Hypertrophic cardiomyopathy (HCM) is a primary disease of cardiac myocytes, characterized mainly by cardiac and myocyte hypertrophy. It is diagnosed clinically by the presence of cardiac hypertrophy in the absence of altered loading conditions that would fully account for the problem.1 Typically, the left ventricle is not dilated and the left ventricular ejection fraction is increased.1 Human HCM is the prototypic and genetic form of pathologic cardiac hypertrophy.
The clinical diagnosis of HCM is neither sufficiently specific (because of the presence of phenocopy) nor completely sensitive (because of incomplete penetrance of the causal mutations). Phenocopies are often indistinguishable from true cases of HCM. The incidence of phenocopy in patients with the clinical diagnosis of HCM is unknown but is estimated at 10%. The distinction between HCM and phenocopy is important, because the treatments of the conditions differ significantly.
Hypertrophic cardiomyopathy is an archetypical single-gene disorder. More than a dozen causal genes and several hundred mutations have been identified in patients and families with HCM.2 The known causal genes encode sarcomeric proteins. Therefore, HCM is commonly recognized as a disease of sarcomeric proteins. The 2 most common genes—each accounting for approximately 25% of cases—are MYH7 and MYBPC3, which encode the β-myosin heavy chain and myosin-binding protein C, respectively. TNNT2, TNNI3, TPM1, and ACTC1 collectively account for about 10% of cases; other causal genes, such as the Z disk proteins MYOZ2 and TCAP, encoding myozenin 2 and telethonin, respectively, are less common. Overall, the causal mutations in approximately two thirds of all patients with HCM have been identified.
Advances in molecular genetics have enabled a genetic-based diagnosis. Genetic screening leads to identification of the causal mutations in approximately half of the cases. In familial cases and in certain clinical situations, genetic testing can provide valuable information, but its impact is diminished in sporadic cases. The value of genetic testing in risk stratification and prognostication is similarly weakened by the involvement of a large number of determinants, in addition to the causal mutation, in the pathogenesis of the phenotype.
Hypertrophic cardiomyopathy is a relatively benign disease. In young athletes, however, HCM is the most common cause of sudden cardiac death (SCD) and is responsible for almost half of all cases.3 Sudden cardiac death is often the first manifestation of HCM in apparently healthy, young individuals.3 Several predictors of the risk of SCD have been identified, including a history of sudden cardiac arrest, a history of recurrent syncope (due to cardiac arrhythmias), and sustained or repetitive nonsustained ventricular tachycardia. The presence of these risk factors warrants the implantation of a defibrillator. A strong family history of SCD (more than 1 family member) and severe cardiac hypertrophy are also important risk factors for SCD that are taken into consideration in evaluating patients for defibrillator implantation.
The fundamental question in HCM, since its modern description about 50 years ago, has been whether cardiac hypertrophy and fibrosis, once established, can be reversed or prevented. The current pharmacologic treatment of human HCM is largely empirical, and none has yet prevented, attenuated, or reversed cardiac hypertrophy or altered the prognosis in cases of HCM. b-Blockers are the mainstay of therapy and are effective for symptomatic relief but not for the reversal of cardiac phenotype. The clinical usefulness of calcium channel blockers is hampered by the risk of hypotension and syncope, at rest or (particularly) during exercise. Likewise, their effectiveness in the reversal of cardiac phenotype remains unestablished.
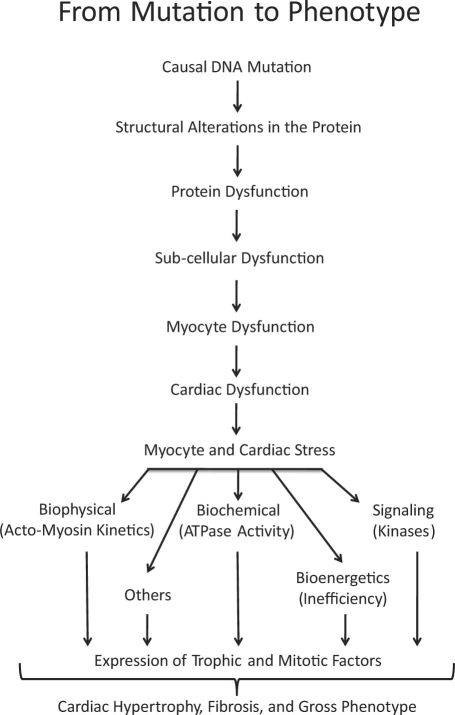
Mechanistic studies suggest that the genetic mutations cause functional defects that activate pro-growth and pro-fibrotic signaling molecules; this signaling leads to cardiac hypertrophy and fibrosis (Fig. 1).4 We have shown that cardiac hypertrophy and fibrosis can be prevented, attenuated, and reversed through genetic and pharmacologic interventions in animal models of HCM.5-9 The most promising among the experimental therapies is N-acetylcysteine (NAC), because it completely reverses cardiac hypertrophy and fibrosis.8,9 The potential usefulness of NAC in the treatment of human HCM requires testing.
Fig. 1 Pathogenesis of hypertrophic cardiomyopathy. The causal mutations alter the structure and function of the sarcomeres and impart various stresses on cardiac myocytes (mechanical, biochemical, bioenergetic, and others). The stress leads to the activation of signaling molecules that mediate the induction of cardiac myocyte hypertrophy, fibrosis, and other aspects of the gross cardiac phenotype.
In summary, HCM is a relatively common disease and is the most common discernible cause of SCD in young athletes. Sudden cardiac arrest in a patient's history, or recurrent syncope, or ventricular tachycardia—any of these is an important risk factor for SCD. Cardiac hypertrophy is an important determinant of the risk of morbidity and death, including the risk of SCD in HCM. None of the currently used pharmacologic therapies attenuates cardiac hypertrophy and fibrosis, prevents SCD, or reduces the mortality rate of HCM. Experimental therapies, such as NAC, can prevent, reverse, or attenuate cardiac hypertrophy and fibrosis in HCM, but their clinical usefulness in human beings remains to be tested.
Footnotes
Address for reprints: Ali J. Marian, MD, 6770 Bertner Ave., DAC 900, Texas Heart Institute at St. Luke's Episcopal Hospital, Houston, TX 77030
E-mail: Ali.J.Marian@uth.tmc.edu
Presented at the 9th Texas Update in Cardiovascular Advancements; Houston, Texas; 4–5 December 2009
Program Director: James T. Willerson, MD
References
- 1.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 2002;287(10):1308–20. [DOI] [PubMed]
- 2.Marian AJ. Genetic determinants of cardiac hypertrophy. Curr Opin Cardiol 2008;23(3):199–205. [DOI] [PMC free article] [PubMed]
- 3.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation 2009;119 (8):1085–92. [DOI] [PubMed]
- 4.Marian AJ. Pathogenesis of diverse clinical and pathological phenotypes in hypertrophic cardiomyopathy. Lancet 2000; 355(9197):58–60. [DOI] [PubMed]
- 5.Patel R, Nagueh SF, Tsybouleva N, Abdellatif M, Lutucuta S, Kopelen HA, et al. Simvastatin induces regression of cardiac hypertrophy and fibrosis and improves cardiac function in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation 2001;104(3):317–24. [DOI] [PMC free article] [PubMed]
- 6.Senthil V, Chen SN, Tsybouleva N, Halder T, Nagueh SF, Willerson JT, et al. Prevention of cardiac hypertrophy by atorvastatin in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res 2005;97(3):285–92. [DOI] [PMC free article] [PubMed]
- 7.Tsybouleva N, Zhang L, Chen S, Patel R, Lutucuta S, Nemoto S, et al. Aldosterone, through novel signaling proteins, is a fundamental molecular bridge between the genetic defect and the cardiac phenotype of hypertrophic cardiomyopathy. Circulation 2004;109(10):1284–91. [DOI] [PMC free article] [PubMed]
- 8.Marian AJ, Senthil V, Chen SN, Lombardi R. Antifibrotic effects of antioxidant N-acetylcysteine in a mouse model of human hypertrophic cardiomyopathy mutation. J Am Coll Cardiol 2006;47(4):827–34. [DOI] [PMC free article] [PubMed]
- 9.Lombardi R, Rodriguez G, Chen SN, Ripplinger CM, Li W, Chen J, et al. Resolution of established cardiac hypertrophy and fibrosis and prevention of systolic dysfunction in a transgenic rabbit model of human cardiomyopathy through thiol-sensitive mechanisms. Circulation 2009;119(10):1398–407. [DOI] [PMC free article] [PubMed]