Abstract
Patients with type 2 diabetes, approximately 85% of whom are overweight or obese, often have an increased incidence of cardiovascular disease (CVD) risk factors such as hypertension and dyslipidemia. Both type 2 diabetes and obesity are independent risk factors for CVD. Unfortunately, many therapies aimed at maintaining and improving glucose control are associated with weight gain. Among the older antidiabetes agents, most, including the insulin secretagogues and sensitizers, can lead to weight gain, except for metformin, which is weight-neutral. Among the newer agents, the dipeptidyl peptidase-4 inhibitors generally are weight-neutral in addition to lowering glucose, while the glucagon-like peptide–1 receptor agonists lead to weight reduction. Patients with type 2 diabetes are at an increased risk for both diabetes-and CV-related outcomes, and weight reduction is an important component of diabetes management. Weight gain in patients with type 2 diabetes can contribute to patient frustration and may negatively impact their compliance to therapeutic regimens. The selection of antidiabetes agents that not only improve glucose control but reduce or have a neutral effect on weight with beneficial effects on lipids are ideal options for managing patients with type 2 diabetes.
Keywords: type 2 diabetes mellitus, weight gain, obesity, cardiovascular disease, incretin therapy, exenatide
Introduction
Type 2 diabetes mellitus, the predominant form of diabetes, is a complex metabolic disorder characterized by hyperglycemia and inextricably linked to both weight gain and cardiovascular disease (CVD). Obesity, which is an independent CV risk factor in itself, is also a factor in the recent increase of newly diagnosed diabetes.1 This review examines the impact of weight gain on the motivation, compliance, and management of patients with type 2 diabetes. Additionally, based on a systematic review of clinical studies, it will provide an overview on the effects of currently approved and future therapies on weight in patients with type 2 diabetes.
Weight Gain/Obesity and CVD Risk in Type 2 Diabetes
According to the Centers for Disease Control and Prevention, 85% of patients with diabetes (of which type 2 diabetes accounts for > 90%) are overweight or obese.2,3 The growing prevalence of excessive visceral obesity and CV risk factors is associated with the increased incidence of type 2 diabetes and CVD.4,5 In fact, the term “diabesity” was coined several decades ago to underscore the strong link between diabetes and obesity,6,7 which are both associated with increased CV risk.8
The Framingham Heart Study showed that weight gain is independently associated with increased CV risk, morbidity, and mortality.9 Weight gain over the 26-year follow-up, that could not have resulted from initial patient weight or the levels of risk factors, was predictive of CVD in both men and women.9 Estimates have shown that a 5-kg weight gain in a man can increase coronary heart disease (CHD) risk as much as 30%.10 Data from the original and offspring cohorts of the Framingham study showed a high lifetime risk of CVD in men and women with diabetes, which was further increased by obesity. The 30-year risk of developing CVD among patients who were obese and had diabetes was 78.8% in women and 86.9% in men.8
A recent Swedish National Diabetes Register study showed that both overweight and obesity independently increase the risk of CVD in patients with type 2 diabetes.11 Analysis of data among overweight/obese type 2 diabetes patients who gained weight during the study showed a 13% increased risk of fatal and nonfatal CHD per 1 unit increase in body mass index (BMI).
Glucose-Lowering, CVD, and Weight: Lessons From Recent Large-Scale Trials
Hyperglycemia, due to type 2 diabetes, is an independent risk factor for CV morbidity and mortality.12,13 The benefits of intensive glycemic control in reducing the risk of microvascular complications have been demonstrated conclusively in several type 2 diabetes CV outcomes studies.14,15 However, the effects of intensive glucose control on macrovascular complications (eg, myocardial infarction, heart failure, stroke) is not as clearly understood.15–17 Data from recently published Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation (ADVANCE)17 and Action to Control Cardiovascular Risk in Diabetes (ACCORD)16 trials have shown inconsistent results regarding the effect of intensive glucose lowering on macrovascular complications.16,17 Approximately one-third of the patients in these studies had concomitant CVD.16,17
While intensive glucose control (defined as the use of the modified-release sulfonylurea, gliclazide, or other agents to achieve a hemoglobin AIC [Hb AIC] ≤ 6.5%) in the ADVANCE study (which had a 5-year median follow-up) was associated with a reduced incidence of major microvascular events and combined major microvascular and macrovascular events, there was no statistically significant difference between groups in the incidence of major macrovascular events, CV-related death, or all-cause mortality.17
In contrast, the ACCORD study found an increased risk of all-cause mortality in patients randomized to intensive therapy (ie, target HbAlc of < 6%), resulting in early termination of this study arm with a shift to standard therapy (ie, target HbAlc of 7%–7.9%) prior to the scheduled end of the study.16 Intensive treatment, over a 3.5-year mean follow-up, was associated with higher rates of CV death but a lower rate of nonfatal myocardial infarction, which led to a nonsignificant decrease in the primary outcome after 3 years.
An analysis to explore the relationship between hypoglycemia and mortality outcomes in ACCORD showed a statistically significant difference between the overall 1.22-fold increase in mortality and the 0.52-fold adjusted mortality ratio in intensive therapy patients who had severe hypoglycemia.18 However, since there was no systematic analysis or collection of low glucose concentrations other than the severe hypoglycemia defined in the study, it was concluded that severe hypoglycemia was not related to the higher mortality. Patients in the intensive treatment group also demonstrated a considerable increase in weight (27.8% gained > 10 kg with a mean gain of 3.5 kg at 3 years). This increase in weight may be attributed to the frequent use of thiazolidinediones (TZDs) (almost entirely rosiglitazone) and insulin.19 It remains undetermined, however, whether the patients with large increases in weight experienced higher rates of clinical events than patients with less weight gain.19
Reduction in Weight and CVD Risk Factors in Patients with Type 2 Diabetes
The Look AHEAD (Action for Health in Diabetes) study suggests that weight reduction in patients with type 2 diabetes is related to improvement in type 2 diabetes and CVD risk factors. One-year data showed that 37.8% of patients in the intensive lifestyle improvement (ILI) group and 3.2% of patients in the diabetes support and education group lost > 10% of their initial weight, with noninsulin users being more likely to achieve weight loss goals.20 The percentage of participants in the ILI group who attained the American Diabetes Association (ADA) HbAlc goal of < 7% increased from 46% to 73%. The decline in fasting serum glucose and HbAlc occurred despite the fact that these patients decreased their use of glucose-lowering medications. Additionally, there was a doubling in the percentage of patients in this group who met the ADA target goals for blood pressure (BP) and lipids. Results from Look AHEAD complement the data from the United Kingdom Prospective Diabetes Study (UKPDS) and the Steno-2 study regarding the benefits of a multifactorial treatment approach (including control of HbAlc, BP, and lipids).15,20–22
Current treatment guidelines highlight the importance of weight management for patients with type 2 diabetes.23–26 A position statement from the ADA, in conjunction with the North American Association for the Study of Obesity and the American Society for Clinical Nutrition, recognizes that even a modest weight loss (5% of body weight) can lead to improvement in insulin action, reduced fasting blood glucose concentrations, and decreased need for diabetes medications.26
The ADA recommends that weight loss programs include reduced carbohydrate and fat intake, increased physical activity, and lifestyle modifications.24,25 Patients with a BMI > 25 kg/m2 should try to reduce their BMI to ≤ 25 kg/m2. Patients with a BMI between 20 kg/m2 and 25 kg/m2 should maintain their present weight.27 Other specific recommendations include limiting saturated fat intake to < 7% of total calories and minimizing trans-fat intake. Carbohydrate intake should be monitored, and use of the glycemic index or glycemic load may be considered.24 Patients should be encouraged to perform ≥ 150 minutes of moderate-to-intense aerobic exercise and, in the absence of contraindications, resistance training 3 times per week.25 Consideration should be given to weight loss medications (combined with lifestyle modification) or bariatric surgery in overweight/obese patients in need of greater weight reduction.24
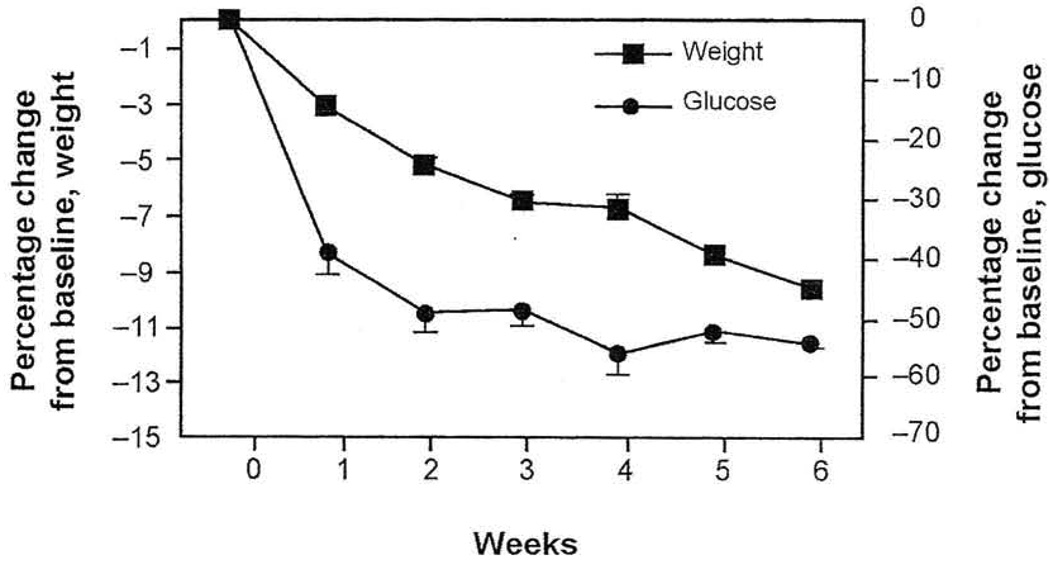
Benefits of weight reduction in overweight/obese patients with type 2 diabetes have been demonstrated in diet therapy and gastric banding studies. One study found that modest weight reduction with very-low energy diets was associated with a decrease in fasting plasma glucose (Figure 1), and significantly greater reductions correlated with increased weight loss.10 In another study, weight loss was associated with long-term improvements in CV risk factors in obese patients with type 2 diabetes who underwent laparoscopic gastric banding. One year after surgery, patients demonstrated a 43% reduction in triglyceride, an 18% increase in high-density lipoprotein cholesterol (HDL-C), improved BP control, and a reduced need for antihypertensive medications.28 Dixon et al28 reported remission in diabetes in these patients following weight loss via improved insulin sensitivity and β-cell function.
Figure 1.
Effects of weight loss (with very low-energy diets) on fasting plasma glucose in patients with obesity and type 2 diabetes mellitus.
Values (with SEM bars) are expressed as percentages of baseline values. Baseline fasting plasma glucose values were 14 mmol/L (95% confidence interval, 12–15.9 mmol/L).
Mean values from 10 studies including 152 subjects.
Reprinted with permission from J Am Coll Nutrition.10
Abbreviation: SEM, standard error of the mean.
Weight management is an ongoing challenge for patients with type 2 diabetes.29 Intensive lifestyle management and weight loss have positive short-term effects on decreasing CV risk, while nutritional education and counseling have low compliance rates, thus making long-term effects difficult to achieve.30–32
Reviewing the Evidence: Weight, Motivation, Adherence, and Compliance in Patients with Type 2 Diabetes
One of the factors related to the success of weight loss programs and the pharmacologic management of type 2 diabetes is patient adherence and compliance.32 Many factors may affect the degree of adherence and compliance, including demographic, psychological, social, health care system, and disease-related factors. Knowledge of a patient’s disease and reasons for the treatment regimen may be lacking; family problems or psychological disorders may affect the patient’s ability to effectively manage their disease, or patients may become overwhelmed and feel they do not have the ability to succeed.32
Diabetes is largely a self-managed and self-monitored disease requiring patient motivation for successful health management.32 Therefore, the concept of patient compliance and adherence should be considered as it pertains to this population and management model. While compliance is described as the extent to which patients follow medical advice, adherence has been defined as active and voluntary patient involvement and a collaborative relationship with their provider to achieve disease management goals.
Motivation is an important tool in the management of patients with type 2 diabetes.33,34 Self-care behaviors play a major role in diabetes control and may be negatively impacted by patient apathy, which is characterized by lack of motivation and initiative.35 Motivated patients are aware of the importance of taking their medication as directed to control their diabetes and to prevent the onset of complications.34 A 12-month study showed that when patients with diabetes perceived their physicians as being autonomy supportive, the patients became more motivated to regulate their diet and exercise regimens, felt more compelled to manage their diabetes, and had significant (P < 0.001) reductions in HbAlc.36 Williams et al37 also showed that autonomy support of patients with diabetes by primary care physicians was related to perceived competence, depressive symptoms, patient satisfaction, and glycemic control. Results from recent research by Daly et al33 showed that family support, physician-patient communication, diabetes knowledge, and motivation and confidence to manage diabetes were keys to self-care behaviors for patients with type 2 diabetes.
It should also be considered that, while patients may not adhere to some aspects of their regimen, they may adopt other components. For diabetes management to be successful and patients to remain adherent, a collaborative care model, where patients are actively involved in setting their goals based on their willingness to change, and ongoing support and motivation are provided by the healthcare team, may be the best approach.32
The Diabetes Attitude, Wishes, and Needs (DAWN) study examined regimen adherence in patients with diabetes and the effect of psychosocial barriers.38 Among patients with type 2 diabetes, there was a 78% rate of medication adherence and a 72% rate of appointment keeping; however, adherence to diet and exercise was low (37% and 35%, respectively). Another study found that persistency rates for type 2 diabetes patients taking oral antidiabetes medications were approximately 68% at 1 year. Lack of adherence had a negative effect on outcomes with the nonpersistent use of oral antidiabetes agents leading to a 20% decrease in probability of attaining HbAlc goals in patients with type 2 diabetes.39
Analysis of data from an open-label, randomized clinical trial showed no significant differences in treatment satisfaction among patients with type 2 diabetes who received either biphasic or basal insulin analogs.40 Most (81%) of the 197 patients who completed the treatment satisfaction assessment did not change their weight status (normal, overweight, or obese), and no significant relationship between change in BMI classification and treatment satisfaction was found. However, there was a systematic improvement in treatment satisfaction across all scores among the 14.5% of patients in whom BMI was reduced, although the only statistically significant (P < 0.05) difference was in the lifestyle flexibility subscale.40
Sacco et al41 studied the impact of depression on the development and worsening of type 2 diabetes using the hypothesis that diabetes symptoms mediate the effect of behavioral adherence and BMI on depression. Patients were assessed for depression using the Patient Health Questionnaire: Nine Symptom Depression Checklist, and for self-efficacy by the diet, exercise, and weight control items of the Multidimensional Diabetes Questionnaire self-efficacy subscale (using a scale of 0 to 100, patients assess their confidence in following their diet and exercise regimen). Results suggest there are 2 independent pathways by which BMI and adherence could impact depression in patients with type 2 diabetes. The first pathway suggests the effects of higher BMI and poor adherence are mediated by lower self-efficacy perceptions, while the second pathway indicates that the effects of higher BMI on depression are mediated by increased diabetes symptoms. While causality cannot be established, these findings may help to explain why overweight/obese individuals with type 2 diabetes are predisposed to experience weight-related negative, self-relevant cognition, medical symptoms, and depression.41
Weight gain can contribute significantly to the frustration patients feel with their current antidiabetes therapies and may contribute to early discontinuation or decreased adherence.42,43 Many established antidiabetes regimens, including insulin secretagogues (eg, sulfonylureas, meglitinides) and insulin sensitizers or insulin-sparing agents (eg, TZDs) are associated with weight gain.6,44 The UKPDS showed that, during the first 12 months of therapy, intensive treatment regimens resulted in better glucose control than conventional interventions, but were associated with significantly (P < 0.0001) greater increases in weight.15,42 Patients may not want to take medications knowing there is a potential for weight gain to begin therapy as they are concerned about the adverse effects.42,43 The initiation of appropriate therapies, such as insulin, may be delayed due to patient fear of weight gain; this may lead to noncompliance while the patient is receiving therapy, leading to poor glucose control.
In patients with type 2 diabetes, weight loss has been associated with improvements in sleep, mental health, appearance evaluation, and health-related quality of life, which demonstrate improved psychosocial health.28 Results from a patient-reported outcomes study suggested that the increased efficacy, and potential for weight reduction, provided by the addition of injectable medications to patients inadequately controlled on oral agents may lead to improved patient outcomes including treatment satisfaction and quality of life.45 These improvements, which have the potential to motivate patients and improve the persistency of therapy, indicate an important place in therapy for effective antidiabetes agents with weight-neutral or weight-reducing effects.6
Reviewing the Evidence: Effects of Antidiabetes Agents on HbAlc, Weight, and Lipids
A literature search was conducted for primary studies on the antidiabetes agents glimepiride, metformin, acarbose, pioglitazone, rosiglitazone, repaglinide, nateglinide, and sitagliptin (all oral), and insulin glargine, insulin aspart, insulin detemir, pramlintide, exenatide, and liraglutide (all parenteral) to specifically examine the effects of antidiabetes therapies on weight in patients with type 2 diabetes. Dates were generally limited to 2005 through 2008 (except for an extension to 2004 in the case of the meglitinides repaglinide and nateglinide for lack of better data using the original search timeframe). Head-to-head studies and/or monotherapy studies were preferred as appropriate, and keywords focused on the generic names of the agents (which were either approved by the US Food and Drug Administration or already had a new drug application filing at the time of the writing of this review) and “weight.” The comparison focused on data related to HbAlc, weight effects, and lipids. Findings related to HbAlc, weight, and lipids among the oral antidiabetes agents are summarized in Table 1,46–61 corresponding data with parenteral therapies are outlined in Table 2.47,48,52,57,62–78
Table 1.
Summary of Clinical Data Evaluating the Effects of Oral Antidiabetes Agents on HbAlc Weight, and Lipid Parameters in Patients with Type 2 Diabetes
| Agent | Study | Patient Population Randomized to Treatment Arm (n) |
Dosage | HbAlc, Percent Change From Baseline in Absolute Units |
Weight, kg Change From Baseline |
Lipids, Change From Baseline |
|---|---|---|---|---|---|---|
| Glimepiride | Yamanouchi 200560 |
37 (treatment-naïve to OAD) |
Glimepiride 1–2 mg/day for 12 mo |
−2.1a | −0.2 (BMI) | (mmol/L) TC, −0.19; HDL-C.−0.01; TG, 10.05 |
| Nauck 200952 |
244 | Glimepiride (in combination with metformin) 4 mg/day for 26 wk |
−1.0 | +1.0 | NR | |
| Garber 200948 (LEAD-3) |
248 (receiving OAD plus diet/exercise) |
Glimepiride 8 mg/day for 52 wk |
−0.51 | +1.02 | NR | |
| Metformin | Yamanouchi 200560 |
39 (treatment-naïve to OAD) |
Metformin 750 mg/day for 12 mo |
−2.1a | −0.7 (BMI) | (mmol/L) TC. −0.18; HDL-C. −0.01; TG, −0.09 |
| Yilmaz 200761 |
17 (receiving insulin) | Metformin 1700 mg/day for 6 mo |
−2.0a | +1.4 | (mg/dL) TC, −2.3; LDL-C, +2.2; HDLC, −0.1; TG, −16.5a |
|
| Kawai 200849 |
35 (Japanese patients who received metformin 500–750 mg/day for 12wk) |
Metformin 500–750 mg/day for 6 mo |
−0.1 | +0.1 | (mg/dL) TC, +0.9; HDL-C, +0.2; TG, −6.0 |
|
| Kusaka 200350 |
17 (Japanese patients treated with diet/exercise alone or with glimepiride 2 mg/day) |
Metformin 750 mg/day for 4 mo |
−1.0a | No change (BMI) | (mmol/L) LDL-C, −0.3; HDL-C. no change: TG, +0.2 |
|
| Acarbose | Yilmaz 200761 |
15 (receiving insulin) | Acarbose 300 mg/day for 6 mo |
−0.6a | +2.7 | (mg/dL) TC, −2.3; LDL-C, +6.9; HDL-C+1.7; TG, −27.4a |
| Pan 200853 | 220 (treatment-naïve) | Acarbose ≤ 300 mg/day for 24 wk |
−1.3 | −1.7 | NR | |
| Pioglitazone | Yamanouchi 200560 |
38 (treatment-naïve to OAD) |
Pioglitazone 30–45 mg/day for 12 mo |
−2.3a | +0.9 (BMI) | (mmol/L) TC, −0.23; HDL-C, +0.11; TG, −0.39 |
| Kawai 200849 |
28 (Japanese patients who received metformin 500–750 mg/day for 12 wk) |
Pioglitazone 15 mg/day for 6 mo |
-1.2a | +2.2a | (mg/dL) TC, + 1.5; HDL-C, +5.6a; TG.-13.5 |
|
| Kusaka 200850 |
16 (Japanese patients treated with diet/exercise alone or with glimepiride 2 mg/day) |
Pioglitazone 15 mg/day (women) or 30 mg/day (men) for 4 mo |
−1.1a | +0.9a (BMI) | (mmol/L) LDL-C, no change; HDL-C, +0.2; TG, +0.2 |
|
| Miyazaki 200851 |
21 (treatment-naïve or receiving sulfonylurea) |
Pioglitazone 45 mg/day for 3 mo |
−1.3a | +2.7a | (mg/dL) LDL-C, +1.0a; HDL-C, +2.0; TG, −47.0a |
|
| Rosiglitazone | Rosen stock 200657 |
112 (insulin-naïve; receiving sulfonylurea plus metformin) |
Rosiglitazone 4 mg/day for 24 wk |
−1.51 | +3.0 | (mg/dL) TC,+19.0; LDL-C, +14.0; HDL-C, +4.4%; TG, +11.0 |
| Yilmaz 200761 |
15 (receiving insulin) | Rosiglitazone 8 mg/day for 6 mo |
−2.5a | +4.6 | (mg/dL) TC, −12.5; LDL-C, −11.9; HDL-C. +3.3; TG, −29.7a |
|
| Miyazaki 200851 |
37 (treatment-naïve or receiving sulfonylurea) |
Rosiglitazone 8 mg/day for 3 mo |
−1.4a | +2.9a | (mg/dL) LDL-C. +15.0a; HDL-C, +4.0; TG, −8.0a |
|
| Scott 200859 |
87 (receiving metformin) | Rosiglitazone 8 mg/day for 18 wk |
−0.79 | +1.5 | (mg/dL, at wk 24) TC, +26.2; LDL-C, +20.4; HDL-C, +3.5; TG.-1.8 |
|
| Repaglinide | Rosenstock 200456 |
76 (treated with diet/ exercise for previous 3 mo) |
Repaglinide 0.5–4.0 mg/meal for 16 wk 120 mg/meal for 16 wk |
−1.57a | +1.8 | NR |
| Nateglinide | Rosenstock 200456 |
74 (treated with diet/ exercise for previous 3 mo) |
Nateglinide 60–120 mg/meal for 16 wk |
−1.04 | +0.7 | NR |
| Sitagliptin | Aschner 200646 |
467 (not receiving an OAD) |
Sitagliptin 100–200 mg/day for 24 wk |
−0.61 to −0.76a | −0.1 to −0.2 | NR |
| Raz 200654 | 392 (uncontrolled by diet/exercise) |
Sitagliptin 100–200 mg/day for 18 wk |
−0.36 to −0.48a | −0.2 to −0.6 | NR | |
| Rosenstock 200658 |
175 (added to pioglitazone) |
Sitagliptin 100 mg/day plus pioglitazone for 24 wk |
−0.85a | +1.8 | (mg/dL) TC, +1.7; LDL-C, +1.2; HDL-C,+0.1; TG, −1.1 |
|
| DeFronzo 200847 |
61 (receiving metformin) | Sitagliptin 100 mg/day for 2 wk (then crossover to exenatide) |
−19 (FPG, mg/dL) | −0.3 | NR | |
| Raz 200855 | 96 (receiving metformin for > 6 wk) |
Sitagliptin 100 mg/day for 30 wk |
−1.0a | −0.5 | No significant between-group differences in TC, LDL-C, HDL-C |
|
| Scott 200859 |
94 (receiving metformin) | Sitagliptin 100 mg/day for 18 wk |
−0.73 | −0.4 | (mg/dL, at wk 24) TC +8.1; LDL-C, +9.2; HDL-C, +1.8: TG, −14.5 |
P < 0.05.
Abbreviations: BMI, body mass index; FPG. fasting plasma glucose: HDL-C. high-density lipoprotein cholesterol; LEAD. Liraglutide Effects and Actions in Diabetes; LDL-C, low-density lipoprotein cholesterol: NR, not reported; OAD. oral antidiabetes (agent); TC. total cholesterol; TG, triglyceride.
Table 2.
Summary of Clinical Data Evaluating the Effects of Parenteral Antidiabetes Agents on HbAlc, Weight, and Lipid Parameters in Patients with Type 2 Diabetes
| Agent | Study | Patient Population Randomized to Treatment Arm (n) |
Dosage | HbAlc, Percent Change From Baseline in Absolute Units |
Weight, kg Change From Baseline |
Lipids, Change From Baseline |
|---|---|---|---|---|---|---|
| Insulin Glargine |
Heine 200567 | 260 (uncontrolled by metformin plus sulfonylurea) |
Insulin glargine QD titrated to FBG < 5.6 mmol/L for 26 wk |
−1.11 | +1.8 | NR |
| Rosenstock 200657 |
104 (insulin-naïve; receiving sulfonylurea plus metformin) |
Insulin glargine 10 U/day titrated to FPG of 5.5–6.7 mmol/L for 24 wk |
−1.66 | +1.7 | (mg/dL) TC, -10.0; LDL-C, -2.0; HDL-C, no change; TG, -4I.0 |
|
| Barnett 200762 | 70 in insulin glargine/ exenatide group (receiving a single OAD) |
Insulin glargine QD titrated to FSG < 5.6 mmol/L for 16 wk (then crossover to exenatide) |
−1.36a | +1.0 in 1st period and +2.3 in 2nd period |
NR | |
| Fakhoury 200866 |
869 (uncontrolled by OAD) |
Insulin glargine 23–68 IU/day for 24 to 52 wk |
−0.76 to −2.36 | +2.57 to +3.7 | NR | |
| Insulin Aspart |
Nauck 200773 | 248 (receiving metformin plus a sulfonylurea) |
Biphasic insulin aspart 30/70 BID for 52 wk |
−0.89a | +2.9a | Greater increase in HDL-C with insulin aspart (LS mean, exenatide minus insulin, −0.04 mmol/L; P = 0.003; absolute values NR) |
| Insulin Detemir |
Fakhoury 200866 |
169 (uncontrolled by OAD) |
Insulin detemir mean dose of 37 IU/day for 20 wk |
−1.48 | +0.69 | NR |
| Pramlintide | Karl 200768 | 166 (uncontrolled by insulin) |
Pramlintide 120 µg BID or TID for 6 mo |
−0.56a | −2.8a | NR |
| Riddle 200774 | 105 (uncontrolled by insulin glargine with or without an OAD) |
Pramlintide 60 or l20 µg BID or TID for 16 wk |
−0.7a | −1.6a | NR | |
| Exenatide BID |
DeFronzo 200564 |
223 (receiving metformin) |
Exenatide 5–10 µg BID for 30 wk |
−0.40 to −0.78a | −1.6 to −2.8a | NR |
| Heine 200567 | 275 (uncontrolled by metformin plus a sulfonylurea) |
Exenatide 10 µg BID for 26 wk |
−l.11 | −2.3 | NR | |
| Kendall 200569 | 486 (receiving metformin plus a sulfonylurea) |
Exenatide 5–10 µg BID for 30 wk |
−0.55 to −0.77a | −1.6a | NR | |
| Barnett 200762 | 68 in exenatide/insulin glargine group (receiving a single OAD) |
Exenatide 10 µg BID for 16 wk (then crossover to insulin glargine) |
−1.36a | −2.0 in 1st period and −2.2 in 2nd period |
NR | |
| Nauck 200773 | 253 (receiving metformin plus a sulfonylurea) |
Exenatide 5 µg BID for 4 wk then 10 µg BID for 48 wk |
−1.04a | −2.5a | Greater increase in HDL-C with insulin aspart (LS mean, exenatide minus insulin, −0.04 mmol/L; P = 0.003; absolute values NR) |
|
| Zinman 200777 | 121 (uncontrolled by a thiazolidinedione with or without metformin) |
Exenatide 10 µg BID for 16 wk |
−0.89a | −1.75 | NR | |
| DeFronzo 200847 |
61 (receiving met- formin) |
Exenatide 5 µg BID for 1 wk then 10 µg BID for 1 wk (then crossover to sitagliptin) |
-15 (FPG, mg/dL) |
−0.8a | NR | |
| Drucker 200365 | 148 (uncontrolled by OAD with or without diet/exercise) |
Exenatide 10 µg BID for 30 wk |
−1.5a | −3.6 | (mmol/L) TC, −0.1; LDL-C, +0.03; HDL-C, −0.03; TG, −11% |
|
| Klonoff 200871 | 217 (uncontrolled by metformin with or without a sulfonyl urea) |
Exenatide 5 µg BID for 4 wk then 10 µg for up to 3.5 y |
−0.8a | −5.3a | (mg/dL) TC, −10.81a; LDL-C, −11.8a; HDL-C, +8.5a; TG, −44.1a |
|
| Exenatide Once Weeklyb |
Kim 200770 | 31 (uncontrolled by metformin with or without diet/exercise) |
Exenatide 0.8 to 2.0 mg/wk for 15 wk |
−1.4 to −1.7a | −3.8a (with 2.0 mg/wk dose) |
NR |
| Drucker 200865 (DURATION-1) |
147 (uncontrolled by OAD With or without diet/exercise) |
Exenatide 2 mg/wk for 30 wk |
−1.9a | −3.7 | (mmol/L) TC, −0.31; LDL-C, −0.13; HDL-C, −0.02; TG, −15% |
|
| Bergenstal 200863 |
120 (52-wk data of DURATION-I) |
Exenatide 2 mg/wk SC for 52 wk |
−2.0 | −4.1 | (mg/dL) TC, −7.9; LDL-C, −2.2; HDL-C, −0.3; TG, −40.1 |
|
| Liraglutideb | Vilsbøll 200776 | 123 (as monotherapy) | Liraglutide 0.65, 1.25, 1.90 mg QD SC for 14 wk |
−0.98 to −1.45 | −2.99 (1.90 mg dose) |
No consistent changes among treatment in TC, LDL-C, HDL-C (no data shown) |
| Seino 200875 | 180 (Japanese patients) |
Liraglutide 0.1−0.9 mg/day SC for 14 wk |
−0.72 to −1.67 | +0.13 to −0.48 | NR | |
| Garber 200948 (LEAD-3) |
498 (receiving an OAD plus diet/exercise) |
Liraglutide 1.2–1.8 mg/day for 52 wk |
−0.84 to −1.14 | −2.0 to −2.5a | NR | |
| Marre 200972 (LEAD-I) |
695 (added to sulfonylurea) |
Liraglutide 0.6, 1.2, 1.8 mg QD SC for 26 wk |
−1.1a | +0.7 to −0.2 | NR | |
| Nauck 200952 (LEAD-2) |
725 (plus metformin) | Liraglutide 0.6–1.8 mg/day for 26 wk |
−0.7 to −1.0a | −1.8 to −2.8a | NR | |
| Zinman 200978 (LEAD-4) |
356 (in combination with metformin and rosiglitazone) |
Liraglutide 1.2–1.8 mg/day for 26 wk |
−1.5 | −1.0 to −2.0 | (mmol/L) TC, −0.20 to −0.21; LDL-C, −0.23 to −0.28a; HDL-C, −0.03 to −0.04; TG, −0.32 to −0.38a |
P < 0.05.
Not currently approved by the US Food and Drug Administration; undergoing regulatory review.
Abbreviations: DURATION-1, Diabetes Therapy Utilization; Researching Changes in AIC, Weight and Other Factors Through Intervention with Exenatide Once Weekly-1; FBG, fasting blood glucose; FPG, fasting plasma glucose; FSG, fasting serum glucose; HDL-C, high-density lipoprotein cholesterol; LEAD, Liraglutide Effects and Actions in Diabetes; LDL-C, low-density lipoprotein cholesterol: LS, least squares: NR, not reported; OAD, oral antidiabetes (agent); SC, subcutaneous: TC, total cholesterol; TG, triglyceride.
Findings in the data review of newer agents and their effects on weight and other CV risk parameters are consistent with and confirm findings from previous reports. Most antidiabetes agents result in weight gain or are weight-neutral despite attempts at lifestyle and diet modification interventions.44 In a 2003 literature review of the effects of antidiabetes agents on weight, insulin, sulfonylureas, and TZDs were associated with increases in BMI and weight gain while biguanides, such as metformin, and α-glucosidase inhibitors, such as acarbose, had a weight-neutral effect.44
A recently published review of newer classes of oral/injectable antidiabetes agents, including the dipeptidyl peptidase–4 (DPP-4) inhibitors, as monotherapy or in combination, reported this class of agents to be weight-neutral and to have no effects on lipid profiles.79 In a review by Bolen et al,80 findings confirm that the majority of available oral antidiabetes agents cause a weight increase of 1 to 5 kg, with the exception of metformin, which does not affect weight in either direction. In addition, effects on lipids were compared with TZDs increasing low-density lipoprotein cholesterol (LDL-C), as well as HDL-C. Metformin has been found to decrease LDL-C while the other agents had no significant effect on LDL-C.80 Rosiglitazone was the only oral agent shown to increase triglycerides.80
The current review expands on the findings of previous studies and provides new insights by including additional data from trials of the newer antidiabetes agents, such as incretin-based therapies—the parenterally administered (ie, subcutaneously) glucagon-like peptide–1 (GLP-I) receptor agonists and the oral DPP-4 inhibitors. Increases in body weight were greatest among patients treated with pioglitazone, rosiglitazone, and insulin glargine. Consistent with the findings of earlier studies, including studies in which these drugs were used as add-on therapy,58,81 the DPP-4 inhibitors (ie, sitagliptin) were found to be generally weight-neutral. The largest reduction in body weight from a therapy prescribed to manage hyperglycemia in patients with type 2 diabetes was seen with the GLP-I receptor agonists (eg, exenatide produced weight reductions ranging from −1.6 kg at 12 weeks to −5.3 kg at 3.5 years).71
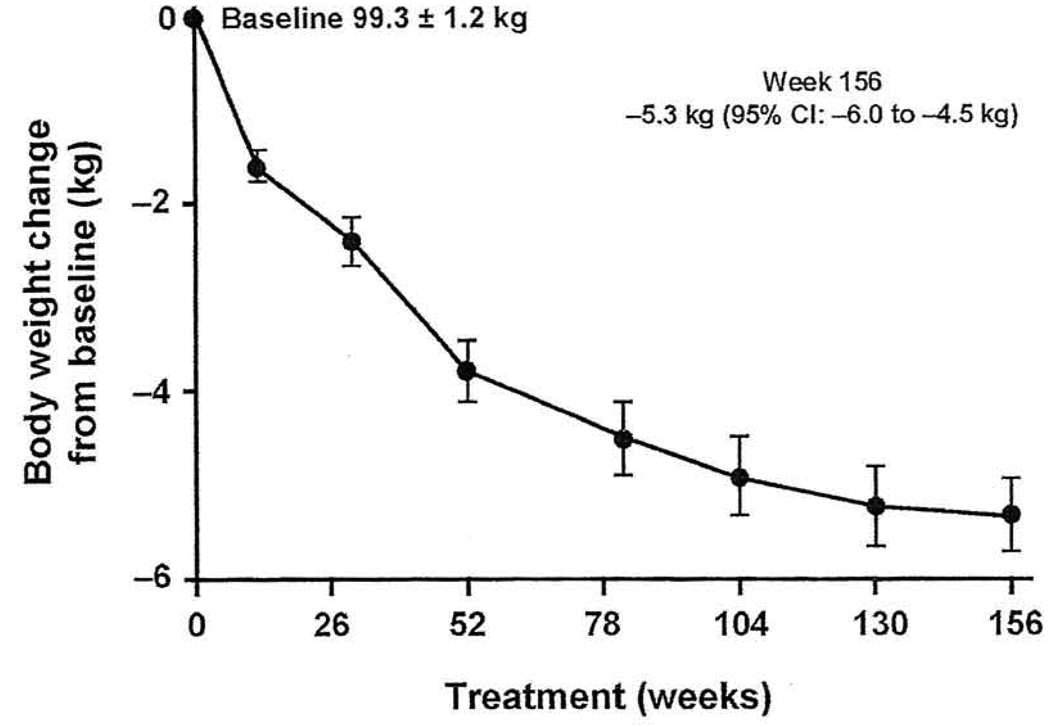
Exenatide may be helpful in patients with the metabolic syndrome because of its effects on glycemic control and other surrogates of CV risk, including weight, BP, and lipids.82 The effects of long-term exenatide therapy on diabetes, obesity, CV risk factors, and hepatic markers were assessed in an open-ended, open-label clinical trial in patients with type 2 diabetes,71 All patients received exenatide 10 µg twice daily for ≥ 3 years. Patients demonstrated significant reductions in HbAlc and fasting glucose as early as week 12 and these were sustained throughout the study. In patients who completed 3 years of therapy, HbAlc was significantly (P < 0.0001) reduced by −1% (46% of patients had an HbAlc ≤ 7%), and fasting plasma glucose was reduced by −23.5 mg/dL. For patients who continued therapy, exenatide was associated with a significant and progressive reduction in weight (Figure 2): −1.6 kg at 12 weeks, −2.1 kg at 30 weeks, −4.7 kg at 2 years, and up to −5.3 kg at 3.5 years (P < 0.0001).71 A total of 84% of patients lost weight over the 3-year follow-up and 50% lost ≥ 5% of baseline body weight.
Figure 2.
Progressive reductions in weight from baseline (P < 0.0001) in patients with type 2 diabetes treated with exenatide 10 µg twice daily.
Abbreviation: CI, confidence interval.
Reprinted with permission from Curr Med Res Opin.71
There are other advances in incretin-based therapies. The DPP-4 inhibitor vildagliptin is approved for use in the European Union and Latin America. Other DPP-4 inhibitors under development or being investigated include alogliptin and saxagliptin.83 Among the GLP-I receptor agonists, a new once-weekly formulation of the currently approved agent exenatide is being investigated in phase III clinical trials, as is liraglutide, an agent which is projected to be administered once daily.83 To date, there have been several published studies for both once-weekly exenatide63,65,70 and once-daily liraglutide (Table 2).48,52,72,75,76,78
Integrated Approach to Antihyperglycemic and CV Risk Factor Management in Type 2 Diabetes
According to data from the National Health and Nutrition Examination Survey (NHANES) between 1993 and 2004, only approximately 13% of adult US patients with diagnosed diabetes achieved target goals for HbAlc, BP, and lipids concurrently. The majority of these patients were overweight or obese.2,84 Unfortunately, most of the traditional hypoglycemic agents for patients with type 2 diabetes are associated with weight gain.44 Weight gain can lead to therapeutic noncompliance and, when extreme, further increase CV risk in a population that already has increased potential for adverse events.30 An open-label study to assess treatment satisfaction was conducted in patients not reaching glycemic targets with insulin who were given adjunctive pramlintide. Treatment satisfaction was highest in patients who reported better clinical outcomes, including improved glycemic control, weight loss, and reduced insulin requirements.85
The comprehensive and goal-oriented management of type 2 diabetes and associated CV risk factors is warranted. When selecting treatment regimens, the focus should not be solely on glucose-lowering effects but also on nonglycemic factors such as weight and other CV risk factors, as well as ease of use, tolerability, and long-term adherence.30 Although lifestyle interventions themselves have failed to show long-term success, lifestyle changes, including increased physical activity and weight reduction, should be part of the management program of patients with type 2 diabetes and reinforced every visit.24,30 The incorporation of appropriate therapies to improve risk factors for diabetes and CV-related events should enhance clinical outcomes of patients with type 2 diabetes.30,86 Given that weight management is an important part of treating patients with type 2 diabetes and given the challenges these patients face in reducing and/or maintaining their weight, agents, such as incretin-based therapies, that can provide beneficial effects on both glucose and weight, represent an important addition to the antidiabetes armamentarium.24,82
Conclusion
Weight reduction remains a significant challenge in patients with type 2 diabetes, the majority of whom are overweight or obese. Additionally, weight gain associated with antidiabetes medications may increase CV risk and reduce adherence to therapy. Weight loss has been associated with improvements in mental health, self-evaluation of appearance, and health-related quality of life in patients with type 2 diabetes, as well as correlated with patient-reported treatment satisfaction. Patient motivation coupled with safe and effective treatment is critical to achieving optimal management of type 2 diabetes.
Appreciating the different effects that antidiabetes agents have on HbAlc, weight, and lipids can lead to better agent selection and potentially improved clinical outcomes. In the literature reviewed, antidiabetes medications were associated with varying effects on weight and other CV risk factors. Given the increased focus on the importance of weight loss in patients with type 2 diabetes, the selection of regimens that not only improve glucose control but have weight-reducing effects (ie, GLP-I receptor agonists), or are at least weight-neutral (ie, DPP-4 inhibitors) may be the best approach to treatment. Therapies that lead to weight reduction in addition to effective glucose lowering may motivate patients with diabetes to remain compliant to their therapies and consequently improve their CV risk profiles.
Acknowledgment
The author thanks Jose Iglesia, MD, for editorial assistance and for support from Amylin Pharmaceuticals, Inc. and Eli Lilly and Company.
Footnotes
Conflict of Interest Statement
F. Xavier Pi-Sunyer, MD discloses conflicts of interest with Amylin Pharmaceuticals, AstraZeneca, Eli Lilly and Co., Novo-Nordisk, and Roche.
References
- 1.Geiss LS, Pan L, Cadwell B, Gregg EW, Benjamin SM, Engelgau MM. Changes in incidence of diabetes in U.S. adults, 1997–2003. Am. J Prev Med. 2006;30(5):371–377. doi: 10.1016/j.amepre.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prevalence of overweight and obesity among adults with diagnosed diabetes—United States, 1988–1994 and 1999–2002. Morb Mortal Wkly Rep. 2004;53(45):1066–1068. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. National diabetes fact sheet, 2007: general information and national estimates on diabetes in the United States, 2007. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 4.Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Hawken S, Ounpuu S, et al. INTERHEART Study Investigators. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 6.Pi-Sunyer FX. The effects of pharmacologic agents for type 2 diabetes mellitus on body weight. Postgrad Med. 2008;120(2):5–17. doi: 10.3810/pgm.2008.07.1785. [DOI] [PubMed] [Google Scholar]
- 7.Sims EA, Danforth E, Jr, Horton ES, Bray GA, Glennon JA, Salans LB. Endocrine and metabolic effects of experimental obesity in man. Recent Prog Horm Res. 1973;29:457–496. doi: 10.1016/b978-0-12-571129-6.50016-6. [DOI] [PubMed] [Google Scholar]
- 8.Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D’Agostino RB., Sr Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care. 2008;31(8):1582–1584. doi: 10.2337/dc08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67(5):968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JW, Kendall CW, Jenkins DJA. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutrition. 2003;22(5):331–339. doi: 10.1080/07315724.2003.10719316. [DOI] [PubMed] [Google Scholar]
- 11.Eeg-Olofsson K, Cederholm J, Nilsson PM, et al. Risk of cardiovascular disease and mortality in overweight and obese patients with type 2 diabetes: an observational study in 13,087 patients. Diabetologia. 2009;52(1):65–73. doi: 10.1007/s00125-008-1190-x. [DOI] [PubMed] [Google Scholar]
- 12.Gerstein HC, Pogue J, Mann JFE, et al. The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia. 2005;48(9):1749–1755. doi: 10.1007/s00125-005-1858-4. [DOI] [PubMed] [Google Scholar]
- 13.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skyler JS, Bergenstal R, Bonow R, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32(1):187–192. doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 16.Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study Group. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 18.Bloomgarden ZT. Glycemic control in diabetes: a tale of three studies. Diabetes Care. 2008;31(9):1913–1919. doi: 10.2337/dc08-zb09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dluhy RG, McMahon GT. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med. 2008;358(24):2630–2633. doi: 10.1056/NEJMe0804182. [DOI] [PubMed] [Google Scholar]
- 20.Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UK Prospective Diabetes Study (UKPDS) Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 22.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 23.American Association of Clinical Endocrinologists Clinical Practice Guidelines Task Force. Medical guidelines for clinical practice for the management of diabetes mellitus. Endocr Pract. 2007;13 Suppl 1:1–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Bantle JP, Wylie-Rosett J, Albright AL, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31 Suppl 1:S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 25.American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care. 2009;32 Suppl 1:S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein S, Sheard NF, Pi-Sunyer X, et al. Weight management through lifestyle modification for the prevention and management of type 2 diabetes: rationale and strategies: a statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care. 2004;27(8):2067–2073. doi: 10.2337/diacare.27.8.2067. [DOI] [PubMed] [Google Scholar]
- 27.Pi-Sunyer FX. Weight and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1996;63(3 Suppl):426S–429S. doi: 10.1093/ajcn/63.3.426. [DOI] [PubMed] [Google Scholar]
- 28.Dixon JB, O’Brien PE. Health outcomes of severely obese type 2 diabetic subjects 1 year after laparoscopic adjustable gastric banding. Diabetes Care. 2002;25(2):358–363. doi: 10.2337/diacare.25.2.358. [DOI] [PubMed] [Google Scholar]
- 29.Pi-Sunyer FX. Weight loss in type 2 diabetic patients. Diabetes Care. 2005;28(6):1526–1527. doi: 10.2337/diacare.28.6.1526. [DOI] [PubMed] [Google Scholar]
- 30.Nathan DM, Buse JB, Davidson MB, et al. American Diabetes Association; European Association for the Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vermeire E, Wens J, Van Royen P, Biot Y, Hearnshaw H, Lindenmeyer A. Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005 Apr;18(2):CD003638. doi: 10.1002/14651858.CD003638.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Delamater AM. Improving patient adherence. Clin Diabetes. 2006;24(2):71–77. [Google Scholar]
- 33.Daly JM, Hartz AJ, Xu Y, et al. An assessment of attitudes, behaviors, and outcomes of patients with type 2 diabetes. J Am Board Fam Med. 2009;22(3):280–290. doi: 10.3122/jabfm.2009.03.080114. [DOI] [PubMed] [Google Scholar]
- 34.Rubak S, Sandbaek A, Lauritzen T, Borch-Johnsen K, Christensen B. General practitioners trained in motivational interviewing can positively affect the attitude to behaviour change in people with type 2 diabetes. Scand J Prim Health Care. 2009;29:1–8. doi: 10.1080/02813430903072876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Padala PR, Desouza CV, Almeida S, et al. The impact of apathy on glycemic control in diabetes: a cross-sectional study. Diabetes Res Clin Pract. 2008;79(1):37–41. doi: 10.1016/j.diabres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Williams GC, Freedman ZR, Deci EL. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 1998;21(10):1644–1651. doi: 10.2337/diacare.21.10.1644. [DOI] [PubMed] [Google Scholar]
- 37.Williams GC, McGregor HA, King D, Nelson CC, Glasgow RE. Variation in perceived competence, glycemic control, and patient satisfaction: relationship to autonomy support from physicians. Patient Educ Couns. 2005;57(1):39–45. doi: 10.1016/j.pec.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the cross-national Diabetes Attitudes, Wishes and Needs (DAWN) study. Diabetes Med. 2005;22(10):1379–1385. doi: 10.1111/j.1464-5491.2005.01644.x. [DOI] [PubMed] [Google Scholar]
- 39.Penning-van Beest FJA, van der Bij S, Erkens JA, Kessabi S, Groot M, Herings RM. Effect of non-persistent use of oral glucose-lowering drugs on HbAlc goal attainment. Curr Med Res Opin. 2008;24(9):2523–2529. doi: 10.1185/03007990802336335. [DOI] [PubMed] [Google Scholar]
- 40.Brod M, Cobden D, Lammert M, Bushnell D, Raskin P. Examining correlates of treatment satisfaction for injectable insulin in type 2 diabetes: lessons learned from a clinical trial comparing biphasic and basal analogues. Health Qual Life Outcomes. 2007;5:8. doi: 10.1186/1477-7525-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacco WP, Wells KJ, Friedman A, Matthew R, Perez S, Vaughan CA. Adherence, body mass index, and depression in adults with type 2 diabetes: the mediational role of diabetes symptoms and self-efficacy. Health Psychol. 2007;26(6):693–700. doi: 10.1037/0278-6133.26.6.693. [DOI] [PubMed] [Google Scholar]
- 42.Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes—causes, effects and coping strategies. Diabetes Obes Metab. 2007;9(6):799–812. doi: 10.1111/j.1463-1326.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 43.Leslie WS, Hankey CR, Lean ME. Weight gain as an adverse effect of some commonly prescribed drugs; a systematic review. Q J Med. 2007;100(7):395–404. doi: 10.1093/qjmed/hcm044. [DOI] [PubMed] [Google Scholar]
- 44.Purnell JQ, Weyer C. Weight effect of current and experimental drugs for diabetes mellitus: from promotion to alleviation of obesity. Treat Endocrinol. 2003;2(1):33–47. doi: 10.2165/00024677-200302010-00004. [DOI] [PubMed] [Google Scholar]
- 45.Secnik Boye K, Matza LS, Oglesby A, et al. Patient-reported outcomes in a trial of exenatide and insulin glargine for the treatment of type 2 diabetes. Health Qual Life Outcomes. 2006;4:80. doi: 10.1186/1477-7525-4-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams-Herman DE. Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29(12):2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 47.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24(10):2943–2952. doi: 10.1185/03007990802418851. [DOI] [PubMed] [Google Scholar]
- 48.Garber A, Henry R, Ratner R, et al. LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 49.Kawai T, Funae O, Shimada A, et al. Effects of preteatment with low-dose metformin on metabolic parameters and weight gain by pioglitazone in Japanese patients with type 2 diabetes. Intern Med. 2008;47(13):1181–1188. doi: 10.2169/internalmedicine.47.0969. [DOI] [PubMed] [Google Scholar]
- 50.Kusaka I, Nagasaka S, Horie H, Ishibashi S. Metformin, but not pioglitazone, decreases postchallenge plasma ghrelin levels in type 2 diabetic patients: a possible role in weight stability? Diabetes Obes Metab. 2008;10(11):1039–1046. doi: 10.1111/j.1463-1326.2008.00857.x. [DOI] [PubMed] [Google Scholar]
- 51.Miyazaki Y, Defronzo RA. Rosiglitazone and pioglitazone similarly improve insulin sensitivity and secretion, glucose tolerance and adipocytokines in type 2 diabetic patients. Diabetes Obes Metab. 2008;10(12):1204–1211. doi: 10.1111/j.1463-1326.2008.00880.x. [DOI] [PubMed] [Google Scholar]
- 52.Nauck M, Frid A, Hermansen K, et al. LEAD-2 Study Group. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32(1):84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan C, Yang W, Barona JP, et al. Comparison of vildagliptin and acarbose monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabet Med. 2008;25(4):435–441. doi: 10.1111/j.1464-5491.2008.02391.x. [DOI] [PubMed] [Google Scholar]
- 54.Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49(11):2564–2571. doi: 10.1007/s00125-006-0416-z. [DOI] [PubMed] [Google Scholar]
- 55.Raz I, Chen Y, Wu M, et al. Efficacy and safety of sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes. Curr Med Res Opin. 2008;24(2):537–550. doi: 10.1185/030079908x260925. [DOI] [PubMed] [Google Scholar]
- 56.Rosenstock J, Hassman DR, Madder RD, et al. Repaglinide Versus Nateglinide Comparison Study Group. Repaglinide versus nateglinide monotherapy: a randomized, multicenter study. Diabetes Care. 2004;27:1265–1270. doi: 10.2337/diacare.27.6.1265. [DOI] [PubMed] [Google Scholar]
- 57.Rosenstock J, Sugimoto D, Strange P, Stewart JA, Soltes-Rak E, Dailey G. Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naive patients. Diabetes Care. 2006;29(3):554–559. doi: 10.2337/diacare.29.03.06.dc05-0695. [DOI] [PubMed] [Google Scholar]
- 58.Rosenstock J, Brazg R, Andryuk PJ, Lu K, Lu P Sitagliptin Study 019 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28(10):1556–1568. doi: 10.1016/j.clinthera.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Scott R, Loeys T, Davies MJ, Engel SS Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2008;10(10):959–969. doi: 10.1111/j.1463-1326.2007.00839.x. [DOI] [PubMed] [Google Scholar]
- 60.Yamanouchi T, Sakai T, Igarashi K, Ichiyanagi K, Watanabe H, Kawasaki T. Comparison of metabolic effects of pioglitazone, metformin, and glimepiride over 1 year in Japanese patients with newly diagnosed type 2 diabetes. Diabet Med. 2005;22(8):980–985. doi: 10.1111/j.1464-5491.2005.01656.x. [DOI] [PubMed] [Google Scholar]
- 61.Yilmaz H, Gursoy A, Sabin M, Guvener Demirag N. Comparison of insulin monotherapy and combination therapy with insulin and metformin or insulin and rosiglitazone or insulin and acarbose in type 2 diabetes. Acta Diabetol. 2007;44(4):187–192. doi: 10.1007/s00592-007-0004-9. [DOI] [PubMed] [Google Scholar]
- 62.Barnett AH, Burger J, Johns D, et al, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Ther. 2007;29(11):2333–2348. doi: 10.1016/j.clinthera.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Bergenstal M, Kim T, Trautmann M, Zhuang D, Okerson T, Taylor K. Exenatide once weekly elicited improvements in blood pressure and lipid profile over 52 weeks in patients with type 2 diabetes. Circulation. 2008;118:S1086. Abstract 1239. [Google Scholar]
- 64.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28(5):1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 65.Drucker DJ, Buse JB, Taylor K, et al. DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 66.Fakhoury W, Lockhart I, Kotchie RW, Aagren M, LeReun C. Indirect comparison of once daily insulin detemir and glargine in reducing weight gain and hypoglycaemic episodes when administered in addition to conventional oral anti-diabetic therapy in patients with type-2 diabetes. Pharmacology. 2008;82(2):156–163. doi: 10.1159/000149569. [DOI] [PubMed] [Google Scholar]
- 67.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143(8):559–569. doi: 10.7326/0003-4819-143-8-200510180-00006. [DOI] [PubMed] [Google Scholar]
- 68.Karl D, Philis-Tsimikas A, Darsow T, et al. Pramlintide as an adjunct to insulin in patients with type 2 diabetes in a clinical practice setting reduced AIC, postprandial glucose excursions, and weight. Diabetes Technol Ther. 2007;9(2):191–199. doi: 10.1089/dia.2006.0013. [DOI] [PubMed] [Google Scholar]
- 69.Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28(5):1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 70.Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30(6):1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 71.Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24(1):275–286. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 72.Marre M, Shaw J, Brandle M, et al. LEAD-I SU study group. Liraglutide, a once-daily human GLP-I analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-I SU) Diabet Med. 2009;26(1):268–278. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50(2):259–267. doi: 10.1007/s00125-006-0510-2. [DOI] [PubMed] [Google Scholar]
- 74.Riddle M, Frias J, Zhang B, et al. Pramlintide improved glycemic control and reduced weight in patients with type 2 diabetes using basal insulin. Diabetes Care. 2007;30(11):2794–2799. doi: 10.2337/dc07-0589. [DOI] [PubMed] [Google Scholar]
- 75.Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;81(2):161–168. doi: 10.1016/j.diabres.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 76.Vilsbøll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30(6):1608–1610. doi: 10.2337/dc06-2593. [DOI] [PubMed] [Google Scholar]
- 77.Zinman B, Hoogwerf BJ, Durán Garcia S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146(7):477–485. doi: 10.7326/0003-4819-146-7-200704030-00003. [DOI] [PubMed] [Google Scholar]
- 78.Zinman B, Gerich J, Buse JB, et al. LEAD-4 Study Investigators. Efficacy and safety of the human GLP-I analog liraglutide in combination with metformin and TZD in patients with type 2 diabetes mellitus (LEAD-4 Met+TZD) Diabetes Care. 2009;32(7):1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henry RR. Evolving concepts of type 2 diabetes management with oral medications: new approaches to an old disease. Curr Med Res Opin. 2008;24(8):2189–2202. doi: 10.1185/03007990802212981. [DOI] [PubMed] [Google Scholar]
- 80.Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386–399. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 81.Charbonnel B, Karasik A, Liu J, Wu M, Meininger G Sitagliptin Study 020 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29(12):2638–2643. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- 82.Stonehouse AH, Holcombe JH, Kendall DM. GLP-I analogues, DPP-IV inhibitors, and the metabolic syndrome. In: Fonseca V, editor. Therapeutic Strategies: Metabolic Syndrome. Oxford, UK: Atlas Medical Publishing Ltd; 2008. pp. 137–157. [Google Scholar]
- 83.Baggio LL, Maida A, Lamont BL. [Accessed December 7, 2008];ADA 2008: Incretin-based therapeutics. Available at: http.//www.medscape.com/viewprogram/15786_pnt. Last updated July 31, 2008.
- 84.Ong KL, Cheung BM, Wong LY, Wat NM, Tan KC, Lam KS. Prevalence, treatment, and control of diagnosed diabetes in the U.S. National Health and Nutrition Examination Survey 1999–2004. Ann Epidemiol. 2008;18(3):222–229. doi: 10.1016/j.annepidem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 85.Rubin RR, Peyrot M. Assessing treatment satisfaction in patients treated with pramlintide as an adjunct to insulin therapy. Curr Med Res Opin. 2007;23(8):1919–1929. doi: 10.1185/030079907X210804. [DOI] [PubMed] [Google Scholar]
- 86.Buse JB, Ginsberg HN, Bakris GL, et al. American Heart Association, American Diabetes Association. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115(1):114–126. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]