Abstract
Native volume-sensitive outwardly rectifying anion channels (VSOACs) play a significant role in cell volume homeostasis in mammalian cells. However, the molecular correlate of VSOACs has been elusive to identify. The short isoform of ClC-3 (sClC-3) is a member of the mammalian ClC gene family and has been proposed to be a molecular candidate for VSOACs in cardiac myocytes and vascular smooth muscle cells. To directly test this hypothesis, and assess the physiological role of ClC-3 in cardiac function, we generated a novel line of cardiac specific inducible ClC-3 knock-out mice. These transgenic mice were maintained on a doxycycline diet to preserve ClC-3 expression; removal of doxycycline activates Cre recombinase to inactivate the Clcn3 gene. Echocardiography revealed dramatically reduced ejection fraction and fractional shortening, and severe signs of myocardial hypertrophy and heart failure in the knock-out mice at both 1.5 and 3 weeks off doxycycline. In mice off doxyclycline, time-dependent inactivation of ClC-3 gene expression was confirmed in atrial and ventricular cells by qRT-PCR and Western blot analysis. Electrophysiological examination of native VSOACs in isolated atrial and ventricular myocytes 3 weeks off doxycycline revealed a complete elimination of the currents, whereas at 1.5 weeks, VSOAC current densities were significantly reduced, compared to age-matched control mice maintained on doxycycline. These results indicate that ClC-3 is a key component of native VSOACs in mammalian heart and plays a significant cardioprotective role against cardiac hypertrophy and failure.
Keywords: regulatory volume decrease (RVD), volume-sensitive outwardly rectifying anion channels (VSOACs), cardiac specific inducible ClC-3 knock-out mice, atrial myocytes, whole-cell patch clamp recordings, echocardiography, cardiac hypertrophy and failure, doxycycline
1. Introduction
Mammalian cells, including cardiac myocytes, have the capability of adjusting their volumes when challenged by a number of different stimuli. Regulatory volume decrease (RVD) is an important and universal physiological process, defined as the decrease of the cell volume in response to stretch or cell swelling [1]. Various ion channels and transporters, including volume-sensitive outwardly rectifying anion channels (VSOACs), have been shown to contribute to the RVD process [2-4]. These channels also have been implicated in proliferation, apoptosis and the regulation of electrical activity [1]. Although the physiological significance of VSOACs, also referred to as volume-regulated anion current (VRAC), has been clearly established, the molecular entity for native VSOACs has been difficult to conclusively identify [2, 5].
ClC-3 is a member of the mammalian ClC gene family and has been proposed to be the molecular candidate for the native VSOAC in certain mammalian cell types, including cardiac myocytes and vascular smooth muscle cells [6, 7]. Although different lines of evidence from several independent studies have provided support for the role of ClC-3 as a volume-regulated chloride channel [8-11], the ClC-3 hypothesis remains controversial. Specifically, the reported presence of native VSOAC currents in at least two different cell types (pancreatic acinal cells and hepatocytes) from ClC-3 global knock-out mice cast considerable doubt on this proposed role for ClC-3 [12]. A later study revealed the remaining VSOACs in cardiac myocytes from ClC-3 global knock-out mice exhibited altered properties and extensive compensatory changes in membrane protein expression [13], complicating a meaningful interpretation of the phenotypic consequences of Clcn3 deletion. Novel transgenic mouse lines with cardiac specific manipulation of the Clcn3 gene could provide useful information on the actual physiological role of ClC-3. Recently, a new transgenic mouse line with heart-specific overexpression of the human short isoform of ClC-3 (sClC-3) exhibited significantly increased VSOAC current densities in isolated atrial myocytes, with biophysical and pharmacological properties characteristic of native wildtype VSOACs [14]. Atrial myocytes overexpressing sClC-3 also exhibited an acceleration of RVD.
In the present study, we used a different transgenic approach to assess the physiological role of ClC-3 in mouse heart. Specifically, a novel line of inducible cardiac specific Clcn3 knock-out mice was developed to further investigate the role of ClC-3 in heart. We hypothesized that using the tetO-Cre inducible approach would provide more specific gene manipulation and allow precise control of the time course of gene deletion in adult mice..
2. Materials and methods
The present study conformed to the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health and was approved by the University of Nevada Institutional Animal Care and Use Committee.
2.1 Generation of heart-specific inducible ClC-3 knock-out mice
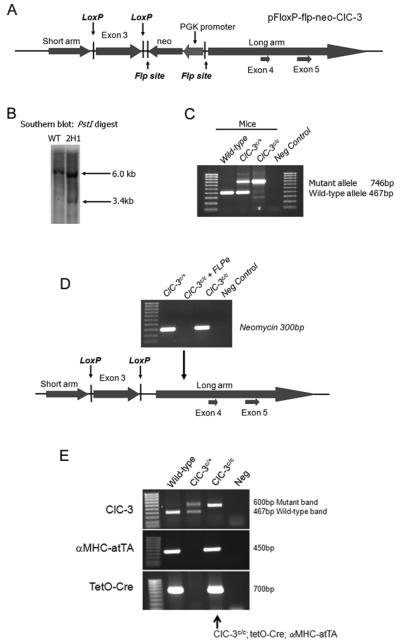
A genomic clone containing a 10.8 kb fragment of the mouse Clcn3 gene was isolated from a mouse 129 genomic DNA library. The short arm, exon 3 and the long arm were amplified from this genomic clone using Pfu turbo (Stratagene, La Jolla, CA). A 1,141bp fragment constituting the short arm was cloned into the XhoI sites of pFloxP-flp-neo vector (provided by Dr. James Shayman, University of Michigan, MI). A 957bp fragment containing exon 3 was cloned into the BamHI sites. Finally, a 3,903bp fragment containing exons 4 and 5 was cloned into the SwaI sites of the pFloxP-flp-neo vector. The pFloxP-flp-neo-Clcn3 targeting vector was verified by DNA sequencing and in the resulting targeting construct exon 3 was flanked by loxP sites (Fig. 1A).
Figure 1.
Production of inducible heart-specific Clcn3 knock-out mice. (A) Diagrammatic representation of the pFloxP-flp-neo-Clcn3 targeting construct with exon 3 flanked by loxP sites. (B) Southern blot of genomic DNA from non-transfected (wild-type) and transfected (2H1) ES cells digested with PstI. A single 6.0kb was detected in wild-type DNA, while a 6.0 and 3.4kb band was detected in the 2H1 targeted cell line. (C) Resultant mice were genotyped for targeted Clc3n gene. The wild-type Clcn3 allele produced a 467bp band while the targeted allele produced a 746bp band. (D) Loss of the neomycin gene after FLPe recombination was confirmed by PCR. (E) PCR was used genotype mice at the Clcn3 locus and for the aMHC-atTA and TetO-Cre transgenes.
Fifty micrograms of linearized plasmid was electroporated into R1 mouse embryonic stem (ES) cells maintained on a feeder layer of irradiated mouse fibroblasts. R1 ES cells were grown in DMEM supplemented with 15% FCS and 1000U/ml recombinant leukemia inhibitory factor (Chemicon, Temecula, CA) and selected with medium that contained 0.3 mg/ml G418 (Invitrogen). DNA was extracted from G418 resistant ES cell colonies which were then digested with PstI and the resultant Southern blot probed using a DNA probe located outside the targeted region. The wild-type allele generated a 6kb band while the target allele produced a 3.4kb band (Fig. 1B). Positive ES cell clones were microinjected into C57BL/6J blastocysts. Chimeric mice were mated with C57BL/6J animals to produce agouti pups that showed germline transmission of the conditional targeted Clcn3 allele by PCR (Fig. 1C). To remove the PGK-neo cassette from the targeted Clcn3 allele, Clcn3c/c animals were crossed with FLPe mice (Jackson Laboratories, Bar Habor, ME). Loss of the neomycin gene was confirmed by PCR (Fig. 1D). Resultant Clcn3c/c mice that lacked the neomycin gene were bred with tetO-Cre (a kind gift from Dr. Jeffrey Gordon, Fred Hutchinson Cancer Research Institute, Seattle, WA) and α-MHC-rTtA (Jackson Laboratories, Bar Habor, ME) and transgenes were followed by PCR (Fig. 1E). Cre expression was driven by the cardiac specific promoter, α-MHC, which restricts homologous recombination to cardiomyocytes [15]. Resultant Clcn3c/c; tetO-Cre; α-MHC-rTtA mice were maintained on chow containing doxycycline (Bio-Serve, Frenchtown, NJ) which prevented expression of Cre recombinase and maintained normal expression of Clcn3 gene in the heart. Mice were placed on chow without doxycycline to activate Cre recombinase expression and inactivate the Clcn3 gene only in the heart.
2.2 Echocardiography
Left ventricular (LV) function was assessed by two-dimensional (2D) and M-mode echocardiography using a GE Vivid 7 Pro Color Ultrasound system with a 13 MHz linear transducer (i13L) and EchoPAC software (GE Healthcare). Briefly, mice were lightly anesthetized with isoflurane until the heart rate was maintained within the range of 550-600 beats per minute (BPM). When clear 2D images of the LV in short-axis and long-axis view were obtained, M-mode images were recorded for the measurement of end-diastolic (d) volume and end-systolic (s) volume, interventricular septum (IVS), left ventricular internal diameter (LVID), and left ventricular posterior wall (LVPW) using the leading edge–to–leading edge convention adopted by the American Society of Echocardiography [16]. The left ventricular ejection fraction (LVEF = [(LVIDd)3 − (LVIDs)3]/LVIDd3) and left ventricular fractional shortening (%FS = [(LVIDd − LVIDs)/LVIDd] × 100%) were calculated with the analysis program incorporated on the ultrasound machine. Left ventricular mass (LVM) was calculated according to the following equation: LVM (mg) = 1.05 × [(IVS+LVID+LVPW)3−(LVID)3], where 1.055 is the specific gravity of the myocardium [17].
2.3 RNA isolation, quantitative reverse transcription polymerase chain reaction (qRT-PCR), Westerns and immunocytochemistry
Mouse atrial and ventricular myocytes were isolated enzymatically from age-matched heart-specific inducible ClC-3 knock-out mice as described before [13]. Brains were isolated from the same mice and placed in a preservative solution (RNALater; Ambion, Austin, TX, USA) prior to use. Total RNA was extracted in TRIzol reagent according to the protocol provided by the manufacturer (Invitrogen, Carlsbad, CA) and treated with 1 U/μl DNase I (Promega Corp., Madison, WI, USA). First-strand cDNA synthesis was performed using the manufacturer's protocol for SuperScriptIII from 2 μg total RNA using 250 ng random hexamers (Invitrogen, Carlsbad, CA, USA). RNAse H (20 U) was added to remove RNA complementary to the cDNA. Myocytes and brain samples were diluted 1:5 in nuclease-free water.
TaqMan® MGB, FAM™-dye labeled probes were obtained from Applied Biosystems TaqMan® Gene Expression Assays database (Applied Biosystems, Foster City, CA, USA) and proceeded according to the manufacturer's protocols. The assay (Mm01348785) detects a 99 base pair amplicon in the exon 2-3 boundary. All reactions were amplified in triplicate on an ABI Prism 7000 Sequence Detection System in 20 μl total volume containing a 1X concentration TaqMan Universal Master Mix (Applied Biosystems, Foster City, CA, USA), 1X TaqMan primer/probe and 2 μL cDNA. Sample amplification was activated by incubation at 95°C for 10 minutes, followed by 50 cycles of 95°C for 15 seconds and 60°C for 1 minute. Changes in gene expression were measured using relative quantitation methods from serial dilutions of cDNA as previously described [14]. All samples were normalized to 18S rRNA amplified from the same samples to control for variations in sample quality.
For Western blotting, whole hearts and whole brains from age-matched cardiac-specific ClC-3 inducible knock-out mice, (6 on doxycycline, 6 off doxycycline for 3 weeks), were individually homogenized in 10 mM Tris, 0.5 KCl, pH 7.4, and centrifuged for 5 min at 1,500 xg. The supernatants were centrifuged at 100,000 xg for 15 minutes. The pellets were resuspended in 10 mM Tris, 0.3 M sucrose, pH 7.4, repelleted and resuspended in the same buffer. 50μg of each crude heart and brain membrane prep was used for Western analysis, and probed with an anti-ClC3 antibody. Blots were reprobed with an anti-GAPDH antibody to confirm equal loading of protein in wells.
For immunocytochemistry, enzymatically dispersed atrial and ventricular cells fixed with 4% paraformaldehyde, were permeabilized with 0.3% Triton X-100. Slides were incubated with a chicken anti-ClC-3 antibody and a mouse anti-alpha actinin antibody. Slides were imaged on a Nikon Radiance 2100 confocal microscope.
2.4 Whole-cell voltage-clamp experiments
Mouse atrial myocytes were enzymatically isolated from age-matched (17–21 weeks old) cardiac specific inducible ClC-3 knock-out mice and their on doxycycline control animals as described previously [13]. Current–voltage relationships for VSOAC currents were generated by voltage steps ranging from −100 mV to +100 mV in 20 mV increments. To study the time-course of changes in VSOAC currents, repetitive voltage steps at ±80 mV were applied every 30 seconds from a holding potential of −40 mV. Symmetrical chloride concentrations were used in the bath and pipette solutions. The hypotonic bath solution (220 mOsmol) contained (in mmol/L): NaCl 90; tetraethylammonium chloride 10; BaCl2 2.0; CaCl2 1.0; MgCl2 0.8; CdCl2 0.2; HEPES 10; glucose 5.5, adjusted to pH 7.4. The isotonic solution (300 mOsmol) was prepared by adding mannitol to the hypotonic solution. The pipette solution contained (in mmol/L): N-methyl-d-glutamine chloride (NMDG-Cl) 108; EGTA 5; γ-ATP 5; HEPES 5, with pH adjusted to 7.3 and osmolarity to 290 mOsmol with mannitol. Patch pipettes (1.5mm o.d. borosilicate glass electrodes) had tip resistances of 1–3Ω_when filled with pipette solutions. Cell membrane capacitances were measured by application of 2.5 ms duration 5 mV voltage steps and the area of the capacitative transient was calculated using pCLAMP software (Molecular Devices, Sunnyvale, CA, USA) following membrane rupture. Due to the small size and slow kinetics of the measured VSOAC currents, no series resistance or capacitance compensation was utilized.
The blocking effects of the protein kinase C (PKC) activator were calculated with the following equation:
where IHypo is the stabilized maximal amplitude of VSOAC currents in hypotonic solutions, IBlocker is the stabilized minimal VSOAC current amplitude after the application of the PKC activator, and IIso is the VSOAC current amplitude in isotonic solutions.
2.5 Statistical analysis
Data are expressed as the mean ± SD. Student's t-test or ANOVA was utilized to assess differences between groups. p < 0.05 was considered significant.
3. Results
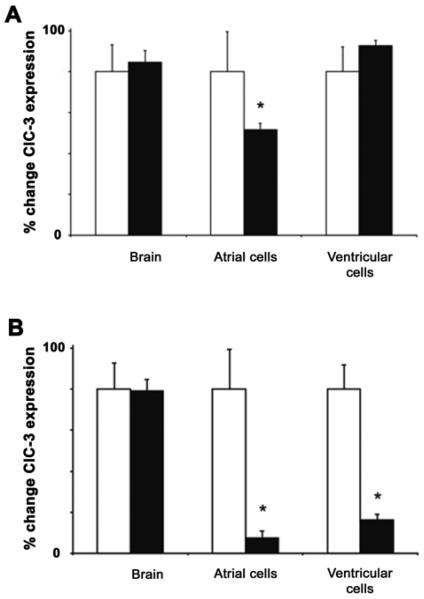
Initially a survey of atrial myocytes from Clcn3c/c; tetO-Cre; α-MHC-rTtA mice at a variety of time points off doxyclycine were examined. In preliminary experiments, it was empirically determined by 3 weeks off doxycline there was a maximal reduction in ClC-3 mRNA expression. Thereafter, we collected enzymatically isolated atrial and ventricular myocytes from age-matched heart-specific inducible ClC-3 knock-out mice at 1.5 weeks and 3 weeks off doxycycline and their relevant on doxycycline control mice for quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis to quantify the targeted ClC-3 mRNA expression levels. The results (Fig. 2) indicated a time-dependent decrease of the ClC-3 mRNA expression levels in atrial cells from the inducible knock-out mice after the animals were no longer maintained on doxycycline. In comparison, the ClC-3 expression in ventricular cells was significantly reduced from the inducible knock-out mice at 3 weeks off doxycycline whereas there was little change at 1.5 weeks off doxycycline, when compared to the age-matched on doxycycline control mice.
Figure 2.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) examination of the freshly isolated atrial and ventricular myocytes, and brain tissues from the heart-specific inducible ClC-3 knock-out, age-matched mice (A) at 1.5 weeks (B) and 3 weeks. Open bars are mice maintained on doxycycline and filled bars are mice off doxycycline. ClC-3 expression was normalized to 18S rRNA and data calculated as the % change in normalized ClC3 expression from mice maintained on doxycycline for each tissue type. In all conditions, error bars represent SEM from these values, n = 4∼7, * =p<0.01usingStudent'st-test.
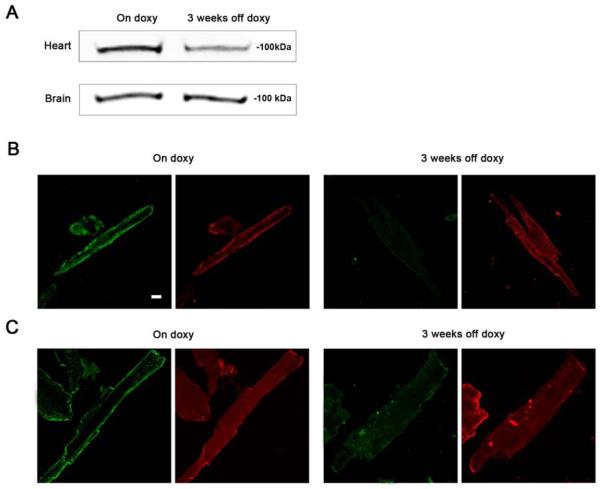
To verify the cardiac specific transgenic manipulations of ClC-3, mouse brain samples were also collected for qRT-PCR analysis (as described in Materials and Methods). As shown, there was almost complete elimination of the ClC-3 gene expression in atrial myocytes and a very significant decrease in ventricular myocytes from the inducible knock-out mice at 3 weeks off doxycycline, compared to the age-matched on doxycycline control animals. In comparison, there were no changes in ClC-3 mRNA expression in the brain tissues from the inducible knock-out mice at 1.5 weeks and 3 weeks off doxycycline and their age-matched on doxycycline controls. These data verify heart-specific knock-out of the ClC-3 gene in this novel line of ClC-3 transgenic mice at 3 weeks off doxycycline. ClC-3 protein expression was also found to be reduced in whole hearts from mice 3 weeks off doxycycline compared to on doxycycline control hearts (Fig. 3), but no differences were observed in mouse brain. ClC-3 expression was not completely eliminated in whole hearts at 3 weeks off doxycycline, since ClC-3 expression is not expected to be affected in other cell types in heart such as nerve, smooth muscle, fibroblasts. Immunocytochemistry verified loss of ClC-3 protein in isolated cardiomyocytes from mice 3 weeks off doxycycline.
Figure 3.
ClC-3 protein expression in heart and brain of cardiac-specific ClC-3 inducible knock-out mice. (A) Western analysis of whole heart and brain from age-matched cardiac-specific ClC-3 inducible knock-out mice, either maintained on doxycycline, or off doxycycline for 3 weeks. Note whole heart was used for the Western, so non-cardiomyocytes cell types are present. (B) isolated atrial and (C) ventricular cardiomyocytes from age-matched cardiac-specific ClC-3 inducible knock-out mice, on doxycycline, or off doxycycline for 3 weeks. In each pair of images the left panel is probed with anti-ClC-3 (green) and the right panel is probed with anti-alpha-actinin (red). Scale bar in B = 5 μm.
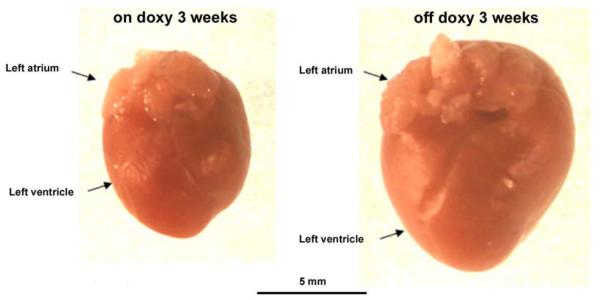
Visual inspection of hearts revealed dramatically enlarged hearts from the ClC-3 knock-out mice 3 weeks off doxycline compared to age-matched on doxycline control mice (Fig. 4). In preliminary studies it became evident that there was also increased mortality of mice the longer they were maintained off doxycline beyond the 3 week time point. Echocardiography was utilized to assess heart function and characterize the cardiac phenotype of this novel line of inducible cardiac specific ClC-3 knock-out mice and their age-matched controls. At the time point of 3 weeks off doxycycline, serious signs of myocardial hypertrophy and heart failure were revealed by echocardiography recordings (Fig. 5 and Table 1). Compared to the age-matched control mice maintained on doxycycline, significantly reduced cardiac function parameters (left ventricular ejection fraction (LVEF) and fractional shortening (%FS)) were observed in the knock-out mice. Additionally, heart mass of the heart-specific inducible ClC-3 knock-out mice turned out to be significantly increased at 3 weeks off doxycycline, compared to the age-matched on doxycycline control animals. While Clcn−/− mice had a significant increase in heart mass, there was no statistically significant difference in body weight between the knock-out mice and their age-matched control animals. In addition, the heart mass:body weight ratio was also significantly increased in the heart-specific inducible ClC-3 knock-out mice at 3 weeks off doxycycline. To study the time-course of cardiac specific ClC-3 gene knock-out on mouse heart function, we also performed echocardiography examination at an intermediate time point of 1.5 weeks off doxycycline.
Figure 4.
Comparison of hearts from inducible cardiac-specific ClC-3 knock-out age-matched mice, 3 weeks on doxycycline (left), and 3 weeks off doxycycline (right).
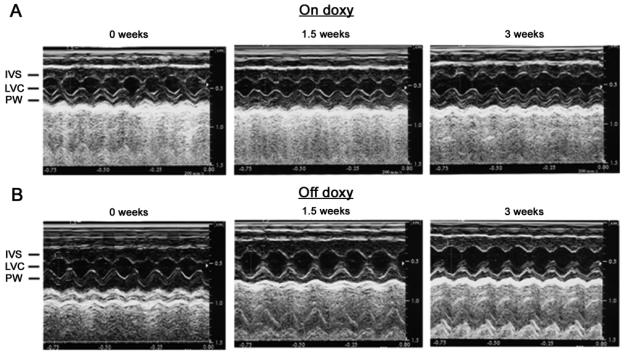
Figure 5.
Representative M-mode echocardiograms of inducible cardiac-specific ClC-3 knock-out age-matched mice (A) maintained on doxycycline (on doxy) or (B) without doxycycline (off doxy) for 1.5 and 3 weeks. IVS: interventricular septum; LVC: left ventricular chamber; PW: left ventricular posterior wall. The average parameters of the measurement are shown in Table 1.
Table 1.
Time-dependent changes in M-mode echocardiogram of age-matched inducible cardiac specific ClC-3 knock-out mice with doxycycline (on Doxy) in the diet or after withdraw of doxycycline (off doxy) from the diet.
| 0 week | 1.5 weeks | 3 weeks | ||||
|---|---|---|---|---|---|---|
| On Doxy | Off Doxy | On Doxy | Off Doxy | On Doxy | Off Doxy | |
| n=8 | n=15 | n=4 | n=12 | n=5 | n=9 | |
| IVSs (mm)1 | 1.6±0.03 | 1.6±0.04 | 1.7±0.01 | 1.5±0.04*,## | 1.6±0.03 | 1.5±0.04* |
| LVIDs (mm) | 1.0±0.1 | 1.0±0.1 | 1.1±0.1 | 1.5±0.11*** | 1.2±0.1 | 1.6±0.1***,# |
| LVPWs (mm) | 1.4±0.1 | 1.5±0.1 | 1.2±0.1 | 1.3±0.04 | 1.3±0.03 | 1.3±0.04 |
| IVSd (mm) | 0.8±0.02 | 0.8±0.02 | 0.8±0.02 | 0.8±0.02 | 0.8±0.02 | 0.8±0.02 |
| LVIDd (mm) | 2.7±0.2 | 2.9±0.1 | 2.7±0.1 | 3.3±0.1*,# | 2.9±0.1 | 3.2±0.1*,# |
| LVPWd (mm) | 1.1±0.1 | 1.1±0.03 | 1.0±0.04 | 1.1±0.04 | 1.0±0.1 | 1.1±0.1 |
| FS (%) | 64.1±1.7 | 67.1±1.8 | 59.3±1.9 | 54.3±1.9*** | 60.5±1.8 | 51.6±1.8***,## |
| LVEF (%) | 95.1±0.7 | 96.0±1.6 | 93.0±0.9 | 89.8±1.2** | 93.6±0.1 | 88.2±1.3**,## |
| Mass (mg/mm2) | 80.0±4.3 | 92.9±3.42 | 72.1±4.1 | 102.5±4.4## | 87.8±5.3 | 102.1±3.0# |
| Body Weight (g) | 34.9±2.6 | 43.1±1.9# | 33.2±3.6 | 41.2±2.1 | 38.2±1.8 | 36.3±2.4 |
| Mass/BW Ratio | 2.4±0.2 | 2.2±0.1 | 2.3±0.2 | 2.5±0.1* | 2.3±0.1 | 2.9±0.1***,# |
systolic and diastolic left ventricular (LV) interventricular septum and posterior wall thickness (IVS and LVPW), chamber dimension (LVID), mass (LVM), LVM/body weight (BW) ratio, and contractile function (LV ejection fraction (LVEF) and percent fractional shorting (%FS)).
P<0.05,
P<0.01,
P<0.001 vs off Doxy 0 week;
P<0.05,
P<0.01,
P<0.001 vs on Doxy at the same time point using ANOVA.
As summarized in Table 1, M-mode echocardiographic evaluation of systolic and diastolic left ventricular (LV) wall thickness (IVS and LVPW), chamber dimension (LVID), mass (LVM) , LVM/body weight (BW) ratio and contractile function (LVEF and %FS) revealed a significant increase in the chamber cavity (LVIDs and LVIDd), LVM, LVM/BW ratio, and a marked decrease in LVEF and %FS in the inducible knock-out mice off doxycycline for 1.5 and 3 weeks while no significant changes were observed in the age-matched control mice with doxycycline kept on doxycycline (on doxycycline control). These data suggest that mice off doxycycline for 1.5 weeks are likely to have a reduction of ClC-3 expression that may be responsible for the observed dilated cardiomyopathy (DCM). The development of DCM was more prominent in mice off doxycycline for 3 weeks. This observation was confirmed by the qRT-PCR data shown in Fig. 2.
The whole-cell patch clamp technique was used to investigate native VSOAC currents from enzymatically dispersed atrial myocytes from the heart-specific inducible ClC-3 knock-out mice at 1.5 weeks and 3 weeks off doxycycline and from control mice maintained on doxycycline. Freshly isolated single mouse atrial myocytes were placed in an isotonic bath solution (300 mOsm) for ∼ 5 minutes to stabilize before the perfusion of hypotonic solution (220 mOsm), which was used to swell the cell and activate native VSOAC currents. At 1.5 weeks off doxycycline, the native VSOAC current densities under hypotonic stimulation were significantly decreased in the knock-out mice, compared to the age-matched on doxycycline control mice, whereas there was no difference in current densities under isotonic conditions prior to cell swelling (Fig. 6). In addition, at 3 weeks off doxycycline, the hypotonic-induced VSOAC currents were completely eliminated in atrial myocytes from cardiac specific ClC-3 inducible knock-out mice. In comparison, hypotonic-induced VSOAC currents were still present in the on doxycycline control mice, and the current densities measured are comparable to those measured previously in atrial cells from normal wildtype mice [14].
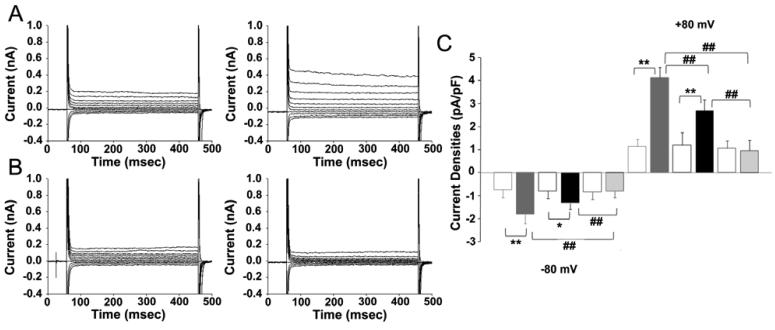
Figure 6.
Representative current traces in isotonic condition and under hypotonic challenge in the freshly isolated atrial myocytes from age-matched cardiac-specific ClC-3 inducible knock-out mice. (A) Mice maintained on doxycycline, and (B) mice after 3 weeks off doxycycline. (C) is a summary of current densities under isotonic and hypotonic solutions, recorded at +80 mV and −80 mV, in freshly isolated atrial myocytes from age-matched cardiac-specific ClC-3 inducible knock-out mice, either maintained on doxycycline (n=14, 5 mice), or off doxycycline for 1.5 weeks (n=11, 3 mice) or 3 weeks (n=17, 4 mice). Open boxes, under isotonic conditions, filled boxes under hypotonic conditions. Grey boxes on doxycycline, black boxes off doxycycline 1.5 weeks and pale grey boxes off doxycycline for 3 weeks. ** p<0.01, hypotonic-induced VSOAC current densities compared to isotonic conditions. ## p<0.01, hypotonic-induced VSOAC current densities compared between on doxycycline and 1.5 weeks off doxycycline, and between 1.5 and 3 weeks off doxycycline using ANOVA.
We also examined native VSOAC currents from enzymatically dispersed ventricular myocytes from the heart-specific inducible ClC-3 knock-out mice at 3 weeks off doxycycline and from control mice maintained on doxycycline. At 3 weeks off doxycycline, the hypotonic-induced VSOAC currents were completely eliminated in ventricular myocytes from cardiac specific ClC-3 inducible knock-out mice (Fig 7B & C), compared to the hypotonic-induced VSOAC currents present in ventricular cells from on doxycycline control mice (Fig. 7A & C). There was no significant difference in membrane capacitance in atrial myocytes at 3 weeks off doxycycline (81.0 ± 6.1 pF, n=17, 4 mice) compared to atrial myocytes from on doxyclycline control mice (84.6 ± 9.1 pF, n=14, 5 mice). In contrast, membrane capacitance was significantly increased in ventricular myocytes from mice 3 weeks off doxyclycline (271.7 ± 24.7 pF, n=16, 4 mice) compared to ventricular myocytes from on doxycycline control mice (178.9 ± 14.2 pF, n=11, 4 mice, p 0.01). The observed increase in ventricular membrane capacitance is consistent with severe ventricular cell swelling which is expected in the hypertrophied hearts from the off doxy mice.
Figure 7.
Representative current traces in isotonic condition and under hypotonic challenge in the freshly isolated ventricular myocytes from age-matched cardiac-specific ClC-3 inducible knock-out mice. (A) Mice maintained on doxycycline, and (B) mice after 3 weeks off doxycycline. (C) is a summary of current densities under isotonic and hypotonic solutions, recorded at +80 mV and −80 mV, in freshly isolated atrial myocytes from age-matched cardiac-specific ClC-3 inducible knock-out mice, either maintained on doxycycline (n=13, 4 mice), or off doxycycline for 3 weeks (n=20, 4 mice). Open boxes, under isotonic conditions, filled boxes under hypotonic conditions. Black boxes on doxycycline, and grey boxes off doxycycline for 3 weeks. ** p<0.01, hypotonic-induced VSOAC current densities compared to isotonic conditions. ## p<0.01, hypotonic-induced VSOAC current densities compared between, on doxycycline, and 3 weeks off doxycycline using ANOVA.
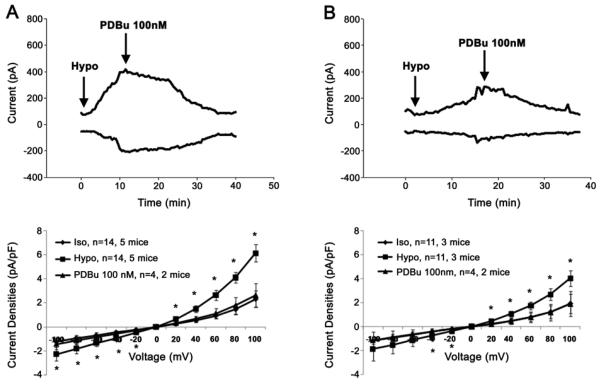
An important property of native VSOAC currents in mammalian cardiac cells is regulation by endogenous protein kinase C (PKC) [6,13]. In our studies, cell swelling is associated with PKC phosphorylation and channel closure [18], although variable effects of PKC regulation have been reported [19] . We therefore tested the sensitivity of native VSOACs to the PKC activator, PDBu. 100 nM PDBu was applied to the bath solution after the stabilization of the hypotonic-induced VSOAC currents. At 1.5 weeks off doxycycline, the residual hypotonic-induced VSOAC currents were totally abolished by activation of PKC in atrial myocytes from cardiac specific ClC-3 inducible knock-out mice (Fig. 8). Similarly, in the on doxycycline control mice, the native VSOAC currents induced by hypotonic cell swelling were also completely eliminated by perfusion of 100 nM PDBu. This indicates that the native VSOACs measured after 1.5 weeks off doxycycline in cells from cardiac specific ClC-3 inducible knock-out mice, although reduced in amplitude, retain their sensitivity to PKC regulation, similar to VSOACs measured in atrial cells from the on doxycycline control mice.
Figure 8.
PKC regulation of hypotonic-induced VSOAC currents in freshly isolated atrial myocytes from cardiac-specific ClC-3 inducible knock-out age-matched mice. (A) On doxycycline control mice, and (B), mice of doxycycline for 3 weeks.
4. Discussion
To determine the actual physiological role of ClC-3 in the heart, we developed a novel line of transgenic mice utilizing the tetO-Cre approach to conditionally inactivate Clcn3 in mouse heart. The advantages of this approach include elimination of possible confounding effects if Clcn3 is required for mouse development, organ specific Clcn3 gene inactivation, and temporal control of Cre expression and Clcn3 gene deletion [20]. We hoped this approach would allow an assessment of the actual phenotypic consequences of cardiac specific Clcn3 gene deletion before major compensatory changes in other proteins occur, as has been shown to occur in global Clcn3−/− mice [13]. The results indicate that at 3 weeks off doxycycline, heart-specific knock-out of Clcn3 in mice resulted in significant increases heart mass, heart mass : body weight ratio, and heart mass : tibia length ratio, compared to the age-matched on doxycycline control mice. Serious signs of myocardial hypertrophy and heart failure were revealed by echocardiography in the cardiac specific inducible ClC-3 knock-out mice 3 weeks off doxycycline, compared to age-matched on doxycycline control mice. There was significantly reduced left ventricular ejection fraction and fractional shortening. These phenotypic consequences are the opposite of those observed in mice with heart-specific overexpression of the human sClC-3 isoform which resulted in significantly reduced heart size and heart weight : body weight ratio, which suggested that ClC-3 normally plays a protective role against the development of myocardial hypertrophy [14]. This is consistent with a previous report that global deletion of the ClC-3 gene in mice facilitated the pathological development of myocardial hypertrophy and heart failure in a pressure overload transverse aortic banding animal model [21].
Myocardial cells are known to swell during ischemia, dilated cardiomyopathy and failure. Native VSOACs have been shown to be persistently activated during these conditions [22, 23] and likely play a protective role in helping maintain cardiac cell volume. The myocardial hypertrophy and dilated cardiomyopathy characteristic of the cardiac specific inducible ClC-3 knock-out mice 3 weeks off doxycycline is precisely the phenotype expected due to loss of an ion channel intimately involved in cell volume homeostasis.
Another important phenotypic consequence of cardiac specific inducible knock-out of Clcn3 in mice was the complete elimination of native VSOAC currents in freshly isolated atrial myocytes 3 weeks off doxycycline. This is consistent with our recent study which demonstrated significantly increased native VSOAC current densities in freshly isolated atrial myocytes from cardiac specific sClC-3 overexpressing mice, compared to age-matched control mice [14]. All of these results taken together provide strong evidence supporting an essential role of endogenous ClC-3 in native VSOAC function in heart. It also has been recently reported that ClC-3 short hairpin small interfering RNA treatment significantly suppressed ClC-3 expression and consequently reduced native VSOAC currents and slowed RVD in human corneal keratocytes and fibroblasts [24]. The present data do not distinguish between whether ClC-3 represents an actual component of the VSOAC channel pore forming protein, or functions as a channel regulator, even though earlier site-directed mutagenesis studies of the short ClC-3 isoform [6,18,27] support the former role. Yet other data from global Clcn3−/− mice [25, 26] supports the latter role for ClC-3. Future studies are needed to conclusively resolve this question and to determine if up-regulation of endogenous ClC-3 may represent a novel therapeutic strategy for the treatment of myocardial hypertrophy and failure.
Acknowledgements
This work was supported by National Institutes of Health grants HL-49254 and P20RR1581 from the National Center for Research Resources. The authors thank Paul Scowen, Susan Tamowski, Phil Keller and Heather Beck for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hoffmann EK, Dunham PB. Membrane mechanisms and intracellular signaling in cell volume regulation. Int Rev Cytol. 1995;161:173–262. doi: 10.1016/s0074-7696(08)62498-5. [DOI] [PubMed] [Google Scholar]
- 2.Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–30. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- 3.Hand M, Morrison R, Strange K. Characterization of volume-sensitive organic osmolyte efflux and anion current in Xenopus oocytes. J Membr Biol. 1997;157:9–16. doi: 10.1007/s002329900211. [DOI] [PubMed] [Google Scholar]
- 4.Stutzin A, Torres R, Oporto M, Pacheco P, Eguiguren AL, Cid LP, et al. Separate taurine and chloride efflux pathways activated during regulatory volume decrease. Am J Physiol. 1999;277:C392–402. doi: 10.1152/ajpcell.1999.277.3.C392. [DOI] [PubMed] [Google Scholar]
- 5.Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: Fresh start to the molecular identity and volume sensor. Am J Physiol. 1997;273:C755–89. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- 6.Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997;390:417–21. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki J, Duan D, Janiak R, Kuenzli K, Horowitz B, Hume JR. Functional and molecular expression of volume-regulated chloride channels in canine vascular smooth muscle cells. J Physiol. 1998;507:729–36. doi: 10.1111/j.1469-7793.1998.729bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang L, Chen L, Jacob TJ. The role of ClC-3 in volume-activated chloride currents and volume regulation in bovine epithelial cells demonstrated by antisense inhibition. J Physiol. 2000;524.1:63–75. doi: 10.1111/j.1469-7793.2000.t01-1-00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin NG, Kim JK, Yang DK, Cho SJ, Kim JM, Koh EJ, et al. Fundamental role of ClC-3 in volume sensitive Cl− channel function and cell volume regulation in AGS cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G938–48. doi: 10.1152/ajpgi.00470.2002. [DOI] [PubMed] [Google Scholar]
- 10.Vessey JP, Shi C, Jollimore C, Stevens KT, Coca-Prados M, Barnes S, et al. Hyposmotic activation of ICl,swell in rabbit nonpigmented ciliary epithelial cells involves increased ClC-3 trafficking to the plasma membrane. Biochem Cell Biol. 2004;82:708–18. doi: 10.1139/o04-107. [DOI] [PubMed] [Google Scholar]
- 11.Zhou JG, Ren JL, Qiu QY, He H, Guan YY. Regulation of intracellular Cl− concentration through volume-regulated ClC-3 chloride channels in A10 vascular smooth muscle cells. J Biol Chem. 2005;280:7301–8. doi: 10.1074/jbc.M412813200. [DOI] [PubMed] [Google Scholar]
- 12.Stobrawa SM, Breiderhoff T, Takamori S, Engel D, Schweizer M, Zdebik AA, et al. Disruption of ClC-3, a chloride channel expressed on synaptic vesicles, leads to a loss of the hippocampus. Neuron. 2001;29:185–96. doi: 10.1016/s0896-6273(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto-Mizuma S, Wang GX, Liu LL, Schegg K, Hatton WJ, Duan D, et al. Altered properties of volume-sensitive osmolyte and anion channels (VSOACs) and membrane protein expression in cardiac and smooth muscle myocytes from Clcn3−/− mice. J Physiol. 2004;557:439–56. doi: 10.1113/jphysiol.2003.059261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiong D, Wang GX, Burkin DJ, Yamboliev IA, Singer CA, Rawat S, et al. Cardiac-specific overexpression of the human short ClC-3 chloride channel isoform in mice. Clin Exp Pharmacol Physiol. 2009;36:386–393. doi: 10.1111/j.1440-1681.2008.05069.x. [DOI] [PubMed] [Google Scholar]
- 15.Moga M-A, Nakamura T, Robbins J. Genetic approaches for changing the heart and dissecting complex syndromes. J Mol Cell Cardiol. 2008;45:148–155. doi: 10.1016/j.yjmcc.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 17.Collins KA, Korcarz CE, Shroff SG, Bednarz JE, Fentzke RC, Lin H, et al. Accuracy of echocardiographic estimates of left ventricular mass in mice. Am J Physiol Heart Circ Physiol. 2001 May;280(5):H1954–62. doi: 10.1152/ajpheart.2001.280.5.H1954. [DOI] [PubMed] [Google Scholar]
- 18.Duan D, Cowley S, Horowitz B, Hume JR. A serine residue in ClC-3 links phosphorylation-dephosphorylation to chloride channel regulation by cell volume. J Gen Physiol. 1999 Jan;113(1):57–70. doi: 10.1085/jgp.113.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Hill JA. Human atrial chloride channels: swelling with pride. J Cardiovas Electrophysiol. 2006;17:69–71. doi: 10.1111/j.1540-8167.2005.00296.x. [DOI] [PubMed] [Google Scholar]
- 20.Utomo ARH, Nikitin AY, Lee W-H. Temporal, spatial, and cell specific control of Cre-mediated DNA recombination in transgenic mice. Nature Biotech. 1999;17:1091–1096. doi: 10.1038/15073. [DOI] [PubMed] [Google Scholar]
- 21.Liu LL, Ye L, McGuckin C, Hatton WJ, Duan D. Disruption of Clcn3 gene in mice facilitates heart failure during pressure overload. J Gen Physiol. 2003;122:6a. (Abstract) [Google Scholar]
- 22.Baumgarten CM, Clemo HF. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog Biophys Mol Biol. 2003;82:25–42. doi: 10.1016/s0079-6107(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 23.Duan DY, Liu LL, Bozeat N, Huang ZM, Xiang SY, Wang GL, et al. Functional role of anion channels in cardiac diseases. Acta Pharmacol Sin. 2005;26:265–78. doi: 10.1111/j.1745-7254.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 24.Yin Z, Tong Y, Zhu H, Watsky MA. ClC-3 is required for LPA-activated Cl− current activity and fibroblast to myofibroblast differentiation. Am J Physiol Cell Physiol. 2008 Feb;294(2):C535–42. doi: 10.1152/ajpcell.00291.2007. [DOI] [PubMed] [Google Scholar]
- 25.Rossow CF, Duan D, Hatton WJ, Britton F, Hume JR, Horowitz B. Functional role of amino terminus in ClC-3 chloride channel regulation by phosphorylation and cell volume. Acta Physio. Scand. 2006;187:5–19. doi: 10.1111/j.1748-1716.2006.01550.x. [DOI] [PubMed] [Google Scholar]
- 26.Gong W, Xu H, Shimizu T, Morishma S, Tanabe S, Tachibe T, et al. ClC-3-independent, PKC-dependent activity of volume-sensitive Cl− channel in mouse ventricular myocytes. Cell Physiol Biochem. 2004;14:213–224. doi: 10.1159/000080330. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Xu H, Morishima S, Tanabe S, Jishage K, et al. Single channel properties of volume-sensitive Cl− channel in ClC-3-deficient cardiomyocytes. Jap J Physiol. 2005;55:379–383. doi: 10.2170/jjphysiol.S655. [DOI] [PubMed] [Google Scholar]