Abstract
Purpose of review
Perinatal asphyxia, intraventricular hemorrhage and stroke are common causes of neonatal brain injury, with hypoxia-ischemia as the final common pathway of injury. Erythropoietin (Epo) has potential to lessen neurologic sequelae due to hypoxia-ischemia. The purpose of this review is to highlight new clinical trials and experimental evidence that expand our understanding of Epo as a potential treatment for perinatal brain injury.
Recent findings
Several trials of Epo treatment are reviewed: Two phase I/II trials of high-dose Epo given to preterm infants established pharmacokinetic and safety profiles, and a trial of Epo treatment for term infants with moderate hypoxic-ischemic encephalopathy found reduced disability. Potential risks and benefits of high-dose Epo are discussed. New evidence related to Epo receptor expression, signal transduction pathways, and mechanisms of neuroprotection are reviewed.
Summary
Cautious optimism is warranted regarding the use of high-dose Epo as a treatment option for neonatal brain injury. To date, Epo has been safe to use in neonatal populations and now studies of neuroprotective efficacy are underway.
Keywords: Neuroprotection, growth factors, newborn
Introduction
Medical advances in neonatology have significantly improved survival statistics, particularly for extremely preterm infants. Similar progress has not been made to improve neurodevelopmental outcomes for brain-injured neonates. Clinical trials of hypothermia have demonstrated benefit for term neonates with mild and moderate brain injury, but none when hypoxia-ischemia is severe or prolonged [1, 2, 3]. Hypothermia is also contraindicated for preterm infants, leaving this group with no proven therapeutic options when hypoxia-ischemia occurs. A neuroprotective pharmaceutical treatment to minimize neonatal brain injury is greatly needed. The optimal therapy will be safe for use in both preterm and term neonates, and effective when administered after an insult. Animal studies support the efficacy and safety of erythropoietin (Epo) as a therapeutic intervention for a variety of brain insults [4*], and Epo is now in the clinical testing phase. We highlight recent clinical trials and experimental reports that further consideration of Epo as a therapy for neonatal hypoxic-ischemic injury.
Epo Trials in Preterm Infants
Both preventative treatment and rescue therapy are possible applications of Epo. For example, all extremely low birth weight (< 1000 g) infants could be treated prophylactically because they are at high risk for poor outcome. In contrast, rescue treatment would occur only after a neurologically deleterious event. The advantage of rescue therapy is that unnecessary exposure is eliminated, but the disadvantage is that treatment may be delayed. With either approach, Epo treatment must be safe.
To evaluate safety, we reviewed the use of Epo as an erythropoietic treatment in prospective randomized trials. Between 1991 and 2006, over 2400 infants were enrolled in 30 randomized controlled trials to evaluate the safety and efficacy of Epo for the prevention or treatment of anemia of prematurity. Treatment regimens ranged from 70 to 5,000 U/kg/week (35 to 750 U/kg/dose), with duration of therapy ranging from 2 weeks to several months [5, 6]. None of these studies reported increased risk for stroke, hemorrhage, clotting, or death. At the outset, erythropoietic Epo dosing for neonates was extrapolated from adults. But that dosing was found to be too low for neonates who have a higher volume of distribution and more rapid clearance than adults. Subsequent trials in preterm infants established the safety, pharmacokinetics and efficacy of higher doses [7, 8, 9]. In contrast, animal Epo neuroprotection studies have generally used high doses (1,000 – 5,000 U/kg) to ensure penetration of the blood-brain barrier (BBB) [10]. The safety of high-dose Epo was recently confirmed in rats [11].
Two single-center phase I/II prospective trials examining the safety and efficacy of high-dose Epo for preterm infants have been published [12**, 13**]. Table 1 compares key design details from these trials. In the study by Fauchère et al. [12**], newborns born 24 – 32 weeks of gestation and < 1500 g were given Epo (3000 U/kg x 3 i.v. doses, n = 30) or placebo (n = 15). The primary outcome was survival without intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), and retinopathy of prematurity (ROP). Care was withdrawn from 5 of 30 Epo-treated infants due to severe IVH (n = 3, one case diagnosed at enrollment) or severe respiratory failure (n = 2). All of these deaths occurred in infants < 26 weeks of gestation. Complication risks were not different for ROP, IVH, sepsis, necrotizing enterocolitis (NEC), and lung disease. A phase III randomized controlled study is ongoing in Switzerland.
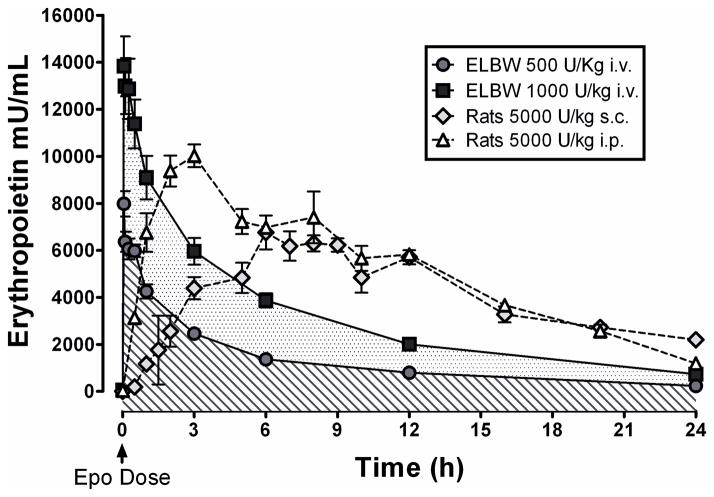
The phase I/II study by Juul et al. [13**] evaluated the safety and pharmacokinetics of Epo given to newborns born 23 – 28 weeks of gestation and < 1000 g. Three Epo doses were tested (500, 1000, and 2500 U/kg, x 3 i.v. doses, n = 10/dose). There were trends towards less IVH (p = 0.07), less severe IVH or PVL (p = 0.06), and fewer infants were diagnosed with stage III NEC (p = 0.018). There were no Epo-related complications. Plasma Epo concentrations 30 min after injection were 5,973, 12,291 and 34,197 mU/mL after 500, 1000, and 2500 U/kg, respectively. Figure 1 compares rat [14] and human [13**] plasma Epo concentrations following a single Epo injection. The circulating concentrations of Epo in rats given 5,000 U/kg Epo (a neuroprotective dose) is comparable to that for infants given 500 or 1000 U/kg, suggesting that 500 – 1000 U/kg is a suitable dosing range. Note that plasma concentrations fall more rapidly after i.v. dosing, so more frequent dosing might be required.
Figure 1.
A comparison of human and rat blood Epo concentrations. Data from [13] and [14]
While there are design differences between these two trials, there is considerable agreement regarding the short-term safety of high-dose Epo. Between the two studies, 60 preterm newborns received high-dose Epo and there were no complications, and no harmful effects on blood pressure or hematological indices. In addition, measures of liver and renal function were unaffected. These trials complement one another by documenting the short-term safety of three injections of high-dose Epo therapy for preterm infants.
Early Epo may improve cognitive function. He et al [15] evaluated the effect of repeated early Epo on the neurobehavioral development of preterm infants. Preterm infants given Epo 250 U/kg i.v. three times/week for 4 weeks (n = 22) were compared to 22 untreated infants. Neurologic assessments were done at term corrected gestational age (CGA), and at 6 and 12 months. At term, the neonatal behavioral neurological assessment was improved in Epo-treated infants. At 6 and 12 months, the Gesell development schedule showed benefits in gross motor, fine motor and language skills (P<0.05).
Two retrospective reports also suggest that early Epo treatment can improve neurodevelopmental outcome. In the first study, designed to test the effects of Epo on erythropoiesis and transfusion needs in preterm infants born ≤ 1250 g, subjects received Epo from day 4 of life until 35 weeks CGA, but there was no effect of Epo on neurodevelopmental outcome when all infants were considered [16]. In a follow-up analysis considering only infants born < 1000 g (n = 12), mental developmental index (MDI) scores were higher at 18 to 22 months for infants with peak serum Epo concentrations > 500 mU/mL [17]. More recently, a retrospective analysis of data from infants born < 1500 g (n = 82) treated with 250 – 400 U/kg/dose Epo 3 times/week for 6 weeks to prevent anemia of prematurity, found that MDI scores at median age 25 months correlated with cumulative Epo exposure [18**]. Although differences in early cognitive function due to Epo exposure are small, they are detectable. Collectively, these data are very encouraging because they indicate that even lower doses of repeated Epo can be beneficial.
Epo Trials in Term Infants
The first trial of Epo therapy for neuroprotection in term infants born > 37 weeks with moderate to severe hypoxic-ischemic encephalopathy (HIE) has now been completed [19**]. Zhu et al. randomized eligible babies to either Epo (n=83) or conventional (n=84) treatment. Epo-treated babies received either 300 U/kg (n=52) or 500 U/kg (n=31), every other day for 2 weeks, with the first dose administered by 48 hours of life. Epo treatment improved neurologic signs at 7, 14, and 28 days as assessed by Thompson Neurologic Assessment, reduced disability for moderate HIE, decreased the overall number of MDI scores below 70, and reduced the incidence of cerebral palsy at 18 months of age. Death or disability at 18 months was present in 43.8% of controls compared to 24.6% of Epo-treated subjects (p < 0.02). There were no discernable differences in outcomes based on Epo doses, and no adverse effects of Epo were identified. Consistent with trials of hypothermia for HIE, Epo was only effective for infants with moderate injury, and did not improve outcome for severely-affected infants. Considering that both hypothermia and Epo are more effective when given proximal to injury, it is possible that severe HIE cases are indicative of an early insult with protracted prenatal injury and this highlights the drawback of rescue therapy.
The observation that low doses of Epo were neuroprotective in the Zhu trial warrants further discussion. Epo concentrations were measured in a subset of patients given 500 U/kg Epo and cerebrospinal fluid (CSF) Epo concentration increased within 3 h, and in parallel with circulating Epo concentrations which were only 14 – 44 mU/mL. The elevated CSF Epo concentration indicates that Epo readily penetrated the BBB. We speculate that hypoxia-ischemia triggers a breach in the BBB that permits greater penetration of Epo, and other factors, into brain tissues like CSF and presumably neuronal structures as well. Under these conditions, perhaps Epo neuroprotection includes systemic effects such as enhanced erythropoiesis which increases iron utilization, thereby decreasing free iron and reducing oxidative brain injury [20]. In that context, we note that Epo recently decreased the systemic inflammatory response in preterm and term infants [21*]. We speculate that systemic effects of Epo such as stabilizing oxygen availability, decreasing free iron, and reducing inflammation, complement the direct neuroprotective effects of Epo and may explain why lower dosing strategies also improve outcome. Figure 2 summarizes known mechanisms that contribute to Epo neuroprotection. The net effect of the acute actions of Epo work to decrease apoptosis. Epo also improves long term brain healing after an insult by providing increased oxygen carrying capacity through erythropoiesis and angiogenesis, and also by increasing neurogenesis.
Figure 2.
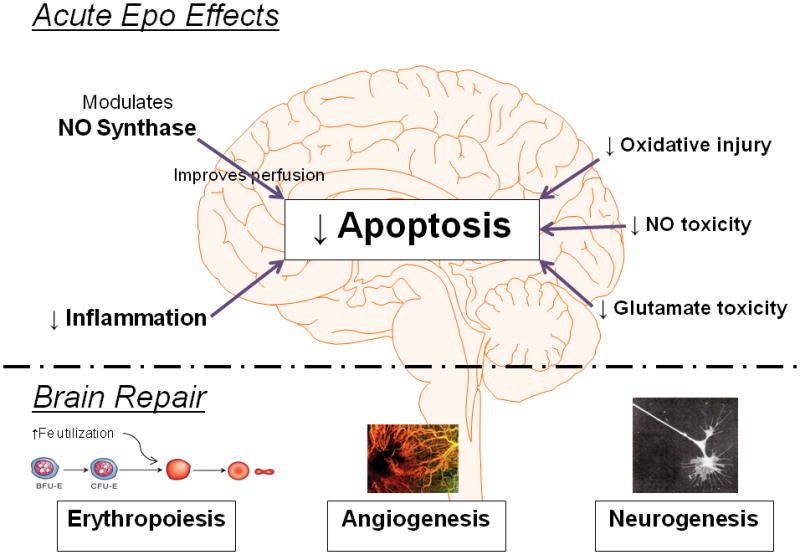
Mechanisms of Epo neuroprotection.
Combination Therapies
Epo has demonstrated neuroprotection in many models of brain injury, but protection has been incomplete. This has led investigators to consider combinations of protective therapies that also deserve discussion.
Hypothermia with Epo
Multiple clinical trials examining safety and efficacy of mild hypothermia (32 – 34°C) have now been published [1, 2, 3, 22]. Despite differences in approach (head cooling vs. total body cooling), there is general agreement that hypothermia improves outcomes for moderately (but not severely) asphyxiated infants, decreasing the combined outcome of death and neurologic dysfunction at 18 – 24 months from approximately 60 to 45%. Combination therapies such as Epo plus hypothermia are being considered to further improve outcomes. It will be important to thoroughly evaluate the safety of combined therapies because unanticipated complications may arise. It will also be important to consider the combined effects of hypoxia-ischemia, hypothermia, and Epo on clotting function. Hypoxia-ischemia increases the risk for disseminated intravascular coagulation. Hypothermia disrupts hemostasis in a dose-dependent manner with clotting disorders present even when hypothermia is mild [23], possibly due to decreased fibrinogen availability and delayed thrombin production [24]. Epo treatment may also influence clotting function because adults exhibit cardiovascular accidents, catheter thrombosis, and clot formation coincident with Epo. No data are available as to how these forces interact. Even though no Epo-induced clotting complications have been reported in neonates, it is important to remain vigilant as clinical studies proceed.
Insulin like Growth Factor-1 (IGF-1) with Epo
IGF-1 is another possible candidate for use with Epo because it is neurotrophic and neuroprotective [25, 26*, 27]. Synergistic effects of IGF-1, mediated by activation of phosphoinositol kinase (PI3-K) have been reported when Epo and IGF-1 treatment are combined in cell culture [28]. IGF-1 augmented Epo neuroprotection and reduced the threshold dose of Epo. Epo and IGF-1 co-treatment also prolonged the therapeutic window so that treatment initiated 9 h after injury was still effective. Preliminary studies are testing the possible intranasal delivery of these combined treatments in rodents [29].
Other Applications of Epo
Research into the neuroprotective properties of Epo is not limited to newborn hypoxia-ischemia. In addition to its neuroprotective effects, Epo stimulates angiogenesis and neurogenesis [30*, 31*]. These properties make Epo a candidate therapy for many disorders in children and adults. For example, Epo has been recently evaluated as a treatment for traumatic brain injury [32, 33*, 34, 35], Parkinson’s disease [36], and depression [37, 38].
Mechanisms of Epo Neuroprotection
Epo receptor (EpoR) activation can trigger different signaling pathways. The conventional understanding is that Epo prevents neuronal apoptosis via Janus kinase/Stat5 activation and NFκB phosphorylation [39]. However, Epo neuroprotection also involves PI3K and protein kinase B (Akt) signaling. Using Stat5 null mutation, Byts et al. found that activation of PI3K/Akt, but not Stat5, was essential for Epo-induced protection against excitotoxicity, while Stat5 and Akt were required for neurotrophic effects of Epo [40**]. Similarly, Epo prevented excitotoxicity in neuronal cultures [41] via a PI3K/Akt-dependent mechanism. Data are beginning to associate specific effects of Epo with specific signaling pathways.
A brain-specific heterodimer composed of EpoR with common beta chain (βc) receptor was proposed as the specific receptor mediating Epo neuroprotection [42]. New data weakens this hypothesis because expression of the βc receptor does not correspond with either Epo or EpoR expression in brain [43*]. Sanchez et al. have definitively evaluated Epo, EpoR, and βc receptor RNA expression and receptor immunolabeling in postnatal and aged rat brain, and also examined neuronal precursor PC12 cells, and their findings refine the localization of EpoR and identify specific patterns of regulation during development.
Epo is reported to stimulate vascular endothelial growth factor secretion and angiogenesis via PI3K/Akt and extracellular-signal-regulated kinases (ERK, a.k.a. MAPK) signaling pathways [31*]. Other Epo effects are now thought to be mediated through Epo stimulation of brain-derived neurotrophic factor (BDNF) [44, 45]. Epo-mediated upregulation of BNDF occurs in hippocampus [46] after experimental autoimmune encephalomyelitis [47], and after spinal ischemia [48]. Electroconvulsive shock applied to rats stimulated both Epo and BDNF expression, suggesting that antidepressive effects of electroconvulsive therapy may involve Epo and BDNF [49].
Schelshorn et al. found that neurons specifically express hemoglobin in response to either hypoxia or Epo, and that neuronal hemoglobin expression is neuroprotective [50**]. This is interesting because local hemoglobin expression could ensure neuronal oxygen availability in the event of hypoxia-ischemia.
Risks of Epo
Complications seen in adults (e.g. hypertension, clotting, seizures, polycythemia, and death) have not been identified in infants. Preterm infants have a long history of Epo treatment, with few reported side effects. Neutropenia was initially thought to be a complication of Epo treatment unique to preterm infants [51, 52], but further experience has shown this not to be the case when erythropoietic doses are used [53, 54]. If higher doses are used for neuroprotection, this will need to be watched. In the 2 pilot studies published to date, this was not a noted complication [12**, 13**]. A concern unique to the preterm population remains whether Epo might increase the risk or severity of ROP. Since a prospective randomized human trial to study this is unlikely, we turn to animal models to help us answer this question. We recently reported that early high-dose Epo (5,000 U/kg/x3) did not exacerbate (or reduce) ROP in a neonatal rat model [55**]. Using a mouse model, Chen et al. suggested that Epo effects on ROP may depend on timing because late Epo exposure exacerbated, but early Epo exposure reduced experimental ROP [56**].
Conclusion
Consideration of Epo as a potential therapeutic agent for brain injury has advanced to a new phase. Clinical studies are ongoing to test the safety and efficacy of Epo in patient populations that span from newborns to adults. Neither term nor preterm infants have exhibited complications after Epo. Nevertheless, it is important to proceed cautiously with clinical trials because risks may vary among specific populations, ages, and disease states. We are cautiously optimistic regarding the use of repeated early high-dose Epo as a neurotherapeutic treatment for neonatal brain injury.
Acknowledgments
Authors have no conflicts of interest to declare. This report was supported by NIH grant R01-HD-52820-01A2, and March of Dimes #12-FY06-236.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 2.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 3.Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med. 2009;361:1349–1358. doi: 10.1056/NEJMoa0900854. [DOI] [PubMed] [Google Scholar]
- 4*.McPherson RJ, Juul SE. Recent trends in erythropoietin-mediated neuroprotection. Int J Dev Neurosci. 2008;26:103–111. doi: 10.1016/j.ijdevneu.2007.08.012. The historical evidence for Epo-mediated neuroprotection, and the rationale for using Epo to treat perinatal asphyxia are reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer MP, Sharma E, Carsons M. Recombinant erythropoietin and blood transfusion in selected preterm infants. Arch Dis Child Fetal Neonatal Ed. 2003;88:F41–45. doi: 10.1136/fn.88.1.F41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haiden N, Klebermass K, Cardona F, et al. A randomized, controlled trial of the effects of adding vitamin B12 and folate to erythropoietin for the treatment of anemia of prematurity. Pediatrics. 2006;118:180–188. doi: 10.1542/peds.2005-2475. [DOI] [PubMed] [Google Scholar]
- 7.Widness JA, Veng-Pedersen P, Peters C, et al. Erythropoietin pharmacokinetics in premature infants: developmental, nonlinearity, and treatment effects. J Appl Physiol. 1996;80:140–148. doi: 10.1152/jappl.1996.80.1.140. [DOI] [PubMed] [Google Scholar]
- 8.Ohls RK, Veerman MW, Christensen RD. Pharmacokinetics and effectiveness of recombinant erythropoietin administered to preterm infants by continuous infusion in total parenteral nutrition solution. J Pediatr. 1996;128:518–523. doi: 10.1016/s0022-3476(96)70363-3. [DOI] [PubMed] [Google Scholar]
- 9.Garcia MG, Hutson AD, Christensen RD. Effect of recombinant erythropoietin on “late” transfusions in the neonatal intensive care unit: a meta-analysis. J Perinatol. 2002;22:108–111. doi: 10.1038/sj.jp.7210677. [DOI] [PubMed] [Google Scholar]
- 10.Juul SE, McPherson RJ, Farrell FX, et al. Erythropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol Neonate. 2004;85:138–144. doi: 10.1159/000074970. [DOI] [PubMed] [Google Scholar]
- 11.McPherson RJ, Demers EJ, Juul SE. Safety of high-dose recombinant erythropoietin in a neonatal rat model. Neonatology. 2007;91:36–43. doi: 10.1159/000096969. [DOI] [PubMed] [Google Scholar]
- 12**.Fauchere JC, Dame C, Vonthein R, et al. An approach to using recombinant erythropoietin for neuroprotection in very preterm infants. Pediatrics. 2008;122:375–382. doi: 10.1542/peds.2007-2591. A phase I trial evaluating the safety of high-dose Epo given to very low birth weight infants at a single center. [DOI] [PubMed] [Google Scholar]
- 13**.Juul SE, McPherson RJ, Bauer LA, et al. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 2008;122:383–391. doi: 10.1542/peds.2007-2711. A phase I/II trial evaluating the safety and pharmacokinetics of 3 doses of Epo (500, 1000 and 2500U/kg) given i.v. to extremely low birth weight infants at a single center. [DOI] [PubMed] [Google Scholar]
- 14.Statler PA, McPherson RJ, Bauer LA, et al. Pharmacokinetics of high-dose recombinant erythropoietin in plasma and brain of neonatal rats. Pediatr Res. 2007;61:671–675. doi: 10.1203/pdr.0b013e31805341dc. [DOI] [PubMed] [Google Scholar]
- 15.He JS, Huang ZL, Yang H, et al. Early use of recombinant human erythropoietin promotes neurobehavioral development in preterm infants. Zhongguo Dang Dai Er Ke Za Zhi. 2008;10:586–588. [PubMed] [Google Scholar]
- 16.Ohls RK, Ehrenkranz RA, Das A, et al. Neurodevelopmental outcome and growth at 18 to 22 months’ corrected age in extremely low birth weight infants treated with early erythropoietin and iron. Pediatrics. 2004;114:1287–1291. doi: 10.1542/peds.2003-1129-L. [DOI] [PubMed] [Google Scholar]
- 17.Bierer R, Peceny MC, Hartenberger CH, Ohls RK. Erythropoietin concentrations and neurodevelopmental outcome in preterm infants. Pediatrics. 2006;118:e635–640. doi: 10.1542/peds.2005-3186. [DOI] [PubMed] [Google Scholar]
- 18**.Brown MS, Eichorst D, Lala-Black B, Gonzalez R. Higher cumulative doses of erythropoietin and developmental outcomes in preterm infants. Pediatrics. 2009;124:e681–687. doi: 10.1542/peds.2008-2701. The largest retrospective analysis to date evaluating preterm infants given 6 weeks of prophylactic neonatal Epo therapy finds that neurodevelopmental scores above one year of age are correlated with early Epo exposure. [DOI] [PubMed] [Google Scholar]
- 19**.Zhu C, Kang W, Xu F, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009;124:e218–226. doi: 10.1542/peds.2008-3553. The first published trial using Epo to treat HIE in term infants finds a number of benefits including reduced death and disability, and there were no adverse effects. [DOI] [PubMed] [Google Scholar]
- 20.Buonocore G, Perrone S, Longini M, et al. Non protein bound iron as early predictive marker of neonatal brain damage. Brain. 2003;126:1224–1230. doi: 10.1093/brain/awg116. [DOI] [PubMed] [Google Scholar]
- 21*.Strunk T, Hartel C, Temming P, et al. Erythropoietin inhibits cytokine production of neonatal and adult leukocytes. Acta Paediatr. 2008;97:16–20. doi: 10.1111/j.1651-2227.2007.00560.x. Using in vitro assay of cytokine production in whole blood, the authors find that Epo significantly reduces inflammatory cytokine production in term and preterm infants, and adults. [DOI] [PubMed] [Google Scholar]
- 22.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr Neurol. 2005;32:11–17. doi: 10.1016/j.pediatrneurol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Eicher DJ, Wagner CL, Katikaneni LP, et al. Moderate hypothermia in neonatal encephalopathy: safety outcomes. Pediatr Neurol. 2005;32:18–24. doi: 10.1016/j.pediatrneurol.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 24.Martini WZ. The effects of hypothermia on fibrinogen metabolism and coagulation function in swine. Metabolism. 2007;56:214–221. doi: 10.1016/j.metabol.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 25.Lin S, Fan LW, Rhodes PG, Cai Z. Intranasal administration of IGF-1 attenuates hypoxic-ischemic brain injury in neonatal rats. Exp Neurol. 2009;217:361–370. doi: 10.1016/j.expneurol.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Duarte AI, Santos P, Oliveira CR, et al. Insulin neuroprotection against oxidative stress is mediated by Akt and GSK-3beta signaling pathways and changes in protein expression. Biochim Biophys Acta. 2008;1783:994–1002. doi: 10.1016/j.bbamcr.2008.02.016. The authors analyze insulin and insulin growth factor-1 receptor signaling pathways in rat cortical neurons and implicate a PI3K/Akt pathway underlying insulin receptor-dependent neuroprotection. [DOI] [PubMed] [Google Scholar]
- 27.Vincent AM, Mobley BC, Hiller A, Feldman EL. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol Dis. 2004;16:407–416. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Digicaylioglu M, Garden G, Timberlake S, et al. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I. Proc Natl Acad Sci U S A. 2004;101:9855–9860. doi: 10.1073/pnas.0403172101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fletcher L, Kohli S, Sprague SM, et al. Intranasal delivery of erythropoietin plus insulin-like growth factor-I for acute neuroprotection in stroke. Laboratory investigation. J Neurosurg. 2009;111:164–170. doi: 10.3171/2009.2.JNS081199. [DOI] [PubMed] [Google Scholar]
- 30*.Esneault E, Pacary E, Eddi D, et al. Combined therapeutic strategy using erythropoietin and mesenchymal stem cells potentiates neurogenesis after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2008;28:1552–1563. doi: 10.1038/jcbfm.2008.40. This is test of systemic delivery of stem cells, with or without Epo cotreatment, to promote neurogenesis and recovery after transient focal cerebral ischemia in the adult rats. [DOI] [PubMed] [Google Scholar]
- 31*.Wang L, Chopp M, Gregg SR, et al. Neural progenitor cells treated with EPO induce angiogenesis through the production of VEGF. J Cereb Blood Flow Metab. 2008;28:1361–1368. doi: 10.1038/jcbfm.2008.32. An in vitro test showing that EPO enhances VEGF secretion in neural progenitor cells via PI3K/Akt signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adembri C, Massagrande A, Tani A, et al. Carbamylated erythropoietin is neuroprotective in an experimental model of traumatic brain injury. Crit Care Med. 2008;36:975–978. doi: 10.1097/CCM.0B013E3181644343. [DOI] [PubMed] [Google Scholar]
- 33*.Liao ZB, Zhi XG, Shi QH, He ZH. Recombinant human erythropoietin administration protects cortical neurons from traumatic brain injury in rats. Eur J Neurol. 2008;15:140–149. doi: 10.1111/j.1468-1331.2007.02013.x. Epo and Epo receptor expression increase in rat cortical neurons within the peritrauma region after TBI, with Epo receptor induction persisting for one week. [DOI] [PubMed] [Google Scholar]
- 34.Xiong Y, Mahmood A, Chopp M. Emerging treatments for traumatic brain injury. Expert Opin Emerg Drugs. 2009;14:67–84. doi: 10.1517/14728210902769601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Xiong Y, Mahmood A, et al. Therapeutic effects of erythropoietin on histological and functional outcomes following traumatic brain injury in rats are independent of hematocrit. Brain Res. 2009;1294:153–164. doi: 10.1016/j.brainres.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadota T, Shingo T, Yasuhara T, et al. Continuous intraventricular infusion of erythropoietin exerts neuroprotective/rescue effects upon Parkinson’s disease model of rats with enhanced neurogenesis. Brain Res. 2009;1254:120–127. doi: 10.1016/j.brainres.2008.11.094. [DOI] [PubMed] [Google Scholar]
- 37.Miskowiak K, O’Sullivan U, Harmer CJ. Erythropoietin reduces neural and cognitive processing of fear in human models of antidepressant drug action. Biol Psychiatry. 2007;62:1244–1250. doi: 10.1016/j.biopsych.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 38.Miskowiak K, Inkster B, Selvaraj S, et al. Erythropoietin improves mood and modulates the cognitive and neural processing of emotion 3 days post administration. Neuropsychopharmacology. 2008;33:611–618. doi: 10.1038/sj.npp.1301439. [DOI] [PubMed] [Google Scholar]
- 39.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kappaB signalling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 40**.Byts N, Samoylenko A, Fasshauer T, et al. Essential role for Stat5 in the neurotrophic but not in the neuroprotective effect of erythropoietin. Cell Death Differ. 2008;15:783–792. doi: 10.1038/cdd.2008.1. A well designed evaluation of Epo effects on hippocampal excitotoxicity in wild type and transgenic Stat5 knockout mice finds that PI3K/Akt signaling mediates Epo neuroprotection. [DOI] [PubMed] [Google Scholar]
- 41.Shang Y, Wu Y, Yao S, et al. Protective effect of erythropoietin against ketamine-induced apoptosis in cultured rat cortical neurons: involvement of PI3K/Akt and GSK-3 beta pathway. Apoptosis. 2007;12:2187–2195. doi: 10.1007/s10495-007-0141-1. [DOI] [PubMed] [Google Scholar]
- 42.Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- 43*.Sanchez PE, Navarro FP, Fares RP, et al. Erythropoietin receptor expression is concordant with erythropoietin but not with common beta chain expression in the rat brain throughout the life span. J Comp Neurol. 2009;514:403–414. doi: 10.1002/cne.22020. A thorough analysis of RNA expression and immunolabeling of rat brain for beta common receptor, Epo, and Epo receptor that finds no overlap and thus disqualifies the EpoR-beta common heteroreceptor hypothesis. [DOI] [PubMed] [Google Scholar]
- 44.Dzietko M, Felderhoff-Mueser U, Sifringer M, et al. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15:177–187. doi: 10.1016/j.nbd.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Zhang Z, Wang Y, et al. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 46.Viviani B, Bartesaghi S, Corsini E, et al. Erythropoietin protects primary hippocampal neurons increasing the expression of brain-derived neurotrophic factor. J Neurochem. 2005;93:412–421. doi: 10.1111/j.1471-4159.2005.03033.x. [DOI] [PubMed] [Google Scholar]
- 47.Zhang J, Li Y, Cui Y, et al. Erythropoietin treatment improves neurological functional recovery in EAE mice. Brain Res. 2005;1034:34–39. doi: 10.1016/j.brainres.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 48.Sonmez A, Kabakci B, Vardar E, et al. Erythropoietin attenuates neuronal injury and potentiates the expression of pCREB in anterior horn after transient spinal cord ischemia in rats. Surg Neurol. 2007;68:297–303. doi: 10.1016/j.surneu.2006.11.045. discussion 303. [DOI] [PubMed] [Google Scholar]
- 49.Girgenti MJ, Hunsberger J, Duman CH, et al. Erythropoietin induction by electroconvulsive seizure, gene regulation, and antidepressant-like behavioral effects. Biol Psychiatry. 2009;66:267–274. doi: 10.1016/j.biopsych.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 50**.Schelshorn DW, Schneider A, Kuschinsky W, et al. Expression of hemoglobin in rodent neurons. J Cereb Blood Flow Metab. 2009;29:585–595. doi: 10.1038/jcbfm.2008.152. An interesting new finding that both hypoxia and Epo trigger neuronal hemoglobin expression that improves brain oxygenation. [DOI] [PubMed] [Google Scholar]
- 51.Ohls RK, Christensen RD. Recombinant erythropoietin compared with erythrocyte transfusion in the treatment of anemia of prematurity. J Pediatr. 1991;119:781–788. doi: 10.1016/s0022-3476(05)80303-8. [DOI] [PubMed] [Google Scholar]
- 52.Christensen RD, Rothstein G. Erythropoietin affects the maturation pattern of fetal G-CSF-responsive progenitors. Am J Hematol. 1992;39:108–112. doi: 10.1002/ajh.2830390207. [DOI] [PubMed] [Google Scholar]
- 53.Aher SM, Ohlsson A. Early versus late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;3:CD004865. doi: 10.1002/14651858.CD004865.pub2. [DOI] [PubMed] [Google Scholar]
- 54.Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;3:CD004863. doi: 10.1002/14651858.CD004863.pub2. [DOI] [PubMed] [Google Scholar]
- 55**.Slusarski JD, McPherson RJ, Wallace GN, Juul SE. High-Dose Erythropoietin Does Not Exacerbate Retinopathy of Prematurity in Rats. Pediatr Res. 2009 doi: 10.1203/PDR.0b013e3181bc33e6. A recent test of the safety of high-dose Epo using a rat model of ROP finds that repeated 5,000 U/kg Epo has no effect on retinal blood vessel development. [DOI] [PubMed] [Google Scholar]
- 56**.Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008;118:526–533. doi: 10.1172/JCI33813. Early Epo administration is shown to decrease ROP by normalizing blood vessel growth after exposure to hyperoxia; late Epo signaling is shown to increase ROP pathology by virtue of its angiogenic effects. [DOI] [PMC free article] [PubMed] [Google Scholar]