Abstract
Betel nut chewing has been reported to increase the risk of cardiovascular disease and all-cause mortality. The reason is unclear. In this study, we investigated the association between betel nut chewing and general obesity (BMI ≥25kg/m2) and central obesity (waist circumference (WC) ≥90 cm). A total of 1,049 male subjects, aged ≥40 years, were recruited from Taichung city in Taiwan in 2004. The relationships between betel nut chewing and general and central obesity were studied by multiple linear and logistic regression analyses. The prevalence of current and former betel nut chewing was 7.0 and 10.5% in our male Taiwanese cohort. Current/former betel nut chewers had a higher prevalence of general and central obesity when compared with individuals who had never chewed betel nut. Adjusted for age, diabetes, hypertension, lipids, smoking, alcohol drinking, physical activity, income, and education level, the odds ratios (ORs; 95% confidence intervals) of general and central obesity among the lower consumption of betel nut chewers were 1.78 (1.07, 2.96) and 1.19 (0.70, 2.02), respectively, compared to 2.01 (1.18, 3.41) and 1.89 (1.10, 3.23), respectively, among higher consumption chewers compared to individuals who had never chewed betel nut. The increasing ORs of general and central obesity with higher betel nut consumption revealed dose–response effects. Using multiple linear regression analyses, after adjusting for potential confounders, betel nut consumption was statistically significantly associated with BMI and WC. In conclusion, betel nut chewing was independently associated with general and central obesity in Taiwanese men. Dose–response effects of the association between betel nut consumption and general obesity as well as central obesity were found.
INTRODUCTION
Obesity is one of the most serious problems in human health and is recognized as an important risk factor for many chronic diseases, such as cardiovascular disease, type 2 diabetes, hypertension, and cancer (1–6). It has also been identified as the second preventable cause of death (7). Obesity has increased dramatically around the world. In US adults, the prevalence of obesity (BMI ≥30kg/m2) doubled between 1986 and 2000 (8). According to the National Bureau of Economic Research in the United States, about three-fourths of Americans ≥20 years are predicted to be overweight and two-fifths to be obese by the year 2020 (9). Although the prevalence of obesity is high and rising in developed countries, the increase is often faster in developing nations. For example, a nationally representative survey in China from 1991 to 1999–2000 showed that the prevalence of overweight and obesity had dramatically increased in all age groups and in both rural and urban areas (10). The increase of obesity (BMI ≥28kg/m2) was more than threefold in men and twofold in women (10). The situation is similar in many other Asia-Pacific regions. In Taiwan, based on the data of the Nutrition and Health Surveys of 1993–1996 and 2000–2001, the prevalence of obesity (BMI ≥25kg/m2) in adult men increased from 24.7 to 33.1% (11). This dramatic increase in obesity during the past two decades underscores that every region has its own specific preferences of food and lifestyle behavior and it is important to identify different risk factors leading to obesity in different regions.
Betel nut (Areca catechu) is the fourth most widely used addictive substance in the world (12, 13). There are ~600 million people who chew betel nut worldwide, most of them resident in Asia-Pacific regions (13). Betel nut chewing is not only related to the development of oral and esophageal cancer (12,14,15) but also to type 2 diabetes mellitus, hypertension, metabolic syndrome, chronic kidney disease, and heart disease (16–20). In a previous study, we found that Taiwanese men who were betel nut chewers (either current chewers or former chewers) had an increased risk of cardiovascular disease and all-cause mortality (21). The main component of betel nut (areca alkaloids) is an inhibitor of γ-aminobutyric acid (GABA) receptor (11). Betel nut chewing was closely associated with BMI and it was proposed that the underlying cause was increased appetite of betel nut chewers through the inhibitory effects of betel nut on the GABA receptor (22). Chang et al. and Mannan et al. also proposed that obesity could be the possible intermediary by which betel nut chewing could increase cardiovascular disease (22,23). However, these studies only focused on the relationship between betel nut chewing and either general or central obesity. Furthermore, these studies did not detail the cumulative betel nut chewing-years, so that the association between betel nut chewing and obesity could be influenced by residual confounding. Because betel nut consumption may increase the risk for obesity and its complications, it is important to assess the association between betel nut chewing and obesity in Asian countries. The World Health Organization has used BMI and waist circumference (WC) to predict obesity (6), so we investigated the association between both general obesity (measured by BMI) and central obesity (measured by WC) and cumulative betel nut chewing-years in Taiwanese men in a metropolitan city.
METHODS AND PROCEDURES
Subjects
The target population was Taiwan citizens aged ≥40 in Taichung city, Taiwan in October 2004. There were a total of 363,543 residents in this area at this time, which represented ~4.09% of the national population of the same age. A stratified, two-stage random sampling approach was used for the selection of the survey sample; and the sampling rate was proportional to size within each stage. A total of 4,280 individuals were selected. During household visits, we identified 750 individuals who were not eligible and we excluded them from the sample. The reasons for exclusion included death (n = 18), hospitalization or imprisonment (n = 14), living abroad (n = 39), moving out of the home (n = 411), living with relatives/children’s house (n = 7), sampling frame errors (n = 59), and not being at home during three visits made by interviewers (n = 202). Among 3,530 individuals selected, only 2,359 agreed to participate. Thus, the overall response rate was 66.8% (24–26). Among the 1,147 male subjects, 98 individuals did not complete the questionnaire items pertaining to betel nut chewing habits. Therefore, the baseline subjects analyzed in the study comprised 1,049 male participants. Female betel nut chewers were excluded because the prevalence of chewers in women was very low (prevalence was 17.4% in men and 0.25% in female in this study). No statistical differences were observed in age, height, weight, BMI, WC, and hip circumference among selected subjects (n = 1,049) and excluded subjects (n = 98).
Anthropometric index and laboratory assays
Trained staff measured height, WC, hip circumference, weight, and blood pressure as described in previous reports (24–26). The cohort was divided into BMI (kg/m2) quartiles. Blood was drawn in the morning after a 12-h overnight fast and was sent for analysis within 4h of collection. Total cholesterol, high-density lipoprotein cholesterol, triglycerides, and fasting glucose level were analyzed by a biochemical autoanalyzer (Beckman Coulter, Fullerton, CA) at the Clinical Laboratory Department (China Medical University Hospital, Taichung, Taiwan).
Sociodemographic factors and lifestyle behaviors
Information on selected demographic (age, gender, marital status, employment, level of education), socioeconomic, lifestyle, and behavioral characteristics (diet history, physical activity) including medical history were collected using self-administered questionnaires. Marital status was coded as single (unmarried), married, and widower/divorce/separate. The cumulative exposure to smoking, alcohol drinking, and betel nut chewing was assessed by recording the duration (years), frequency (times/day), quantity (number/times), and ethanol content (percent). Former users were also asked for their age at quitting. Cumulative pack-years of smoking were calculated as smoking-years multiplied by average daily cigarette use divided by 20. Cumulative pack-years for smokers were categorized based on an equal distribution (low: ≤19.5 pack-years; high: >19.5 pack-years), so smoking status was categorized as none (0 pack-years), low (0–19.5 pack-years), and high (>19.5 pack-years). History of alcohol consumption was coded as drink-years (drink/day × years) in which a “drink” was defined as consuming 0.5 oz (13.7g) of ethanol (27). Average alcohol consumption for each time was defined as the average amount of alcohol consumed each time across the interviews. The average frequency of alcohol drinking was recorded as limes/week. It was divided by 7 to get times/day. The average content of alcohol drinking was recorded as the average percent of different type of alcohol which subjects usually drink. Daily alcohol amount for a consumed beverage was calculated as follows: “drink” = average alcohol consumption per times × average frequency per day × average content of alcohol drinking divided by 13.7. Drink-years for alcohol drinkers were categorized based on an equal distribution (low: ≤3.4 drink-year; high: >3.4 drink-years), so alcohol drinking status was divided as none, low, and high.
Average betel nut chewing was defined as the average amount of betel nut consumed each time across the interviews (quids/times). The average frequency of betel nut chewing was recorded as times/day. Cumulative quids/day-years of betel nut chewing was calculated as follows: betel nut consumed each time × daily average frequency of betel nut chewing × exposure years. Betel nut chewing-years for chewers were categorized based on an equal distribution (low: ≤76 quids/day × year; high: >76 quids/day × year). Finally, betel nut chewing status was divided as none, low, and high. Income was divided into three levels: low (<USD 15,000/year, rate = 32:1), middle (15,000–37,500/year), and high (>37,500/year). Education was also divided into three levels: low (elementary school and below), middle (junior and senior high school), and high (college/university and above).
Definitions of obesity, diabetes, hypertension, and mean arterial pressure
Obesity was defined as BMI ≥25kg/m2 and central obesity as WC ≥90cm, according to the definition of the World Health Organization for Asia-Pacific area (5). Diabetes was defined as fasting plasma glucose concentration ≥7.0 mmol/l or on drug treatment for diabetes. Hypertension was defined as systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg or on drug treatment for hypertension. Mean arterial pressure was calculated as ((2 × diastolic BP) + systolic BP)/3.
Statistical analysis
The data are presented as means and s.d. unless indicated otherwise. Student’s t-test and Pearson’s χ2-test were used to compare mean values for continuous and categorical variables. Log transformation was used for variables with significant deviation from normal distribution, assessed by the Kolmogorov–Smirnov test before further analyses. ANOVA test was used to compare the continuous variables across betel nut chewing groups. The Pearson’s χ2-test was used to compare the differences in the lifestyle variables (smoking, alcohol drinking, betel nut chewing, and physical activity) and socioeconomic factors (income and education level). Multivariate logistic regression analyses were used to estimate the adjusted odds ratios (ORs) and their 95% confidence intervals for the presence of general and central obesity in relation to betel nut chewing. Three different models were derived by adjusting for different confounders (such as age, lifestyle behavior, and laboratory assays) to minimize residual confounding. Multiple linear regression analyses were also used to assess the association between BMI/WC and cumulative exposure of betel nut chewing with adjustment for potential confounders. All statistical tests were two sided at the 0.05 significance level. These statistical analyses were performed using the PC version of SPSS statistical software (13th version; SPSS, Chicago, IL).
Ethics approval for patient recruitment and data analyses was obtained from the Institutional Review Board of the China Medical University Hospital.
RESULTS
The prevalence of current and former betel nut chewing was 7.0 and 10.5% in male Taiwanese. The prevalence of general and central obesity was 43.5 and 33.9%, respectively. Subjects with general obesity were younger and had higher body weight, BMI, WC, hip circumference, systolic BP, diastolic BP, fasting glucose, triacylglycerol, and prevalence of hypertension and lower high-density lipoprotein cholesterol than nongeneral obesity subjects (Table 1). Central obesity subjects were older and had greater height, body weight, BMI, WC, hip circumference, systolic BP, diastolic BP, triacylglycerol, and the prevalence of diabetes and hypertension and lower high-density lipoprotein cholesterol than noncentral obesity subjects (Table 1). Chewers with higher betel nut consumption were younger and had higher body weight, BMI, WC, smoking pack-years, alcohol drink-years, triacylglycerol, income level, and education level than nonchewers (Table 2). Using multiple logistic regression analyses with adjustment for potential confounders, the ORs for general and central obesity were statistically higher among chewers with higher betel nut consumption than among nonchewers (models 1–3 in Table 3). Among these models, there was no interaction (P > 0.05) between betel nut chewing and smoking or alcohol drinking status for predicting the risk of general and central obesity. Adjusting for age, diabetes, hypertension, lipids, smoking, alcohol drinking, physical activity, income, and education level, the adjusted ORs (95% confidence intervals) of general and central obesity among the lower consumption of betel nut chewers were 1.78 (1.07, 2.96) and 1.19 (0.70, 2.02), respectively, and 2.01 (1.18, 3.41) and 1.89 (1.10, 3.23), respectively, among higher consumption chewers compared with individuals who had never chewed betel quid (model 3 in Table 3). To better understand the association between obesity and cumulative betel nut chewing exposure, we also used multiple linear regression analyses to clarify the relationship between BMI and/or WC and betel nut chewing-years (Table 4). Log transformation for mean arterial pressure, fasting glucose, and triacylglycerol were done for normal distribution. The distribution about cumulative betel nut chewing, smoking, and alcohol drinking was not normal, so we divided them into two dummy variables (high vs. none and low vs. none). After adjusting for potential confounders, we found that BMI and WC were strongly associated with cumulative betel nut exposure (betel nut chewing-years) (Table 4). Smoking is another risk factor of general and central obesity. To further clarify the effect of smoking and betel nut chewing on obesity, we analyzed the relationship between betel nut chewing and general and/or central obesity in never smokers. Figure 1 compares those who never smoked to never chewers. Using multiple logistic regression analyses with adjustment for potential confounders, the adjusted ORs of general obesity (Figure 1a) and central obesity (Figure 1b) among the lower consumption of betel nut chewers were 3.64 (0.71, 18.6) and 1.57 (0.40, 6.22), respectively, and 2.97 (0.50, 17.9) and 6.32 (1.00, 39.7), respectively, among higher consumption chewers. The adjusted ORs for the presence of general and central obesity in relation to betel nut chewing were higher among never smokers than among all subjects (Figure 1).
Table 1.
Baseline characteristics according to status of obesity
| General obesity (BMI ≥25 kg/m2) (n = 456) | Nonobesity (BMI <25 kg/m2) (n = 593) | Centrally obese (WC ≥90cm) (n = 356) | Noncentrally obese (WC<90cm) (n = 693) | |
|---|---|---|---|---|
| Age (years) | 57.3 ± 11.8‡ | 59.6± 12.4† | 59.8± 12.4* | 58.1 ±12.1* |
| Height (cm) | 166.8±6.2 | 166.2 ± 6.1 | 167.8±6.3‡ | 165.8 ± 6.0‡ |
| Body weight (kg) | 76.9±8.7‡ | 62.6 ± 6.7‡ | 78.0±9.3‡ | 64.2 ± 7.4‡ |
| BMI (kg/m2) | 27.6±2.3‡ | 22.7 ± 1.8‡ | 27.7 ± 2.7‡ | 23.3±2.3‡ |
| WC (cm) | 92.9± 7.0‡ | 81.3 ± 6.5‡ | 95.8±5.6‡ | 81.5 ± 5.8‡ |
| HipC (cm) | 101.5 ± 5.9‡ | 93.5±4.6‡ | 102.6±5.6‡ | 94.0±4.8‡ |
| Systolic BP (mmHg) | 143.0 ± 20.3‡ | 134.4 ± 20.1‡ | 145.8 ± 20.5‡ | 134.2 ± 19.5‡ |
| Diastolic BP (mmHg) | 85.6 ± 11.3‡ | 80.0± 11.2‡ | 86.5±11.1‡ | 80.3 ± 11.2‡ |
| Fasting glucose (mmol/l) | 6.00 ± 1.49* | 5.79 ± 1.65* | 6.14±1.65‡ | 5.74± 1.53‡ |
| TCHOL (mmol/l) | 5.20±0.93 | 5.14±0.96 | 5.23±0.96 | 5.13±0.93 |
| Triacylglycerol (mmol/l) | 1.80± 1.39‡ | 1.36 ± 1.19‡ | 1.85 ± 1.41‡ | 1.41±1.21‡ |
| HDL-C (mmol/l) | 1.01 ± 0.25‡ | 1.12 ± 0.30‡ | 1.00 ±0.24‡ | 1.11 ±0.30‡ |
| Diabetes (%) | 15.4 | 13.0 | 19.1‡ | 11.4‡ |
| Hypertension (%) | 64.5‡ | 43.3‡ | 70.2‡ | 43.4‡ |
| Alcohol drinking (%) | ||||
| Never | 52.4 | 55.6 | 52.8 | 55.0 |
| Former | 9.6 | 8.3 | 10.4 | 8.1 |
| Current | 37.9 | 36.1 | 36.8 | 36.9 |
| Smoking(%) | † | † | ||
| Never | 45.8 | 52.1 | 42.7 | 52.8 |
| Former | 26.1 | 20.4 | 27.0 | 20.8 |
| Current | 28.1 | 27.5 | 30.3 | 26.4 |
| Betel nut chewing (%) | † | † | * | * |
| Never | 77.9 | 86.2 | 79.2 | 84.3 |
| Former | 14.0 | 7.8 | 13.8 | 8.8 |
| Current | 8.1 | 6.1 | 7.0 | 6.9 |
| Physical activity (%) | ||||
| None/seldom | 32.2 | 29.2 | 30.3 | 30.6 |
| Regular | 67.8 | 70.8 | 69.7 | 69.4 |
| Income(%) | * | * | ||
| Low | 44.5 | 50.6 | 46.9 | 48.5 |
| Middle | 41.9 | 39.0 | 40.9 | 39.9 |
| High | 13.6 | 10.4 | 12.3 | 11.5 |
| Education (%) | ||||
| Low | 19.6 | 16.8 | 20.6 | 16.6 |
| Middle | 38.2 | 40.1 | 38.3 | 39.8 |
| High | 42.2 | 43.1 | 41.1 | 43.6 |
| Marital status (%) | ||||
| Single (unmarried) | 1.8 | 2.5 | 1.4 | 2.6 |
| Married | 89.9 | 87.1 | 90.7 | 87.1 |
| Widower/divorce/separate | 8.3 | 10.3 | 7.9 | 10.3 |
Student’s t-test was used for comparing mean values of continuous variables between groups (general obesity vs. nonobesity group and centrally obese vs. noncentrally obese group); data were shown as means ± s.d. Pearson’s χ2-test was used for categorical data; data were shown as percentage.
BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; HipC, hip circumference; TCHOL, total cholesterol; WC, waist circumference.
P < 0.05;
P < 0.01;
P < 0.001.
Table 2.
Demographic and laboratory characteristics according to different categories of betel nut use
| None (n = 866) | Low (≤76quids/day × year; n = 91) | High (>76 quids/day × year; n = 92) | P value | |
|---|---|---|---|---|
| Age (years) | 59.6 ± 12.4 | 51.9 ± 9.2 | 56.5±10.8 | <0.001 |
| Height (cm) | 166.6±6.2 | 166.5±5.7 | 165.3±5.8 | 0.179 |
| Body weight (kg) | 68.4 ± 10.4 | 71.1 ±9.7 | 70.8±11.0 | 0.012 |
| BMI (kg/m2) | 24.6±3.2 | 25.6±2.9 | 25.8±3.4 | <0.001 |
| WC(cm) | 86.1 ±8.8 | 85.9± 7.9 | 89.5±9.4 | 0.001 |
| HipC(cm) | 96.9±6.5 | 96.8± 6.9 | 97.7±6.7 | 0.502 |
| Smoking (pack-years) | 9.31 ± 16.6 | 21.4± 17.9 | 27.2 ±20.5 | <0.001 |
| Alcohol (drink-years) | 8.79±39.2 | 25.7 ± 123.5 | 32.1 ±71.5 | <0.001 |
| Systolic BP (mm Hg) | 138.3± 20.3 | 134.0±19.6 | 140.5±23.7 | 0.085 |
| Diastolic BP (mm Hg) | 82.1 ± 11.3 | 82.9±12.3 | 84.9± 12.9 | 0.081 |
| Fasting glucose (mmol/l) | 5.88± 1.58 | 5.74±1.58 | 6.03± 1.60 | 0.455 |
| TCHOL (mmol/l) | 5.17±0.92 | 5.16± 1.01 | 5.13 ± 1.13 | 0.907 |
| Triacylglycerol (mmol/l) | 1.48± 1.17 | 1.72± 1.11 | 2.15 ± 2.16 | <0.001 |
| HDL-C (mmol/l) | 1.08±0.28 | 1.06±0.26 | 1.06±0.32 | 0.737 |
| Diabetes (%) | 14.3 | 11.0 | 14.1 | 0.684 |
| Hypertension (%) | 53.1 | 42.9 | 56.5 | 0.127 |
| Physical activity (%) | <0.001 | |||
| None/seldom | 26.8 | 46.2 | 50.0 | |
| Regular | 73.2 | 53.8 | 50.0 | |
| Income(%) | <0.001 | |||
| Low | 45.2 | 52.7 | 69.7 | |
| Middle | 41.9 | 37.4 | 27.0 | |
| High | 12.9 | 9.9 | 3.4 | |
| Education (%) | <0.001 | |||
| Low | 15.4 | 16.5 | 43.5 | |
| Middle | 36.7 | 53.8 | 48.9 | |
| High | 47.9 | 29.7 | 7.6 | |
| Marital status (%) | 0.016 | |||
| Single (unmarried) | 1.9 | 4.4 | 3.3 | |
| Married | 89.8 | 83.5 | 79.3 | |
| Widower/divorce/separate | 8.3 | 12.1 | 17.4 |
ANOVA test was used for comparing mean values of continuous variables between groups; data were shown as means± s.d.; Pearson’s χ2-test was used for categorical data; data were shown as percentage.
BP, blood pressure; HDL-C, high-density lipoprotein cholesterol; HipC, hip circumference; TCHOL, total cholesterol; WC, waist circumference.
Table 3.
Odds ratios (95% confidence interval) of having general and/or central obesity in several different models derived from a multiple logistic regression analysis using age, diabetes, hypertension, lipid profile, smoking, alcohol drinking, physical activity, income, and educational level as independent variables
| Betel nut chewing-years (n) | Obesity | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| None (n = 866) | General | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Low (n = 91) | General | 1.68 (1.09, 2.59)* | 1.53 (0.95, 2.48) | 1.78 (1.07, 2.96)* |
| High (n = 92) | General | 1.87 (1.21, 2.89)† | 1.90 (1.16, 3.10)* | 2.01 (1.18, 3.41)* |
| None (n = 866) | Central | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Low (n = 91) | Central | 1.07 (0.68, 1.69) | 1.03 (0.62, 1.71) | 1.19 (0.70, 2.02) |
| High (n = 92) | Central | 1.82 (1.18, 2.80)† | 1.75 (1.06, 2.87)* | 1.89 (1.10, 3.23)* |
Betel nut chewing-years for chewers were categorized based on an equal distribution (low: ≤76 quids/day × year; high: >76 quids/day × year). Model 1: unadjusted. Model 2: adjusted for age, alcohol drinking, smoking, physical activity, income, and educational level. Model 3: adjusted for age, diabetes, hypertension, total cholesterol, high-density lipoprotein cholesterol, triacylglycerol, smoking, alcohol drinking, physical activity, income, and educational level. No interaction between betel nut chewing and smoking status for predicting the risk of general obesity (P = 0.517) and central obesity (P = 0.148); no interaction between betel nut chewing and alcohol drinking status for predicting the risk of general obesity (P = 0.407) and central obesity (P = 0.725).
P < 0.05;
P < 0.01;
P < 0.001.
Table 4.
Multiple linear regression analyses of betel nut chewing-years as a risk factor for the BMI and/or waist circumference
| BMI |
WC |
|||
|---|---|---|---|---|
| Coefficient | P valuea | Coefficient | P valuea | |
| Betel nut chewing (quids/day-years)b,c | ||||
| High vs. none | 0.867 ± 0.342 | 0.011 | 2.294 ± 0.947 | 0.016 |
| Low vs. none | 0.708 ± 0.332 | 0.033 | −0.442 ± 0.921 | 0.631 |
| Smoking (pack-years)b,c | ||||
| High vs. none | −0.113±0.241 | 0.640 | 0.840 ± 0.668 | 0.209 |
| Low vs. none | 0.040 ± 0.226 | 0.858 | 0.666 ± 0.627 | 0.289 |
| Alcohol drinking (drink-years)b,c | ||||
| High vs. none | 0.134 ± 0.240 | 0.577 | 0.524 ± 0.665 | 0.431 |
| Low vs. none | 0.282 ±0.227 | 0.216 | 0.312 ± 0.630 | 0.621 |
| Age (years) | −0.030±0.008 | <0.001 | 0.033 ± 0.023 | 0.161 |
| LnMAP (mm Hg)d | 6.572 ±0.690 | <0.001 | 18.04± 1.912 | <0.001 |
| LnFasting glucose (mmol/l)d | 0.788 ±0.440 | 0.073 | 2.918± 1.219 | 0.017 |
| TCHOL (mmol/l) | 0.069 ± 0.110 | 0.528 | 0.365 ± 0.304 | 0.229 |
| HDL-C (mmol/l) | −2.543 ± 0.373 | <0.001 | −7.317± 1.033 | <0.001 |
| LnTriacylglycerol (mmol/l)d | 0.774±0.187 | <0.001 | 2.227±0.519 | <0.001 |
HDL-C, high-density lipoprotein cholesterol; MAP, mean arterial pressure; TCHOL, total cholesterol; WC, waist circumference.
Using multiple linear regression analyses after adjusted for physical activity, income, and education level.
Betel nut chewing-years for chewers, pack-years for smokers, and drink-years for drinkers were categorized based on an equal distribution (low: ≤76 quids/day × year; high: >76 quids/day × year for chewers; low: ≤19.5 pack-years; high: > 19.5 pack-years for smokers; low: ≤3.4 drink-year; high: >3.4 drink-years for drinkers).
Cumulative betel nut chewing, smoking, and alcohol drinking was divided into two dummy variables: high vs. none and low vs. none.
Log transformation was done for normal distribution.
Figure 1.
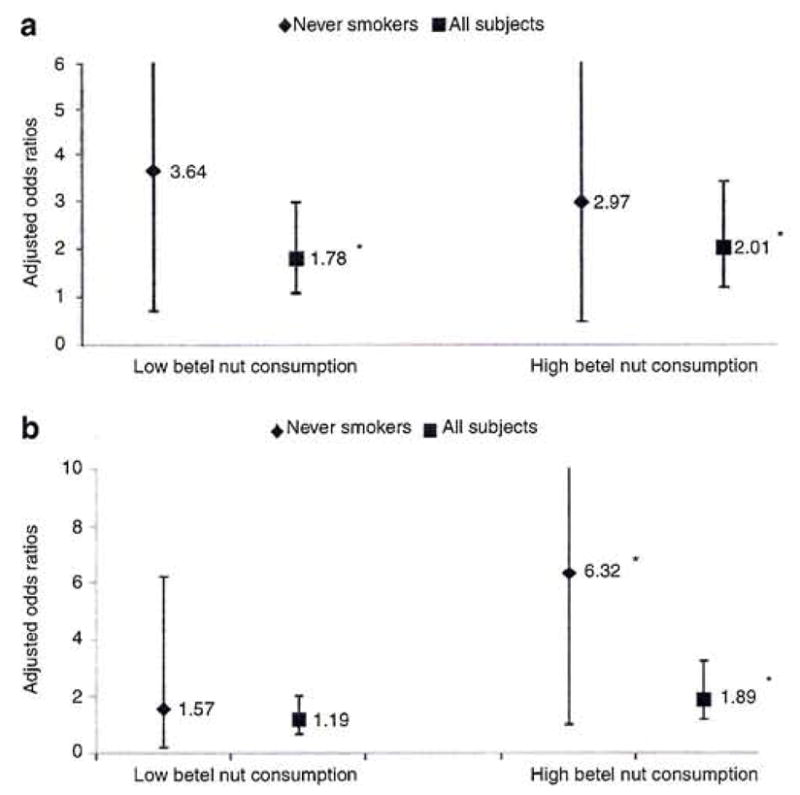
Odds ratios between obesity and betel nut chewing in all subjects and never smokers. Adjusted odds ratios of having (a) general obesity and (b) central obesity in all subjects and never smokers derived from a multiple logistic regression analysis using age, diabetes, hypertension, lipid profile, alcohol drinking, physical activity, income, and educational level as independent variables. In never smokers, compared to never chewers, the adjusted odds ratios of general and central obesity among the lower consumption of betel nut chewers were 3.64 (0.71, 18.6) and 1.57 (0.40, 6.22), respectively, and 2.97 (0.50, 17.9) and 6.32 (1.00, 39.7), respectively, among higher consumption chewers. *P < 0.05.
DISCUSSION
We have demonstrated that both general obesity and central obesity are closely associated to cumulative betel nut chewing-years in a population-based study. Dose-response effects were also found. Furthermore, the effects of betel nut chewing on obesity are remarkably increased in never smokers. Our finding has important implications because there is a large population in the world who is obese and chews betel nuts. A great challenge in public health in Taiwan, as well as in other countries with a high prevalence of betel nut chewing, is to develop useful strategies for the cessation of betel nut chewing.
Few studies have addressed the association between obesity and betel nut chewing. Previous animal studies have shown inconsistent results. For example, 2-week-old hamsters which were fed betel quid or areca nut for 18 months showed decreased body weight (28). On the contrary, young adult CD1 mice which were fed betel nut in standard feed for 2–6 days developed central obesity later (29). Two epidemiological human studies support our result. In one, Yen et al. found betel-quid chewing to be associated with central obesity in a population-based study (16). In another, Mannan et al. found that Asian betel nut chewers in East London increased their WC (23). Chang et al. also found betel nut chewing associated with general obesity in Taiwan (22). However, there are no population-based studies which investigated the relationship between betel nut chewing and both general and central obesity. Our population -based study demonstrates that both general and central obesity (using either multiple logistic regression or multiple linear regression analyses) are strongly associated with betel nut chewing. Although our study is a cross-sectional one, our population is large enough to allow us to adjust for many potential confounders, such as lifestyle factors, diabetes, hypertension, and lipid profile, which have been shown to increase the risk of obesity, Finally, we also have found the adjusted ORs for general and central obesity increased with the increase of cumulative betel nut chewing-years dose, which supports the possibility of a causal association between betel nut chewing and obesity. The dose–response relationship exists numerically, but did not reach statistical significance between the high and low doses of betel nut chewing.
There are two possible mechanisms linking obesity and betel nut chewing. First, the main component of betel nut (areca alkaloids) is an inhibitor of GABA receptor (12). The inhibitory effects of betel nut on the GABA receptor may result in an increase of appetite with eventual fat accumulation (12). Second, the areca nitrosamines derived from areca alkaloids are diabetogenic (12,29). Tung et al. have found that betel nut chewing independently contributes to the risk of hyperglycemia and type 2 diabetes in Taiwanese men (19). Hyperglycemia and type 2 diabetes are closely related to insulin resistance. Betel nut chewing may induce the development of insulin resistance, which is associated with an increase in obesity over time (30). The exact mechanism linking betel nut and obesity is unknown and this merits further study.
Cigarette smokers tend to have a lower BMI than nonsmokers (31). However, cigarette smokers accumulate significantly more abdominal adiposity than nonsmokers of a similar BMI (32–34). Although quitting smoking leads to weight gain, less upperbody fat deposition occurs than would be expected (34). In our study, we found that subjects with central obesity had a higher percent of current/former smokers than subjects with noncentral obesity, but this was not true for the general obesity group (Table 1). In Table 4, even though there was no statistically significant difference between BMI/WC and smoking status, the relationship between WC and smoking status trends positive and for BMI, it trends negative. This is similar to other studies (32,33). Both betel nut chewing and smoking are independently associated with central obesity in this study. We also analyzed the effect of betel nut chewing and smoking on obesity. In Figure 1, we found that the adjusted ORs are higher in never smokers than in all subjects. It seems that betel nut chewing is more important than smoking on increasing obesity, especially in central obesity. Furthermore, the adjusted ORs for central obesity increased with an increase of cumulative betel nut chewing dose, revealing a dose–response effect. Developing useful strategies for the cessation of both smoking and betel nut chewing are important future initiatives to prevent central obesity. In our previous report, we found that 83 and 64% of current betel nut chewers are concurrent smokers and alcohol drinkers, respectively (21). From Table 2, we found that subjects with a higher cumulative exposure of betel nut chewing tended to have a higher cumulative exposure of smoking and alcohol. It is likely that successful smoking cessation will reduce the rate of betel nut chewing in areas with a high prevalence of both smoking and betel nut chewing.
Smoking rate is increasing in most developing countries, especially in China, India, and Indonesia which also have a high prevalence of betel nut chewing (35). Successful smoking cessation strategies have been developed in the United States. According to the Centers for Disease Control and Prevention, cigarette smoking has been dropping steadily among American adults from 42.4% in 1965 to 28.8% in 1987, and 19.8% in 2007 (36). Applying similar smoking cessation strategies developed in United States on betel nut cessation may result in dramatically lowering the prevalence of cigarette smoking and betel nut chewing in Asia-Pacific countries.
We also found that physical inactivity, low income level, and low education level were associated with cumulative betel nut chewing in this population-based study. This is similar to previous studies (16,21).
Although we have shown that betel nut chewing is closely related to obesity, there are several limitations to this study. First, this study is a cross-sectional one, so causality cannot be demonstrated, Future prospective cohort studies are necessary. Second, the exposure to betel nut chewing was from self-report questionnaires, the potential misclassification of the exposure is possible which would probably have biased the result toward the null. However, using the cumulative exposure of betel nut chewing to assess the relationship to obesity can minimize the residual confounding. Furthermore, we show dose–response effects between betel nut chewing and obesity. Thus, it is impossible to cause a biased result but the true strength of association may be weakened.
In conclusion, we have demonstrated that betel nut chewing is closely associated with general and central obesity. Increasing cumulative doses of betel nut chewing relate to general and central obesity, revealing a dose–response effect. As the prevalence of obesity has increased dramatically worldwide in the past decades, it is important to identify different risk factors in varied regions, so useful local strategies can be developed. In this study, we have identified that betel nut chewing is a risk factor for obesity. Useful strategies for betel nut chewing cessation should be initiated to prevent further increases in chronic diseases which may be caused by obesity.
Acknowledgments
This study is supported by grants from National Science Council of Taiwan (NSC 93-2314-B-039-025, NSC 94-2314-B-039-024) and from US National Institutes of Health (DK 026687). We thank all the staffs who helped the data collection in China Medical University.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Ko GT, Tang JS. Waist circumference and BMI cut-off based on 10-year cardiovascular risk: evidence for “central pre-obesity”. Obesity (Silver Spring) 2007;15:2832–2839. doi: 10.1038/oby.2007.336. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan S, Rosenberg L, Djousse L, Cupples LA, Palmer JR. Overall and central obesity and risk of type 2 diabetes in U.S. black women. Obesity (Silver Spring) 2007;15:1860–1866. doi: 10.1038/oby.2007.220. [DOI] [PubMed] [Google Scholar]
- 3.Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization, International Association for the Study of Obesity and International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment. Health Communications; Sydney: 2000. [Accessed 14 January 2009]. < http://www.iotf.org/asiapacific/>. [Google Scholar]
- 6.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 7.Flegal KM, Williamson DF, Pamuk ER, Rosenberg HM. Estimating deaths attributable to obesity in the United States. Am J Public Health. 2004;94:1486–1489. doi: 10.2105/ajph.94.9.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sturm R. Increases in clinically severe obesity in the United States, 1986–2000. Arch Intern Med. 2003;163:2146–2148. doi: 10.1001/archinte.163.18.2146. [DOI] [PubMed] [Google Scholar]
- 9.Ruhm CJ. Current and Future Prevalence of Obesity and Severe Obesity in the United States. National Bureau of Economic Research; Cambridge, MA: 2007. [Google Scholar]
- 10.Wildman RP, Gu D, Muntner P, et al. Trends in overweight and obesity in Chinese adults: between 1991 and 1999–2000. Obesity (Silver Spring) 2008;16:1448–1453. doi: 10.1038/oby.2008.208. [DOI] [PubMed] [Google Scholar]
- 11.Chu NF. Prevalence of obesity in Taiwan. Obes Rev. 2005;6:271–274. doi: 10.1111/j.1467-789X.2005.00175.x. [DOI] [PubMed] [Google Scholar]
- 12.Boucher BJ, Mannan N. Metabolic effects of the consumption of Areca catechu. Addict Biol. 2002;7:103–110. doi: 10.1080/13556210120091464. [DOI] [PubMed] [Google Scholar]
- 13.Gupta PC, Warnakulasuriya S. Global epidemiology of areca nut usage. Addict Biol. 2002;7:77–83. doi: 10.1080/13556210020091437. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Reichart PA. A review of betel quid chewing, oral cancer and precancer in Mainland China. Oral Oncol. 2007;43:424–430. doi: 10.1016/j.oraloncology.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Wu IC, Lu CY, Kuo FC, et al. Interaction between cigarette, alcohol and betel nut use on esophageal cancer risk in Taiwan. Eur J Clin Invest. 2006;36:236–241. doi: 10.1111/j.1365-2362.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 16.Yen AM, Chiu YH, Chen LS, et al. A population-based study of the association between betel-quid chewing and the metabolic syndrome in men. Am J Clin Nutr. 2006;83:1153–1160. doi: 10.1093/ajcn/83.5.1153. [DOI] [PubMed] [Google Scholar]
- 17.Guh JY, Chen HC, Tsal JF, Chung LY. Betel-quid use is associated with heart disease in women. Am J Clin Nutr. 2007;85:1229–1235. doi: 10.1093/ajcn/85.5.1229. [DOI] [PubMed] [Google Scholar]
- 18.Guh JY, Chuang LY, Chen HC. Betel-quid use is associated wrth the risk of the metabolic syndrome in adults. Am J Clin Nutr. 2006;83:1313–1320. doi: 10.1093/ajcn/83.6.1313. [DOI] [PubMed] [Google Scholar]
- 19.Tung TH, Chiu YH, Chen LS, et al. A population-based study of the association between areca nut chewing and type 2 diabetes mellitus in men (Keelung Community-based Integrated Screening programme No. 2) Diabetologia. 2004;47:1776–1781. doi: 10.1007/s00125-004-1532-2. [DOI] [PubMed] [Google Scholar]
- 20.Kang IM, Chou CY, Tseng YH, et al. Association between betelnut chewing and chronic kidney disease in adults. J Occup Environ Med. 2007;49:776–779. doi: 10.1097/JOM.0b013e318095a48a. [DOI] [PubMed] [Google Scholar]
- 21.Lin WY, Chiu TY, Lee LT, et al. Betel nut chewing is associated with increased risk of cardiovascular disease and all-cause mortality in Taiwanese men. Am J Clin Nutr. 2008;87:1204–1211. doi: 10.1093/ajcn/87.5.1204. [DOI] [PubMed] [Google Scholar]
- 22.Chang WC, Hsiao CF, Chang HY, et al. Betel nut chewing and other risk factors associated with obesity among Taiwanese male adults. Int J Obes (Lond) 2006;30:359–363. doi: 10.1038/sj.ijo.0803053. [DOI] [PubMed] [Google Scholar]
- 23.Mannan N, Boucher BJ, Evans SJ. Increased waist size and weight in relation to consumption of Areca catechu (betel-nut); a risk factor for increased glycaemia in Asians in east London. Br J Nutr. 2000;83:267–275. doi: 10.1017/s0007114500000349. [DOI] [PubMed] [Google Scholar]
- 24.Lin CC, Liu CS, Li TC, et al. Microalbuminuria and the metabolic syndrome and its components in the Chinese population. Eur J Clin Invest. 2007;37:783–790. doi: 10.1111/j.1365-2362.2007.01865.x. [DOI] [PubMed] [Google Scholar]
- 25.Lin CC, Liu CS, Lal MM, et al. Metabolic syndrome in a Taiwanese metropolitan adult population. BMC Public Health. 2007;7:239. doi: 10.1186/1471-2458-7-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang PC, Li TC, Wu MT, et al. Association between television viewing and the risk of metabolic syndrome in a community-based population. BMC Public Healths; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 28.Chiang CP, Chang MC, Lee JJ, et al. Hamsters chewing betel quid or areca nut directly show a decrease in body weight and survival rates with concomitant epithelial hyperplasia of cheek pouch. Oral Oncol. 2004;40:720–727. doi: 10.1016/j.oraloncology.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Boucher BJ, Ewen SW, Stowers JM. Betel nut (Areca catechu) consumption and the induction of glucose intolerance in adult CD1 mice and in their F1 and F2 offspring. Diabetologia. 1994;37:49–55. doi: 10.1007/BF00428777. [DOI] [PubMed] [Google Scholar]
- 30.Gould AJ, Williams DE, Byrne CD, Hales CN, Wareham NJ. Prospective cohort study of the relationship of markers of insulin resistance and secretion with weight gain and changes in regional adiposity. Int J Obes Relat Metab Disord. 1999;23:1256–1261. doi: 10.1038/sj.ijo.0801060. [DOI] [PubMed] [Google Scholar]
- 31.Pisinger C, Jorgensen T. Weight concerns and smoking in a general population: the Inter99 study. Prev Med. 2007;44:283–289. doi: 10.1016/j.ypmed.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Canoy D, Wareham N, Luben R, et al. Cigarette smoking and fat distribution in 21, 828 British men and women: a population-based study. Obes Res. 2005;13:1466–1475. doi: 10.1038/oby.2005.177. [DOI] [PubMed] [Google Scholar]
- 33.Shimokata H, Muller DC, Andres R. Studies in the distribution of body fat. III. Effects of cigarette smoking. JAMA. 1989;261:1169–1173. [PubMed] [Google Scholar]
- 34.Lissner L, Bengtsson C, Lapidus L, Bjorkelund C. Smoking initiation and cessation in relation to body fat distribution based on data from a study of Swedish women. Am J Public Health. 1992;82:273–275. doi: 10.2105/ajph.82.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization. WHO report on the global tobacco epidemic, 2008– The MPOWER package. WHO; Geneva: 2008. [Accessed 14 January 2009]. The global tobacco crisis; pp. 14–22. < http://www.who.int/tobacco/mpower/mpower_report_full_2008.pdf>. [Google Scholar]
- 36.Centers for Disease Control and Prevention. Slightly lower adult smoking rates. CDC; Atlanta, GA: 2008. [Accessed 14 January 2009]. < http://www.cdc.gov/media/pressref/2008/r081113.htm>. [Google Scholar]


