Abstract
Obesity is at epidemic proportions in the United States and in other developed and developing countries. The prevalence of obesity is increasing not only in adults, but especially among children and adolescents. In the United States in 2003 to 2004, 17.1% of children and adolescents were overweight, and 32.2% of adults were obese. Obesity is a significant risk factor for and contributor to increased morbidity and mortality, most importantly from cardiovascular disease (CVD) and diabetes, but also from cancer and chronic diseases, including osteoarthritis, liver and kidney disease, sleep apnea, and depression. The prevalence of obesity has increased steadily over the past 5 decades, and obesity may have a significant impact on quality-adjusted life years. Obesity is also strongly associated with an increased risk of all-cause mortality as well as cardiovascular and cancer mortality. Despite the substantial effects of obesity, weight loss can result in a significant reduction in risk for the majority of these comorbid conditions. Those comorbidities most closely linked to obesity must be identified to increase awareness of potential adverse outcomes. This will allow health care professionals to identify and implement appropriate interventions to reduce patient risk and mortality. A systematic search strategy was used to identify published literature between 1995 and 2008 that reported data from prospective longitudinal studies of obesity and comorbid medical conditions. This article will review evidence for significant associations of obesity with comorbidities to provide information useful for optimal patient management.
Keywords: cancer, cardiovascular disease, diabetes, obesity, cornorbidity, mortality
Introduction
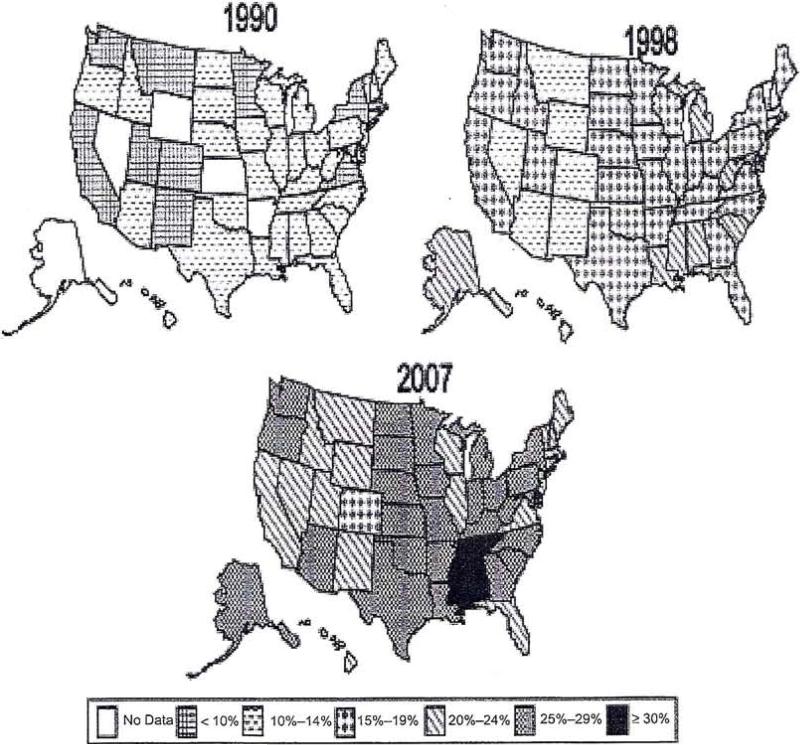
Obesity, defined as a body mass index (BMI) ≥ 30 kg/m2,1,2 is a medical condition encountered daily by physicians throughout the United States. The prevalence of obesity is increasing and reaching epidemic proportions (Figure 1),3 although recent data suggest that the prevalence is leveling off among children and adolescents.4 From 2003 to 2004 in the United States, 32.2% of adults were obese and 17.1% of children and adolescents were overweight.5
Figure 1.
Trends in obesity among US adults over 17 years (1990–2007).3
A major concern for physicians who care for patients who are overweight or obese is the high risk of accompanying comorbid disorders, such as diabetes, cardiovascular disease (CVD), and cancer. An important need for primary care physicians is to identify these comorbidities and the resulting adverse outcomes. Awareness of the disorders with the strongest associations with obesity is important to allow early diagnosis and treatment of these conditions, and to identify the patients most likely to benefit from weight loss. This will allow early identification and assessment of risk so that appropriate interventions can be implemented to reduce risk and mortality.
This article will review significant associations of obesity with comorbidities to provide the clinician with the information necessary to offer optimal patient management. A systematic search strategy was used to identify English language articles cited in PubMed between 1995 and 2008 that reported data from prospective, longitudinal studies of obesity and comorbid medical conditions. Relevant citations were comprehensively reviewed to determine those that confirmed a significant relationship between obesity and comorbid conditions.
Overview of the Epidemiology of Obesity
Numerous large, long-term epidemiological studies have shown that obesity is strongly associated with an increased risk of all-cause, cardiovascular, and cancer mortality (Figure 2).6 In the National Health and Nutrition Examination Study (NHANES) III, obesity was associated with an increased prevalence of type 2 diabetes, gallbladder disease, coronary heart disease (CHD), hypertension, osteoarthritis (OA), and high blood cholesterol among > 16 000 participants.7 Results from other studies have shown a strong association between obesity and the prevalence of comorbid illnesses, health complaints, and physical disability.8–11 A 10-year follow up from Nurses’ Health Study (N > 121000) and the Health Professionals Follow-up Study (N > 51000) evaluated the risk of diabetes, gallstones, and hypertension in obese (BMI ≥ 30 kg/m2) men and women compared with those with a normal BMI.12 The risk of diabetes, gallstones, and hypertension was increased in women, while the risk of diabetes, gallstones, hypertension, heart disease, and stroke was increased in men. Based on the available data, The Obesity Society concluded that obesity is causally associated with functional impairment and reduced quality of life, serious disease, and greater mortality.13
Figure 2.
Relative risk of death from all causes, CVD, and cancer according to BMI in men (top) and women (bottom).4
The association between obesity and comorbid conditions, which will be discussed in the section that follows, is illustrated in Figure 3, where chronic conditions such as kidney disease, OA, cancer, diabetes, sleep apnea, nonalcoholic fatty liver disease (NAFLD), hypertension, and most importantly, CVD, are directly related to obesity.11,12 Further, many of these comorbidities also are directly associated with CVD.
Figure 3.
Association of obesity and important comorbidities.
Many of the epidemiological studies have been confirmed by observations that weight loss improves patient outcomes. The results from the Swedish Obesity Study showed that weight loss from bariatric surgery reduced most cardiovascular risk factors.11 An American Heart Association Committee also concluded that weight loss and physical activity could prevent and treat obesity-related CHD risk factors.14 Data reporting that a specific outcome is improved by weight loss will also be reviewed.
Obesity and Comorbidity
Diabetes
The long-term risk of type 2 diabetes increases significantly with increasing weight. In the Nurses’ Health Study, the effect of weight change on the risk for clinical diabetes was evaluated in 114281 women.15 After adjusting for age, body weight was the major risk factor for diabetes during a 14-year follow-up. Among women with a 5- to 7.9-kg weight gain, the relative risk for diabetes was 1.9 and for those with an 8- to 10.9-kg weight gain, the relative risk was 2.7. In contrast, a 5-kg weight loss resulted in a 50% reduction in the risk of diabetes.
Consistent with this observation, several studies have shown that weight loss is associated with a significant reduction in the risk of diabetes.16 In a prospective, 20-year study of 7176 British men, the rate of new diabetes was 11.4 per 1000 person-years among obese subjects versus 1.6 among normal-weight subjects (P < 0.0001), but the effect of weight change during a 5-year follow-up on the development of diabetes found a relative risk of 0.62 among those losing weight compared with 1.0 for stable weight and 1.76 among those gaining > 10% body weight (P < 0.0001). Similarly, a Health Technology Assessment that examined the effect of weight loss in patients with diabetes found significant improvement in the risk of developing diabetes.17 Long-term weight loss was also associated with a reduction in the risk of type 2 diabetes in the Diabetes Prevention Program.18 Thus, despite the known risk of type 2 diabetes associated with obesity, weight loss has the potential to improve outcomes.
Weight loss was also associated with improved diabetes control in the Look AHEAD (Action for Health in Diabetes) study19 Look AHEAD is a randomized trial of intensive lifestyle intervention versus usual support and education in 5145 patients with type 2 diabetes andBMI > 25 kg/m2. The intensive group lost 8.6% of body weight compared with 0.7% in supportive group (P < 0.001). At 1 year, intensive intervention resulted in clinically significant weight loss in people with type 2 diabetes, which was associated with improved diabetes control and reduction in CVD risk factors and medication use.
Cardiovascular Disease
Obesity is an independent risk factor for CVD, defined as including CHD, myocardial infarction (MI), angina pectoris, congestive heart failure (CHF), stroke, hypertension, and atrial fibrillation.7,14 Overall, results from large prospective and observational studies confirm the marked adverse effects of obesity on CVD.
Numerous large-scale, long-term studies in the United States have investigated the role of obesity in CVD risk and on the development of CVD. The Multiethnic Study of Atherosclerosis evaluated the effects of obesity on CVD risk factors and on subclinical signs of CVD in 6814 participants who were free of CVD at baseline.20 Hypertension and diabetes as well as subclinical cardiovascular findings were more prevalent in obese (BMI ≥ 30 kg/m2) than nonobese participants. In addition, data collected from the original cohort of 5209 participants of the Framingham Heart Study over 44 years were used to evaluate the effect of obesity (BMI ≥ 30 kg/m2) on the risk of CVD (angina, MI, CHD, or stroke), diabetes, hypertension, and hypercholesterolemia.21 During follow-up, the age-adjusted relative risk for CVD was 1.46 in men and 1.64 in women, and the age-adjusted relative risk for hypertension was even higher among obese men and women (2.21 and 2.75, respectively). In a separate analysis of the Framingham Heart Study, the lifetime risk of CVD was assessed among obese men and women with diabetes versus nonobese subjects.22 During a 30-year follow-up, the risk of CVD was 54.8% in normal-weight women versus 78.8% among obese women with diabetes and 78.6% versus 86.9% among normal and obese men with diabetes, respectively.22
Similar results have been obtained in studies performed outside of the United States. The International Day for the Evaluation of Abdominal Obesity (IDEA) study evaluated waist circumference, CVD, and diabetes mellitus in 168000 primary care patients in 63 countries.23 Overall, 24% of men and 27% of women were obese, and the risk of CVD and diabetes was strongly associated with BMI and waist circumference. Lastly, among 7176 British men followed for 20 years, the rate of major CVD was 24.9/1000 in obese (BMI ≥ 30 kg/m2) subjects versus 13.9/1000 among normal-weight (BMI < 25 kg/m2) subjects.16
Results from the Framingham Heart Study also showed that obesity increases the risk of atrial fibrillation.24 Among 5282 participants (of whom 55% were women) without atrial fibrillation at baseline, subjects were classified as normal (BMI < 25 kg/m2), overweight, and obese (BMI ≥ 30 kg/m2). During a mean follow-up of 13.7 years, a 4% increase in risk of atrial fibrillation/1-unit increase in BMI was observed in men and women after adjustment for cardiovascular risk factors. Compared with normal-weight individuals, in obese subjects the hazard ratio for atrial fibrillation was 1.52 for men and 1.46 for women.
Among 111847 patients with non–ST-segment myocardial infarction (NSTEMI) who were included in the CRUSADE registry,25 excess BMI was also strongly associated with an earlier age of first NSTEMI. The registry collected data from January 2001 to January 2007 in high-risk patients with unstable angina and NSTEMI. Extreme obesity (BMI > 40 kg/m2) had the strongest association with age at first MI after adjustment for baseline factors. After adjustment for baseline demographic data, cardiac risk factors, and medications, the first NSTEMI occurred 3.5, 6.8, 9.4, and 12 years (P < 0.0001) earlier with increasing adiposity (BMI 25.1–30 kg/m2, 30.1–35 kg/m2, 35.1–40 kg/m2, and > 40 kg/m2, respectively).
Hypertension, a risk factor for CVD, is related to obesity. An analysis from the Women's Health Study found a significant association between obesity, the development of hypertension, and diabetes.26 In this analysis of 38172 women who were free of diabetes and CVD at baseline with a mean 10.2 years of follow-up, the age-adjusted incidence rate/1000 of diabetes in obese women (BMI ≥ 30 kg/m2) was 7.58 among normotensive patients (120/75) versus 20.53 among hypertensive patients. Further, a significant association between BMI and hypertension was observed in a prospective study from Norway, the Nord-Trondelag Health Study.27 Among> 15900 women and > 13800 men at least 20 years old without hypertension, diabetes, or CVD at baseline, the risk for hypertension was increased ≥ 1.4-fold among men and women whose BMI increased from baseline compared with those who maintained a stable BMI.
Metabolic Syndrome
A combination of commonly associated cardiovascular risk factors is known as metabolic syndrome (MetS). Metabolic syndrome represents a group of cardiometabolic risk factors that include abdominal obesity combined with elevated blood pressure, fasting plasma glucose, and triglycerides, and reduced high-density lipoprotein cholesterol levels. Metabolic syndrome is associated with an increased risk of cardiovascular mortality.28 Guidelines for the diagnosis and management of MetS are available from a number of professional organizations including the American Heart Association and the International Union of Angiology.29,30
As discussed previously, abdominal obesity, a key part of the constellation of risk factors for MetS, is strongly associated with the risk of diabetes.31,32 An analysis of the associations between risk factors for MetS in 2735 participants from the Dallas Heart Study showed that higher BMI was significantly associated with MetS in both diabetic and nondiabetic patients.31 In a prospective cohort study that examined the association between MetS and type 2 diabetes among 4022 patients with atherosclerosis, abdominal obesity was the component most strongly associated with the risk of type 2 diabetes.32 Data from 9 European studies were examined to determine the association between MetS and abdominal adiposity in > 15000 men and women.28 The definition of MetS was satisfied in 41 % of men and 37.9% of women, and those with MetS were more often obese and had a higher prevalence of diabetes than nonobese participants. A prospective study of 3051 elderly men with diabetes or CHD also found that obesity and physical inactivity as well as cigarette smoking and high carbohydrate diet were significantly associated with a greater risk of MetS.33
In contrast, weight reduction alone or combined with lifestyle intervention is associated with a significant reduction in the prevalence of MetS.34,35 The prevalence of MetS and abdominal obesity was significantly reduced from 74% to 58% in a lifestyle intervention group versus to 67.7% in a standard care group (P = 0.025).34 In a separate study, a moderate 8-kg reduction in weight after 1 year resulted in a significant (P < 0.05) reduction in the prevalence ofMetS from 35% to 27%.35
Cancer
A number of large-scale, prospective studies have confirmed a significant association between obesity and cancer. The strongest association is between an elevated BMI and cancer risk. A prospective cohort study in the United States found a significant association between obesity and cancer.36 This prospective study involved > 900000 subjects from the American Cancer Prevention Study II who were free from cancer in 1982 and had a mean follow up of 16 years. Among those with a BMI ≥ 40 kg/m2, mortality from all causes of cancer was 52% higher in men and 62% higher in women compared with those with a normal BMI. Body mass index was also significantly associated with higher rates of death due to cancer of the esophagus, colon and rectum, liver, gallbladder, pancreas, kidney, non-Hodgkin lymphoma, and multiple myeloma.
In the Million Women Study from the United Kingdom, increasing BMI was associated with a significant increase in risk for 10 out of 17 of the most common types of cancer.37 Over 1.2 million UK women, aged 50 to 64 years during 1996 to 2001, were recruited into the Million Women Study and followed for a mean of 5.4 years for cancer incidence and 7 years for cancer mortality. Increasing BMI was associated with an increased incidence of all cancers combined in addition to endometrial cancer, adenocarcinoma of the esophagus, kidney cancer, leukemia, multiple myeloma, pancreatic cancer, non-Hodgkin lymphoma, ovarian cancer, breast cancer in postmenopausal women, and colorectal cancer in premenopausal women (Figure 4).
Figure 4.
Relative risk of cancer according to the presence of obesity (BMI ≥ 30 kg/m2).37 Reference value was 1.0 for participants with a BMI of 22.5–24.9 kg/m2.
A prospective study evaluated the effect of BMI and weight gain on prostate cancer incidence and mortality among 287700 men in the NIH-AARP Diet and Health Study.38 During a mean follow-up of 5 to 6 years, the relative risk for mortality from prostate cancer was 1.46 and 2.l2 for a BMI ≥ 30 kg/m2 and ≥ 35 kg/m2, respectively. In a separate study of 69991 men, the risk of high-grade nonmetastatic and metastatic prostate cancer was increased with obesity (1.2- and 1.5-fold, respectively), and the risk of high-grade nonmetastatic cancer was reduced to 0.58 with > 11-lb weight loss.39
In the Health Professionals Follow-up Study, a significant association between obesity and colon cancer was observed in men.40 This 18-year, prospective follow-up study of 46349 men who were cancer-free at baseline found a multivariate hazard ratio (HR) for colon cancer was increased at a BMI > 22.5 kg/m2, but was highest (HR, 2.29) at a BMI > 30 kg/m2. An estimated 30% of all colon cancer cases were attributed to overweight and obesity.
Pischon et al41,42 evaluated the association between the risk of colon and rectal cancer and renal cell carcinoma and body weight in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. More than 368000 men and women who were cancer-free at baseline were followed for a mean of 6.1 years in the EPIC Study. Body weight and BMI (≥ 29.4 kg/m2) were significantly associated with the risk of colon cancer in men but not women (relative risk [RR], 1.55; P = 0.006). Among 348500 men and women with a 6-year follow-up, the RR for renal cell carcinoma associated with increased BMI in women was 2.25 (P = 0.009; BMI ≥ 29 kg/m2) but no significant increase was observed for men (RR, 1.22; P = 0.51).
Arthritis and Disability
Osteoarthritis has a major impact on patient mobility, disability, lost productivity, and patients may become disabled from OA early in life.43,44 Obesity is strongly associated with an increased risk of OA of the knee but only a moderate association with OA of the hip has been found.45 Because OA strongly impacts patient lifestyle and function, it is important to recognize this effect of obesity and the potential need for weight loss and rehabilitation.
The relationship between OA of the hip and knee and obesity was examined in The Rotterdam Study.45 Radiographic confirmation of OA was established in 3585 participants at baseline, and patients were followed for a mean of 6.6 years. A BMI > 27 kg/m2 was associated with a 3.3-fold greater risk of OA and progression of OA of the knee but not the hip. In a longitudinal study of 715 women in the Chingford population over 4 years, mean age 54 years at baseline, those in the top BMI tertile had an increased risk of knee OA compared with women in lower BMI tertiles.46 In The Framingham Heart Study, the effect of obesity on the increased risk of knee OA was determined in elderly patients without knee OA at baseline.47 Among 598 patients who developed OA over a 1O-year follow-up, the risk for OA was increased by 1.6 for each 5-unit increase of BMI.
The association between obesity and OA of the knee is thus widely recognized. A number of prospective studies have examined the relationship between obesity and disability in patients with knee OA.48–52 A prospective cohort study of 5784 participants at least 50 years old was conducted to examine the effect of obesity on knee pain and disability.49 Obesity accounted for a substantial proportion of severe disabling knee pain in this cohort, and the authors concluded that health interventions to avoid obesity would have a major impact on improving disability associated with knee OA. Another cross-sectional study of 3664 participants > 25 years old found that obesity was associated with a higher risk of OA of the hip or knee, chronic pain, and a mobility disability.52 In 56 obese adults,51 knee OA was significantly associated with reduced exercise capacity, ambulatory capacity, and quality of life.
Importantly, weight loss has been shown to significantly improve signs and symptoms of OA and improve disability and function in obese patients.53–58 A meta-regression analysis that included 4 trials including a total of 454 patients was conducted on the effect of weight loss on OA.53 Mean baseline BMI ranged from 29 to 36 kg/m2 in each of 5 intervention groups, and weight loss ranged from 1.7 to 6.7 kg over 6 weeks to 18 months. Modest weight loss (5.1%) improved physical disability among patients with knee OA. A randomized study of 87 obese (BMI ≥ 30 kg/m2) adults at least 60 years old with symptomatic knee OA was also undertaken to evaluate the effect of weight loss intervention.57 At 6 months, those randomized to intervention had lost a mean of 8.7% of body weight compared with no weight loss in the control group. Functional status was significantly (P < 0.05) improved in the intervention group with greater improvements observed with more weight loss. Others have found significant improvements in function and pain with weight loss and/or exercise among patients with knee OA.55,56,58 Thus, recognition of the impact of obesity among patients with knee OA offers an opportunity to significantly improve associated disability and pain by encouraging weight loss.
Gallbladder Disease
Gallbladder disease is a common cause of hospitalization, especially among women, and has a considerable impact on health care costs.59 An epidemiologic study from the National Health Service in England and Scotland found a significant association between obesity and gallbladder disease among women.59 In this study, data for 1.3 million women (mean age, 56 years), representing 7.8 million person-years of follow-up, were evaluated. After adjusting for age, socioeconomic status, and other factors, women with higher BMI at study entry were more likely to be admitted and spend more days in the hospital for gallbladder disease. For each 1000 person-years of follow-up, women with BMI in the lowest BMI category (18.5–24.9 kg/m2) spent a mean of 16.5 days hospitalized versus 44 days for women in the obese category (BMI 30–39.9 kg/m2). Overall, 25% of hospital days for gallbladder disease were attributed to obesity.
In a prospective evaluation from the Health Professionals Follow-up Study, the association between abdominal obesity and the incidence of symptomatic gallstone disease was determined in a cohort of 29 847 men who were free of prior gallstone disease and who provided complete data on waist and hip circumferences.60 Men with BMI ≥ 28.5 kg/m2 had a 2.49-fold greater risk of gallstones compared with men with a normal BMI (< 22.2 kg/m2) Similar findings were observed in the Swedish Twin Registry Study.61 The Swedish Twin Registry study assessed the effects of overweight and obesity (BMI > 30 kg/m2) on symptomatic gallstones in 58400 participants. Overweight and obesity were both associated with a significant increase in the risk of symptomatic gallstones (OR = 1.86 and 3.38, respectively).
Acute Pancreatitis
Acute pancreatitis is closely associated with obesity, and a number of studies have shown that obesity increases the severity of and mortality from acute pancreatitis.62–65 Obesity is a primary risk factor for local complications, organ failure, and death from acute pancreatitis.
In a meta-analysis of 5 studies including a total of 739 patients, obesity (BMI ≥ 30 kg/m2) was identified as a risk factor for the development of local and systemic complications in acute pancreatitis and was also associated with increased mortality.64 Among these patients from the 5 studies, severe acute pancreatitis was significantly associated with obesity (OR 2.9, 95% CI 1.8–4.6). Among these obese patients, significantly more systemic (OR 2.3, 95% CI 1.4–3.8) and local complications occurred (OR 3.8, 95% CI 2.4–6.6), and mortality was higher (OR 2.1, 95% CI 1.0–4.8).
Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease (NAFLD) represents a spectrum of disorders that range from steatosis to nonalcoholic steatohepatitis and ultimately cirrhosis and hepatocellular carcinoma.66 Nonalcoholic fatty liver disease is associated with obesity, dyslipidemia, hypertension, and insulin resistance, components of the MetS that increase cardiovascular risk.67 It affects approximately 15%–30% of the general population, and has a prevalence of approximately 70% in people with type 2 diabetes.67
Studies have indentified obesity as a predictor of NAFLD.68–70 In a multivariate analysis among 832 Chilean participants, the primary variable associated with NAFLD was BMI > 26.9 kg/m2.68 The diagnosis of NAFLD was based on ultrasound and no history of alcohol abuse or hepatitis C infection. Multivariate analysis found that obesity was significantly and independently associated with NAFLD with odds ratio of 6.2. In a cross-sectional study of 326 Israelis who participated in a National Health survey, the prevalence of NAFLD was 30%; NAFLD was more common in men (38%) than in women (21%), and obesity (BMI ≥ 30 kg/m2) was independently associated with NAFLD (odds ratio 2.9).69 A study of 218 nonsmoking, healthy men found that 24 met criteria for NAFLD.70 Lack of fitness and BMI ≥ 30 kg/m2 were significantly (P < 0.001) and independently associated with NAFLD.
Increased physical activity and bariatric surgery in selected cases may be effective therapy for NAFLD.71,72 Although evidence is limited, weight loss may be beneficial for reducing the risk of NAFLD in obese patients.71
Pulmonary Complications
Obstructive sleep apnea (OSA) potentially results in a number of complications including pulmonary hypertension, right heart failure, drug-resistant hypertension, stroke, and arrhythmias.73–78 Obstructive sleep apnea is characterized by upper airway obstruction that occurs as repetitive episodes during sleep.74 Among the typical features of OSA are loud snoring, fragmented sleep, repetitive hypoxemia/hypercapnia, daytime sleepiness, and cardiovascular complications. Although the prevalence of OSA is 2% to 3% among middle-aged women and 4% to 5% among middle-aged men, the prevalence among obese patients is > 30% and among the morbidly obese ranges from 50% to 98%.75–77 Thus, obesity is the most important risk factor for the development of OSA, where 60% to 90% of adults are overweight, and the relative risk in obese patients (BMI > 29 kg/m2) is ≥ 10.74
The independent association between sleep-disordered breathing and weight gain was evaluated in a population-based, prospective study of 690 randomly selected residents of Wisconsin.76 Participants had a mean age of 46 years, a mean baseline BMI of 29–30 kg/m2, and were evaluated twice at 4-year intervals. A 10% weight gain predicted a 32% increase in the apnea-hypopnea index and a 6-fold increase in the odds of developing moderate-to-severe sleep-disordered breathing.
Similarly, the effect of weight gain on sleep-disordered breathing was determined in a prospective study of 2968 men and women in the United States.77 Baseline mean BMI was approximately 29 kg/m2, mean age was 62 years, and participants were examined at baseline and 5 years. An increased number of respiratory events was associated with weight increases. Men with a 10-kg increase in weight had 5.21-fold increased risk of developing > 15 events/hour and women had a 2.5-fold increased risk.
A reduction in OSA was observed in The Swedish Obesity Study among patients with diabetes who lost weight.78 This study evaluated 1729 patients with a baseline BMI > 40 kg/m2 undergoing bariatric surgery and 1748 given conservative medical therapy as the control group. A significant (P < 0.001) reduction in symptoms of OSA was observed among the bariatric surgery group at 2 years including apnea (24% to 8%), snoring (44.5% to 10.8%), and daytime sleepiness (25.8% to 12.7%). Those with a mean 31% weight loss had a 2- to 13-fold decrease in the risk of developing new OSA, and those with OSA were 2.5 to 7 times less likely to report continuing OSA symptoms.
Depression
An association between obesity and major depressive disorder (MDD) has long been recognized although a causal association is uncertain. Importantly, many antidepressant drugs are associated with weight gain. The National Epidemiologic Survey on Alcohol and Related Conditions evaluated the relationship between BMI and psychiatric disorders in 41 654 respondents.79 Among participants, BMI was significantly associated with mood, anxiety, and personality disorders. The odds ratio for a psychiatric disorder was 1.21- to 2.08-fold greater among obese (BMI 30-39.9 kg/m2) and extremely obese (BMI ≥ 40 kg/m2) subjects, and the odds ratio for a lifetime prevalence of MDD was 1.53 and 2.02 among obese and extremely obese compared with normal weight subjects.
Others have found similar results. The 2006 Behavioral Risk Factor Surveillance System (N = 217 379) found that adults with current depression or a lifetime diagnosis of depression or anxiety were significantly more likely to have unhealthy behaviors including smoking, obesity, physical inactivity, binge drinking, and heavy drinking.80 The adjusted odds ratio for depression and obesity (BMI ≥ 30 kg/m2) was 1.6 vs 1 for nonobese subjects, and the odds ratio increased with increasing severity of MDD. Among 4641 middle-aged women, MDD was strongly and consistently associated with obesity, lower physical activity, and among the obese, higher caloric intake.81 The prevalence of moderate or severe MDD increased from 6.5% with a BMI < 25 kg/m2 to 25.9% with a BMI > 35 kg/m2. The prevalence of obesity increased from 25.4% to 57.8% among those with no MDD versus those with moderate-to-severe MDD. The odds ratio for having MDD was 4.4 for a BMI of 30 to 35 kg/m2 and 4.95 for a BMI of ≥ 35 kg/m2. Using standard criteria for MDD, the odds ratios were 1.92 for a BMI of 25 to 30 kg/m2, 2.92 for 20–35 kg/m2, and 5.72 for a BMI of ≥ 35 kg/m2.
Despite the absence of a clear causal relationship between obesity and MDD, an awareness of this relationship and the opportunity to improve depression and quality of life by recommending appropriate weight loss interventions is needed.
The Impact of Obesity on Mortality
The net impact of the increased burden of disease associated with obesity is increased mortality, which is well established in this population. An extensive number of epidemiological studies have established a significant increase in cardiovascular and non cardiovascular mortality associated with obesity (Table 1). Overall, large-scale studies such the Nurses’ Health Study, NHANES, Women's Health Initiative Observational Study, American Cancer Society Prevention studies, and others have documented the adverse effects of obesity on mortality from CVD, cancer, and other comorbidities.6,82-89 An increase in years of life lost was found among obese versus nonobese subjects in an analysis of the NHANES database.90 Overall, years of life lost were 1 to 9 for those with low BMI (< 17–19 kg/m2) compared with 9 to 13 for those with a high BMI (≥ 35 kg/m2).
Table 1.
Effect of Obesity on Mortality: A Summary of Controlled Trials
| Reference | Study Population | Endpoints | Weight Categories | Outcomes |
|---|---|---|---|---|
| Flegal (2007)83 | NHANES I, II, III cohort 571 000 years of follow-up | CV mortality, cancer mortality | Underweight, overweight (BMI 25–30 kg/m2), obese (BMI ≥ 30 kg/m2) | Significant ↑ in CVD and obesity-related cancer mortality |
| Manson (1995)84 | Nurses’ Health Study 115 195 women, aged 30–55 years, free of CVD and cancer at baseline; 16-year follow-up | All-cause mortality, CV mortality, cancer mortality | Overweight (BMI 29–31.9 kg/m2) Obese (BMI ≥ 32 kg/m2) | P < 0.001, trend for ↑ all-cause mortality; for BMI ≥ 32 kg/m2 vs < 19 kg/m2. RR of CV mortality 4.1 and cancer 2.1 |
| McTigue (2006)85 | Women's Health Initiative Observational Study 90 185 women; mean follow-up of 7 years | All-cause mortality | Normal (BMI 18.5–24.9 kg/m2) Obese (BMI ≥ 30 kg/m2) | All = cause mortality ↑ 68.4/10 000 normal 84.5 for obese |
| Stevens (1998)87 | ACS Prevention Study I; 324 000 healthy, never smoking men and women; 12-year follow-up | All-cause mortality, CV mortality | Overweight (BMI 29—31.9 kg/m2) Obese (BMI ≥ 32 kg/m2) | 50% ↑ in all-cause mortality associated with BMI > 27 kg/m2 in men aged 30–84 years and ≥ 29 in women aged 30–74 years |
| Adams (2006)82 | NIH-AARP cohort 527 265 men and women, aged 50–71 years; 10-year follow-up | All-cause mortality | Obese (BMI 30–34.9 kg/m2) | 1.1-fold ↑ in mortality compared with normal BMI; obesity = 18% of excess mortality |
| Calle (1999)6 | > 1 million in ACS Study II 14-year follow-up | All-cause mortality | Obese (BMI 30–32 kg/m2) | Among healthy nonsmokers risk of death ↑ 17%–32% |
| Fontaine (2003)90 | NHANES cohort adults aged 18–85 years | Years of life lost | Low BMI (< 17–19 kg/m2), high BMI (≥ 35 kg/m2) | Years of life lost were 1–9 for low BMI vs 9–13 for BMI ≥ 35 kg/m2 |
| Yan (2006)89 | 17 643 men and women aged 31–64 years; no risk factors at baseline | Mortality from CHD, CVD, or diabetes | Obese (BMI ≥ 30 kg/m2) | ↑ risk of hospitalization and mortality from CHD, CV disease, and diabetes vs normal weight |
| Thomas (2006)88 | 243 000 men and women aged 18–95 years, followed for up to 25 years | Total mortality, CVD mortality | Obese (BMI ≥ 30 kg/m2) | Total and CVD mortality was > 2-fold higher among obese vs nonobese |
Abbreviations: ACS, acute coronory syndrome; CHD, coronary heart disease; CV, cardiovascular; CVD, cardiovascular disease; NHANES, National Health and Nutrition Examination Survey; RR, relative risk.
Importantly, studies of patients undergoing gastric bypass surgery for morbid obesity have demonstrated significant reductions in mortality with substantial weight loss.91,92 Adams91 reported a retrospective cohort study of mortality in 7925 surgical patients and 7925 severely obese control subjects who were matched for age, sex, and BMI. During a mean follow-up of 7.1 years, all-cause mortality decreased by 40% (57.1 to 37.6/10 000 patient-years), and mortality decreased by 56% for CAD, 92% for diabetes, and 60% for cancer (P < 0.01 for each). The Surgical Obesity Study was a prospective evaluation of gastric surgery (n = 20lO) or conventional treatment (n = 2037) of patients with morbid obesity.92 Overall mortality was reported after 10.9 years of follow-up, where average weight change was 2% in the control group and 14% to 25% in the surgery group depending on the procedure. The adjusted hazard ratio for mortality was 0.71 (P = 0.01) in the surgery group versus the control group.
Summary
Obesity is at epidemic proportions in the United States and other developed countries, but, importantly, even in developing countries. Large, high-quality longitudinal or prospective studies have confirmed that obesity is a significant risk factor for and contributor to increased morbidity and mortality, primarily from CVD and diabetes, but also from cancer and other acute and chronic diseases, including osteoarthritis, liver and kidney disease, sleep apnea, and depression (Figure 3). For the majority of these comorbid conditions, weight loss can result in a significant reduction in risk.
The economic costs of obesity are substantial. A model based on NHANES and the Framingham Heart Study was used to assess the lifetime health and economic consequences of obesity.93 The analysis showed substantial effects on lifetime health and economic consequences of obesity, and the authors suggested that significant benefits could be expected from interventions to prevent or reduce obesity.93 Disease risks and costs increased with increasing BMI. For instance, the risk of hypertension was 2-fold higher and diabetes was 3-fold higher among 45- to 54-year-old obese men compared with nonobese men.93 Lifetime medical costs increased incrementally with increased BMI and age by approximately 2-fold for each group. Obesity also has a significant impact on quality-adjusted life years and reduces years oflife.90,93,94 In an analysis of quality-adjusted life years, obese men and women lost 1.9 million and 3.4 million quality-adjusted life years and experienced lower health-related quality of life compared with normal weight subjects.94 A model of the economic costs of obesity found a substantial impact could be reduced with effective measures to prevent weight gain.93
Recognition of the association between obesity and comorbidities is critical for patient diagnosis and management by primary care physicians. Physicians need to be aware of comorbidities and their implications for outcomes and patient management of the obese patient. Global efforts to control obesity and minimize factors that contribute to obesity are essential to improving health status and life expectancy worldwide.
Acknowledgments
The author would like to acknowledge the assistance of Richard S. Perry, PharmD in the preparation of this manuscript, which was funded by Amylin Pharmaceutics, Inc.
Footnotes
Conflict of Interest Statement
Xavier Pi-Sunyer, MD discloses conflicts of interest with Amylin Pharmaceuticals, Arena Pharmaceuticals, Novartis, Novo Nordisk, Orexigen Therapeutics, and VIVUS, Inc.
References
- 1.National Heart, Lung, and Blood Institute [October 10, 2008];Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. doi: 10.1093/ajcn/68.4.899. http://www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 3.Centers for Disease Control (CDC) [October 10, 2008];BRFSS, Behavioral Risk Factor Surveillance System Survey Data, Atlanta, Georgia: US Department of Health and Human Services. http://www.cdc.gov/brfss. [Google Scholar]
- 4.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006. JAMA. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U .S. adults. N Engl J Med. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 7.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 8.Luo W, Morrison H, de Groh M, et al. The burden of adult obesity in Canada. Chronic Dis Can. 2007;27(4):135–144. [PubMed] [Google Scholar]
- 9.Kress AM, Hartzel MC, Peterson MR. Burden of disease associated with overweight and obesity among U.S. military retirees and their dependents, aged 38-64, 2003. Prey Med. 2005;41(1):63–69. doi: 10.1016/j.ypmed.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 10.Peytremann-Bridevaux I, Santos-Eggimann B. Health correlates of overweight and obesity in adults aged 50 years and over: results from the Survey of Health, Ageing and Retirement in Europe (SHARE). Obesity and health in Europeans aged > or = 50 years. Swiss Med Wkly. 2008;138(17-19):261–266. doi: 10.4414/smw.2008.12067. [DOI] [PubMed] [Google Scholar]
- 11.Rydén A, Torgerson JS. The Swedish Obese Subjects Study—what has been accomplished to date? Surg Obes Relat Dis. 2006;2(5):549–560. doi: 10.1016/j.soard.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 13.Allison DB, Downey M, Atkinson RL, et al. Obesity as a disease: a white paper on evidence and arguments commissioned by the Council of the Obesity Society. Obesity (Silver Spring) 2008;16(6):1161–1177. doi: 10.1038/oby.2008.231. [DOI] [PubMed] [Google Scholar]
- 14.Klein S, Burke LE, Bray GA, et al. American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2004;110(18):2952–2967. doi: 10.1161/01.CIR.0000145546.97738.1E. [DOI] [PubMed] [Google Scholar]
- 15.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122(7):481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 16.Wannamethee SG, Shaper AG, Walker M. Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes. J Epidemiol Community Health. 2005;59(2):134–139. doi: 10.1136/jech.2003.015651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avenell A, Broom J, Brown TJ, et al. Systematic review of the long-term effects and economic consequences of treatments for obesity and implications for health improvement. Health Technol Assess. 2004;8(21):1–182. doi: 10.3310/hta8210. [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto WY, Jablonski KA, Bray GA, et al. Diabetes Prevention Program Research Group. Body size and shape changes and the risk of diabetes in the diabetes prevention program. Diabetes. 2007;56(6):1680–1685. doi: 10.2337/db07-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Look AHEAD Research Group. Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke GL, Bertoni AG, Shea S, et al. The impact of obesity on cardiovascular disease risk factors and subclinical vascular disease: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168(9):928–935. doi: 10.1001/archinte.168.9.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson PW, D'Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–1872. doi: 10.1001/archinte.162.16.1867. [DOI] [PubMed] [Google Scholar]
- 22.Fox CS, Pencina MJ, Wilson PW, Paynter NP, Vasan RS, D' Agostino RB., Sr Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care. 2008;31(8):1582–1584. doi: 10.2337/dc08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balkau B, Deanfield JE, Després JP, et al. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007;116(17):1942–1951. doi: 10.1161/CIRCULATIONAHA.106.676379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292(20):2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 25.Madala MC, Franklin BA, Chen AY, et al. Obesity and Age of First Non–ST-Segment Elevation Myocardial Infarction. J Am Coll Cardiol. 2008;52(12):979–985. doi: 10.1016/j.jacc.2008.04.067. [DOI] [PubMed] [Google Scholar]
- 26.Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ. Blood pressure and risk of developing type 2 diabetes mellitus: the Women's Health Study. Eur Heart J. 2007;28(23):2937–2943. doi: 10.1093/eurheartj/ehm400. [DOI] [PubMed] [Google Scholar]
- 27.Drøyvold WB, Midthjell K, Nilsen TI, Holmen J. Change in body mass index and its impact on blood pressure: a prospective population study. Int J Obes (Land) 2005;29(6):650–655. doi: 10.1038/sj.ijo.0802944. [DOI] [PubMed] [Google Scholar]
- 28.Gao W, DECODE Study Group Does the constellation of risk factors with and without abdominal adiposity associate with different cardiovascular mortality risk? Int J Obes. 2008;32(5):757–762. doi: 10.1038/sj.ijo.0803797. [DOI] [PubMed] [Google Scholar]
- 29.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association; National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 30.Novo S, Balbarini A, Belch JJ, et al. Guidelines Committee of the International Union of Angiology; Scientific Committee of the International Union of Angiology; Council of Vascular Medicine of the International Union of Angiology. The metabolic syndrome: definition, diagnosis and management. Int Angiol. 2008;27(3):220–231. [PubMed] [Google Scholar]
- 31.Chen K, Lindsey JB, Khera A, et al. Independent associations between metabolic syndrome, diabetes mellitus and atherosclerosis: observations from the Dallas Heart Study. Diab Vasc Dis Res. 2008;5(2):96–101. doi: 10.3132/dvdr.2008.016. [DOI] [PubMed] [Google Scholar]
- 32.Wassink AM, Van Der Graaf Y, Soedamah-Muthu SS, Spiering W, Visseren FLJ, Smart Study Group Metabolic syndrome and incidence of type 2 diabetes in patients with manifest vascular disease. Diab Vasc Dis Res. 2008;5(2):114–122. doi: 10.3132/dvdr.2008.019. [DOI] [PubMed] [Google Scholar]
- 33.Wannamethee SG, Shaper AG, Whincup PH. Modifiable lifestyle factors and the metabolic syndrome in older men: Effects of lifestyle changes. J Am Geriatr Soc. 2006;54(12):1909–1914. doi: 10.1111/j.1532-5415.2006.00974.x. [DOI] [PubMed] [Google Scholar]
- 34.Ilanne-Parikka P, Eriksson JG, Lindström J, et al. Finnish Diabetes Prevention Study Group. Effect of lifestyle intervention on the occurrence of metabolic syndrome and its components in the Finnish Diabetes Prevention Study. Diabetes Care. 2008;31(4):805–807. doi: 10.2337/dc07-1117. [DOI] [PubMed] [Google Scholar]
- 35.Phelan S, Wadden TA, Berkowitz RI, et al. Impact of weight loss on the metabolic syndrome. Int J Obes. 2007;31:1442–1448. doi: 10.1038/sj.ijo.0803606. [DOI] [PubMed] [Google Scholar]
- 36.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 37.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, Million Women Study Collaboration Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109(4):675–684. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez C, Freedland SJ, Deka A, et al. Body mass index, weight change, and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(1):63–69. doi: 10.1158/1055-9965.EPI-06-0754. [DOI] [PubMed] [Google Scholar]
- 40.Thygesen LC, Grønbaek M, Johansen C, Fuchs CS, Willett WC, Giovannucci E. Prospective weight change and colon cancer risk in male US health professionals. Int J Cancer. 2008;123(5):1160–1165. doi: 10.1002/ijc.23612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98(13):920–931. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 42.Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. 2006;118(3):728–738. doi: 10.1002/ijc.21398. [DOI] [PubMed] [Google Scholar]
- 43.Sharma L, Chang A. Overweight: advancing our understanding of its impact on the knee and the hip. Ann Rheum Dis. 2007;66(2):141–142. doi: 10.1136/ard.2006.059931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lievense AM, Bierma-Zeinstra SM, Verhagen AP, van Baar ME, Verhaar JA, Koes BW. Influence of obesity on the development of osteoarthritis of the hip: a systematic review. Rheumatology (Oxford) 2002;41(10):1155–1162. doi: 10.1093/rheumatology/41.10.1155. [DOI] [PubMed] [Google Scholar]
- 45.Reijman M, Pols HA, Bergink AP, et al. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: the Rotterdam Study. Ann Rheum Dis. 2007;66(2):158–162. doi: 10.1136/ard.2006.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hart DJ, Doyle DV, Spector TD. Incidence and risk factors for radiographic knee osteoarthritis in middle-aged women: the Chingford Study. Arthritis Rheum. 1999;42(1):17–24. doi: 10.1002/1529-0131(199901)42:1<17::AID-ANR2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 47.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40(4):728–733. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 48.Creamer P, Lethbridge-Cejku M, Hochberg MC. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology. 2000;39(5):490–496. doi: 10.1093/rheumatology/39.5.490. [DOI] [PubMed] [Google Scholar]
- 49.Jinks C, Jordan K, Croft P. Disabling knee pain—another consequence of obesity: results from a prospective cohort study. BMC Public Health. 2006;6:258. doi: 10.1186/1471-2458-6-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marks R. Obesity profiles with knee osteoarthritis: correlation with pain, disability, disease progression. Obesity. 2007;15(7):1867–1874. doi: 10.1038/oby.2007.221. [DOI] [PubMed] [Google Scholar]
- 51.Sutbeyaz ST, Sezer N, Koseoglu BF, Ibrahimoglu F, Tekin D. Influence of knee, osteoarthritis on exercise capacity and quality of life in obese adults. Obesity. 2007;15(8):2071–2076. doi: 10.1038/oby.2007.246. [DOI] [PubMed] [Google Scholar]
- 52.Tukker A, Visscher T, Picavet H. Overweight and health problems of the lower extremities: osteoarthritis, pain and disability. Public Health Nutr. 2009;12(3):359–368. doi: 10.1017/S1368980008002103. [DOI] [PubMed] [Google Scholar]
- 53.Christensen R, Bartels EM, Astrup A, Bliddal H. Effect of weight reduction in obese patients diagnosed with knee osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2007;66(4):433–439. doi: 10.1136/ard.2006.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fransen M. Dietary weight loss and exercise for obese adults with knee osteoarthritis: modest weight loss targets, mild exercise, modest effects. Arthritis Rheum. 2004;50(5):1366–1369. doi: 10.1002/art.20257. [DOI] [PubMed] [Google Scholar]
- 55.Huang MH, Chen CH, Chen TW, Weng MC, Wang WT, Wang YL. The effects of weight reduction on the rehabilitation of patients with knee osteoarthritis and obesity. Arthritis Care Res. 2000;13(6):398–405. doi: 10.1002/1529-0131(200012)13:6<398::aid-art10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 56.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50(5):1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 57.Miller GD, Nicklas BJ, Davis C, Loeser RF, Lenchik L, Messier SP. Intensive weight loss program improves physical function in older obese adults with knee osteoarthritis. Obesity. 2006;14(7):1219–1230. doi: 10.1038/oby.2006.139. [DOI] [PubMed] [Google Scholar]
- 58.van Gool CH, Penninx BW, Kempen GI, et al. Effects of exercise adherence on physical function among overweight older adults with knee osteoarthritis. Arthritis Rheum. 2005;53(1):24–32. doi: 10.1002/art.20902. [DOI] [PubMed] [Google Scholar]
- 59.Liu B, Balkwill A, Spencer E, Beral V, Million Women Study Collaborators Relationship between body mass index and length of hospital stay for gallbladder disease. J Public Health (Oxf) 2008;30(2):161–166. doi: 10.1093/pubmed/fdn011. [DOI] [PubMed] [Google Scholar]
- 60.Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Prospective study of abdominal adiposity and gallstone disease in US men. Am J Clin Nutr. 2004;80(1):38–44. doi: 10.1093/ajcn/80.1.38. [DOI] [PubMed] [Google Scholar]
- 61.Katsika D, Tuvblad C, Einarsson C, Lichtenstein P, Marschall HU. Body mass index, alcohol, tobacco and symptomatic gallstone disease: a Swedish twin study. J Intern Med. 2007;262(5):581–587. doi: 10.1111/j.1365-2796.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- 62.Al Mofleh IA. Severe acute pancreatitis: pathogenetic aspects and prognostic factors. World J Gastroenterol. 2008;14(5):675–684. doi: 10.3748/wjg.14.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Waele B, Vanmierlo B, Van Nieuwenhove Y, Delvaux G. Impact of body overweight and class I, II and III obesity on the outcome of acute biliary pancreatitis. Pancreas. 2006;32(4):343–345. doi: 10.1097/01.mpa.0000220857.55378.7b. [DOI] [PubMed] [Google Scholar]
- 64.Martínez J, Johnson CD, Sánchez-Payá J, de Madaria E, Robles-Díaz G, Pérez-Mateo M. Obesity is a definitive risk factor of severity and mortality in acute pancreatitis: an updated meta-analysis. Pancreatology. 2006;6(3):206–209. doi: 10.1159/000092104. [DOI] [PubMed] [Google Scholar]
- 65.Papachristou GI, Papachristou DJ, Avula H, Slivka A, Whitcomb DC. Obesity increases the severity of acute pancreatitis: performance of APACHE-O score and correlation with the inflammatory response. Pancreatology. 2006;6(4):279–285. doi: 10.1159/000092689. [DOI] [PubMed] [Google Scholar]
- 66.Preiss D, Sattar N. Non-alcoholic fatty liver disease: an overview of prevalence, diagnosis, pathogenesis and treatment considerations. Clin Sci (Lond) 2008;115(5):141–150. doi: 10.1042/CS20070402. [DOI] [PubMed] [Google Scholar]
- 67.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191(2):235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 68.Riquelme A, Arrese M, Soza A, et al. Non-alcoholic fatty liver disease and its association with obesity, insulin resistance and increased serum levels of C-reactive protein in Hispanics. Liver Int. 2009;29(1):82–88. doi: 10.1111/j.1478-3231.2008.01823.x. [DOI] [PubMed] [Google Scholar]
- 69.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. Prevalence of primary non-alcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int. 2006;26(7):856–863. doi: 10.1111/j.1478-3231.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 70.Church TS, Kuk JL, Ross R, Priest EL, Biltoft E, Blair SN. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130(7):2023–2030. doi: 10.1053/j.gastro.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 71.Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006;82(967):315–322. doi: 10.1136/pgmj.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duvnjak M, Lerotić I, Barsić N, Tomasić V, Virović Jukić L, Velagić V. Pathogenesis and management issues for non-alcoholic fatty liver disease. World J Gastroenterol. 2007;13(34):4539–4550. doi: 10.3748/wjg.v13.i34.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 74.Pillar G, Shehadeh N. Abdominal fat and sleep apnea: the chicken or the egg? Diabetes Care. 2008;31(suppl 2):S303–S309. doi: 10.2337/dc08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Resta O, Foschino-Barbaro MP, Legari G, et al. Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. Int J Obes Relat Metab Disord. 2001;25(5):669–675. doi: 10.1038/sj.ijo.0801603. [DOI] [PubMed] [Google Scholar]
- 76.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 77.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165(20):2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 78.Grunstein RR, Stenlöf K, Hedner JA, Peltonen M, Karason K, Sjöström L. Two year reduction in sleep apnea symptoms and associated diabetes incidence after weight loss in severe obesity. Sleep. 2007;30(6):703–710. doi: 10.1093/sleep/30.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petry NM, Barry D, Pietrzak RH, Wagner JA. Overweight and obesity are associated with psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosom Med. 2008;70(3):288–297. doi: 10.1097/PSY.0b013e3181651651. [DOI] [PubMed] [Google Scholar]
- 80.Strine TW, Mokdad AH, Dube SR, et al. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry. 2008;30(2):127–137. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 81.Simon GE, Ludman EJ, Linde JA, et al. Association between obesity and depression in middle-aged women. Gen Hosp Psychiatry. 2008;30(1):32–39. doi: 10.1016/j.genhosppsych.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763–778. doi: 10.1056/NEJMoa055643. [DOI] [PubMed] [Google Scholar]
- 83.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298(17):2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 84.Manson JE, Willett WC, Stampfer MJ, et al. Body weight and mortality among women. N Engl J Med. 1995;333(11):677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 85.McTigue K, Larson JC, Valoski A, et al. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296(1):79–86. doi: 10.1001/jama.296.1.79. [DOI] [PubMed] [Google Scholar]
- 86.Murphy NF, MacIntyre K, Stewart S, Hart CL, Hole D, McMurray JJ. Long-term cardiovascular consequences of obesity: 20-year follow-up of more than 15000 middle-aged men and women (the Renfrew-Paisley study). Eur Heart J. 2006;27(1):96–106. doi: 10.1093/eurheartj/ehi506. [DOI] [PubMed] [Google Scholar]
- 87.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338(1):1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 88.Thomas F, Bean K, Pannier B, Oppert JM, Guize L, Benetos A. Cardiovascular mortality in overweight subjects: the key role of associated risk factors. Hypertension. 2005;46(4):654–659. doi: 10.1161/01.HYP.0000184282.51550.00. [DOI] [PubMed] [Google Scholar]
- 89.Yan LL, Daviglus ML, Liu K, et al. Midlife body mass index and hospitalization and mortality in older age. JAMA. 2006;295(2):190–198. doi: 10.1001/jama.295.2.190. [DOI] [PubMed] [Google Scholar]
- 90.Fontaine KR, Redden DT, Wang C, Westfall AO, Allison DB. Years of life lost due to obesity. JAMA. 2003;289(2):187–193. doi: 10.1001/jama.289.2.187. [DOI] [PubMed] [Google Scholar]
- 91.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357(8):753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 92.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish Obese Subjects. N Engl J Med. 2007;357(8):741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 93.Thompson D, Edelsberg J, Colditz GA, Bird AP, Oster G. Lifetime health and economic consequences of obesity. Arch Intern Med. 1999;159(18):2177–2183. doi: 10.1001/archinte.159.18.2177. [DOI] [PubMed] [Google Scholar]
- 94.Muennig P, Lubetkin E, Jia H, Franks P. Gender and the burden of disease attributable to obesity. Am J Public Health. 2006;96(9):1662–1668. doi: 10.2105/AJPH.2005.068874. [DOI] [PMC free article] [PubMed] [Google Scholar]