Abstract
Platelets are crucial in the pathogenesis of acute coronary syndrome. Treatment for acute coronary syndrome usually involves antiplatelet, anticoagulant, and antithrombotic therapy, and the performance of percutaneous coronary intervention. All of the medications are associated with bleeding sequelae and are typically withheld from patients who have thrombocytopenia. The safety of antiplatelet therapy and percutaneous coronary intervention in patients who have acute coronary syndrome and thrombocytopenia is unknown, and there are no guidelines or randomized studies to suggest a treatment approach in such patients.
Acute coronary syndrome is uncommon in patients who have thrombocytopenia; however, it occurs in up to 39% of patients who have both thrombocytopenia and cancer. Herein, we present the cases of 5 patients with acute coronary syndrome, thrombocytopenia, and cancer who underwent percutaneous coronary intervention with stenting. Before intervention, their platelet counts ranged from 17 to 72 × 109/L. One patient underwent preprocedural platelet transfusion. All were given aspirin, alone or with clopidogrel. One patient experienced melena (of colonic origin). No other patient experienced bleeding sequelae.
Aside from the occasional use of antiplatelet and thrombolytic agents in patients with thrombocytopenia, no therapeutic recommendation can be made until data are available on a larger patient population. Until then, treatment should conform to specific clinical circumstances. Approaches to the treatment of acute coronary syndrome in patients with thrombocytopenia might be better directed toward the evaluation of platelet function rather than toward absolute platelet count, and the risk–benefit equation of invasive procedures and antithrombotic therapies may need to incorporate this information.
Key words: Angioplasty, transluminal, percutaneous coronary; anticoagulants/adverse effects; aspirin/therapeutic use; blood platelets/drug effects/physiology; coronary artery disease/physiopathology/prevention & control; hemorrhage/complications; myocardial infarction/drug therapy; neoplasms/complications; platelet aggregation inhibitors/administration & dosage/therapeutic use; platelet count/drug effects; thrombocytopenia/complications/drug therapy/etiology/prevention & control
Platelets play a crucial role in the pathogenesis of acute coronary syndrome (ACS).1 Although ACS in patients with thrombocytopenia is uncommon, it is found in up to 39% of patients who have both thrombocytopenia and cancer.2 The standard treatment for ACS involves therapy with antiplatelet, anticoagulant, and thrombolytic agents, and the performance of percutaneous coronary intervention (PCI). All of these medications are associated with bleeding sequelae and are typically withheld from patients who have thrombocytopenia. The safety of antiplatelet therapy and PCI in patients who have ACS and thrombocytopenia is unknown, and there are no guidelines or randomized studies to suggest treatment approaches in such patients.
Herein, we present a case series of patients with ACS, thrombocytopenia, and cancer who underwent PCI with stenting, and we discuss the treatment options.
Patient 1
A 60-year-old woman with multiple myeloma and a history of coronary artery disease presented at the hospital with chest pain and dyspnea. Her heart rate was 120 beats/min, and her blood pressure was 154/64 mmHg. Auscultation revealed a systolic ejection murmur and crackles in the left lung base. A 12-lead electrocardiogram (ECG) showed sinus tachycardia. Laboratory results included a platelet count of 21 ×109/L, a hemoglobin level of 8.1 g/dL, a creatinine level of 0.8 mg/dL, and normal levels of cardiac enzymes. A transthoracic echocardiogram (TTE) showed normal left ventricular (LV) function and an ejection fraction (LVEF) of 0.60. The diagnosis of unstable angina was made, and she was treated with nitrates, aspirin, β-blockers, and a 3-hydroxy-3-methylglutaryl coenzyme-A reductase inhibitor. After a coronary angiogram showed an 80% stenosis of the 1st diagonal branch of the left anterior descending coronary artery (LAD), PCI with stenting was performed, with no bleeding sequelae. Before the procedure, she was given a transfusion of 20 units of platelets. During the procedure, she was given 11,500 units of intravenous heparin, 200 μg of intracoronary nitroglycerin, and 300 mg of oral clopidogrel. The activating clotting time (ACT) recorded during the procedure was 190 sec. No platelet count was obtained after the procedure. The patient was discharged from the hospital and died of sepsis 4 years later.
Patient 2
A 70-year-old man with a history of coronary artery disease, diabetes mellitus, and acute myeloid leukemia presented at the hospital with chest pain and palpitations. His heart rate was 90 beats/min, and his blood pressure was 152/72 mmHg. A 12-lead ECG showed sinus rhythm and LV hypertrophy. Laboratory results included a platelet count of 46 ×109/L, a hemoglobin level of 8.6 g/dL, and normal levels of cardiac enzymes. A TTE showed normal LV systolic function and an LVEF of 0.60. The diagnosis of unstable angina was made. Coronary angiography showed a 50% stenosis of the proximal circumflex artery, 95% stenosis of the distal circumflex artery, 75% stenosis of the proximal 1st obtuse marginal branch, and 100% occlusion of the proximal right coronary artery (RCA). During PCI, he received 7,500 units of intra-arterial heparin, 200 μg of intracoronary nitroglycerin, and 300 mg of oral clopidogrel. The maximum ACT recorded during the procedure was 238 sec. The patient underwent PCI and stenting of the circumflex artery, and he was given clopidogrel and aspirin for a month. However, he experienced chronic recurrent melena after PCI, and the aspirin and clopidogrel were temporarily discontinued. An upper gastrointestinal endoscopy and a colonoscopy showed colonic polyps, diverticulosis, and hemorrhoids, but no active bleeding. Chemotherapy was initiated a few days after the coronary revascularization, and the patient was discharged from the hospital.
Patient 3
A 69-year-old man with a history of diabetes mellitus, hypertension, and mantle-cell lymphoma presented at the hospital with recurrent episodes of chest pain. On examination, his heart rate was 60 beats/min, and his blood pressure was 130/70 mmHg. Splenomegaly was detected. A 12-lead ECG showed sinus rhythm. A TTE showed normal LV systolic function and an LVEF of 0.58. Laboratory results included a platelet count of 28 ×109/L, a hemoglobin level of 11.1 g/dL, a creatinine level of 0.9 mg/dL, and an elevated troponin I level. The diagnosis of ACS was made, and the patient was given aspirin and clopidogrel. Coronary angiography revealed an 80% stenosis of the RCA, for which the patient underwent PCI with stent placement. During the procedure, he was given 8,500 units of intra-arterial heparin, 300 μg of intracoronary nitroglycerin, and 300 mg of oral clopidogrel. The maximum ACT during the procedure was 358 sec. He continued to take aspirin and clopidogrel without bleeding sequelae, and he experienced no further coronary events. He died of pneumonia 3 years later.
Patient 4
A 75-year-old man with a history of hypertension, diabetes mellitus, hypercholesterolemia, coronary artery bypass grafting (CABG), and chronic myeloid leukemia presented at the hospital with chest pain. His heart rate was 72 beats/min, and his blood pressure was 98/40 mmHg. A 12-lead ECG showed a 1-mm ST elevation in the inferior leads, a 1-mm ST depression in leads I and aVL, and a right bundle branch block. Laboratory results included a platelet count of 72 ×109/L, a hemoglobin level of 8.5 g/dL, a creatinine level of 1.2 mg/dL, and normal levels of cardiac enzymes. A TTE showed an LVEF of 0.45. The diagnosis of ACS was made, and the patient was started on aspirin, clopidogrel, and nitroglycerin. Coronary angiography showed a 60% to 70% stenosis of the native circumflex artery, 100% occlusion of the native LAD, and 99% stenosis at the anastomosis site of the saphenous vein graft (SVG) to the LAD. The patient underwent PCI with stent placement at the anastomosis site and experienced no bleeding sequelae. During the procedure, he was given 6,000 units of heparin intravenously, an intravenous integrilin bolus of 12.4 mg followed by an integrilin infusion at a dose of 2.037 μg/kg/min, oral clopidogrel of 150 mg, and (by injection into the LAD graft) 200 μg of nitroglycerin and 100 μg of adenosine. The recorded ACT during the procedure was 206 sec. He died 1 month later of sepsis.
Patient 5
An 80-year-old man with a history of hypertension, hypercholesterolemia, and acute myeloid leukemia presented at the hospital with chest pain. His heart rate was 65 beats/min, and his blood pressure was 120/62 mmHg. A 12-lead ECG showed atrial fibrillation with a left bundle branch block. Laboratory results included a platelet count of 17 ×109/L, a hemoglobin level of 8.3 g/dL, a creatinine level of 1.2 mg/dL, and an elevated troponin I level with a peak value of 11.91 ng/mL (normal value, <0.4 ng/mL). A TTE showed normal LV systolic function and an LVEF of 0.54. The diagnosis of ACS was made, and the patient was treated with aspirin, nitroglycerin, and β-blockers. Coronary angiography showed a 90% lesion in the obtuse marginal branch of the circumflex coronary artery and a 40% to 50% stenosis of the distal RCA. The patient underwent PCI without stent placement in the obtuse marginal branch and experienced no bleeding sequelae. During the procedure, he was given 5,000 units of intravenous heparin, 400 μg of intracoronary nitroglycerin, and 325 mg of oral aspirin. The ACT recorded during the procedure was 262 sec. Seven days after PCI, he experienced an episode of noncardiac chest pain, after completion of a platelet transfusion. One week later, a temporary pacemaker was inserted because of severe bradycardia. His general condition deteriorated, and he died 22 days after PCI of multiorgan failure, pneumonia, and diffuse alveolar hemorrhage.
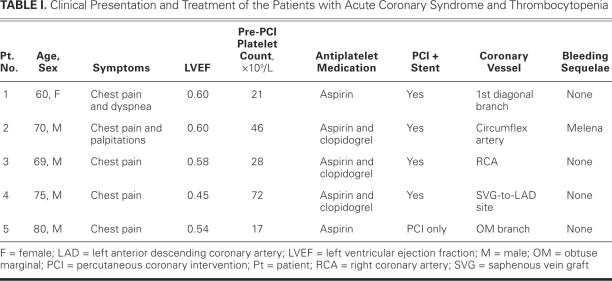
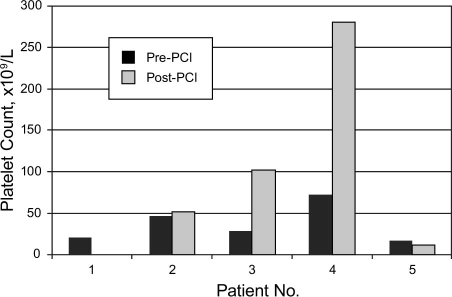
Table I shows the clinical data on the 5 patients. Figure 1 shows the available pre- and post-PCI platelet counts.
TABLE I. Clinical Presentation and Treatment of the Patients with Acute Coronary Syndrome and Thrombocytopenia
Fig. 1 Platelet counts before and after percutaneous coronary intervention (PCI). No post-PCI platelet count was obtained in patient 1. In the other 4 patients, the post-PCI platelet counts were obtained from 24 hours to 20 days after PCI.
Discussion
Platelets play an important role in the atherosclerotic process and are intrinsically involved in the pathogenesis of ACS.1 Thrombocytosis may be a risk factor for acute myocardial infarction (AMI): in 1 study, the incidence of AMI in patients with essential thrombocythemia was 9.4%.3 However, thrombocytopenia itself is not protective, and AMI has been reported in patients who have thrombocytopenia that is associated with various conditions.4-12 In fact, in patients with Kawasaki disease, thrombocytopenia is considered a risk factor in the development of AMI.13 This implies that some factor other than platelet numbers alone is involved.
The cause of coronary thrombosis in patients with thrombocytopenia is debatable and multifactorial. However, the development and effect of occlusive thrombus in these patients are similar to those of atherosclerotic disease: for example, at autopsy, a fibrin-platelet thrombus occluded the left coronary artery in a young thrombocytopenic patient.14
Independently of their thrombocytopenia, patients may be predisposed to coronary thrombosis because their platelets are larger and more adhesive to the vascular surface. In AMI or stroke patients, mean platelet volume is significantly increased despite a concomitant decrease in platelet count.15,16 In a rodent model, large platelets appeared in the circulation within 12 hours of the induction of thrombocytopenia, and at 18 hours, nearly half of all platelets were large.17 Evidence supports the concept that large platelets have a higher thrombotic potential. In 1 study, the platelet aggregation velocity that was induced with adenosine-5′-diphosphate, collagen, or epinephrine was directly in proportion to the platelet volume and correlated best with the megathrombocyte index.18 Because platelet production is naturally regulated to maintain platelet mass (the product of platelet count and mean platelet volume), it can be inferred that the larger platelets not only provide a hemostatic plug and prevent bleeding via high activity, but also that they may be linked to coronary thrombosis in patients with thrombocytopenia.
There is substantial evidence of the involvement of microparticles in the activation of the coagulation cascade. Some of these microparticles are derived from platelets.19 Levels of platelet microparticles (PMPs) are significantly higher in patients who have idiopathic thrombocytopenic purpura (ITP) than in control groups.20 Patients who were free of petechiae and mucosal bleeding were found to have significantly high PMP levels.20 Elevated PMP levels have been observed in patients who have ITP and neurologic complications20 and in patients who have ACS and thrombocytopenia.21 This suggests that PMPs play an important hemostatic role in patients who have thrombocytopenia and that hemostatically active PMPs may be thrombogenic at high concentrations and in certain clinical circumstances.20
There are isolated reports of the use of antiplatelet agents with no bleeding sequelae in patients with AMI and thrombocytopenia.11,12 In 1 patient, streptokinase was used locally in the LAD, but without success: postmortem examination revealed occlusion of the vascular lumen by the fibrin-platelet thrombus.14 Even in patients who have been treated with both aspirin and clopidogrel, thrombotic stent occlusion has been reported.12 In patients with ITP, successful PCI22 and CABG with supportive platelet transfusion23,24 have been reported.
Aspirin therapy in the presence of thrombocytopenia is a matter of debate,25,26 and there are no widely accepted protocols. One guideline is to avoid giving aspirin after CABG to patients who have thrombocytopenia (defined as platelet count <50 ×109/L).27 This recommendation (for patients who are undergoing CABG) cannot be extrapolated to patients who have AMI and thrombocytopenia, because a more recent study showed that aspirin is safe and reduces mortality rates in such patients.2
The safety and tolerability of anticoagulants and antiplatelet agents have been well characterized in the general ACS population. However, few data are available with regard to the safety and tolerability of such medications in patients who have thrombocytopenia and cancer, or thrombocytopenia from bone-marrow suppression. Bleeding is the most common adverse event related to therapy with antithrombotic and antiplatelet agents. Therefore, the risks and benefits of this therapy should be critically weighed in order to achieve optimal outcomes and minimize bleeding sequelae in patients who have thrombocytopenia. The outcomes of antiplatelet therapy in patients with thrombocytopenia, ACS, and cancer have been reported.2 However, these data are from a retrospective study and warrant validation in prospective trials.
Vanderschueren and colleagues28 evaluated a large series of patients with thrombocytopenia in terms of their prognosis in the intensive care unit. Severity of thrombocytopenia served as a biomarker for high rates of bleeding and death. The incidence of bleeding was 4.1% in nonthrombocytopenic patients, 21.4% in patients who had platelet counts between 101 and 149 ×109/L, and 52.6% in patients with platelet counts below 100 ×109/L. However, in this last group, there was no correlation between initial platelet count and risk of bleeding28; this is probably due to the routine use of prophylactic platelet transfusion, which was given to as many as 75% of patients with platelet counts below 20 ×109/L.28 In patients undergoing coronary angiography, those with low platelet counts (<100 ×109/L) were 9 times more likely to develop a medium or large hematoma than were patients with platelet counts above that level.29
In patients with thrombocytopenia, the risk of bleeding varies and may depend upon the underlying cause. For instance, in chemotherapy-induced thrombocytopenia, spontaneous clinical bleeding (none life-threatening or intracranial) was seen only in individuals whose platelet counts were below 10 ×109/L.30 The risk of bleeding was much higher in patients who had myelodysplastic syndrome.31 In 1 institution, among patients with myelodysplastic syndrome for whom a cause-of-death code was assigned, hemorrhage contributed to the death of 20% and was the only cause of death in 10%.31 Despite the lack of data, some investigators advocate the use of thrombolytic therapy in patients with thrombocytopenia and AMI provided that there is no evidence of bleeding sequelae.32 However, aside from occasional use of antiplatelet and thrombolytic agents, no recommendation can be made until data are available on a larger patient population. Until then, each patient should be treated in accordance with the specific clinical circumstances. Approaches to the treatment of AMI in thrombocytopenic patients might be better directed toward the evaluation of platelet function than toward platelet count, and the risk–benefit equation of invasive procedures and antithrombotic therapies may need to take this information into account.
Footnotes
Address for reprints: Syed Wamique Yusuf, MBBS, MRCPI, Department of Cardiology, University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Unit 1451, Houston, Texas 77030
E-mail: syusuf@mdanderson.org
References
- 1.Willerson JT. Conversion from chronic to acute coronary heart disease syndromes. Role of platelets and platelet products. Tex Heart Inst J 1995;22(1):13–9. [PMC free article] [PubMed]
- 2.Sarkiss MG, Yusuf SW, Warneke CL, Botz G, Lakkis N, Hirch-Ginsburg C, et al. Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes. Cancer 2007;109(3):621–7. [DOI] [PubMed]
- 3.Rossi C, Randi ML, Zerbinati P, Rinaldi V, Girolami A. Acute coronary disease in essential thrombocythemia and polycythemia vera. J Intern Med 1998;244(1):49–53. [DOI] [PubMed]
- 4.Varbella F, Bongioanni S, Gagnor A, Nannini C, La Brocca A, Badali A, Conte MR. Primary angioplasty in a patient with the May-Hegglin anomaly, a rare heredity thrombocytopenia. A case report and review of the literature [in Italian]. Ital Heart J Suppl 2005;6(4):214–7. [PubMed]
- 5.Schoenfeld MR, Goldberger E. Acute myocardial infarction in the presence of thrombocytopenia [letter]. Can Med Assoc J 1962;86(1):464. [PMC free article] [PubMed]
- 6.Kim JH, Park KU, Chun WJ, Kim SH, Nah DY. Primary percutaneous coronary intervention for acute myocardial infarction with idiopathic thrombocytopenic purpura: a case report. J Korean Med Sci 2006;21(2):355–7. [DOI] [PMC free article] [PubMed]
- 7.Fruchter O, Blich M, Jacob G. Fatal acute myocardial infarction during severe thrombocytopenia in a patient with idiopathic thrombocytopenic purpura. Am J Med Sci 2002;323 (5):279–80. [DOI] [PubMed]
- 8.Hasper D, Schrage D, Niesporek S, Knollmann F, Barckow D, Oppert M. Extensive coronary thrombosis in thrombotic-thrombocytopenic purpura. Int J Cardiol 2006;106(3):407–9. [DOI] [PubMed]
- 9.Yen CH, Lee PY, Hou CJY, Chou YS, Tsai CH. Acute myocardial infarction in systemic lupus erythematosus with antiphospholipid syndrome and severe thrombocytopenia–a case report and literature review [in Chinese]. J Intern Med Taiwan 2005;16:246–50.
- 10.Fireman Z, Yust I, Zahavi J, Kahn Y, Abramov LA. Subendocardial infarction and thrombocytopenia. Postgrad Med J 1979;55(639):36–8. [DOI] [PMC free article] [PubMed]
- 11.Pervez H, Potti A, Mehdi SA. Challenging and unusual cases: Case 1. Simultaneous presentation of acute myelogenous leukemia and myocardial infarction. J Clin Oncol 2003;21(7): 1416–7. [DOI] [PubMed]
- 12.Jachmann-Jahn U, Cornely OA, Laufs U, Hopp HW, Meuthen I, Krakau M, O'Brien B. Acute anterior myocardial infarction as first manifestation of acute myeloid leukemia. Ann Hematol 2001;80(11):677–81. [DOI] [PubMed]
- 13.Niwa K, Aotsuka H, Hamada H, Uchishiba M, Terai M, Niimi H. Thrombocytopenia: a risk factor for acute myocardial infarction during the acute phase of Kawasaki disease. Coron Artery Dis 1995;6(11):857–64. [PubMed]
- 14.Solomons HD, Stanley A, King PC, Pienaar N, Atkinson PM. Acute promyelocytic leukaemia associated with acute myocardial infarction. A case report. S Afr Med J 1986;70(2):117–8. [PubMed]
- 15.Cameron HA, Phillips R, Ibbotson RM, Carson PH. Platelet size in myocardial infarction. Br Med J (Clin Res Ed) 1983;287(6390):449–51. [DOI] [PMC free article] [PubMed]
- 16.O'Malley T, Langhorne P, Elton RA, Stewart C. Platelet size in stroke patients. Stroke 1995;26(6):995–9. [DOI] [PubMed]
- 17.Odell TT, Murphy JR, Jackson CW. Stimulation of megakaryocytopoiesis by acute thrombocytopenia in rats. Blood 1976;48(5):765–75. [PubMed]
- 18.Karpatkin S. Heterogeneity of human platelets. VI. Correlation of platelet function with platelet volume. Blood 1978;51 (2):307–16. [PubMed]
- 19.VanWijk MJ, VanBavel E, Sturk A, Nieuwland R. Microparticles in cardiovascular diseases. Cardiovasc Res 2003;59(2): 277–87. [DOI] [PubMed]
- 20.Jy W, Horstman LL, Arce M, Ahn YS. Clinical significance of platelet microparticles in autoimmune thrombocytopenias. J Lab Clin Med 1992;119(4):334–45. [PubMed]
- 21.Ozner MD, Ahn YS, Horstman LL, Jy W, Kolodny L, Myerberg RJ. Chronic platelet activation and acute coronary syndromes in 13 middle-aged patients. Clin Appl Thromb Hemost 1997;3(1):46–53.
- 22.Fuchi T, Kondo T, Sase K, Takahashi M. Primary percutaneous transluminal coronary angioplasty performed for acute myocardial infarction in a patient with idiopathic thrombocytopenic purpura. Jpn Circ J 1999;63(2):133–6. [DOI] [PubMed]
- 23.Crouch ED, Watson LE. Intravenous immunoglobulin-related acute coronary syndrome and coronary angiography in idiopathic thrombocytopenic purpura–a case report and literature review. Angiology 2002;53(1):113–7. [DOI] [PubMed]
- 24.Mathew TC, Vasudevan R, Leb L, Pezzella SM, Pezzella AT. Coronary artery bypass grafting in immune thrombocytopenic purpura. Ann Thorac Surg 1997;64(4):1059–62. [DOI] [PubMed]
- 25.Kohl BA. Con: Should aspirin be continued after cardiac surgery in the setting of thrombocytopenia? J Cardiothorac Vasc Anesth 2006;20(1):114–6. [DOI] [PubMed]
- 26.Faraday N. Pro: Should aspirin be continued after cardiac surgery in the setting of thrombocytopenia? J Cardiothorac Vasc Anesth 2006;20(1):112–3. [DOI] [PMC free article] [PubMed]
- 27.Ferraris VA, Ferraris SP, Moliterno DJ, Camp P, Walenga JM, Messmore HL, et al. The Society of Thoracic Surgeons practice guideline series: aspirin and other antiplatelet agents during operative coronary revascularization (executive summary). Ann Thorac Surg 2005;79(4):1454–61. [DOI] [PubMed]
- 28.Vanderschueren S, De Weerdt A, Malbrain M, Vankersschaever D, Frans E, Wilmer A, Bobbaers H. Thrombocytopenia and prognosis in intensive care. Crit Care Med 2000;28 (6):1871–6. [DOI] [PubMed]
- 29.Darcy MD, Kanterman RY, Kleinhoffer MA, Vesely TM, Picus D, Hicks ME, Pilgram TK. Evaluation of coagulation tests as predictors of angiographic bleeding complications. Radiology 1996;198(3):741–4. [DOI] [PubMed]
- 30.Goldberg GL, Gibbon DG, Smith HO, DeVictoria C, Runowicz CD, Burns ER. Clinical impact of chemotherapy-induced thrombocytopenia in patients with gynecologic cancer. J Clin Oncol 1994;12(11):2317–20. [DOI] [PubMed]
- 31.Kantarjian H, Giles F, List A, Lyons R, Sekeres MA, Pierce S, et al. The incidence and impact of thrombocytopenia in myelodysplastic syndromes. Cancer 2007;109(9):1705–14. [DOI] [PubMed]
- 32.Conti CR. Thrombolytic therapy in thrombocytopenic patients. Clin Cardiol 1995;18(6):299–300. [DOI] [PubMed]