-kit+ cells prepared from the hearts of adult ACT-EGFP/MHC-nLAC double transgenic mice. After 7 days of co-culture, the samples were fixed, reacted with X-GAL, and processed for EGFP and α-actinin immune reactivity. Cells with green EGFP immune reactivity were readily identified, indicating that the adult cardiac-resident c-kit+ cells survived in co-culture. However, these cells lacked detectable α-actinin immune reactivity (Figure 5) and failed to activate the MHC-nLAC reporter transgene (Figure 5, inset). No cardiomyogenic events were detected when more than 18,000 EGFP-expressing c-kit+ cells were screened (Table 1).
Figure 5. Cardiac-resident c-kit+ cells from normal adult hearts fail to undergo cardiomyogenic differentiation when co-cultured with fetal cardiomyocytes.
Non-transgenic fetal cardiomyocyte cultures were seeded with c-kit+ cells from adult ACT-EGFP/MHC-nLAC double transgenic hearts and cultured for 7 days. The cultures were then fixed and processed for β-galactosidase activity (blue), EGFP (green) and α-actinin (red) immune reactivity. EGFP-expressing cells were readily detected; however these cells lacked sarcomeric structure when examined by fluorescence microscopy, and lacked β-galactosidase activity when visualized under bright field illumination (inset). Bar = 20 microns in the fluorescent image, 20 microns in the bright field inset. Bright field, single color fluorescence and merged images for these cells are shown in the Supplemental Data section.
To determine if the adult heart provides an environment more conducive to their cardiomyogenic differentiation, MACS-isolated c-kit+ cells prepared from normal adult ACT-EGFP/MHC-nLAC double transgenic mice were engrafted into normal or infarcted (via permanent coronary artery ligation) recipient hearts. Syngenic [C57Bl/6J × DBA/2J]F1 or immune compromised NOD/SCID-gamma recipients were used. The hearts were harvested at 10 to 21 days post-engraftment, fixed and sectioned. The sections were then reacted with X-GAL and processed for EGFP and α-actinin immune reactivity. Although cells with EGFP immune reactivity were detected within the engrafted hearts, they lacked α-actinin immune reactivity (Figure 6) and failed to activate the MHC-nLAC reporter (Figure 6, inset). No cardiomyogenic events were detected in 14 hearts successfully engrafted with adult heart-derived c-kit+ cells (Supplemental Table 1). Control experiments with double transgenic fetal cardiomyocyte donor cells confirmed the fidelity of the reporter transgenes for these analyses (see Supplemental Figure 2).
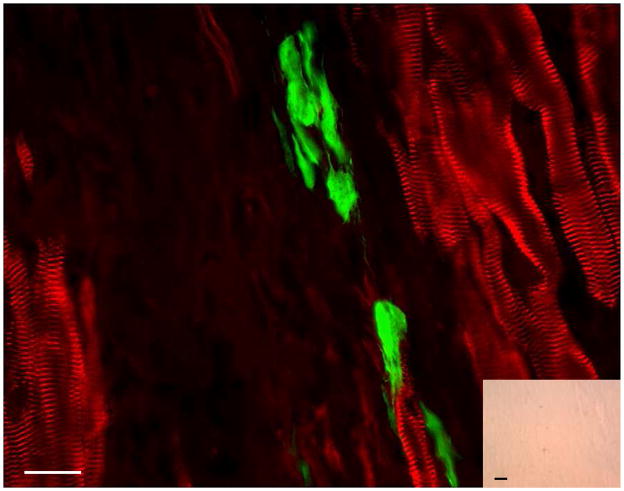
Figure 6. Cardiac-resident c-kit+ cells from normal adult hearts fail to undergo cardiomyogenic differentiation when transplanted into infarcted adult mouse hearts.
c-kit+ cells from ACT-EGFP/MHC-nLAC adult double transgenic hearts were transplanted into an infarcted non-transgenic recipient heart. The heart was harvested 13 days later, fixed and processed for β-galactosidase activity (blue), EGFP (green) and α-actinin (red) immune reactivity. EGFP-expressing cells were readily detected; however these cells lacked sarcomeric structure when examined by fluorescence microscopy and also lacked β-galactosidase activity when visualized under bright field illumination (inset). Bar = 20 microns in the fluorescent image, 20 microns in the bright field inset. Bright field, single color fluorescence and merged images for these cells are shown in the Supplemental Data section.
Finally, to determine if myocardial infarction enhances the cardiomyogenic potential of cardiac-resident c-kit+ cells, adult ACT-EGFP/MHC-nLAC double transgenic mice were subjected to permanent coronary artery ligation. Seven days later, c-kit+ cells were prepared via MACS purification and seeded onto cultures of fetal cardiomyocytes from non-transgenic mice which were prepared 24 hours earlier. After 7 days of co-culture, the samples were fixed and processed as described above. Once again, the EGFP-expressing c-kit+ cells readily survived in co-culture. A single EGFP+ cell exhibited α-actinin immune reactivity and MHC-nLAC reporter activity was observed (Figure 7). The rest of the >37,000 c-kit+ cells examined in the co-cultures lacked α-actinin immune reactivity and failed to activate the MHC-nLAC reporter (Figure 8, Table 1).
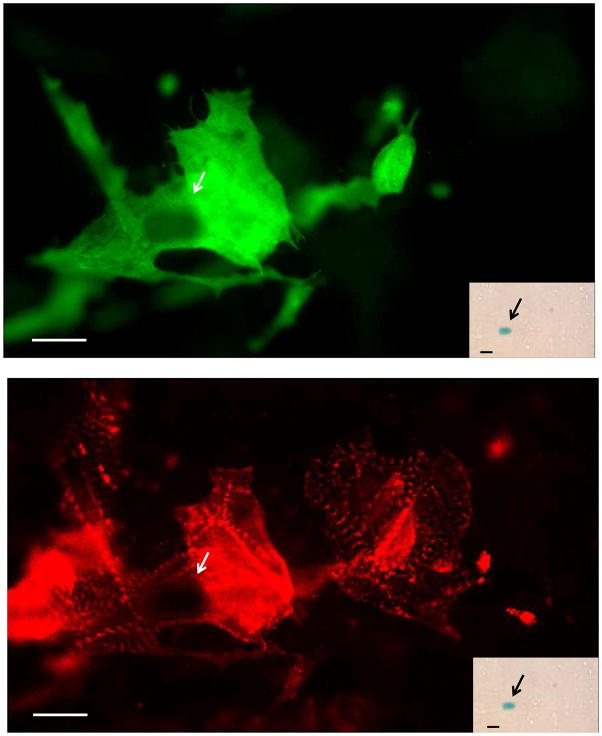
Figure 7. Low levels of apparent cardiomyogenic differentiation from cardiac-resident c-kit+ cells from infarcted adult hearts when co-cultured with fetal cardiomyocytes.
Non-transgenic fetal cardiomyocyte cultures were seeded with c-kit+ cells from infarcted adult ACT-EGFP/MHC-nLAC double transgenic hearts and cultured for 7 days. The cultures were then fixed and processed for β-galactosidase activity (blue), EGFP (green) and α-actinin (red) immune reactivity. A single EGFP-expressing cardiomyocyte exhibiting mature sarcomeric structure (anti-EGFP immune reactivity, green, upper panel; anti-α-actinin immune reactivity, red, lower panel) was observed; this cell also exhibited β-galactosidase activity when visualized under bright field illumination (inset). Bar = 20 microns in the fluorescent image, 20 microns in the bright field inset. Arrows mark β-galactosidase+ nuclei. Bright field, single color fluorescence and merged images for these cells are shown in the Supplemental Data section.
Figure 8. The vast majority of cardiac-resident c-kit+ cells from infarcted adult hearts fail to undergo cardiomyogenic differentiation when co-cultured with fetal cardiomyocytes.
Non-transgenic fetal cardiomyocyte cultures were seeded with c-kit+ cells from infarcted adult ACT-EGFP/MHC-nLAC double transgenic hearts and cultured for 7 days. The cultures were then fixed and processed for β-galactosidase activity (blue), EGFP (green) and α-actinin (red) immune reactivity. EGFP-expressing cells were readily detected; however these cells lacked sarcomeric structure when examined by fluorescence microscopy, and lacked β-galactosidase activity when visualized under bright field illumination (inset). Bar = 20 microns in the fluorescent image, 20 microns in the bright field inset. Bright field, single color fluorescence and merged images for these cells are shown in the Supplemental Data section.
Discussion
The data presented here indicate that a sub-population of the cardiac-resident c-kit+ cells isolated from neonatal mouse heart exhibit an overt cardiomyogenic phenotype when co-cultured with fetal cardiomyocytes. This phenotype was largely absent in cardiac-resident c-kit+ cells isolated from normal or infarcted adult mouse hearts. Importantly, the use of reporter transgenes permitted straightforward quantitation of c-kit+ cell survival and differentiation, and there was extremely high concordance between the results obtained via X-GAL reaction (i.e., monitoring induction of the MHC-nLAC reporter) and α-actinin immune reactivity (Table 1). Thus, the fidelity of the reporter transgene read-out is quite high for identifying well-differentiated cardiomyocytes. Collectively, these data suggest a developmental loss of cardiomyogenic activity in c-kit+ cells in the transition from neonatal to adult life in mice, or alternatively a loss of the cardiomyogenic population.
Previous studies suggested that cell fusion could contribute to apparent transdifferentiation events in vitro and in vivo.21, 22 Although cell fusion events could be detected when MHC-nLAC fetal cardiomyocytes were co-cultured with neonatal ACT-EGFP c-kit+ cells, the frequency of these events was >50-fold lower than the frequency of putative cardiomyogenic events (0.05% vs. 3%, Table 1). Carry-over of differentiated cells from the neonatal c-kit+ heart preparations could also contribute to the apparent cardiomyogenic events. However, immune cytology analyses immediately following MACS isolation indicated that only 0.11% of the cells exhibited alpha-actinin immune reactivity (9.82% exhibited smooth muscle actin immune reactivity and 0.24% exhibited isolectin 4B immune reactivity; see Supplemental Figure 9, Supplemental Table 2). The observation that the percentage of cells with alpha actinin immune reactivity post-MACS (0.11%) was 22- to 27-fold lower than the percentage of alpha-actinin positive cells expressing the reporter transgenes following co-culture with non-transgenic fetal cardiomyocytes (2.4 to 3%, see Table 1) suggests that simple carry-over of neonatal cardiomyocytes does not account for the observed results. Moreover, the presence of a roughly similar content of α-actinin+ cells immediately following MACS isolation versus 7 days of single culture or NIH-3T3 co-culture (Table 1) argues against a role of enhanced survival and/or seeding of carry-over neonatal cardiomyocytes in the fetal cardiomyocyte co-cultures. Collectively, the data are consistent with the notion that a small portion of cardiac-resident c-kit+ cells from neonatal hearts can undergo cardiomyogenic differentiation when co-cultured with fetal cardiomyocytes. The data do not distinguish if these cells represent a true cardiomyogenic stem cell, or alternatively a c-kit+ developmental intermediate which requires a co-culture environment to acquire a cardiomyogenic phenotype. FACS followed by co-culture assay (Supplemental Figure 10, Supplemental Table 3) revealed that the apparent cardiomyogenic activity is present in the c-kit+/CD45− sub-population of cells isolated from the neonatal hearts.
In contrast, the ability of c-kit+ cells isolated from normal or infarcted adult mouse hearts to acquire a cardiomyogenic phenotype was limited, with only a single potential cardiomyogenic event detected when more than 56,000 cardiac-resident c-kit+ cells were screened. Moreover, the origin of the single EGFP+/α-actinin+/β-galactosidase+ cardiomyocyte observed in co-cultures with c-kit+ cells from infarcted adult heart is not clear. It is equally likely that this cell originated from carryover of a border zone cardiomyocyte which re-induced c-kit expression8, from a fusion event between a c-kit+ cell and a fetal cardiomyocyte, or from a bona fide cardiomyogenic differentiation event. Unfortunately, the rarity of the event precludes a systematic assessment of its origin. These data suggest that the cardiomyogenic c-kit+ sub-population present in neonatal hearts is lost upon maturation, or alternatively, loses its ability to undergo cardiomygeinc conversion when co-cultured with fetal cardiomyocytes.
These results differ from several in vitro studies using adult heart-derived c-kit+ cells from rat and human. Although single culture of adult heart-derived c-kit+ cells resulted in only a rudimentary cardiomyocyte phenotype (as evidenced by the induction of a limited number of myocyte markers and the absence of myofiber structure),12 co-culture with cardiomyocytes resulted in more robust cardiomyogenic differentiation.13, 14, 23 It is noteworthy that prior to the establishment of co-cultures, these latter experiments all employed varying degrees of in vitro manipulation of the c-kit+ cells, including prolonged amplification of the cells or exposure to adenoviruses in suspension culture. It is possible that these manipulations imparted a certain degree of re-programming which enhanced cardiomyogenic potential. Indeed, increased expression of GATA-4 was observed in long-term cultures of adult heart-derived c-kit+ cells.23 Although subtle differences in methodologies might have altered our ability to observe overt cardiomyogenic induction in adult c-kit+ cells, the observation that neonatal c-kit+ cells were cardiomyogenic when subjected to the same protocols underscores a fundamental difference between heart-derived c-kit+ cell populations prepared from different developmental stages. Adult heart-derived c-kit+ cells also failed to give rise to overt cardiomyogenesis following engraftment into normal or infarcted mouse hearts in our hands, despite survival of the donor cells. These results contrast with previous data reporting extensive cardiac regeneration after direct injection of c-kit+ derived cells from rat or human hearts.12, 13, 24 Once again, in vitro manipulation of the cells in these latter studies may have contributed to the discrepant results.
The observations reported here are supported by other studies. For example, Talinni and colleagues showed that c-kit+ cells from neonatal mouse hearts could be clonally-amplified and give rise to cardiomyocytes, smooth muscle and endothelial cells.8 In addition, c-kit expression was observed to mark cardiomyocyte and smooth muscle precursors in fetal hearts and differentiating ESC cultures.5, 6 Thus, the apparent cardiomyogenic activity in c-kit+ cells derived from neonatal hearts observed here is perhaps not surprising. It is likely that the co-culture experiments employed in the current study mimicked the cardiomyogenic conditions developed by Talinni and colleagues. Although these authors did not examine the cardiomyogenic potential of c-kit+ cells isolated from adult hearts, they did demonstrate that expression of a c-kit-promoted reporter transgene was predominantly associated with smooth muscle and endothelial cells following cryo-injury of the heart. Reporter gene expression observed in border zone cardiomyocytes was attributed to a previously described reactivation of c-kit expression following myocardial injury.9 The absence of overt cardiomyogenic activity in adult heart derived c-kit+ cells following co-culture or intra-cardiac injection in the current study is consistent with these results. These results are also consistent with the low levels of cumulative cardiomyocyte renewal observed in individuals alive during above-ground nuclear testing.25 Presently it is not clear if the cells with apparent cardiomyogenic activity are cardiac resident or if they populate the heart following migration from an extra-cardiac site. The absence of cardiomyogenic induction in co-cultures with c-kit+ cells isolated from neonatal day 2 (Supplemental Figure 11, Supplemental Table 3) or adult19 bone marrow suggests that the cells are not from the marrow, with the caveat that if residence in a cardiac niche was required to acquire cardiomyogenic activity in marrow-derived cells, this assay would not detect it.
The normal adult mouse heart contains approximately 1.5 × 106 cardiomyocytes, which comprise 14.2% of the total cell number;26, Soonpaa, unpublished consequently there are approximately 9 × 106 non-cardiomyocytes in the adult mouse heart. FACS analyses revealed that approximately 0.5% of the non-cardiomyocytes are c-kit+ (Figure 1) in the uninjured adult heart. Thus, there should be approximately 45,000 c-kit+ cells/heart. Since we failed to see any cardiomyogenic events when 18,866 c-kit+ cells from normal hearts were screened, these data suggest that there would likely be very few (i.e., less than 3) cardiomyogenic c-kit+ stem cells in the normal adult mouse heart. In infarcted mouse hearts, the c-kit+ population increases to 1.4% at 7 days post-injury. Assuming a roughly similar number of total cells, this would translate to 126,000 c-kit+ cells per infarcted adult heart. Since we observed only a single potential cardiomyogenic event (caveats mentioned above notwithstanding) when 37,372 cells were screened, these data suggest that there would likely be fewer than 4 cardiomyogenic c-kit+ cells per adult mouse heart at 7 days post-infarction.
Limitations of the study
The present study does not exclude the possibility of a cardiomyogenic c-kit+ population in vivo in the normal or injured adult mouse heart. However, the quantitative analyses suggest that if such cells do exist, they are extremely rare (within the limits of the cell isolation protocols and cardiomyogenic assays employed). It is possible that long-standing heart failure is required to obtain c-kit+ cells with cardiomyogenic activity. Although Kubo and colleagues demonstrated that c-kit+ cells from failing human hearts were cardiomyogenic in co-culture, it is not clear if cells from non-failing hearts were similarly examined. It is possible that our results reflect an intrinsic species difference, as all of the in vitro differentiation and cell transplantation studies reported to date used rat or human cells; thus far, only studies with circumstantial data implying cardiomyogenic activity in cardiac-resident c-kit+ cells from adult mouse hearts in the absence of genetic enhancement have been reported.27–29 It is also possible that our fetal cardiomyocyte cultures may lack the factors needed to promote differentiation of adult cardiac-resident c-kit+ cells, despite being sufficient to induce differentiation of neonatal-derived cells (although co-culture with neonatal cardiomyocyte preparations also failed to promote differentiation, Supplemental Figure 12). Finally, our experiments did not address benefits of c-kit+ cells on ventricular function after infarction which results from non-cardiomyogenic activity.
Supplementary Material
Acknowledgments
We thank Dorothy Field for technical support.
Funding
Supported by the National Institutes of Health (HL083126) and the German Research Foundation (DFG ZA 575/1-1).
Footnotes
Conflict of Interests
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rubart M, Field LJ. Cardiac regeneration: repopulating the heart. Annual Review Of Physiology. 2006;68:29–49. doi: 10.1146/annurev.physiol.68.040104.124530. [DOI] [PubMed] [Google Scholar]
- 2.Edling CE, Hallberg B. c-Kit--a hematopoietic cell essential receptor tyrosine kinase. Int J Biochem Cell Biol. 2007;39:1995–1998. doi: 10.1016/j.biocel.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- 4.Ronnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004;61:2535–2548. doi: 10.1007/s00018-004-4189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental Origin of a Bipotential Myocardial and Smooth Muscle Cell Precursor in the Mammalian Heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Christoforou N, Miller RA, Hill CM, Jie CC, McCallion AS, Gearhart JD. Mouse ES cell-derived cardiac precursor cells are multipotent and facilitate identification of novel cardiac genes. J Clin Invest. 2008;118:894–903. doi: 10.1172/JCI33942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Tallini YN, Greene KS, Craven M, Spealman A, Breitbach M, Smith J, Fisher PJ, Steffey M, Hesse M, Doran RM, Woods A, Singh B, Yen A, Fleischmann BK, Kotlikoff MI. c-kit expression identifies cardiovascular precursors in the neonatal heart. Proc Natl Acad Sci U S A. 2009;106:1808–1813. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li M, Naqvi N, Yahiro E, Liu K, Powell PC, Bradley WE, Martin DIK, Graham RM, Dell’Italia LJ, Husain A. c-kit Is Required for Cardiomyocyte Terminal Differentiation. Circ Res. 2008;102:677–685. doi: 10.1161/CIRCRESAHA.107.161737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouly J, Bruneval P, Mandet C, Proksch S, Peyrard S, Amrein C, Bousseaux V, Guillemain R, Deloche A, Fabiani JN, Menasche P. Cardiac stem cells in the real world. J Thorac Cardiovasc Surg. 2008;135:673–678. doi: 10.1016/j.jtcvs.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 12.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 13.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D’Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo H, Jaleel N, Kumarapeli A, Berretta RM, Bratinov G, Shan X, Wang H, Houser SR, Margulies KB. Increased cardiac myocyte progenitors in failing human hearts. Circulation. 2008;118:649–657. doi: 10.1161/CIRCULATIONAHA.107.761031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31− but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 16.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol. 2004;265:262–275. doi: 10.1016/j.ydbio.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 17.Soonpaa MH, Koh GY, Klug MG, Field LJ. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science. 1994;264:98–101. doi: 10.1126/science.8140423. [DOI] [PubMed] [Google Scholar]
- 18.Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, Fischer R, Krieg L, Hirsch E, Huber B, Nathan P, Israel L, Imhof A, Herbach N, Assmann G, Wanke R, Mueller-Hoecker J, Steinbeck G, Franz WM. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell stem cell. 2009;4:313–323. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 20.Hadjantonakis AK, Macmaster S, Nagy A. Embryonic stem cells and mice expressing different GFP variants for multiple non-invasive reporter usage within a single animal. BMC Biotechnol. 2002;2:11. doi: 10.1186/1472-6750-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- 22.Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto S, Kawaguchi N, Ellison GM, Matsuoka R, Shin’oka T, Kurosawa H. Characterization of long-term cultured c-kit+ cardiac stem cells derived from adult rat hearts. Stem Cells Dev. 2010;19:105–116. doi: 10.1089/scd.2009.0041. [DOI] [PubMed] [Google Scholar]
- 24.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–116. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soonpaa MH, Field LJ. Assessment of cardiomyocyte DNA synthesis in normal and injured adult mouse hearts. Am J Physiol. 1997;272:H220–226. doi: 10.1152/ajpheart.1997.272.1.H220. [DOI] [PubMed] [Google Scholar]
- 27.Fransioli J, Bailey B, Gude NA, Cottage CT, Muraski JA, Emmanuel G, Wu W, Alvarez R, Rubio M, Ottolenghi S, Schaefer E, Sussman MA. Evolution of the c-kit-positive cell response to pathological challenge in the myocardium. Stem Cells. 2008;26:1315–1324. doi: 10.1634/stemcells.2007-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gude N, Muraski J, Rubio M, Kajstura J, Schaefer E, Anversa P, Sussman MA. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ Res. 2006;99:381–388. doi: 10.1161/01.RES.0000236754.21499.1c. [DOI] [PubMed] [Google Scholar]
- 29.Limana F, Zacheo A, Mocini D, Mangoni A, Borsellino G, Diamantini A, De Mori R, Battistini L, Vigna E, Santini M, Loiaconi V, Pompilio G, Germani A, Capogrossi MC. Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circ Res. 2007;101:1255–1265. doi: 10.1161/CIRCRESAHA.107.150755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.