Abstract
Generalized cognitive impairments are stable deficits linked to schizophrenia and key factors associated with functional disability in the disorder. Preclinical data suggest that second-generation antipsychotics could potentially reduce cognitive impairments; however, recent large clinical trials indicate only modest cognitive benefits relative to first-generation antipsychotics. This might reflect a limited drug effect in humans, a differential drug effect due to brain alterations associated with schizophrenia, or limited sensitivity of the neuropsychological tests for evaluating cognitive outcomes. New adjunctive procognitive drugs may be needed to achieve robust cognitive and functional improvement. Drug discovery may benefit from greater utilization of translational neurocognitive biomarkers to bridge preclinical and clinical proof-of-concept studies, to optimize assay sensitivity, enhance cost efficiency, and speed progress in drug development.
Keywords: antipsychotic medication, biomarker, cognition, neuropsychology, schizophrenia
Cognition in schizophrenia
Over the past few decades schizophrenia has been characterized with increasing precision along three independent dimensions, including positive symptoms, negative symptoms and cognitive impairment. Using the tools of clinical neuropsychology, many studies have documented moderate-to-marked impairments across a wide range of cognitive abilities [1,2]. Indeed, diffuse and persistent cognitive impairments, on the order of 1–2 standard deviations below psychiatrically healthy groups [3], are now recognized as fundamental and highly prevalent deficits in schizophrenia. Not only are cognitive impairments present during the prodrome phase [4] and during the first episode of psychosis, they also endure with minimal change in the early years after illness onset [5,6] and have been linked to long-term functional disability in schizophrenia [7,8].
The financial cost of schizophrenia extends beyond drug and psychosocial treatments to include lost income, public aid, and other government expenditures. Estimates of the lifetime cost of the illness range from US$1.12 to 2.31 million per case in North America [9], and global expenditures for antipsychotic medications are over US$15 billion per year [10]. Cognitive deficits are the best established predictors of functional disability in the disorder. Thus, ameliorating cognitive deficits has the potential to meaningfully improve quality of life and occupational function, and reduce illness burden and societal costs. For this reason there is significant interest in the development of pharmacological and psychosocial interventions to enhance cognitive function in this population. To this end, a major initiative in the USA involved the US FDA, National Institute of Mental Health (NIMH), and industry partners, working together to develop a framework of criteria for approving drugs with a treatment indication for enhancing cognition in schizophrenia. This resulted in a mandate for demonstrating not only improved performance on cognitive tests, but also a significant improvement in functional outcomes in clinical trials.
The aim of this paper is to describe the modest cognition-enhancing outcomes of first-generation antipsychotic (FGA) and second-generation antipsychotic (SGA) drugs, and to review the strategies that address gaps in our current knowledge and can better guide the development of procognitive drugs for this population.
First-generation antipsychotics
First-generation antipsychotic medications, also known as ‘typical’ antipsychotics, were first brought to wide clinical use in the 1950s. The ability of these drugs to reduce positive symptoms (e.g., delusions, hallucinations and disorganization) and risk for relapse markedly changed clinical outcomes for schizophrenia patients [11]. All FGAs possess prominent dopamine D2 receptor antagonism effects. In addition to producing adverse motor system effects, D2 blockade can have adverse effects on higher level cognitive skills. Such adverse effects on working memory are well established in animal models [12–14]. Similar effects have been reported in both psychiatrically healthy human subjects [15] and in schizophrenia patients for working memory, processing speed, motor skills, and other higher order cognitive abilities [16–19]. Some members of this class, called low-potency agents, such as chlorpromazine, also possess high affinities for histamine H1 receptors, muscarinic M1 receptors, and α adrenergic NEα1 receptors. This general pharmacodynamic profile in low-potency agents can result in cognitive deficits, sedation, and anticholinergic effects. While a number of factors come into play when evaluating the cognitive effects of FGAs in clinical studies, the general consensus is that FGAs do not improve cognition and can have some specific adverse cognitive effects [20–23].
Second-generation antipsychotics
Second-generation antipsychotic medications, sometimes referred to as ‘atypical’ antipsychotics, have a lower risk for extrapyramidal side effects and tardive dyskinesia than their first-generation counterparts. This class of drugs includes aripiprazole, clozapine, iloperidone, olanzapine, paliperidone, quetiapine, risperidone and ziprasidone. Although the exact definition of ‘atypical’ remains an area of ongoing debate, a common characteristic of these drugs is prominent serotonin 5HT2A antagonism combined with D2 antagonism. In addition to commonalities in 5HT2A and D2 receptor blockade, each drug in this class has a unique receptor-binding profile for histamine, muscarinic, α and serotonin receptors, which can result in partially distinctive and overlapping side effect profiles.
Early clinical studies with SGAs suggested that they had procognitive benefits. An early report noted that clozapine reduced negative symptoms and improved ratings of cognitive deficit [24]. In addition to comparable, if not superior, control of positive symptoms with fewer extrapyramidal symptoms and less risk for tardive dyskinesia, SGAs were seen as holding the promise of meaningful benefits for schizophrenia patients in terms of quality of life and daily function via cognitive improvements.
Cognitive effects of second-generation antipsychotics
Animal studies
Extensive animal research has been conducted with clozapine and other SGAs to advance our understanding of the different mechanisms and the effects of these drugs relative to FGAs. Several animal models of cognitive dysfunction in schizophrenia have been used for evaluating the cognitive effects of antipsychotic medications, including: administration of phencyclidine, an N-methyl-d-aspartic acid glutamate receptor antagonist; administration of amphetamine or methamphetamine, which primarily effects dopamine systems, but also impacts serotonin and norepinephrine systems; neurochemically induced hippocampal cell death; and isolation rearing [25–36].
Second-generation antipsychotics have often outperformed their first-generation counterparts in reversing or attenuating phencyclidine- or methamphetamine-induced deficits in reversal learning, conditional learning, attention, spatial memory, spatial learning, and long-term potentiation in rodents and nonhuman primates [37–42]. In rodents, neuropathological changes in the hippocampus, induced by stress or neurotoxins, are associated with impairments in spatial learning and spatial memory, and these too are attenuated by SGAs [43–46]. For clozapine these beneficial effects have been attributed to an augmentation of synaptic plasticity in the hippocampal–prefrontal pathways via effects on D1 receptors [47], normalized dopamine turnover in the dorsolateral prefrontal cortex [48], and modulation of muscarinic and glutamatergic neurotransmission [49].
Early clinical studies
Early clinical studies generated enthusiasm for the potential cognitive benefits of SGAs relative to FGAs [50–52]. Several studies reported improved cognitive function at retest following a medication switch from FGAs to SGAs. Early reviews concurred that SGAs appeared to be better for neurocognition than first-generation medications [53–55]. A meta-analysis of the initial wave of open-label (n = 12) and double-blind (n = 3) studies indicated robust cognitive improvement associated with SGA treatments [56]. More recently, a meta-analysis of 27 open-label and 14 double-blind investigations (many of which involved a switch from prior treatments) comparing FGAs and SGAs, reported the overall effect size of the difference between SGAs and FGAs at the study end was relatively small (0.00–0.24). Within-group analysis of SGAs indicated somewhat larger effect sizes (0.17 to 0.46) for pre- and post-cognitive treatment effects (within-group effect sizes were not examined for FGAs) [57]. However, it is difficult to draw firm conclusions from ‘switch’ studies, in which participants who have been maintained on FGAs are tested before and after switching to a SGA, because a nonrepresentative sample of patients less likely to be responsive to FGAs might be included. Second, as discussed in detail below, practice effects for many cognitive measures may artificially raise performance gains at retest and thus artificially elevate the perceived medication benefit suggested by pre- to post-test differences.
Critical methodological issues
Relative dosing of investigational & comparator drugs & dosing of antipsychotics & anticholinergic medications before change in medication status
Some switch designs favor SGA outcomes as a function of taking patients from a high dose of a FGA to a low dose SGA, and an over-representation in study samples of patients who were previously nonresponsive to FGA treatment. In the case of higher dose FGA treatment, the apparent advantages of SGAs observed in both switch studies and better designed trials have been hypothesized to be a byproduct of the the adverse cognitive effects of the high-dose FGA [58]. Higher dosing of the FGA may lead to a higher incidence of impaired cognitive and motor performance as well as increased use of anticholinergics that, in turn, may have adverse cognitive effects, particularly on memory. Thus, the impact of prior drug exposure on outcome measures may confound early studies employing a switch design. Too often, research reports carefully described the antipsychotic medication regimens but did not fully report the other medications patients were receiving. In many studies, a nontrivial number of patients were prescribed adjunctive anticholinergic medications to address extrapyramidal system side effects, and the adverse effects of anticholinergics on cognitive performance are well established [21,59,60]. Nonequivalent D2 blockade from differential dosing of FGA and SGA drugs is another potential confounder. One approach to reducing confounds associated with the cumulative effects of disease progression, prior antipsychotic exposure, and adjunctive (anticholinergic) medication has been to study antipsychotic-naive patients or early-phase patients who undergo a medication washout period prior to randomization. One pivotal investigation that prompted greater interest in the cognitive effects of SGAs reported a significantly greater benefit of olanzapine relative to both haloperidol and risperidone for immediate recall memory after 6 weeks of treatment [61]. Olanzapine also showed a modest advantage after a 12-week trial as both olanzapine and low-dose haloperidol were associated with significant overall increases in cognitive test scores relative to baseline, with some improvements statistically greater with olanzapine [62]. The magnitude of the initial advantage for olanzapine (ES = 0.36) over haloperidol (ES = 0.20) on overall cognitive benefit was approximately one sixth of a standard deviation. However, when longer periods of treatment were evaluated there was no longer a cognitive advantage for olanzapine as all treatment groups displayed significant, but similar, test score increases after 1 and 2 years of treatment [63]. A modest advantage for the second-generation agent risperidone over haloperidol was observed for overall cognitive performance after 3 months of treatment for first-episode schizophrenia [64]. Overall findings in first-episode and early-phase psychosis samples were generally consistent with studies of chronic schizophrenia patients who were studied over a longer period of time, reporting no differential response to the type of antipsychotic medication after 1 year of treatment with olanzapine, risperidone or haloperidol.
Randomized, double-blind, controlled studies, rather than open-label or switch studies
Open-label and switch studies are susceptible not only to recruitment biases, but also to biases that can impact not only clinical ratings but also the administration and scoring of neuropsychological tests. The best design for clinical trials involves random assignment to multiple study arms in which treatment is double blinded. Beginning around the year 2000, following the first wave of open-label and switch studies examining the cognitive effects of SGA drugs, a number of randomized, double-blind controlled studies investigated the cognitive effects of several SGAs relative to FGAs. In a meta-analysis of 14 prospective double-blind studies with random assignment to either FGA or SGA treatment, SGAs were found to improve overall cognition, with effect sizes ranging from 0.20 to 0.40 [57]. Within the class there was no separation among the four treatments reviewed (clozapine, olanzapine, quetiapine and risperidone) in overall performance. Greater scores at follow-up, relative to baseline, were noted in just two cognitive domains (i.e., learning and processing speed). In another meta-analysis focusing on memory measures, SGAs improved performance relative to first-generation treatments, with an effect size for differential improvement of less than 0.20 [65]. Thus, the magnitude of the putative cognitive advantages of SGAs have regressed from ‘robust’ and medium effect sizes in early open-label and switch studies, to modest and at times negligible effect sizes in double-blind controlled trials. In addition to the observation that effect sizes for SGA superiority have diminished with better controlled studies, the performance improvements at retest (relative to baseline) do not differ significantly from practice effects observed in psychiatrically healthy people retested over similar time frames without drug treatment in both clinical trials [66] and naturalistic studies [5,67,68].
Practice effects
Industry sponsored clinical trials investigating the cognitive effects of antipsychotic drugs have rarely addressed the potential confound of practice effects. Without a matched healthy control group that is retested over a similar time period, or a group of demographically similar patients who are retested with no change in treatment (preferably when their anticipation of treatment change is similar to that of other patients in the trial), it is difficult to determine whether test score improvements at retest result from true enhancement of cognitive abilities or from learning to perform tests more efficiently due to familiarity with the testing procedures. Concern for this issue was brought to the forefront by a recent study that reported improvements associated with SGAs in first-episode schizophrenia patients were no greater on most measures than practice effects in psychiatrically healthy controls [66]. Whereas performance gains exceeding practice effects in controls have been reported in schizophrenia groups for select measures (Visual Reproduction and Trail Making Test [66], Finger Tapping, Trail Making Test – part B and Rey Complex Figure [69]), the overall pattern of treatment-related performance gains falling within or below the range of practice effects in healthy groups is consistent with prior reports from NIMH-funded first-episode studies that followed matched healthy control groups in parallel [5,6]. Likewise, a meta-analysis of the cognitive changes associated with haloperidol treatment indicated that, except for very large doses (≥25 mg), improvements were similar to practice effects in normative samples [70]. To further complicate matters, the expected range of practice effects in schizophrenia relative to psychiatrically healthy samples is unclear and may be task- or time-to-retest-dependent. An argument can be made that practice effects in psychiatrically healthy groups may exceed those in clinical samples owing to illness-related learning deficits. Moreover, it is unclear whether equivalent practice effects should be expected of first-episode and early-phase patients relative to older, chronic patients with long-standing schizophrenia diagnoses, as well as aging patients. Clearly, more work dealing with practice effects as a cause of improved performance at retest is needed to lay the groundwork for interpreting performance gains in trials evaluating SGAs or adjunctives targeting cognition.
Additional methodological considerations
There are a number of methodological considerations that warrant discussion beyond the scope of this review, but select considerations will be covered here briefly. Treatment status prior to initiation of a new medication is an important consideration when evaluating medication effects on cognition. For example, prior treatment with a FGA increases the likelihood of extra-pyramidal symptoms that may require adjunctive anticholinergic treatment, which is known to have negative effects on cognition. Additionally, patients who have recently undergone a change in medication status exhibit more side effects and symptom variability, which may negatively impact cognitive performance. Thus, baseline assessments should typically be performed following a stable medication regimen on the order of 4–6 weeks.
It is unclear whether enhancement of specific neurocognitive systems by drugs targeting specific receptors will have a generalized or specific effect. While preclinical studies strongly suggest that specific behavioral effects would result from specific drug treatments, the literature in schizophrenia does not provide a clear answer to this question, and a composite index of generalized deficits across tasks is a commonly used treatment outcome. To be sensitive to specific effects, a multifactorial assessment of cognition with tests specifically evaluating particular cognitive abilities would be essential for drug evaluation. Yet, there is longstanding controversy regarding whether neuropsychological tests are sensitive to specific domains or generalized abilities in schizophrenia [71,72]. Only recently has this issue been carefully investigated and there is now strong evidence that the neuropsychological approach is primarily sensitive to a generalized cognitive factor and not multiple cognitive domains [73,74]. Furthermore, as assessed by neuropsychological tests, cognitive effects of SGAs also appear to be generalized [74]. Thus, clustering neuropsychological test scores to assess distinct cognitive functions in schizophrenia trials is not supported by ‘construct validity’ studies. Although meaningful estimates of generalized cognitive abilities can be achieved with shorter batteries (which offers several advantages including fewer missing data points, reduced costs associated with administration time, and increased tolerability), lengthier multifactorial neuropsychological batteries may have limited sensitivity to pharmacological effects on specific functional circuits and the cognitive domains they support.
Trial duration is an important issue given that positive and negative symptom improvement may extend several months after initiation of antipsychotic treatment. It is possible that cognitive effects may also be evident over the course of several months, and the time course of these effects for different drugs is largely unknown. Moreover, both higher order cognitive processes and many neuropsychological measures are dependent on adequate skills across several areas and it may take longer for broad effects to take place. For example, until attention-related impairments are reduced, it may be difficult to see improvements in memory and problem-solving skills. In a naturalistic study examining cognitive change over a 1-year treatment period, improved response inhibition was nonsignificant at 6 weeks, but significant improvements were observed after 26 and 52 weeks of treatment [75]. Thus, when assessing potential cognitive treatment effects, long-term studies may be preferable, if not necessary, despite the escalating costs and susceptibility to attrition.
The Clinical Antipsychotic Trials of Intervention Effectiveness project
The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project was designed to study antipsychotic treatment effectiveness in the community. It included studies of cognitive outcomes, and addressed potential sources of bias such as:
Adverse effects of high-dose FGA comparator drugs
Concomitant anticholinergic treatment
Practice effects
Industry sponsorship of studies in which sponsor’s drugs have had a tendency to outperform comparator drugs (albeit in modest ways)
This landmark study directly compared the cognitive effects of several SGAs to a representative FGA in a large ‘real-world’ sample. Comparison of olanzapine, quetiapine, risperidone, ziprasidone and perphenazine indicated small but significant improvements in cognitive test scores from baseline but no significant performance differences between treatment groups [76]. Moreover, the cognitive performance gains in each treatment group were small and similar to practice effects observed in NIMH-funded longitudinal studies (in which healthy controls are typically tested in parallel) [5,6,66–68].
Findings from the CATIE project were consistent with the broader pattern throughout the literature in which improvements to the scientific rigor of clinical trials coincided with a reduction in apparent beneficial cognitive effects of SGAs. That is, against a background of diffuse cognitive impairments in schizophrenia, on the order of 1–2 standard deviations below psychiatrically healthy groups (depending on the chronicity and illness severity in the schizophrenia samples), the magnitude of observed cognitive improvements associated with SGA treatment are relatively small and similar to practice effects in healthy controls. More recent higher quality open-label studies have also shown a negligible cognitive advantage of SGAs relative to low-dose haloperidol [77]. Most importantly, the minimal cognitive gains associated with SGAs have not been sufficient to reduce disability in a clinically meaningful way, although there is some suggestion that even modest improvement may be beneficial to some patients in reducing functional disability [78].
Current methodological issues
Proof-of-principle not established for neuropsychological measures
The advantages of the neuropsychological approach for evaluating cognitive outcomes, especially in large multisite clinical trials, include ease of use, portability, established reliability and normative data, wide availability of psychologists experienced with such testing and interpreting test findings, low cost, and modest equipment requirements. The NIMH-sponsored Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) program, in collaboration/consultation with the FDA and the pharmaceutical industry, worked to develop a consensus neuropsychological battery for evaluating cognitive change in clinical trials. This battery is longer but somewhat more comprehensive than the Brief Assessment of Cognition in Schizophrenia (BACS) battery developed by Richard Keefe and has been used in many clinical trials [79]. However, proof-of-principle for the MATRICS program and other neuropsychological batteries has yet to be established in clinical trials.
Neuropsychological tests are often multidimensional and require integrated function of numerous cognitive and related brain systems. Thus, the neuropsychological tests themselves may lack the specificity to link specific behavioral outcomes with the discrete neural system changes induced by drugs targeting specific neurotransmitter systems. Given the class effect of antipsychotic treatments on dopaminergic systems, one would expect specific effects on working memory, attention, and other domains where performance is largely mediated by dopamine modulation of the frontostriatal brain systems, rather than in generalized cognitive ability. However, the latent variable structure of many neuropsychological test batteries is characterized by a single generalized ability factor rather than discrete and independent cognitive abilities [73,80]. This factor structure is present both before antipsychotic treatment and in the changes that occur after SGA treatment [74]. Thus, standard neuropsychological measures widely used as primary cognitive outcomes in trials may be too global and integrative to detect specific cognitive changes resulting from particular drug effects on specific neurotransmitter systems. Additionally, the utility of neuropsychological methods for detecting drug effects are constrained by the psychometric properties of many tests. For example, many neuropsychological tests were designed to discriminate individual differences in ability around the mean of healthy subject performance, and this may limit sensitivity to change at lower levels of function (floor effects). This issue may be particularly relevant to studies of cognitively impaired populations. Most importantly, the neuropsychological testing strategy has not been validated with regard to sensitivity to drug effects in psychiatric research. In fact, several well-controlled industry sponsored studies of novel cognitive-enhancing drugs in schizophrenia have yet to demonstrate robust effects using the MATRICS battery with a variety of compounds [81]. As opposed to detecting cognitive change, neuropsychological tests were designed to measure stable traits rather than variability over time. In this context, the conflicting findings of an absence of robust cognitive benefits from second-generation drugs in clinical studies versus positive effects in animal models raises concern that neuropsychological testing may be a weak assay for detecting cognitive outcomes with SGAs and novel adjunctive treatments.
Functional outcomes
For treatment of cognitive disability in a disorder such as schizophrenia, the ultimate objective is more than just improved performance on neuropsychological tests. Instead, the degree of cognitive benefit must have a positive impact on day-to-day functional ability. In line with this, the FDA has indicated that improved functional outcome, not only improved cognitive test data, is needed to justify a treatment indication in this area. Despite some mixed findings [82–89], the impact of SGAs on functional outcome, much like their impact on cognitive performance, has been below the level hoped for, and less than what would be required to fundamentally improve the quality of life of a large majority of schizophrenia patients [90–92].
Improving community function may take time
In the event that changes in basic cognitive skills result from short-term drug therapy, one might expect lagged rather than parallel changes for both higher order integrative cognitive skills and community function. Whereas improvements in basic cognitive skills (e.g., reaction time, processing speed and attention) may be apparent early on, comparable improvements in higher order cognitive skills (e.g., problem solving and abstraction) may require recurring feedback and consolidation over time before functional improvements are possible. This may be particularly problematic for chronically disabled patients, who must learn or relearn atrophied functional skills by re-engaging social and occupational environments so that newly enhanced cognitive skills can begin to support the development of a fresh skill set. The attention deficit hyperactivity disorder (ADHD) field is an excellent example of a cognition enhancing drug resulting in relatively rapid improvements in basic cognition, but where improved higher order functioning (i.e., school performance) takes much longer and requires additional interventions. Thus, the notion that basic cognitive improvements, potentially apparent in the course of a typical 8- or 12-week clinical trial, will be paralleled in time by a significant change in community outcome may be overly optimistic and simplistic.
The potential for functional improvement may be greater if rehabilitation training is provided after cognitive skills have improved. Indeed, the cognitive rehabilitation literature has shown that under certain circumstances, schizophrenia patients can reliably demonstrate improved cognitive performance independent of pharmacological interventions. However, these newly learned skills often do not generalize beyond the laboratory [93]. When functional abilities have been disabled for years, the application of enhanced cognitive abilities to real-world situations may require a higher level of support services and psychosocial scaffolding to facilitate use of an enhanced cognitive skill set. Nonetheless, even successful cognitive enhancement from drug treatments may require rehabilitative interventions to yield optimal real-world social and occupational gains. To date, however, the potential synergistic effects of drug therapies and cognitive rehabilitation, if there are any, are unknown.
Functional capacity versus functional outcome
Bearing in mind the longer term treatment goal of not only enhancing cognitive test scores, but also improving day-to-day function, two strategies for evaluating functional changes have emerged. Interview-based measures of cognition are one option that offers the advantage of ‘real-world’ changes as reported by either the patient or informants with whom patients have regular contact in the community. However, these measures rely on subjective reports that can be susceptible to bias. An alternative option is provided by proxy measures of functional skills during simulated activities. Improved performance on proxy measures signify that patients have the ability to perform tasks in the community, but this does not necessarily mean these skills will be utilized in daily life. More work is needed to better clarify the link between proxy measures of functional ability and real-world community function. More importantly, understanding the relative utility of proxy measures for evaluating meaningful changes in community function is critical to obtaining a treatment indication from the FDA. The field has yet to determine which functional outcome measures are the most useful for evaluating pharmacological treatments, but work in several laboratories evaluating different approaches is making progress in this area [94].
Directions for future research
Adjunctive treatments
With a growing recognition of the limited cognitive benefits apparently associated with SGAs, novel interventions targeting the chronic and debilitating cognitive impairments associated with schizophrenia are the focus of drug development efforts in several pharmaceutical companies. Given the limited procognitive effects of available antipsychotic medications, many plans to develop new pharmacological agents to enhance cognition in schizophrenia are taking the form of adjunctive agents to be added to current antipsychotic treatments.
Preclinical research
To enhance speed and efficiency in the drug-development pathway, one broad need for future work in this area is to enhance the translational bridge from preclinical to Phase II proof-of-concept studies to make earlier and more informed ‘go’ or ‘no-go’ decisions about the future development of a drug. This requires a closer parallel between preclinical outcome measures and those used in human studies – approaches that are widely divergent at the present time. In this context, it is worth considering some inherent challenges in this translational enterprise. First, translation of preclinical models of cognitive processes to the clinic depends on the degree to which the preclinical models sufficiently parallel the neurobiology of schizophrenia and the relevance of behavioral paradigms to the cognitive deficits seen clinically in the disorder. While the challenge for preclinical investigators is to establish clinical significance of cognitive outcomes, the challenge for clinical translational investigators is to establish that outcomes evaluated in humans closely track preclinical outcomes and are modulated by similar mechanisms. Second, it is difficult to determine whether a statistically significant reversal of deficits in animal studies can be expected to be robust in the face of diffuse neuropsychological impairments and the still poorly understood neurobiology of schizophrenia [3]. Improved preclinical models are important in this regard, as are efforts to determine how disease-related alterations affect responsivity to drugs that effectively modulate cognitive function in healthy subjects.
Developments in translational neuroscience are needed to bridge preclinical and proof-of-concept studies so that they can use similar outcome measures to the best extent possible. There are many possible approaches to be pursued in this regard using neurophysiology, neuroimaging and novel behavioral tests. A critical step forward needs to be made in developing these outcomes so that they have high sensitivity and specificity to discrete neurochemical systems and the specific cognitive processes they subserve, rather than trying to improve ‘global cognition’ as a primary study outcome. The level of behavioral specificity of paradigms typically used in preclinical research needs to be brought to early-phase clinical trials.
An example of such an approach is provided by recent work following postmortem findings of abnormalities in specific classes of prefrontal GABAergic interneurons. Synchronization of subsets of dorsolateral prefrontal pyramidal neurons is essential for working memory and depends on the chandelier subclass of parvalbumin-positive GABA neurons, which synapse exclusively on the axon initial segments of pyramidal neurons [95,96]. Continuous excitatory drive of GABA neurons is necessary, in computational models, for synchronized pyramidal cell firing [97]. In schizophrenia, a reduced density of chandelier neurons [98] may be related to decreased synchronous activity during delay periods of working memory tasks [99]. By enhancing continuous GABA neuron drive, synchronous dorsolateral prefrontal activity might be sufficiently sustained to insulate the task-relevant neural signals from competing inputs, and enhance working memory capacity. Of note, EEG and translational paradigms selected to measure this effect in a small clinical trial were more sensitive to drug effects than standard neuropsychological testing [100].
Translational research
With more direct links to neural systems, it is possible that translational behavioral paradigms and neurophysiological measures may be more sensitive to antipsychotic and adjunctive treatments than neuropsychological testing [101,102]. Several cognitive biomarkers have promise in this regard, including oculomotor neurophysiology assessments, electroencephalography (EEG) measures of cortical activity, and functional MRI (fMRI). Utilizing these types of biomarkers may be especially important for proof-of-concept studies because of their ability to directly link preclinical findings to human clinical data, and to use the preclinical data to make highly specific predictions about behavioral drug effects in proof-of-concept trials. Indeed, against a background of modest generalized neuropsychological change, our laboratory showed considerably larger effect-size changes on neurophysiological measures, relative to effect-size changes on neuropsychological tests, after treatment with risperidone in first-episode schizophrenia patients [101].
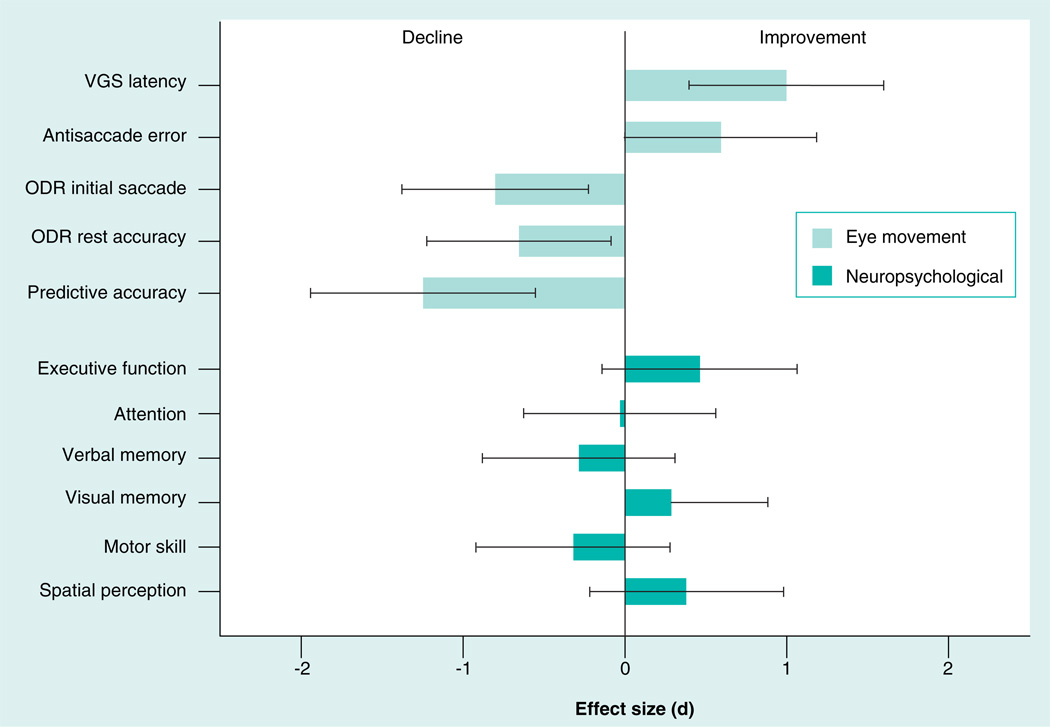
Paradigms from the field of oculomotor neurophysiology have been used to study neural systems in ‘behaving’ nonhuman primates, focal brain lesion studies in neurology, and functional brain imaging studies of healthy individuals. As opposed to the limited neuropsychological changes in patients treated with SGAs, eye movement studies revealed a more robust, but also mixed, pattern of beneficial and adverse effects of risperidone for initially drug-naive patients (Figure 1), including slowed reaction time of saccades from an initially faster level, minimal effects on inhibitory behavioral control during antisaccade tasks after 6 weeks of antipsychotic treatment (but gradual improvement when treatment was continued to 52 weeks [75]), and reduced ability to hold spatial location information over time in working memory [103]. Of note, while the effect size of deficits in neurophysiological and neuropsychological testing were similar in this sample prior to antipsychotic treatment, the effect size of the change after treatment was two- to three-fold greater for the translational neurophysiological measures (Figure 1).
Figure 1. Oculomotor neurophysiological measurements may be more sensitive to the beneficial and adverse neurocognitive changes after acute antipsychotic treatment of first-episode schizophrenia patients when compared with neuropsychological tests.
ODR: Oculomotor delayed response; VGS: Visually guided saccades.
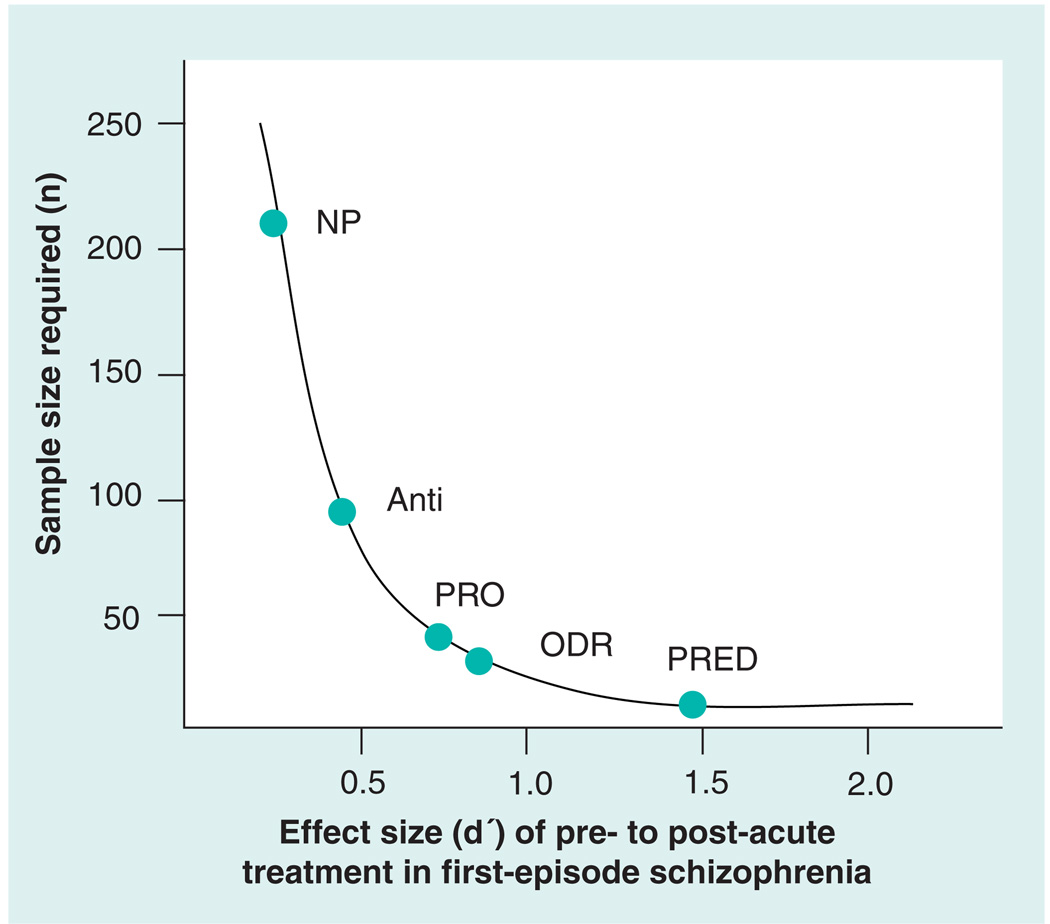
While neurophysiological measures may be more sensitive to SGA effects, it is not clear whether they will have a similar advantage in evaluating new drug classes. Furthermore, it is not clear that translational measures will predict community function as well as clinical neuropsychological tests, although some early data are promising [104]. Still, the issue of sensitivity to the drug effect is especially important for early proof-of-concept studies, not only because of the need to constrain the risk for Type II error, but also for facilitating better decisions about the selection of promising new drugs for further development based on a rational strategy that moves systematically forward from preclinical findings. A benefit of the greater sensitivity of neurophysiological measures to pharmacological treatments is increased power for studies (Figure 2), which translates into reduced costs of early clinical trials and therefore an expanded ability to explore the efficacy of more promising compounds.
Figure 2. Based on larger effect sizes, similar power to detect treatment effects can be achieved with fewer subjects when utilizing neurophysiological biomarker outcomes relative to neuropsychological outcomes.
Thus, smaller and less costly proof-of-concept studies with selected drug-relevant neurophysiological measures can be used to evaluate drug outcomes in clinical trials. At a conservative cost of US$20,000–25,000 per subject, the low end of potential savings when using biomarkers in a proof-of-concept study would be US$2–3 million. More importantly, this does not include the value of identifying ill-fated agents during proof-of-concept studies and avoiding costly clinical trial failures.
Anti: Antisaccade [75]; NP: Neuropsychological battery [113]; ODR: Oculomotor delayed response [19]; PRED: Predictive saccade test [116]; PRO: Prosaccade [114,115].
Similar to oculomotor paradigms, EEG measures and studies of prepulse inhibition effects may be more sensitive to specific drug effects than measures of attention from clinical neuropsychology. In the P50 paradigm, two auditory signals are presented approximately 500 msec apart. When paired consistently, the first signal attenuates the response to the second signal (producing a P50 or positive EEG wave 50 msec after the tone presentation). Relative to low rates of P50 suppression observed in patients treated with FGAs, some studies suggest that those treated with SGAs show some normalization in sensory suppression in this paradigm [102]. Functional neuroimaging may also be sensitive to both beneficial and adverse effects of SGAs. In a recent fMRI study using a saccadic eye movement task, first episode schizophrenia patients showed decreased pretreatment activation in frontal and parietal areas related to visual attention, which improved after risperidone treatment. However, at the same time, and despite no signs of pretreatment abnormalities, there was reduced activity at post-treatment in the dorsal prefrontal cortex, dorsal striatum, and dorsomedial thalamus [105]. These findings highlight the potential sensitivity of neuroimaging biomarkers to both beneficial and adverse effects of a SGA on functional brain systems. Such approaches may not only be especially useful for Phase II proof-of-concept studies, but eventually also for larger registration trials in parallel with neuropsychological testing (Figure 2).
In contrast to neuropsychological tests, neurophysiological and functional neuroimaging biomarker approaches can directly evaluate how regional brain function is altered after drug treatment, and how that parallels changes in animal models. Furthermore, clinical biomarkers with a foundation in animal models can more precisely link discrete drug-induced changes in the neural systems that regulate cognitive processes of interest, and thus test far more specific predictions about how drugs of interest will impact specific brain and cognitive functions, and doing so in a systematic translational framework. This raises the question about what is the right neurobehavioral target for evaluating the cognitive effects of pharmacological treatments at various points along the drug development pathway? In early proof-of-concept Phase II studies, the use of translational biomarkers might be emphasized given their potentially greater power for detecting effects and to provide a stronger bridge to preclinical findings that are driving drug development, while in registration trials a reliance on a combination of translational measures (selected based on early clinical trials), standard neuropsychological tests, and measures of functional ability may be most appropriate. A close working relationship between industry and academia in developing useful translational measures will be important to the guide selection of outcome measures for proof-of-concept studies.
A very conservative view of targets for Phase II studies maintained by many clinical investigators is that there needs to be a sign of generalized cognitive improvement and enhanced functional abilities to justify proceeding to Phase III trials. However, given the complexity and limited understanding of the neurobiology of schizophrenia, the lack of established sensitivity of neuropsychological testing to drug effects without very large samples, the lack of an established rationale for generating generalized cognitive improvement from alteration in specific neurotransmitter systems, and the limited understanding of the time course of inducing changes in community function relative to the onset of drug-induced cognition enhancement, hoping for such positive and reliable outcomes from small Phase II studies may be overly optimistic and too unlikely to succeed. For these studies, it may be necessary to put aside these broad and ambitious hopes in favor of a more incremental strategy in which iterative translational animal and Phase II studies are conducted to establish parallel or discrepant cognitive outcomes using comparable measures in the animal laboratory and in the clinic. Indeed, recent advances in behavioral neuroscience have the advantage of being translatable into human studies using receptor imaging, fMRI, MR spectroscopy, EEG, magnetoencephalogram, and other indicators of brain function. Many neurophysiological biomarkers can be studied using either homologous or analogous processes in animal models [106,107]. Such data can provide important inputs to optimize decisions about continuing or discontinuing the development of a certain drug. As a practical matter, by virtue of closer links to preclinical brain physiology and animal model work that supported progress along the drug development pathway, biomarker approaches linked to preclinical methods can increase our confidence in the sensitivity and trustworthiness of outcome measures and their neurobiological correlates as well as have behavioral significance in relation to known mechanisms of a drug’s effect.
Translational methods are required to bridge preclinical and Phase II proof-of-concept studies to facilitate drug development for cognitive enhancement in schizophrenia. Several steps are needed to achieve this goal, including (but not limited to):
Building on animal models that have characterized the neural systems supporting cognitive processes and behavioral performance on specific tasks. Utilizing translational methods (e.g., eye movement, prepulse inhibition, mismatch negativity and other EEG paradigms and functional neuroimaging) in proof-of-concept studies to confirm specific predicted drug effects on cognition and brain function early in the drug development pathway;
When several possible drugs in a class emerge from preclinical studies, neurophysiological and neuroimaging biomarkers can be utilized more actively in Phase II studies to profile human dose–response effects and thus guide more informed decisions regarding the selection of drugs for use in larger clinical studies;
One concern about moving from preclinical to clinical studies is the degree to which animal models accurately model human cognitive processes and the illness-related abnormalities seen in schizophrenia patients. Studies are needed to validate animal models in relation to the biological features and cognitive processes in the human clinical situation, especially with respect to dose–response relationships and pharmacokinetic–pharmacodynamic relationships;
As noted previously, there is considerable contrast between the modest generalized cognitive improvement in schizophrenia after treatment with SGAs relative to animal model data showing that cognitive impairments are robustly reduced by SGAs. This could be due to a poor translation of animal models to the human clinical condition, or a failure of clinical neuropsychological tests to detect SGA advantages. Greater translational integration of preclinical and Phase II studies will make it possible to address such questions empirically.
Clinical research
The ultimate goal of improving cognitive function is reduced disability and improved in quality of life and community function. Although neuropsychological measures are currently the best established predictors of functional outcome, available evidence suggests they may be less sensitive to drug effects than cognitive neurophysiology. Recent data suggest that neurophysiological measures may also be strong predictors of functional status [104]. By directly linking neurophysiological measures to functional outcomes and utilizing small sample studies with translational paradigms in the early stages of drug development, investigators may be better positioned to evaluate new drugs in a translational context with smaller patient samples. Thus, translational biomarkers may be especially useful for Phase II studies at this point, and their relation to longer term community function remains to be established.
While efficacy and effectiveness studies provide useful views of typical treatment outcomes, the clinical reality is that outcomes differ across patients. Tailoring psychosocial services to support different types of cognitive improvements may be one area where individualized care is needed. Individualizing treatments in relation to genetic variations, as is becoming more common for other therapeutic areas such as oncology, cardiovascular diseases and gastroesophageal reflux, among others, may prove to be another means of individualizing care for schizophrenia patients. Cognitive response to pharmacological treatments may be determined by a variety of factors including genetics, overall cognitive level, availability and nature of social supports, and chronicity of deficits, to name just a few possibilities. These considerations have only begun to be explored.
An important future therapeutic challenge in psychopharmacology is to identify genetic variations that predispose patients to both favorable and adverse responses, and subsequently to tailor individual treatment regimens. Today, pharmacogenetic studies of the cognitive and neurophysiological effects of antipsychotics are in their infancy. One key to advancing the treatment of schizophrenia is improved understanding of the variations in genes encoding factors related to specific transmitter receptor systems that are blocked or stimulated by a specific antipsychotic drug. Many genes associated with cognition in nontreatment studies (e.g., dopamine system: COMT, DRD1, DRD2, DRD4, DAT; Serotonin system: HTR2A and HTR1A; glutamate system: GRM3, GRIK1, GRIK4, GRIK5, AMPA, GAD, DAAO, NRGs, ERB4, G72; and others such as BDNF and CHRNA7) have the potential to enhance the prediction of the cognitive response to both current and new treatments.
Pharmacogenetic research for antipsychotics has evolved from studies focused on drug metabolism to more focused studies of pharmacodynamics and receptor regulation that affect specific aspects of the drug response. Research investigating the role of genetic variants regulating dopamine and glutamate disposition in the brain has set the stage for this by enhancing our understanding of the determinants of interindividual variations in cognitive processes [108,109]. However, early attempts to identify pharmacogenetic predictors of cognitive response to antipsychotic agents have been hampered by small samples, variable compliance, prior treatment exposure, treatment heterogeneity and the nonspecific nature of the neurocognitive assessments used.
By focusing on polymorphisms in genes that are associated with cognition in the general population, candidate-oriented pharmacogenetic approaches have excellent potential for facilitating bottom-up advances in drug development and platforms for individualized care. A prototypical genetic marker for these studies has been the catechol-o-methyltransferase (COMT) gene. The COMT enzyme accounts for 60% of dopamine metabolism in prefrontal cortex [110]. The Val158Met single nucleotide polymorphism causes thermal instability of this enzyme, with Met/Met homozygosity experiencing a three- to four-fold reduction in enzymatic activity. This reduced activity results in higher prefrontal concentrations of dopamine and enhanced performance on executive function tasks, brain activity, and potentially greater symptom response for some patients. The Val/Met alleles are codominant, resulting in three levels of COMT activity (Val/Val>Val/Met>Met/Met). The 158Met allele has been associated with a more positive cognitive response to clozapine and olanzapine [111,112].
While pharmacogenetics has excellent potential for understanding individual responses to medication, adequate study design and identification of phenotypes that are sufficiently sensitive to genotype effects are essential for significant advancements in this area. This is a challenging area, given the need for large samples requiring multisite studies, the difficulty in maintaining data equivalence across sites, the lack of data linking genotype with daily function, and the costs of genome-wide scans. However, exploiting the potential of genetic investigations with larger samples to predict drug response combined with the development of translational biomarkers for evaluating CNS effects of drugs offers the greatest potential for producing rapid advances that fundamentally alter treatment delivery for both antipsychotic drugs and adjunctive cognitive-enhancing agents.
Expert commentary
The advent of FGAs provided symptom relief and relapse prevention to many schizophrenia patients, so that many could live independently in the community. More recently, it has become clear that cognitive deficits are highly persistent into remission and are the best established cause of functional disability in schizophrenia patients. With preclinical work demonstrating the cognitive advantages of SGAs and the initial clinical reports that SGAs compared with FGAs may have greater cognitive benefit [24], many large clinical trials were conducted to investigate the extent to which second-generation drugs may reduce cognitive deficit in patients with schizophrenia. The moderate effect sizes observed for greater cognitive benefit from SGAs relative to FGAs in early open-label and medication ‘switch’ studies essentially have been cut in half with later, more controlled trials. The best available data now indicate that improvement in neuropsychological retest performance after treatment with SGAs is no greater than improvement seen in healthy controls retested over similar time intervals. This raises sobering questions about whether there is any significant and clinically meaningful cognitive improvement associated with SGAs.
As limitations of early studies were reduced with improved study methodology, a significant dischord between preclinical and clinical studies became evident. This raises questions about whether neuropsychological tests are an inefficient assay for detecting drug effects seen in preclinical models, whether illness effects on brain systems render patients less responsive to beneficial cognitive effects of drugs for reasons we have yet to fully understand, whether animal models of schizophrenia pathology are too imprecise, whether paradigms used to assess cognition in preclinical work bear insufficient relationship to domains of cognitive deficits in schizophrenia, or whether there is a species transfer problem of animal models to cognition in humans. These remain open questions for future research. Resolving these issues is crucial for the development of translational approaches in early-phase drug discovery.
Efforts to develop novel treatments for cognitive deficits in schizophrenia are approaching a critical juncture. The reality is that as long as detailed behavioral phenotyping of drug responses in preclinical models is used to guide decisions about initiating large expensive trials aiming to induce generalized cognitive improvement in patients, while so many questions remain unanswered about ‘disconnects’ in translational models, there may be too much risk and uncertainty in the system for the pharmaceutical industry to continue aggressively pursuing drug development efforts in this area. From the perspective of cognitive assessment in trials, the notion that any drug targeting a specific neurotransmitter system will cause a generalized improvement in neuropsychological test performance, which is the standard outcome for clinical trials [73,74,80], may be both limited in terms of its potential and ill advised in terms of its rationale. Decades of behavioral pharmacology research shows that drugs targeting specific neurotransmitter systems have specific rather than generalized effects on cognition and behavior. We urgently need better translational platforms for detecting these specific effects. While it is desirable that patients demonstrate a robust and generalized cognitive improvement, it remains possible that meaningful positive pharmacological effects on receptor systems and the functional brain systems that selectively modulate particular cognitive operations may be missed if the primary treatment outcome is generalized cognitive improvement. It may be more rational to learn more about how specific drugs improve specific brain cognitive operations in preclinical studies, and then work forward to build on that knowledge to design translational Phase II studies to test the applicability of those data in proof-of-concept studies, and finally to use that information to select drugs for large-scale registration trials. Thus, it may be wise to consider the drug development pathway from preclinical to Phase II to registration as a multistep process in which different outcomes (e.g., animal models, translational outcomes, neuropsychological performance, and functional outcomes) are needed at different points along the pathway to optimize drug development. specifically, early phase clinical studies might best emphasize translational models, while later stage trials might focus more on broader neuropsychological outcomes and impact on community function as primary outcomes. It would be optimal if translational measures predicted functional outcomes, or if standard neuropsychological measures were able to detect specific drug effects on cognition, but at this point neither of these possibilities are close to being established. Some key steps in this pathway will be to develop translational neurophysiological biomarker strategies [107], develop more efficient and sophisticated neuropsychological measures rooted in modern cognitive neuroscience, and establish links between biomarkers and functional outcomes.
Five-year view
In many short-duration Phase II studies, the success of a trial is judged on robust improvements on broad neuropsychological tests, such as those compromising the MATRICS battery, and related changes in functional capacity. This approach faces very high hurdles given the problems raised previously with practice effects, unresolved concerns about optimal measurement strategies for proof-of-concept studies, and the potential need for longer term follow-up with low attrition so that psychosocial interventions can foster the use of enhanced cognitive abilities in real-world functions. To avoid prematurely dismissing promising drugs early in the developmental pathway and the high cost of Phase III trials for ill-fated compounds, a more systematic translational approach may be needed to bridge the gap between preclinical and Phase II studies to optimize selection of compounds for extensive development and larger trials. First, outcome measures must be validated as capable of detecting changes due to treatment effects in clinical trials, or being ‘fit for purpose’. Early phase work, especially in small sample/brief follow-up studies, may need to be shifted away from seeking to detect ‘globally improved cognition’ or improved community function as a primary outcome, to more realistic targets of improvements in specific cognitive processes based on drug pharmacology, pre-clinical findings, and the functional brain systems targeted by the drug of interest. Second, an improved understanding of the pathophysiology of schizophrenia is needed to guide the development and selection of new compounds for further investigation. Furthermore, studies of drugs targeting dysfunctional systems may be informative regarding which elements of cognition can be effectively enhanced in schizophrenia, what is the best way to enhance dysfunctional systems in terms of drug and dosing, whether cognitive profiling can guide individualized care, and to what extent enhanced function in specific cognitive abilities is related to generalized improvements on neuropsychological test batteries and functional status in the community. Another area likely to undergo rapid advances is the development of individualized treatments based on pharmacogenetics. Last, not only is cognitive phenotyping needed to evaluate who is most likely to respond to certain treatments and how well they will respond, but effective, validated psychosocial interventions may be needed to help patients take advantage of cognitive improvements resulting from drug treatments, so that increased cognitive ability can lead to improved role function.
Key issues.
Cognition is a significant focus as a treatment target in schizophrenia.
Typically, neither first- nor second-generation antipsychotics have a clinically meaningful beneficial effect on cognition in schizophrenia.
There is a gap between the beneficial effects of second-generation antipsychotics observed in animal studies using tests of very specific behavioral abilities and the modest to negligible effects in global neuropsychological function seen in clinical studies that remains to be fully understood.
There is a need for greater validation of standardized neuropsychological batteries (i.e., the Measurement and Treatment Research to Improve Cognition in Schizophrenia program) as an approach for assessing cognitive outcomes in comparison to other approaches rooted in cognitive neuroscience.
Other valid methodologies for evaluating cognitive treatment effects, such as those grounded in translational neuroscience, have not been utilized frequently or effectively.
For future research and drug development, a translational/biomarker approach may be more effective.
Significant hurdles to obtaining an indication for cognitive enhancement include validation of functional outcome measures and understanding the time and psychosocial supports needed for cognitive changes to translate into improved community function.
Developing approaches for using cognitive profiles and pharmacogenetics to individualize treatment selection are promising strategies for potential advancement in this area.
Footnotes
Financial & competing interests disclosure
Donna Palumbo is employed by Pfizer; John Sweeney consults with Pfizer; and John Sweeney and Jeffrey Bishop have received research funding from Janssen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
S Kristian Hill, Center for Cognitive Medicine (M/C 913), University of Illinois at Chicago, 912 South Wood Street, Suite 235, Chicago, IL 60612, USA, Tel.: +1 312 355 1582, Fax: +1 312 413 8837, | shill@psych.uic.edu.
Jeffrey R Bishop, Center for Cognitive Medicine (M/C 913), University of Illinois at Chicago, 912 South Wood Street, Suite 235, Chicago, IL 60612, USA.
Donna Palumbo, Adjunct Associate Professor of Neurology, Psychiatry, and Pediatrics, University of Rochester Medical Center & Pfizer Global Research and Development, Rochester, NY, USA.
John A. Sweeney, Center for Cognitive Medicine (M/C 913), University of Illinois at Chicago, 912 South Wood Street, Suite 235, Chicago, IL 60612, USA.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Blanchard JJ, Neale JM. The neuropsychological signature of schizophrenia: generalized or differential deficit? Am. J. Psychiatry. 1994;151(1):40–48. doi: 10.1176/ajp.151.1.40. [DOI] [PubMed] [Google Scholar]
- 2.Saykin AJ, Shtasel DL, Gur RE, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch. Gen. Psychiatry. 1994;51(2):124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 3.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 4.Hawkins KA, Keefe RS, Christensen BK, et al. Neuropsychological course in the prodrome and first episode of psychosis: findings from the PRIME North America Double Blind Treatment study. Schizophr. Res. 2008;105(1–3):1–9. doi: 10.1016/j.schres.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naive patients with schizophrenia. Schizophr. Res. 2004;68(1):49–63. doi: 10.1016/S0920-9964(03)00213-5. [DOI] [PubMed] [Google Scholar]
- 6.Hoff AL, Sakuma M, Wieneke M, Horon R, Kushner M, DeLisi LE. Longitudinal neuropsychological follow-up study of patients with first-episode schizophrenia. Am. J. Psychiatry. 1999;156(9):1336–1341. doi: 10.1176/ajp.156.9.1336. [DOI] [PubMed] [Google Scholar]
- 7.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am. J. Psychiatry. 1996;153(3):321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 8.Sevy S, Davidson M. The cost of cognitive impairment in schizophrenia. Schizophr. Res. 1995;17(1):1–3. doi: 10.1016/0920-9964(95)00025-h. [DOI] [PubMed] [Google Scholar]
- 9.Blomqvist AG, Leger PT, Hoch JS. The cost of schizophrenia: lessons from an international comparison. J. Ment. Health Policy Econ. 2006;9(4):177–183. [PubMed] [Google Scholar]
- 10.Hoenberg K, Goetz K. Antipsychotics: Analysis of disease markets and emerging agents. Waltham, MA, USA: Decision Resources, Inc.; 2006. [Google Scholar]
- 11.Davis JM, Casper R. Antipsychotic drugs: clinical pharmacology and therapeutic use. Drugs. 1977;14(4):260–282. doi: 10.2165/00003495-197714040-00002. [DOI] [PubMed] [Google Scholar]
- 12.Arnsten AF, Cai JX, Steere JC, Goldman-Rakic PS. Dopamine D2 receptor mechanisms contribute to age-related cognitive decline: the effects of quinpirole on memory and motor performance in monkeys. J. Neurosci. 1995;15:3429–3439. doi: 10.1523/JNEUROSCI.15-05-03429.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castner SA, Williams GV, Goldman-Rakic PS. Reversal of antipsychotic-induced working memory deficits by short-term dopamine D1 receptor stimulation. Science. 2000;287:2020–2022. doi: 10.1126/science.287.5460.2020. [DOI] [PubMed] [Google Scholar]
- 14.Sawaguchi T, Goldman-Rakic PS. The role of D1-dopamine receptor in working memory: local injections of dopamine antagonists into the prefrontal cortex of rhesus monkeys performing an oculomotor delayed-response task. J. Neurophysiol. 1994;71:515–528. doi: 10.1152/jn.1994.71.2.515. [DOI] [PubMed] [Google Scholar]
- 15.Ramaekers JG, Louwerens JW, Muntjewerff ND, et al. Psychomotor, cognitive, extrapyramidal, and affective functions of healthy volunteers during treatment with an atypical (amisulpride) and a classic (haloperidol) antipsychotic. J Clin. Psychopharmacol. 1999;19:209–221. doi: 10.1097/00004714-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am. J. Psychiatry. 2000;157(4):514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 17.Purdon SE, Woodward N, Lindborg SR, Stip E. Procedural learning in schizophrenia after 6 months of double-blind treatment with olanzapine, risperidone, and haloperidol. Psychopharmacology. 2003;169((3–4)):390–397. doi: 10.1007/s00213-003-1505-z. [DOI] [PubMed] [Google Scholar]
- 18.Bedard MA, Scherer H, Stip E, Cohen H, Rodriguez JP, Richer F. Procedural learning in schizophrenia: further consideration on the deleterious effect of neuroleptics. Brain Cogn. 2000;43:31–39. [PubMed] [Google Scholar]
- 19.Reilly JL, Harris MS, Keshavan MS, Sweeney JA. Adverse effects of risperidone on spatial working memory in first-episode schizophrenia. Arch. Gen. Psychiatry. 2006;63(11):1189–1197. doi: 10.1001/archpsyc.63.11.1189. [DOI] [PubMed] [Google Scholar]
- 20.Blyler CR, Gold JM. Cognitive effects of typical antipsychotic treatment: another look. In: Sharma T, Harvey PD, editors. Cognition in Schizophrenia. NY, USA: Oxford University Press; 2000. pp. 241–265. [Google Scholar]
- 21.Spohn HE, Strauss ME. Relation of neuroleptic and anticholinergic medication to cognitive functions in schizophrenia. J. Abnorm. Psychol. 1989;98:367–380. doi: 10.1037//0021-843x.98.4.367. [DOI] [PubMed] [Google Scholar]
- 22.Cassens G, Inglis AK, Appelbaum PS, Gutheil TG. Neuroleptics: effects on neuropsychological function in chronic schizophrenic patients. Schizophr. Bull. 1990;16(3):477–499. doi: 10.1093/schbul/16.3.477. [DOI] [PubMed] [Google Scholar]
- 23.Bilder RM, Turkel E, Lipschutz-Broch L, Lieberman JA. Antipsychotic medication effects on neuropsychological functions. Psychopharmacol. Bull. 1992;28:353–366. [PubMed] [Google Scholar]
- 24.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry. 1988;45(9):789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 25.Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23(3):223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 26.Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J. Neurosci. 1998;18(14):5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cochran SM, Kennedy M, McKerchar CE, Steward LJ, Pratt JA, Morris BJ. Induction of metabolic hypofunction and neurochemical deficits after chronic intermittent exposure to phencyclidine: differential modulation by antipsychotic drugs. Neuropsychopharmacology. 2003;28(2):265–275. doi: 10.1038/sj.npp.1300031. [DOI] [PubMed] [Google Scholar]
- 28.Kargieman L, Santana N, Mengod G, Celada P, Artigas F. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proc. Natl Acad. Sci. USA. 2007;104(37):14843–14848. doi: 10.1073/pnas.0704848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jentsch J. Pre-clinical models of cognitive dysfunction in schizophrenia: new avenues to addressing unmet needs. Clin. Neurosci. Res. 2003;3:303–315. [Google Scholar]
- 30.Abekawa T, Ito K, Nakagawa S, Nakato Y, Koyama T. Olanzapine and risperidone block a high dose of methamphetamine-induced schizophrenia-like behavioral abnormalities and accompanied apoptosis in the medial prefrontal cortex. Schizophr. Res. 2008;101(1–3):84–94. doi: 10.1016/j.schres.2007.12.488. [DOI] [PubMed] [Google Scholar]
- 31.Toua C, Brand L, Mo Ller M, Emsley RA, Harvey BH. The effects of sub-chronic clozapine and haloperidol administration on isolation rearing induced changes in frontal cortical N-methyl-d-aspartate and D(1) receptor binding in rats. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.10.039. DOI:10.1016/j.neuroscience.2009.10.039 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 32.Koike H, Ibi D, Mizoguchi H, et al. Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav. Brain Res. 2009;202(1):114–121. doi: 10.1016/j.bbr.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 33.Li N, Wu X, Li L. Chronic administration of clozapine alleviates reversal-learning impairment in isolation-reared rats. Behav. Pharmacol. 2007;18(2):135–145. doi: 10.1097/FBP.0b013e3280d3ee83. [DOI] [PubMed] [Google Scholar]
- 34.Barr AM, Powell SB, Markou A, Geyer MA. Iloperidone reduces sensorimotor gating deficits in pharmacological models, but not a developmental model, of disrupted prepulse inhibition in rats. Neuropharmacology. 2006;51(3):457–465. doi: 10.1016/j.neuropharm.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 35.Cilia J, Cluderay JE, Robbins MJ, et al. Reversal of isolation-rearing-induced PPI deficits by an a7 nicotinic receptor agonist. Psychopharmacology (Berl.) 2005;182(2):214–219. doi: 10.1007/s00213-005-0069-5. [DOI] [PubMed] [Google Scholar]
- 36.Kinkead B, Dobner PR, Egnatashvili V, Murray T, Deitemeyer N, Nemeroff CB. Neurotensin-deficient mice have deficits in prepulse inhibition: restoration by clozapine but not haloperidol, olanzapine, or quetiapine. J. Pharmacol. Exp. Ther. 2005;315(1):256–264. doi: 10.1124/jpet.105.087437. [DOI] [PubMed] [Google Scholar]
- 37.Abdul-Monim Z, Reynolds GP, Neill JC. The atypical antipsychotic ziprasidone, but not haloperidol, improves phencyclidine-induced cognitive deficits in a reversal learning task in the rat. J. Psychopharmacol. 2003;17(1):57–66. doi: 10.1177/0269881103017001700. [DOI] [PubMed] [Google Scholar]
- 38.Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology. 2007;193(4):521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- 39.He J, Xu H, Yang Y, Rajakumar D, Li X, Li XM. The effects of chronic administration of quetiapine on the phencyclidine-induced reference memory impairment and decrease of Bcl-X-sub(L)/ Bax ratio in the posterior cingulate cortex in rats. Behav. Brain Res. 2006;168(2):236–242. doi: 10.1016/j.bbr.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 40.He J, Yang Y, Yu Y, Li X, Li XM. The effects of chronic administration of quetiapine on the methamphetamine-induced recognition memory impairment and dopaminergic terminal deficit in rats. Behav. Brain Res. 2006;172(1):39–45. doi: 10.1016/j.bbr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 41.Hagiwara H, Fujita Y, Ishima T, et al. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the antipsychotic drug perospirone: role of serotonin 5-HT-sub(1A) receptors. Eur. Neuropsychopharmacol. 2008;18(6):448–454. doi: 10.1016/j.euroneuro.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Dunn MJ, Killcross SClozapine. SCH 23390 and α-flupenthixol but not haloperidol attenuate acute phencyclidine-induced disruption of conditional discrimination performance. Psychopharmacology. 2007;190(4):403–414. doi: 10.1007/s00213-006-0605-y. [DOI] [PubMed] [Google Scholar]
- 43.Luo C, Xu H, Li XM. Quetiapine reverses the suppression of hippocampal neurogenesis caused by repeated restraint stress. Brain Res. 2005;1063(1):32–89. doi: 10.1016/j.brainres.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 44.He J, Yang Y, Xu H, Zhang X, Li XM. Olanzapine attenuates the okadaic acid-induced spatial memory impairment and hippocampal cell death in rats. Neuropsychopharmacology. 2005;30(8):1511–1520. doi: 10.1038/sj.npp.1300757. [DOI] [PubMed] [Google Scholar]
- 45.Bardgett ME, Griffith MS, Foltz RF, Hopkins JA, Massie CM, O’Connell SM. The effects of clozapine on delayed spatial alternation deficits in rats with hippocampal damage. Neurobiol. Learning Memory. 2006;85(1):86–94. doi: 10.1016/j.nlm.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Hou Y, Wu CF, Yang JY, Guo T. Differential effects of haloperidol, clozapine and olanzapine on learning and memory functions in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30(8):1486–1495. doi: 10.1016/j.pnpbp.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Matsumoto M, Shikanai H, Togashi H, et al. Characterization of clozapine-induced changes in synaptic plasticity in the hippocampal-mPFC pathway of anesthetized rats. Brain Res. 2008;1195:50–55. doi: 10.1016/j.brainres.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Elsworth JD, Jentseh JD, Morrow BA, Roth RH, Redmond DE., Jr Clozapine normalizes prefrontal cortex dopamine transmission in monkeys subchronically exposed to phencyclidine. Neuropsychopharmacology. 2008;33(3):491–496. doi: 10.1038/sj.npp.1301448. [DOI] [PubMed] [Google Scholar]
- 49.Sur C, Mallorga PJ, Wittmann M, et al. N-desmethylclozapine, an allosteric agonist at muscarinic 1 receptor, potentiates N-methyl-d-aspartate receptor activity. Proc. Natl Acad. Sci. USA. 2003;100(23):13674–13679. doi: 10.1073/pnas.1835612100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchanan RW, Holstein C, Breier A. The comparative efficacy and long-term effect of clozapine treatment on neuropsychological test performance. Biol. Psychiatry. 1994;36(11):717–725. doi: 10.1016/0006-3223(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 51.Lee MA, Thompson PA, Meltzer HY. Effects of clozapine on cognitive function in schizophrenia. J. Clin. Psychiatry. 1994;55 Suppl. B:82–87. [PubMed] [Google Scholar]
- 52.Green MF, Marshall BD, Jr, Wirshing WC, et al. Does risperidone improve verbal working memory in treatment-resistant schizophrenia? Am. J. Psychiatry. 1997;154(6):799–804. doi: 10.1176/ajp.154.6.799. [DOI] [PubMed] [Google Scholar]
- 53.Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophrenia Bull. 1999;25(2):233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- 54.Green MF. Recent studies on the neurocognitive effects of second-generation antipsychotic medications. Curr. Opin. Psychiatry. 2002;15(1):25–29. [Google Scholar]
- 55.Meltzer HY, McGurk SR. Effect of atypical antipsychotic drugs on cognitive function in schizophrenia. In: Brenner HD, Boker W, Genner R, editors. The treatment of schizophrenia: status and emerging trends. Ashland, OH, USA: Hogrefe & Huber Publishers; 2001. pp. 49–59. [Google Scholar]
- 56.Keefe RSE, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr. Bull. 1999;25(2):201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- 57.Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int. J. Neuropsychopharmacol. 2005;8(3):457–472. doi: 10.1017/S146114570500516X. [DOI] [PubMed] [Google Scholar]
- 58. Carpenter WT, Gold JM. Another view of therapy for cognition in schizophrenia. Biol. Psychiatry. 2002;51(12):969–971. doi: 10.1016/s0006-3223(02)01399-9.. • Presents the indirect action hypothesis that the beneficial cognitive effects of second-generation antipsychotics (SGAs) observed in early studies were merely a release from the adverse effects of first-generation antipsychotics.
- 59.Perlick D, Stastny P, Katz I, Mayer M, Mattis S. Memory deficits and anticholinergic levels in chronic schizophrenia. Am. J. Psychiatry. 1986;143(2):230–232. doi: 10.1176/ajp.143.2.230. [DOI] [PubMed] [Google Scholar]
- 60.Tune LE, Strauss ME, Lew MF, Breitlinger E, Coyle JT. Serum levels of anticholinergic drugs and impaired recent memory in chronic schizophrenic patients. Am. J. Psychiatry. 1982;139(11):1460–1462. doi: 10.1176/ajp.139.11.1460. [DOI] [PubMed] [Google Scholar]
- 61.Purdon SE, Jones BDW, Stip E, et al. Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. Arch. Gen. Psychiatry. 2000;57(3):249–258. doi: 10.1001/archpsyc.57.3.249. [DOI] [PubMed] [Google Scholar]
- 62.Keefe RSE, Seidman LJ, Christensen BK, et al. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am. J. Psychiatry. 2004;161(6):985–995. doi: 10.1176/appi.ajp.161.6.985. [DOI] [PubMed] [Google Scholar]
- 63.Keefe RS, Seidman LJ, Christensen BK, et al. Long-term neurocognitive effects of olanzapine or low-dose haloperidol in first-episode psychosis. Biol. Psychiatry. 2006;59(2):97–105. doi: 10.1016/j.biopsych.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 64.Harvey PD, Rabinowitz J, Eerdekens M, Davidson M. Treatment of cognitive impairment in early psychosisa comparison of risperidone and haloperidol in a large long-term trial. Am. J. Psychiatry. 2005;162(10):1888–1895. doi: 10.1176/appi.ajp.162.10.1888. [DOI] [PubMed] [Google Scholar]
- 65.Thornton AE, Van Snellenberg JX, Sepehry AA, Honer WG. The impact of atypical antipsychotic medications on long-term memory dysfunction in schizophrenia spectrum disorder: a quantitative review. Psychopharmacology. 2006;20(3):335–346. doi: 10.1177/0269881105057002. [DOI] [PubMed] [Google Scholar]
- 66. Goldberg TE, Goldman RS, Burdick KE, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia: is it a practice effect? Arch. Gen. Psychiatry. 2007;64(10):1115–1122. doi: 10.1001/archpsyc.64.10.1115.. •• Provides empirical support for the notion that treatment-related cognitive improvements may not be greater than expected practice effects
- 67.Albus M, Hubmann W, Mohr F, et al. Neurocognitive functioning in patients with first-episode schizophrenia: results of a prospective 5-year follow-up study. Eur. Arch. Psychiatry Clin. Neurosci. 2006;256(7):442–451. doi: 10.1007/s00406-006-0667-1. [DOI] [PubMed] [Google Scholar]
- 68.Hill SK, Reilly JL, Harris MS, et al. A comparison of neuropsychological dysfunction in first-episode psychosis patients with unipolar depression, bipolar disorder, and schizophrenia. Schizophr. Res. 2009;113(2–3):167–175. doi: 10.1016/j.schres.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Crespo-Facorro B, Rodriguez-Sanchez JM, Perez-Iglesias R, et al. Neurocognitive effectiveness of haloperidol, risperidone, and olanzapine in first-episode psychosis:a randomized, controlled 1-year follow-up comparison. J. Clin. Psychiatry. 2009;70(5):717–729. doi: 10.4088/JCP.08m04634. [DOI] [PubMed] [Google Scholar]
- 70. Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of cognitive change with haloperidol in clinical trials of atypical antipsychotics: dose effects and comparison to practice effects. Schizophr. Res. 2007;89((1–3)):211–224. doi: 10.1016/j.schres.2006.08.021.. ••This is a meta-analysis that separately evaluates open-label and randomized double-blind controlled trials. Perhaps more interesting is an upcoming meta-analysis from this group, which includes Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) findings and is expected to report a small mean effect size (~0.10) for the beneficial cognitive effects of SGAs
- 71.Chapman LJ, Chapman JP. The measurement of differential deficit. J. Psychiatry Res. 1978;14:303–311. doi: 10.1016/0022-3956(78)90034-1. [DOI] [PubMed] [Google Scholar]
- 72.Strauss ME. Demonstrating specific cognitive deficits: a psychometric perspective. J. Abnorm. Psychol. 2001;110(1):6–14. doi: 10.1037//0021-843x.110.1.6. [DOI] [PubMed] [Google Scholar]
- 73. Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol. Psychiatry. 2008;64(9):823–827. doi: 10.1016/j.biopsych.2008.04.005.. •• Presents the hypothesis that rather than tapping into domain-specific processes, clinical neuropsychological tests converge on a generalized cognitive factor
- 74. Hill SK, Sweeney JA, Hamer RM, et al. Efficiency of the CATIE and BACS neuropsychological batteries in assessing cognitive effects of antipsychotic treatments in schizophrenia. J. Int. Neuropsychol. Soc. 2008;14(2):209–221. doi: 10.1017/S1355617708080570.. • Uses clinical trial data to show that neuropsychological measures primarily assess a generalized cognitive factor, both prior to and after treatment.
- 75.Harris MS, Reilly JL, Keshavan MS, Sweeney JA. Longitudinal studies of antisaccades in antipsychotic-naive first-episode schizophrenia. Psychol. Med. 2006;36(4):485–494. doi: 10.1017/S0033291705006756. [DOI] [PubMed] [Google Scholar]
- 76.Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Arch. Gen. Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 77.Davidson M, Galderisi S, Weiser M, et al. Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: a randomized, open-label clinical trial (EUFEST) Am. J. Psychiatry. 2009;166(6):675–682. doi: 10.1176/appi.ajp.2008.08060806. [DOI] [PubMed] [Google Scholar]
- 78. Keefe RS, Sweeney JA, Gu H, et al. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am. J. Psychiatry. 2007;164(7):1061–1071. doi: 10.1176/ajp.2007.164.7.1061.. •• Reports Comparison of Atypicals for First-Episode Psychosis (CAFE) findings regarding cognitive outcomes
- 79.Keefe RS, Harvey PD, Goldberg TE, et al. Norms and standardization of the Brief Assessment of Cognition in Schizophrenia (BACS) Schizophr. Res. 2008;102(1–3):108–115. doi: 10.1016/j.schres.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 80.Dickinson D, Ragland JD, Calkins ME, Gold JM, Gur RC. A comparison of cognitive structure in schizophrenia patients and healthy controls using confirmatory factor analysis. Schizophr. Res. 2006;85(1–3):20–29. doi: 10.1016/j.schres.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Freedman R, Olincy A, Buchanan RW, et al. Initial Phase 2 trial of a nicotinic agonist in schizophrenia. Am. J. Psychiatry. 2008;165(8):1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liberman RP, Gutkind D, Mintz J, et al. Impact of risperidone versus haloperidol on activities of daily living in the treatment of refractory schizophrenia. Comprehensive Psychiatry. 2002;43(6):469–473. doi: 10.1053/comp.2002.33499. [DOI] [PubMed] [Google Scholar]
- 83.Manschreck TC, Redmond DA, Candela SF, Maher BA. Effects of clozapine on psychiatric symptoms, cognition, and functional outcome in schizophrenia. J. Neuropsych. Clin. Neurosci. 1999;11(4):481–489. doi: 10.1176/jnp.11.4.481. [DOI] [PubMed] [Google Scholar]
- 84.Hofer A, Baumgartner S, Bodner T, et al. Patient outcomes in schizophrenia II: the impact of cognition. Eur. Psychiatry. 2005;20(5–6):395–402. doi: 10.1016/j.eurpsy.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 85.Corrigan PW, Reinke RR, Landsberger SA, Charate A, Toombs GA. The effects of atypical antipsychotic medications on psychosocial outcomes. Schizophr. Res. 2003;63(1–2):97–101. doi: 10.1016/s0920-9964(02)00379-1. [DOI] [PubMed] [Google Scholar]
- 86.Hofer A, Kemmler G, Eder U, Edlinger M, Hummer M, Fleischhacker WW. Quality of life in schizophrenia: the impact of psychopathology, attitude toward medication, and side effects. J. Clin. Psychiatry. 2004;65(7):932–939. [PubMed] [Google Scholar]
- 87.Perlick DA, Rosenheck RA, Kaczynski R, Collins J, Bingham S. Association of symptomatology and cognitive deficits to functional capacity in schizophrenia. Schizophr. Res. 2008;99(1–3):192–199. doi: 10.1016/j.schres.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 88.Norman RM, Malla AK, Cortese L, et al. Symptoms and cognition as predictors of community functioning: a prospective analysis. Am. J. Psychiatry. 1999;156(3):400–405. doi: 10.1176/ajp.156.3.400. [DOI] [PubMed] [Google Scholar]
- 89.Dickerson F, Boronow JJ, Ringel N, Parente F. Social functioning and neurocognitive deficits in outpatients with schizophrenia: a 2-year follow-up. Schizophr. Res. 1999;37(1):13–20. doi: 10.1016/s0920-9964(98)00134-0. [DOI] [PubMed] [Google Scholar]
- 90.Swartz MS, Perkins DO, Stroup TS, et al. Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am. J. Psychiatry. 2007;164(3):428–436. doi: 10.1176/ajp.2007.164.3.428. [DOI] [PubMed] [Google Scholar]
- 91.Awad AG, Voruganti LN. Impact of atypical antipsychotics on quality of life in patients with schizophrenia. CNS Drugs. 2004;18(13):877–893. doi: 10.2165/00023210-200418130-00004. [DOI] [PubMed] [Google Scholar]
- 92.Lipkovich IA, Deberdt W, Csernansky JG, Sabbe B, Keefe RS, Kollack-Walker S. Relationships among neurocognition, symptoms and functioning in patients with schizophrenia: a path-analytic approach for associations at baseline and following 24 weeks of antipsychotic drug therapy. BMC Psychiatry. 2009;9(44) doi: 10.1186/1471-244X-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Velligan DI, Kern RS, Gold JM. Cognitive rehabilitation for schizophrenia and the putative role of motivation and expectancies. Schizophr. Bull. 2006;32(3):474–485. doi: 10.1093/schbul/sbj071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Harvey PD, Keefe RS, Patterson TL, Heaton RK, Bowie CR. Abbreviated neuropsychological assessment in schizophrenia: prediction of different aspects of outcome. J. Clin. Exp. Neuropsychol. 2009;31(4):462–471. doi: 10.1080/13803390802251386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch. Neurol. 2006;63(10):1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 96. Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr. Bull. 2008;34(5):944–961. doi: 10.1093/schbul/sbn070.. •• One of the few bottom-up, translational neuroscience approaches capable of leading to effective procognitive pharmacological treatments
- 97.Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int. J. Psychophysiol. 2000;38(3):315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 98.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 99.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical γ synchrony and cognitive control in schizophrenia. Proc. Natl Acad. Sci. USA. 2006;103(52):19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lewis DA, Cho RY, Carter CS, et al. Subunit-selective modulation of GABA type A receptor neurotransmission and cognition in schizophrenia. Am. J. Psychiatry. 2008;165(12):1585–1593. doi: 10.1176/appi.ajp.2008.08030395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hill SK, Reilly JL, Harris MS, Khine T, Sweeney JA. Oculomotor and neuropsychological effects of antipsychotic treatment for schizophrenia. Schizophr. Bull. 2007;34(3):494–506. doi: 10.1093/schbul/sbm112.. •• This is a unique report directly comparing the sensitivity of oculomotor and neuropsychological measures to antipsychotic treatments
- 102.Light GA, Geyer MA, Clementz BA, Cadenhead KA, Braff DL. Normal P50 suppression in schizophrenia patients treated with atypical antipsychotic medications. Am. J. Psychiatry. 2000;157(5):767–771. doi: 10.1176/appi.ajp.157.5.767. [DOI] [PubMed] [Google Scholar]
- 103. Reilly JL, Lencer R, Bishop JR, Keedy S, Sweeney JA. Pharmacological treatment effects on eye movement control. Brain Cogn. 2008;68(3):415–435. doi: 10.1016/j.bandc.2008.08.026.. •• A comprehensive review of the beneficial and adverse effects of antipsychotics on oculomotor paradigms
- 104.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch. Gen. Psychiatry. 2005;62(2):127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 105.Keedy SK, Rosen C, Khine T, Rajarethinam R, Janicak PG, Sweeney JA. An fMRI study of visual attention and sensorimotor function before and after antipsychotic treatment in first-episode schizophrenia. Psychiatry Res. 2009;172(1):16–23. doi: 10.1016/j.pscychresns.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hajos M. Targeting information-processing deficit in schizophrenia: a novel approach to psychotherapeutic drug discovery. Trends Pharmacol. Sci. 2006;27(7):391–398. doi: 10.1016/j.tips.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 107.Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat. Rev. Drug Discov. 2008;7(1):68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40(5):881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- 109.Tamminga CA. The neurobiology of cognition in schizophrenia. J. Clin. Psychiatry. 2006;67 Suppl. 9:9–13. [PubMed] [Google Scholar]
- 110.Weinberger DR, Egan MF, Bertolino A, et al. Prefrontal neurons and the genetics of schizophrenia. Biol. Psychiatry. 2001;50(11):825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 111.Bertolino A, Caforio G, Blasi G, et al. Interaction of COMT Val108/158 Met genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am. J. Psychiatry. 2004;161(10):1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- 112.Woodward ND, Jayathilake K, Meltzer HY. COMT Val108/158Met genotype, cognitive function, and cognitive improvement with clozapine in schizophrenia. Schizophr. Res. 2007;90(1–3):86–96. doi: 10.1016/j.schres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 113.Hill SK, Keshavan MS, Thase ME, Sweeney JA. Neuropsychological dysfunction in antipsychotic-naive first-episode unipolar psychotic depression. Am. J. Psychiatry. 2004;161(6):996–1003. doi: 10.1176/appi.ajp.161.6.996. [DOI] [PubMed] [Google Scholar]
- 114.Reilly JL, Harris MSH, Keshavan MS, Sweeney JA. Abnormalities in visually guided saccades suggest corticofugal dysregulation in never-treated schizophrenia. Biol. Psychiatry. 2005;57(2):145–154. doi: 10.1016/j.biopsych.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 115.Reilly JL, Harris MS, Khine TT, Keshavan MS, Sweeney JA. Reduced attentional engagement contributes to deficits in prefrontal inhibitory control in schizophrenia. Biol. Psychiatry. 2008;63(8):776–783. doi: 10.1016/j.biopsych.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Harris MS, Wiseman CL, Reilly JL, Keshavan MS, Sweeney JA. Effects of risperidone on procedural learning in antipsychotic-naive first-episode schizophrenia. Neuropsychopharmacology. 2009;34(2):468–476. doi: 10.1038/npp.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]