Abstract
The prognostic value of pulse pressure has been investigated in heart-failure patients. Low pulse pressure in advanced heart failure and high pulse pressure in mild heart failure have been separately linked to increased mortality rates. We prospectively investigated an association between pulse pressure and 2-year cardiovascular death in an entire heart-failure population.
We prospectively enrolled 225 heart-failure patients (New York Heart Association [NYHA] functional class, I–IV; mean age, 56.5 ± 12.3 yr; 188 men). The patients' blood pressures were measured in accordance with recommended guidelines. Pulse pressures were calculated as the difference between systolic and diastolic blood pressure values. The patients were monitored for a mean period of 670 ± 42 days for the occurrence of cardiovascular death.
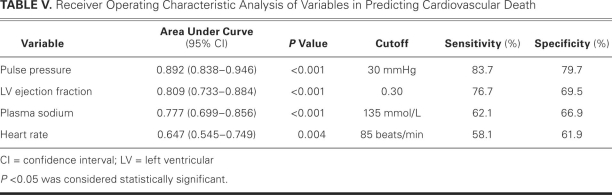
All patients were divided into quartiles according to their pulse pressures (<35, 35–45, 46–55, and >55 mmHg). Pulse pressure decreased as NYHA class worsened (P <0.001). Patients in the <35-mmHg quartile had the lowest plasma sodium concentrations, left ventricular ejection fractions, and systolic myocardial velocities upon echocardiography; and the highest left ventricular dimensions, early diastolic/late diastolic filling velocity ratios, and peak early/peak late diastolic myocardial velocity ratios. Pulse pressure independently predicted death in the patients with advanced heart failure and in the entire population. Upon receiver operating characteristic analysis, a 30-mmHg cutoff value for pulse pressure predicted death with 83.7% sensitivity and 79.7% specificity.
Pulse pressure is easily calculated and enables the prediction of cardiovascular death in patients with mild to advanced heart failure. Pulse pressure can be used reliably as a prognostic marker in clinical practice.
Key words: Blood pressure/physiology, cardiovascular diseases/mortality/physiopathology, epidemiologic methods, heart failure/epidemiology/etiology/physiopathology, multivariate analysis, predictive value of tests, prospective studies, pulse/physiology, reference values, risk factors
Pulse pressure (PP) is the difference between systolic and diastolic blood pressure (BP) values. Pulse pressure markedly rises after the 5th decade of life, due to arterial stiffening with increasing age.1,2 Several studies have shown a close relationship between high PP and the occurrence of cardiovascular (CV) death.3-5 Furthermore, high PP is a risk factor for the development of coronary heart disease, myocardial infarction, and heart failure in normotensive and hypertensive persons.6-10
Data regarding the prognostic value of PP in patients with heart failure are limited and controversial. The importance of PP was investigated in 2 large studies. The SAVE11 (Survival and Ventricular Enlargement) trial revealed a worse prognosis in patients with high PP and symptomatic or asymptomatic left ventricular (LV) systolic dysfunction. The SOLVD12 (Studies of Left Ventricular Dysfunction) trial found that high PP independently predicted total and CV death in mild heart failure. However, in both studies, patients in New York Heart Association (NYHA) functional classes I and II constituted most of the population, and few patients had advanced heart failure (NYHA classes III and IV). In other studies involving patients with advanced heart failure, low PP was associated with high CV mortality rates.13-16 We believed that further study was warranted in order to elucidate the prognostic value of PP in an entire heart-failure population. Accordingly, we investigated the association between PP and 2-year CV death in patients in whom the severity of heart failure ranged from mild to advanced.
Patients and Methods
We enrolled 225 NYHA class I–IV heart-failure patients (mean age, 56.5 ± 12.3 yr; 188 men) in this prospective study. Inclusion criteria were substantially depressed LV systolic function (LV ejection fraction [EF], <0.40) upon echocardiographic examination and impaired exercise capacity before or at the time of enrollment. Patients with renal failure (creatinine, >2 mg/dL), chronic obstructive pulmonary disease, atrial or ventricular arrhythmias, left bundle branch block, a history of recent acute coronary syndrome, or congenital or valvular heart disease were excluded. Qualifying patients were in stable overall condition for 4 weeks or longer before enrollment, and all were on optimal medical regimens. Seventy-two patients (32%) had undergone coronary artery bypass surgery before enrollment. Our local ethics committee approved the study, and written informed consent was obtained from all participants.
We recorded detailed histories of the 225 patients, including demographic characteristics, CV risk factors, and medication usage. The patients were divided into 2 groups according to their NYHA class (mild heart failure [class I–II] and advanced heart failure [class III–IV]) and then into 4 NYHA classes in accordance with their medical histories and the findings upon physical examination.17 Levels of serum lipids, glucose, high-sensitivity C-reactive protein, blood urea nitrogen, creatinine, sodium, and potassium were measured by routine laboratory methods. Blood pressures were measured by sphygmomanometer in accordance with published guidelines.18 Pulse pressure was calculated as the difference between systolic and diastolic BP, and the patients were divided accordingly into 4 quartiles (PP of <35, 35–45, 46–55, or >55 mmHg).14,16
Echocardiographic Examination
All echocardiographic measurements were obtained with the patients at rest. Standard echocardiographic examination, pulsed-wave Doppler, and tissue Doppler imaging were performed on an ACUSON Sequoia™ ultrasound machine (Siemens Medical Solutions USA, Inc.; Mountain View, Calif) with a 2.5-or 3.5-MHz phased-array transducer. The mean of all recordings from 3 consecutive cycles was used to drive the measurements. M-Mode measurements of LV end-diastolic and end-systolic dimensions and volumes, ventricular septal and posterior wall thicknesses, and left atrial end-diastolic dimensions were made in accordance with the recommendations of the American Society of Echocardiography.19 Left ventricular EF was calculated by use of the modified Simpson technique. Left ventricular diastolic function was evaluated in the apical 4-chamber view by means of pulsed-wave and tissue Doppler imaging. The pulsed-wave Doppler imaging was performed in order to measure transmitral flow values, including the peak early diastolic filling velocity (E), the peak late diastolic filling velocity (A), the early diastolic/late diastolic filling velocity (E/A) ratio, the E-wave deceleration time, and the isovolumic relaxation time. The tissue Doppler imaging was performed in order to measure systolic myocardial velocity (Sm), peak early diastolic myocardial velocity (Em), and peak late diastolic myocardial velocity (Am). An Em/Am ratio was calculated at the end of expiration.
Follow-Up
The patients were monitored for 625 to 720 days (mean, 670 ± 42 d) for the occurrence of CV death (sudden cardiac death or death due to decompensated heart failure, acute coronary syndromes, or arrhythmia).
Statistical Analysis
All analyses were performed with use of the SPSS 15.0 statistical software package (SPSS, Inc.; Chicago, Ill). Continuous variables were presented as mean ± SD. Analysis of continuous variables according to NYHA class or PP quartile was performed by means of 1-way analysis of variance or the Kruskal-Wallis test; post hoc tests (Scheffé or Tamhane) were applied when indicated. When dependent variables were binary, the Student t test or Mann-Whitney test was used. Discrete variables were compared by χ2 analysis. Correlations between continuous variables were evaluated by means of Pearson or Spearman rank correlation analysis. Multivariate logistic regression analysis was performed to determine significant predictors of CV death and advanced heart failure. Variables that were significant in univariate analysis at a P <0.1 level were entered into our logistic regression analysis. A linear regression analysis was applied for LVEF. Receiver operator characteristic (ROC) curve analysis was performed to identify the optimal cutoff point of PP (at which sensitivity and specificity would be maximal) for the prediction of CV death. Areas under the curve (AUC) were calculated as measures of the accuracy of the tests. We compared the AUC with use of the Z test. A value of P <0.05 was considered statistically significant. The data conformed to each test by which they were analyzed.
Results
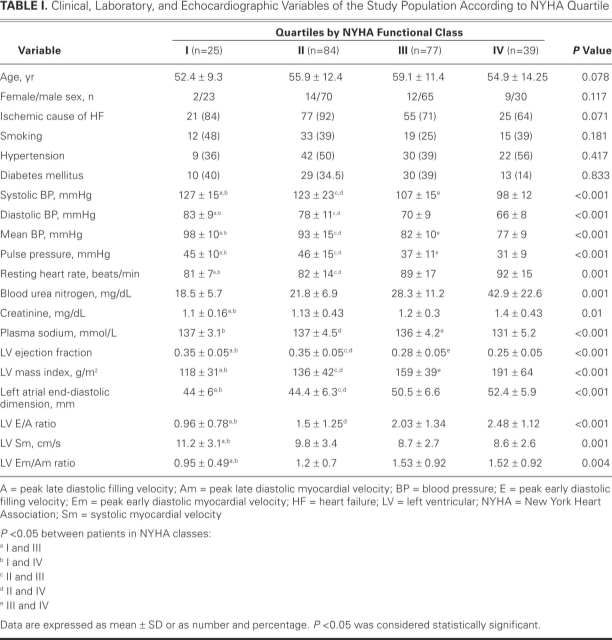
Table I shows the clinical, laboratory, and echocardiographic characteristics of the study population according to NYHA quartile. Systolic BP, diastolic BP, mean BP, and PP decreased as NYHA class worsened (each P <0.001). Severity of NHYA class was also associated with echocardiographic values of impaired systolic and diastolic function. Among the study population, 142 patients were taking diuretics (63%); 128, β-blockers (57%); 198, angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (88%); 72, digitalis (32%); and 92, spironolactone (41%). Upon multivariate logistic regression analysis, independent predictors of advanced heart failure were determined to be LVEF (odds ratio [OR]=0.76; 95% confidence interval [CI], 0.7–0.83; P <0.001) and systolic BP (OR=0.93; 95% CI, 0.9–0.97; P <0.001).
TABLE I. Clinical, Laboratory, and Echocardiographic Variables of the Study Population According to NYHA Quartile
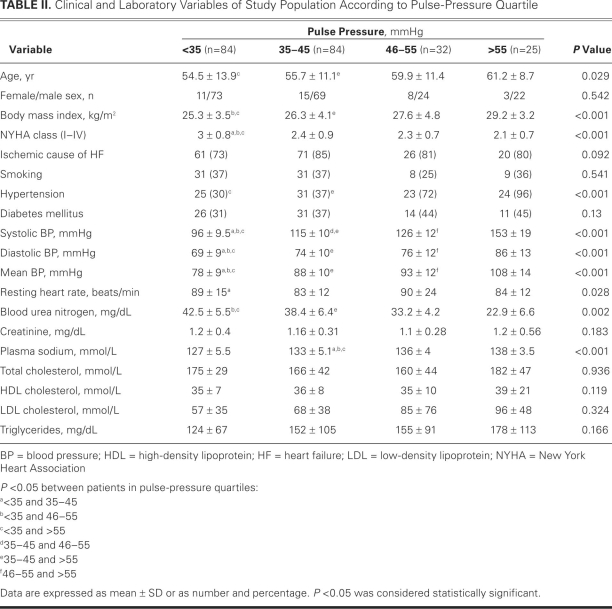
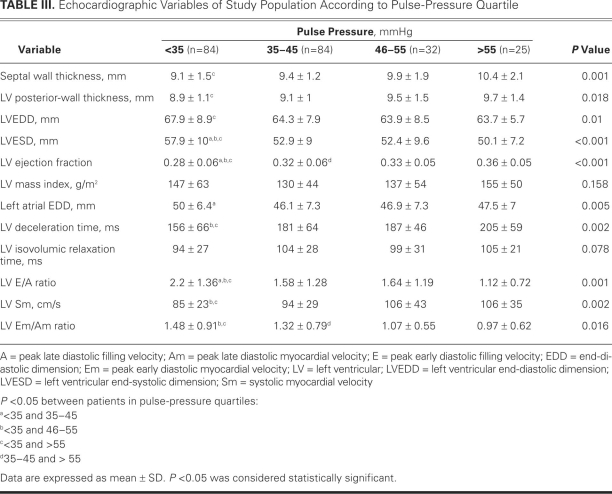
The median PP in the study population was 40 mmHg (range, 20–110 mmHg). Table II shows the clinical characteristics and laboratory variables of the patients according to PP quartile; Table III shows the echocardiographic variables.
TABLE II. Clinical and Laboratory Variables of Study Population According to Pulse-Pressure Quartile
TABLE III. Echocardiographic Variables of Study Population According to Pulse-Pressure Quartile
Significant positive correlations were found between LVEF and BP (systolic, diastolic, and mean), PP, resting heart rate, body mass index, plasma sodium concentration, LV deceleration time, and LV Sm (each P <0.001). Multivariate linear regression analysis showed that the most important predictors of LVEF were systolic BP (β=0.268, P <0.001), body mass index (β=0.156, P=0.008), left atrial end-diastolic dimension (β=–0.411, P <0.001), and LV Sm (β=0.161, P=0.004).
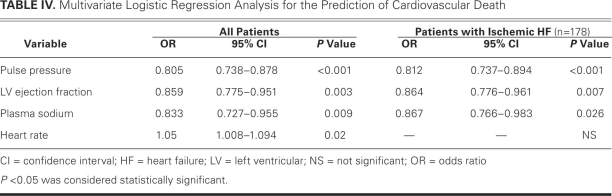
Twelve patients were lost to follow-up during the study period. In the remaining 213 patients, 56 cardiac-related deaths (26.3%) occurred: 42 in patients with a PP of less than 35 mmHg, 9 in those with a PP of 35 to 45 mmHg, 3 in those with a PP of 46 to 55 mmHg, and 2 in those with a PP greater than 55 mmHg. Upon univariate analysis, predictors of death in the entire population were high heart rate, NYHA class, LV and left atrial dimensions, blood urea nitrogen and creatinine, lower body mass index, PP, BP (systolic, diastolic, and mean), and LVEF (each P <0.01). Older age, ischemic heart failure, digoxin use, and lack of β-blocker or ACE-inhibitor therapy were also related to CV death (each P <0.01). Multivariate logistic regression analysis revealed independent predictors in the entire population to be PP, LVEF, plasma sodium level, and heart rate, adjusted for univariate predictors (Table IV). Every 1-mmHg decrease in PP increased the risk of death by 24.2%. Furthermore, PP, LVEF, and plasma sodium level independently predicted death in the 178 patients with ischemic heart failure (Table IV).
TABLE IV. Multivariate Logistic Regression Analysis for the Prediction of Cardiovascular Death
In the patients with advanced heart failure, 48 cardiac-related deaths occurred, and the only independent predictor of death in this group was PP (OR=0.85; 95% CI, 0.79–0.916; P <0.001). However, in the group with mild heart failure, 8 patients died of CV causes, and no independent predictor of death was found.
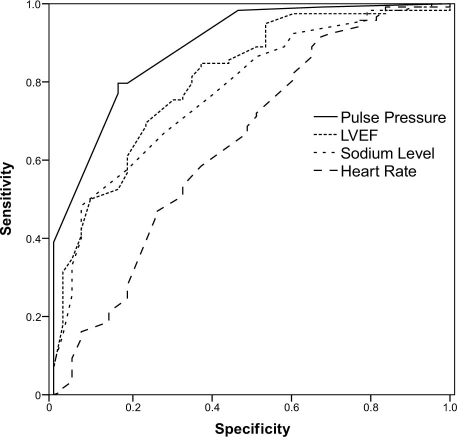
Upon ROC curve analysis, PP had the best discriminatory power among variables that were investigated for the occurrence of death in the entire population. The AUC value in predicting death was 0.892 for PP, 0.809 for LVEF, 0.777 for plasma sodium level, and 0.647 for resting heart rate (Table V). Figure 1 shows the corresponding ROC curves. Statistically significant AUC differences were found between the variables, except between LVEF and plasma sodium level. The ROC curve analysis for PP had the highest AUC value, and heart rate had the lowest. Therefore, the most significant AUC difference was found between PP and heart rate (P <0.001). A 30-mmHg cutoff value for PP predicted death with a sensitivity of 83.7% and a specificity of 79.7%.
TABLE V. Receiver Operating Characteristic Analysis of Variables in Predicting Cardiovascular Death
Fig. 1 The receiver operator characteristic curve for pulse pressure, left ventricular ejection fraction (LVEF), plasma sodium level, and heart rate in the prediction of cardiovascular death.
Discussion
Our study showed that low PP independently predicted CV death in patients with mild to advanced heart failure. Furthermore, low PP was closely associated with worsening echocardiographic and clinical values.
Pulse pressure has been previously correlated with arterial compliance and with hemodynamic factors such as stroke volume and peak aortic blood flow. Left ventricular systolic dysfunction reduces stroke volume and therefore also PP and systolic BP. Several studies have shown a positive correlation between low PP and diminished cardiac index (<2.2 L/min/m2).6,13,20,21 Fagard and colleagues15 reported a positive and independent association between low PP and low LVEF, and our results confirm these observations. We also found a positive correlation between LVEF and BP (systolic, diastolic, and mean), and between LVEF and PP. A recent study reported a correlation between PP and LVEF, but only in patients with nonischemic heart failure.16 In our study, lower systolic BP positively correlated with lower LVEF in all patients, and patients with a PP below 35 mmHg had the lowest LVEFs and the highest heart rates. Our findings are consistent with those of the recent study.16
Well-recognized factors that affect the mortality rate in heart failure are older age, diabetes mellitus, renal failure, severe NYHA class, low LVEF, maximal oxygen consumption, low levels of plasma sodium, and high levels of natriuretic peptide.22 Our results revealed that PP, LVEF, plasma sodium level, and heart rate are independent predictors of CV death—and that PP is the most important.
Available data on the relationship between PP and the prognostication of heart failure are limited and controversial. To our knowledge, there are no reports of PP's use in clinical practice for the prediction of heart failure. In 2 large studies,11,12 high PP predicted adverse CV outcomes in patients with mild heart failure: the SAVE investigators showed that high PP is a predictor of worse outcome in patients who have asymptomatic LV systolic dysfunction,11 and the SOLVD investigators reported that high PP was an independent predictor of total and CV death in patients with mild heart failure.12 However, relatively few patients in either study had advanced heart failure. Other investigators reported that low PP independently predicted higher CV mortality rates in patients with advanced and decompensated heart failure, but not in patients with mild heart failure.13-16 After observing significantly short survival durations in patients with advanced heart failure who had a PP of 35 mmHg or less, Voors and co-authors14 proposed that low PP indicated decreased cardiac function, and that adverse outcome in these patients was linked to worsening of that function. In another study,16 although PP independently predicted hospitalization rates of patients with ischemic and nonischemic heart failure, it independently predicted death only in the nonischemic group.
The different results regarding the prognostic value of PP may be due to different characteristics of the study populations. Higher PP has been associated with higher mortality rates in patients who were in NYHA class I–II, and lower PP has been associated with higher mortality rates in patients who were in NYHA class III–IV. In mild heart failure, a high PP is probably the result of vascular stiffening or decreased aortic elasticity, which indicates atherosclerosis and therfore a poorer prognosis, whereas in advanced heart failure, low PP chiefly indicates decreased cardiac function and an associated adverse prognosis. Previous studies of the prognostic value of PP in heart failure were performed only in specific patient groups—those with only mild or only advanced heart failure. Therefore, the prognostic value of PP is less clear when all NYHA classes are considered. In our study, we included subjects from all NYHA classes, but we observed low PP only in patients who were experiencing advanced heart failure. We found an independent association between low PP and CV mortality rates, not just in patients with advanced heart failure, but in the entire study population. In addition, we discovered that low PP independently predicts death in ischemic heart failure—a novel finding. Other investigators who used PP quartiles or tertiles and regression analysis reported that patients in the low PP quartile (<35 mmHg) experienced higher CV mortality rates.12-14,16 However, no ROC curve analysis was performed in those trials, and no optimal PP cutoff point was identified for the prediction of CV death. In our analysis of predictors of CV death, the ROC curve analysis for PP had the highest AUC value and was more statistically significant than LVEF, heart rate, and plasma sodium level. The cutoff PP value of 30 mmHg was highly predictive of CV death.
Our study has some limitations. The sample size of the study is relatively small. The number of patients with mild heart failure was too small to determine statistical significance for CV death; therefore, our results cannot be used to predict death in that group. Finally, we did not consider N-terminal pro-B-type natriuretic peptide and maximal oxygen consumption, which might have some influence on prognosis.22,23
Conclusion
Pulse pressure is easily calculated, and it enables the prediction of CV death in patients who have mild to advanced heart failure. We believe that PP can be used reliably as a prognostic marker in daily clinical practice. However, our results need to be confirmed in larger study populations.
Footnotes
Address for reprints: Tansel Yildiran, MD, Department of Cardiology, Cukurova University, 01330 Adana, Turkey
E-mail: drtansel@yahoo.com
References
- 1.Franklin SS, Gustin W 4th, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation 1997;96(1):308–15. [DOI] [PubMed]
- 2.Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation 1989;80(6):1652–9. [DOI] [PubMed]
- 3.Darne B, Girerd X, Safar M, Cambien F, Guize L. Pulsatile versus steady component of blood pressure: a cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension 1989;13(4):392–400. [DOI] [PubMed]
- 4.Benetos A, Safar M, Rudnichi A, Smulyan H, Richard JL, Ducimetieere P, Guize L. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension 1997;30(6):1410–5. [DOI] [PubMed]
- 5.Domanski MJ, Sutton-Tyrrell K, Mitchell GF, Faxon DP, Pitt B, Sopko G. Determinants and prognostic information provided by pulse pressure in patients with coronary artery disease undergoing revascularization. The Balloon Angioplasty Revascularization Investigation (BARI). Am J Cardiol 2001; 87(6):675–9. [DOI] [PubMed]
- 6.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham heart study. Circulation 1999;100(4): 354–60. [DOI] [PubMed]
- 7.Fang J, Madhavan S, Cohen H, Alderman MH. Measures of blood pressure and myocardial infarction in treated hypertensive patients. J Hypertens 1995;13(4):413–9. [PubMed]
- 8.Chae CU, Pfeffer MA, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA 1999;281(7):634–9. [DOI] [PubMed]
- 9.Vaccarino V, Holford TR, Krumholz HM. Pulse pressure and risk for myocardial infarction and heart failure in the elderly. J Am Coll Cardiol 2000;36(1):130–8. [DOI] [PubMed]
- 10.Kostis JB, Lawrence-Nelson J, Ranjan R, Wilson AC, Kostis WJ, Lacy CR. Association of increased pulse pressure with the development of heart failure in SHEP. Systolic Hypertension in the Elderly (SHEP) Cooperative Research Group. Am J Hypertens 2001;14(8 Pt 1):798–803. [DOI] [PubMed]
- 11.Mitchell GF, Moye LA, Braunwald E, Rouleau JL, Bernstein V, Geltman EM, et al. Sphygmomanometrically determined pulse pressure is a powerful independent predictor of recurrent events after myocardial infarction in patients with impaired left ventricular function. SAVE investigators. Survival and Ventricular Enlargement. Circulation 1997;96(12):4254–60. [DOI] [PubMed]
- 12.Domanski MJ, Mitchell GF, Norman JE, Exner DV, Pitt B, Pfeffer MA. Independent prognostic information provided by sphygmomanometrically determined pulse pressure and mean arterial pressure in patients with left ventricular dysfunction. J Am Coll Cardiol 1999;33(4):951–8. [DOI] [PubMed]
- 13.Aronson D, Burger AJ. Relation between pulse pressure and survival in patients with decompensated heart failure. Am J Cardiol 2004;93(6):785–8. [DOI] [PubMed]
- 14.Voors AA, Petrie CJ, Petrie MC, Charlesworth A, Hillege HL, Zijlstra F, et al. Low pulse pressure is independently related to elevated natriuretic peptides and increased mortality in advanced chronic heart failure. Eur Heart J 2005;26(17):1759–64. [DOI] [PubMed]
- 15.Fagard RH, Pardaens K, Vanhaecke J. Is the predictive power of a low-pulse pressure independent of peak oxygen uptake in advanced chronic heart failure? J Hum Hypertens 2008;22 (1):57–9. [DOI] [PubMed]
- 16.Petrie CJ, Voors AA, van Veldhuisen DJ. Low pulse pressure is an independent predictor of mortality and morbidity in non ischaemic, but not in ischaemic advanced heart failure patients. Int J Cardiol 2009;131(3):336–44. [DOI] [PubMed]
- 17.The Criteria Committee of the New York Heart Association, Inc. Diseases of the heart and blood vessels: nomenclature and criteria for diagnosis. 6th ed. Boston: Little, Brown & Co.; 1964.
- 18.O'Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, et al. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens 2005;23(4):697–701. [DOI] [PubMed]
- 19.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2(5):358–67. [DOI] [PubMed]
- 20.Stevenson LW, Braunwald E. Recognition and management of patients with heart failure. In: Goldman L, Braunwald E, editors. Primary cardiology. 1st ed. Philadelphia: WB Saunders Co.; 1998. p. 310–29.
- 21.Shah MR, Hasselblad V, Stinnett SS, Gheorghiade M, Swedberg K, Califf RM, O'Connor CM. Hemodynamic profiles of advanced heart failure: association with clinical characteristics and long-term outcomes. J Card Fail 2001;7(2):105–13. [DOI] [PubMed]
- 22.Eichhorn EJ. Prognosis determination in heart failure. Am J Med 2001;110 Suppl 7A:14S-36S. [DOI] [PubMed]
- 23.Koc M, Bozkurt A, Acarturk E, Sahin DY, Unal I. Usefulness of N-terminal pro-B-type natriuretic peptide increase with exercise for predicting cardiovascular mortality in patients with heart failure. Am J Cardiol 2008;101(8):1157–62. [DOI] [PubMed]