Abstract
Functional magnetic resonance imaging (fMRI) and functional connectivity MRI (fcMRI) studies of autism spectrum disorders (ASD) have suggested atypical patterns of activation and long-distance connectivity for diverse tasks and networks in ASD. We explored the regional homogeneity (ReHo) approach in ASD, which is analogous to conventional fcMRI, but focuses on local connectivity. FMRI data of 26 children with ASD and 29 typically developing (TD) children were acquired during continuous task performance (visual search). Effects of motion and task were removed and Kendall‟s coefficient of concordance (KCC) was computed, based on the correlation of the blood oxygen level dependent (BOLD) time series for each voxel and its 6 nearest neighbors. ReHo was lower in the ASD than the TD group in superior parietal and anterior prefrontal regions. Inverse effects of greater ReHo in the ASD group were detected in lateral and medial temporal regions, predominantly in the right hemisphere. Our findings suggest that ReHo is a sensitive measure for detecting cortical abnormalities in autism. However, impact of methodological factors (such as spatial resolution) on ReHo require further investigation.
Keywords: Autism Spectrum Disorder, functional MRI, functional connectivity, regional homogeneity
Autism spectrum disorders (ASD) are pervasive neurodevelopmental conditions characterized by sociocommunicative deficits. FMRI studies have shown atypical activation patterns for diverse tasks in children and adults with ASD [1,2]. There is also evidence of atypical functional connectivity, as detected by spatiotemporal correlations between distal brain regions [3].
While long-distance interregional connectivity has been examined in many fcMRI studies, much less attention has been paid to local connectivity. This is surprising in view of conjectures that local connectivity and functional differentiation may reflect autistic pathology in different patterns than long-distance connectivity [4-6]. Regional homogeneity (ReHo) provides an approach for using fMRI to investigate local connectivity [7].
The ReHo method tests for local correlations in BOLD time series, using Kendall‟s coefficient of concordance (KCC) [8]. KCC is based on time course correlations between a voxel and its neighbors. Mathematically, ReHo is comparable to the conventional fcMRI approach described above. However, whereas fcMRI typically tests for BOLD time series correlations between distal voxels or regions of interest, focusing on long-distance interregional connectivity, ReHo targets connectivity at the local level. Atypical (mostly reduced) ReHo has been reported in Alzheimer‟s disease [9], Parkinson‟s disease [10], schizophrenia [11], and depression [12], attention deficit hyperactivity disorder (ADHD) [13,14], and healthy aging [15]. Clinical correlations have also been reported. For example, correlations were detected between ReHo in the right insula and anxiety severity in depressed patients [12] and between ReHo in medial parietal lobe and scores on the Mini-Mental State Exam in patients with Alzheimer‟s disease [9]. These findings suggest that the ReHo technique may be sensitive to regional cortical pathology in disease-specific ways.
The present study investigated local functional connectivity using the ReHo method in children with ASD and typically developing (TD) children. Specifically, we examined whether in ASD, similar to other clinical and developmental disorders (as described above), ReHo would be overall reduced in comparison with matched controls; or whether increased local connectivity as suggested by some [4,6] might result in generally elevated ReHo in ASD.
Twenty-six children with ASD (25 males, 1 female; handedness (right/left) 24/2; mean age 13.7±0.6 years (range 9-18 years); verbal IQ 109.6±3.1; non-verbal IQ 112.1±2.8 [mean±sem]) and twenty-nine TD children (28 males, 1 female; handedness (right/left) 27/2; mean age 13.8±0.55 years (range 8-19 years); verbal IQ 109.6±2.4; non-verbal IQ 110.8±2.3) were included for the current study. Clinical diagnoses were confirmed by an expert clinical psychologist using the Autism Diagnostic Interview-Revised (ADI-R) [16] and the Autism Diagnostic Observation Schedule (ADOS) [17]. Children with associated medical conditions were excluded. TD children had no reported personal or family history of autism or any other neurological or psychiatric conditions. Independent-sample t-tests confirmed that ASD and TD groups were matched on age [t(53)=0.14; p=.88] and full-scale IQ [t(53)=0.04; p=.96] as determined using the Wechsler Abbreviated Scale of Intelligence (WASI) [18]. The research protocol was approved by the Institutional Review Boards of the University of California, San Diego and San Diego State University. Written informed consent was obtained from all participants.
FMRI data were acquired on a 3T GE MRI scanner (Signa Excite HD), using a standard eight-channel head coil and an echo-planar imaging pulse sequence (repetition time 2500ms, echo time 30ms, flip angle 90°, matrix 64 64, slice thickness 3.2mm, in-plane voxel size 3.4 3.4mm2). A visual search experimental task was presented during the scan in which the target was an upright “T” and distracters were Ts rotated in three cardinal orientations (for details, see [19]). The experiment consisted of four runs, with a total of 480 time points.
FMRI data for individual runs were preprocessed using SPM2, including motion correction, within-subject registration, and slice time correction. Functional volumes with excessive motion (>2mm) were discarded (on average 27 and 24 volumes in ASD and TD groups, respectively). The task-related components were removed from voxel time series using linear least squares. Head motion, as determined by the root mean square of the translational and rotational motion in three cardinal directions, was compared between groups. No significant group differences were detected for translational motion (.55±.08mm [mean±sem] ASD and .59±.07mm TD; p=.73) or rotational motion (.014±.003rad ASD and .010±.002rad TD; p=.29).
Kendall's coefficient of concordance (KCC) [8] was used for measuring the correlation of the time series of a given voxel with the time series of its 6 nearest neighbors. Data were temporally band-pass filtered (0.01<f<0.1 Hz) to reduce low-frequency drift and high-frequency respiratory and cardiac noise. Linear trends were removed. Individual ReHo maps were generated by calculating KCC (with values from 0 to 1) in a voxel-wise way, using a group-averaged gray matter mask, by the REST software (www.bic.mni.mcgill.ca/users/yonghe). AFNI [20] software was used for the remaining analysis. KCC maps were transformed into Talairach space. Spatial smoothing was conducted on final ReHo maps with a Gaussian kernel of 6 mm3 full-width at half-maximum. In each participant, KCC maps were normalized by dividing KCC in each voxel by the mean gray matter KCC. The mean KCC map of four runs was obtained for each participant (except two ASD participants with only three runs and two TD participants with only two runs, due to excessive motion in the remaining runs). A two-sample t-test was performed on the normalized individual KCC maps. The resultant statistical map was set at a combined threshold of p<0.01 and a minimum cluster size of 4050 mm3, corresponding to a corrected threshold of p<0.05, as determined by AlphaSim in AFNI.
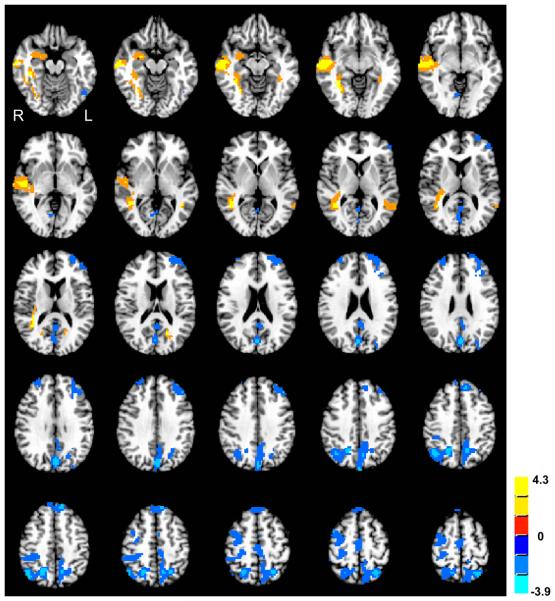
Group differences in ReHo are shown in Figure 1 and Table 1. Decreased ReHo in the ASD group compared to the TD group was found in bilateral middle and superior frontal gyri, left superior parietal lobule, and right precuneus. Increased ReHo in the ASD group compared to the TD group was found in bilateral middle temporal and right parahippocampal gyri.
Figure 1.
Clusters of significant group differences in ReHo (normalized KCC). Positive values (yellow clusters) represent greater ReHo in the ASD than in the TD group; negative values (blue clusters) indicate inverse effects (TD>ASD; all clusters p<0.05, corrected for multiple comparisons). Color scale reflects T-scores.
Table 1.
Clusters of significant group differences (p<.05; corr.) for normalized KCC. (Abbreviations: L, left; R, right).
| Peak Location Additional regions (% volume of cluster) |
Hemisphere | Talairach coordinates |
Volume (μl) | T-score | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| ASD<TD | Superior parietal lobule | L | 31 | −67 | 59 | 23652 | 3.9 |
| Precuneus (14.3) | |||||||
| Cuneus (8.9) | |||||||
| Posterior cingulate cortex (3.2) | |||||||
| Paracentral lobule (3.0) | |||||||
| Precuneus | R | −19 | −61 | 47 | 19494 | 3.8 | |
| Superior parietal lobule (33) | |||||||
| Postcentral gyrus (15.2) | |||||||
| Angular gyrus (11.4) | |||||||
| Middle frontal gyrus | L | 46 | 37 | 44 | 12447 | 3.4 | |
| Superior frontal gyrus (7.5) | |||||||
| Inferior frontal gyrus (3.6) | |||||||
| Superior frontal gyrus | L | 13 | 46 | 50 | 5454 | 3.3 | |
| Superior frontal gyrus | R | −43 | 5 | 59 | 5292 | 3.2 | |
| Middle frontal gyrus | R | −31 | 55 | 32 | 4158 | 2.7 | |
| Superior frontal gyrus (29.9) | |||||||
| Precentral gyrus (10.7) | |||||||
|
| |||||||
| ASD>TD | Middle temporal gyrus | R | −61 | −17 | −12 | 33048 | 4.3 |
| Fusiform gyrus (14.3) | |||||||
| Superior temporal gyrus (13.1) | |||||||
| Inferior temporal gyrus (4.2) | |||||||
| Middle temporal gyrus | L | 34 | −49 | 5 | 10206 | 3.7 | |
| Superior occipital gyrus (4.4) | |||||||
| Cuneus (2.5) | |||||||
| Parahippocampal gyrus | R | −25 | −5 | −10 | 8667 | 2.9 | |
| Amygdala (13.2) | |||||||
| Temporal pole (6.9) | |||||||
| Hippocampus (2.9) | |||||||
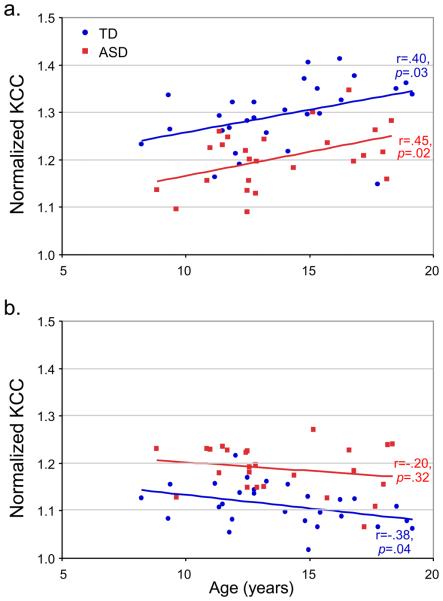
In clusters for which significant group differences had been detected, we also tested for possible correlations between ReHo and diagnostic scores (ASD group only), age, and IQ, for a richer characterization of our regional findings. In clusters showing reduced ReHo in the ASD group (blue in Figure 1), mean KCC was positively correlated with age in the total sample (r=.36, p=.008), as well as in each group separately (ASD: r=.45, p=.02; TD: r=.40, p=.03; Figure 2a). In post hoc analyses, we found that this correlation was significant in both groups only for the cluster in the precuneus, whereas in the other fronto-parietal clusters concordant but non-significant trends were seen.
Figure 2.
Pearson correlation analyses examining the relation between normalized KCC values and age (a) in regions with reduced ReHo in the ASD group compared to the TD group, and (b) for clusters with inverse effects (ASD>TD).
In clusters with increased ReHo in the ASD group (yellow in Figure 1), a marginally significant negative correlation was found between KCC and age in both groups combined (r=−.23, p=0.09). This negative correlation was significant in the TD group (r=−.38, p=.04), but not in the ASD group (r=−.20, p=.32; Figure 2b). In the same clusters (ReHo ASD>TD), KCC was negatively correlated with verbal IQ in the TD group (r=−.44, p=.02), and marginally with non-verbal IQ, and full-scale IQ (both r=−.36, p=.06), but not in the ASD group (ps>.9). No significant correlations were detected between regional KCC and ADOS scores (ps>.23) or ADI-R scores (ps>.30) in the ASD group.
Our observation of decreased ReHo for the ASD group in portions of frontal, parietal, and occipital lobes parallels findings of reduced ReHo in other clinical populations, such as Alzheimer's disease [9], schizophrenia [11], and depression [12]. In our study, these effects of reduced ReHo in ASD had a distinct regional pattern, occurring primarily in prefrontal and superior and medial parietal lobes.
Frontal lobe impairment in ASD has been suggested by postmortem [21], structural MRI [22], magnetic resonance spectroscopy [23], diffusion tensor imaging [24], cerebral blood flow [25] and glucose metabolic studies [26], and is indirectly supported by executive impairments in ASD [27]. Reduced ReHo in our study was found in dorsolateral prefrontal cortex, possibly consistent with executive findings, and in frontopolar superior frontal gyrus (area 10), considered crucial for highly integrative multimodal cognitive processing [28]. Parietal lobe compromise has been suggested in some anatomical [29] and functional [30] studies, accompanied by reports of cognitive impairment of attention reflective of parietal lobe dysfunction [31]. Carper et al. [32] found that both frontal and parietal lobes were affected by atypical early white matter overgrowth and subsequently reduced growth, suggesting early-onset impairments of connectivity.
Frontal and parietal regions showing reduced ReHo in the ASD group were further characterized by age-dependent increases in ReHo. This increase, which was significant in both TD and ASD groups, may reflect effects of maturation and cognitive development. Reduced ReHo in the ASD group further suggests that such age-dependent changes are present, but occur at lower levels in ASD. Normative ReHo data are not available for TD children. The only study that has examined age-dependent changes to date [15] reported widespread decreases of ReHo in healthy aging across all for lobes, indicating that ReHo may show continuous change across the lifespan.
We also detected clusters of significantly greater ReHo in the ASD (compared to the TD) group, which occurred exclusively in the temporal lobes. Most of these temporal effects were seen in the right hemisphere where they occurred both laterally (superior and middle temporal gyri) and medially (parahippocampal gyrus and amygdala). While these effects may appear inconsistent with results from other brain disorders showing predominantly reduced ReHo, they do not necessarily indicate absence of cortical compromise. A few previous ReHo studies have shown similarly mixed effects [9,10]. For example, Cao [13] observed decreased ReHo in fronto-parietal regions, but atypically increased ReHo in temporo-occipital cortex in boys with ADHD. The regional pattern of ReHo increases observed in the present study may relate to EEG findings by Murias et al. [33] of greater short distance connectivity (EEG coherence) within the temporal lobe in ASD in the 3-6 Hz frequency band.
The temporal regions of increased ReHo in ASD were characterized by negative correlations of KCC with verbal IQ and age, observed only in the TD group. The significance of this finding, especially in the context of the age-dependent ReHo increases in widespread frontoparietal regions discussed above, remains unclear. Figure 2b suggests that this finding may reflect a typical maturational change that is diminished in ASD, similar in principle – though different in direction – to the findings for frontoparietal cortices depicted in Figure 2a.
The regional pattern of our findings differs from results for adolescents with ASD recently reported by Paakki et al. [34], who found mostly decreased ReHo in right lateral temporal and prefrontal cortex, and increased ReHo in left inferior frontal lobe. Among potential explanations are age differences, although mean age was only slightly higher than in our ASD group. Paakki et al. stated only a lower cutoff for full-scale IQ (>75) and an overall higher functional level in our cohort cannot be ruled out. More likely reasons for differences in findings are methodological. Paakki et al. acquired data at lower field strength (1.5 vs. 3T) and lower spatial resolution (70.4μl vs. 37.0μl voxels). More importantly, they computed KCC using clusters of 27 neighboring voxels (1971μl), whereas we only included 6 neighboring voxels (259μl). This suggests that ReHo results presented by Paakki et al. address a more macroscopic level of organization than was examined in our study (see further discussion of spatial resolution below). Another important difference relates to cognitive condition. Paakki et al. used resting state fMRI data, whereas we acquired data during continuous task performance (visual search) and regressed out task effects. Low-frequency BOLD fluctuations that both studies examined were therefore most likely affected by the ‘default mode’ in the study by Paakki et al., but not in ours. While the resting condition is considered to prompt such a default mode in neurotypical populations [35], variability in response to the resting state is probably large [36-38]. The cognitive response to lying in a noisy and confined space without any explicit task may differ even more substantially between TD and ASD participants, as a reflection of atypical socio-cognitive function and suspected abnormality of the default mode network in ASD [39,40]. Resting state data can therefore not be considered mentally “neutral” and protected from abnormalities in cognitive response that may exist in ASD. Such differential response may have driven some of the ReHo findings in the study by Paakki et al., whereas in our study continuous task performance did not allow either TD or ASD participants to go into a default mode.
Casanova and colleagues [41,42] reported reduced size and increased numbers of cortical minicolumns in autism postmortem studies for several cortical regions. Minicolumns are excitatory vertical circuits [43] and their relative density in the autistic brain would be associated with atypically enhanced excitatory cortical function [6]. There is convergent evidence for an abnormally increased excitation/inhibition ratio in the autistic brain [44,45], including increased prevalence of epilepsy [46] and epileptiform EEG [47] as well as atypically enhanced high-frequency EEG oscillations in the beta and gamma bands (13-70Hz) [33]. These findings could theoretically account for atypically increased ReHo in ASD, since enhanced local excitatory circuits and reduced intercolumnar inhibition would result in overall more highly correlated local patterns of the BOLD signal.
However, it is obvious that the ReHo measure relies crucially on spatial resolution. Our voxel volumes of 37μl were relatively small compared to most available fMRI studies of ASD and we maintained spatial resolution by computing KCC in native space and avoiding any explicit smoothing in the preprocessing pipeline. Nonetheless, with minicolumnar spacing of <100μm [48], each cortical voxel will contain tens or hundreds of minicolumns and it remains unknown whether the hypothesized reduced lateral inhibition would have a measurable effect on ReHo. The very selective regional patterns of increased ReHo observed in our ASD study, with inverse effects (reduced ReHo) in frontal and parietal cortices, suggest caution in linking ReHo findings with the model of local overexcitation in ASD.
Furthermore, voxel size and the number of time series included in the computation of KCC relate to the issue of partial volume effects. As mentioned above, Paakki et al. [34] determined KCC for each voxel and its 27 neighbors, whereas we only included 6 neighboring voxels. However, even with this reduced cluster volume, partial volume effects are unavoidable. Maximized spatial resolution therefore appears commendable in future ReHo studies, which may also investigate the relation between cortical thickness and ReHo.
It should be noted that the ReHo measure is not sensitive to global increases or decreases in homogeneity of BOLD time series, as it implements normalized KCC (i.e., KCC detected for a given voxel divided by mean KCC for all gray matter voxels). While this has been standard procedure for reducing noise and variability in previous ReHo studies [10,12,13], it may be argued that such normalization increases the risk of type II error when global abnormalities of ReHo are hypothesized. We therefore ran identical analyses using non-normalized KCC. Regional patterns of group differences (supplementary Figure 3) were almost identical to those shown in Figure 1. Global gray matter KCC did not differ between groups (mean KCC: ASD=.60, TD=.61; p=.4).
Unlike some previous clinical ReHo studies, ours did not use resting state data. Recent studies have shown that task-independent fluctuations of the BOLD signal in low frequency domains, as examined here, are contained in fMRI time series acquired during task performance and can be isolated through low-pass filtering and the use of appropriate orthogonal task regressors [49,50]. As discussed above, task-guided datasets provide better control over cognitive state than unguided “rest”, which may be especially important in ASD. However, it cannot be entirely ruled out that despite low-pass filtering and removal of modeled task effects in our study, some residual effects of cognitive state related to the visual search task performed during scanning could have survived. While this is unlikely for robust effects of increased ReHo we saw in right lateral temporal lobe – a region for which no effects of the visual search task were detected by Keehn and colleagues [19] – it may be more likely for parietal regions showing reduced ReHo in the ASD group, some of which occurred in the vicinity of regions found to activate during visual search.
The current study aimed to explore the use of the ReHo method in the study of local functional connectivity in ASD. Our results suggest that ReHo is sensitive to abnormalities of cortical organization in ASD, but the precise nature of underlying mechanisms and links between atypical local connectivity detected by ReHo and abnormal long-distance connectivity [3] remain to be investigated.
Supplementary Material
Clusters of significant group differences in ReHo (non-normalized KCC). Positive values (yellow clusters) represent greater ReHo in the ASD than in the TD group; negative values (blue clusters) indicate inverse effects (TD>ASD; all clusters p<0.05, corrected for multiple comparisons). Color scale reflects T-scores.
Acknowledgments
This study was supported by the National Institutes of Health, R01-DC006155, R01-MH081023, and 1T32 DC007361-03 (BK). Thanks to the children and families who participated, to Yufeng Zang, Chao-Gan Yan, and Xiang-Yu Long for technical support, and to Patricia Shih for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schultz RT, Robins DL. Functional neuroimaging studies of autism spectrum disorders. In: Volkmar FR, Paul R, Klin A, Cohen D, editors. Handbook of Autism and Pervasive Developmental Disorders. Wiley; New York: 2005. pp. 515–533. [Google Scholar]
- 2.Müller RA. Functional neuroimaging of developmental disorders: Lessons from autism research. In: Hillary FD, DeLuca J, editors. Functional Neuroimaging in Clinical Populations. Guilford; 2007. pp. 145–184. [Google Scholar]
- 3.Rippon G, Brock J, Brown C, Boucher J. Disordered connectivity in the autistic brain: challenges for the “new psychophysiology”. Int. J. Psychophysiol. 2007;63:164–172. doi: 10.1016/j.ijpsycho.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Belmonte MK, Cook EH, Jr., Anderson GM, Rubenstein JL, Greenough WT, Beckel-Mitchener A, Courchesne E, Boulanger LM, Powell SB, Levitt PR, Perry EK, Jiang YH, DeLorey TM, Tierney E. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol. Psychiatry. 2004;9:646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- 5.Courchesne E, Pierce K. Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int. J. Dev. Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Casanova M, Trippe J. Radial cytoarchitecture and patterns of cortical connectivity in autism. Philos. Trans. R. Soc. Lond B Biol. Sci. 2009;364:1433–1436. doi: 10.1098/rstb.2008.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Kendall M, Gibbons J. Rank Correlation Methods. Oxford University Press; Oxford: 1990. [Google Scholar]
- 9.He Y, Wang L, Zang Y, Tian L, Zhang X, Li K, Jiang T. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35:488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 10.Wu T, Long X, Zang Y, Wang L, Hallett M, Li K, Chan P. Regional homogeneity changes in patients with Parkinson's disease. Hum. Brain Mapp. 2009;30:1502–1510. doi: 10.1002/hbm.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu H, Liu Z, Liang M, Hao Y, Tan L, Kuang F, Yi Y, Xu L, Jiang T. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- 12.Yao Z, Wang L, Lu Q, Liu H, Teng G. Regional homogeneity in depression and its relationship with separate depressive symptom clusters: a resting-state fMRI study. J. Affect. Disord. 2009;115:430–438. doi: 10.1016/j.jad.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Cao Q, Zang Y, Sun L, Sui M, Long X, Zou Q, Wang Y. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17:1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- 14.Zhu CZ, Zang YF, Cao QJ, Yan CG, He Y, Jiang TZ, Sui MQ, Wang YF. Fisher discriminative analysis of resting-state brain function for attention-deficit/hyperactivity disorder. Neuroimage. 2008;40:110–120. doi: 10.1016/j.neuroimage.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Wu T, Zang Y, Wang L, Long X, Li K, Chan P. Normal aging decreases regional homogeneity of the motor areas in the resting state. Neurosci. Lett. 2007;423:189–193. doi: 10.1016/j.neulet.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 16.Rutter M, Le Couteur A. Lord, Autism Diagnostic Interview - Revised. Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- 17.Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule. Western Psychological Services; Los Angeles, CA: 2001. [Google Scholar]
- 18.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- 19.Keehn B, Brenner L, Palmer E, Lincoln AJ, Müller RA. Functional brain organization for visual search in ASD. J. Int. Neuropsychol. Soc. 2008;14:990–1003. doi: 10.1017/S1355617708081356. [DOI] [PubMed] [Google Scholar]
- 20.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 21.Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicopathological study of autism. Brain. 1998;121(Pt 5):889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- 22.Herbert MR, Ziegler DA, Makris N, Filipek PA, Kemper TL, Normandin JJ, Sanders HA, Kennedy DN, Caviness VS., Jr. Localization of white matter volume increase in autism and developmental language disorder. Ann. Neurol. 2004;55:530–540. doi: 10.1002/ana.20032. [DOI] [PubMed] [Google Scholar]
- 23.Kleinhans NM, Schweinsburg BC, Cohen DN, Müller RA, Courchesne E. N-acetyl aspartate in autism spectrum disorders: regional effects and relationship to fMRI activation. Brain Res. 2007;1162:85–97. doi: 10.1016/j.brainres.2007.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundaram SK, Kumar A, Makki MI, Behen ME, Chugani HT, Chugani DC. Diffusion tensor imaging of frontal lobe in autism spectrum disorder. Cereb. Cortex. 2008;18:2659–2665. doi: 10.1093/cercor/bhn031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zilbovicius M, Garreau B, Samson Y, Remy P, Barthelemy C, Syrota A, Lelord G. Delayed maturation of the frontal cortex in childhood autism. Am. J. Psychiatry. 1995;152:248–252. doi: 10.1176/ajp.152.2.248. [DOI] [PubMed] [Google Scholar]
- 26.Hazlett EA, Buchsbaum MS, Hsieh P, Haznedar MM, Platholi J, LiCalzi EM, Cartwright C, Hollander E. Regional glucose metabolism within cortical Brodmann areas in healthy individuals and autistic patients. Neuropsychobiology. 2004;49:115–125. doi: 10.1159/000076719. [DOI] [PubMed] [Google Scholar]
- 27.Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat. Rev. Neurosci. 2004;5:184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 29.Brieber S, Neufang S, Bruning N, Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, Fink GR, Konrad K. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. J. Child Psychol. Psychiatry. 2007;48:1251–1258. doi: 10.1111/j.1469-7610.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 30.Haist F, Adamo M, Westerfield M, Courchesne E, Townsend J. The functional neuroanatomy of spatial attention in autism spectrum disorder. Dev. Neuropsychol. 2005;27:425–458. doi: 10.1207/s15326942dn2703_7. [DOI] [PubMed] [Google Scholar]
- 31.Townsend J, Courchesne E. Parietal damage and narrow “spotlight” spatial attention. Journal of Cognitive Neuroscience. 1994;6:220–232. doi: 10.1162/jocn.1994.6.3.220. [DOI] [PubMed] [Google Scholar]
- 32.Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 33.Murias M, Webb SJ, Greenson J, Dawson G. Resting state cortical connectivity reflected in EEG coherence in individuals with autism. Biol. Psychiatry. 2007;62:270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paakki JJ, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, Starck T, Remes J, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Mattila ML, Zang Y, Kiviniemi V. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain Res. 2010 doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- 35.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10841–10846. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilbert SJ, Dumontheil I, Simons JS, Frith CD, Burgess PW. Comment on “Wandering minds: the default network and stimulus-independent thought”. Science. 2007;317:43. doi: 10.1126/science.317.5834.43. [DOI] [PubMed] [Google Scholar]
- 38.Waites AB, Stanislavsky A, Abbott DF, Jackson GD. Effect of prior cognitive state on resting state networks measured with functional connectivity. Hum Brain Mapp. 2005;24:59–68. doi: 10.1002/hbm.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 40.Monk CS, Peltier SJ, Wiggins JL, Weng SJ, Carrasco M, Risi S, Lord C. Abnormalities of intrinsic functional connectivity in autism spectrum disorders. Neuroimage. 2009;47:764–772. doi: 10.1016/j.neuroimage.2009.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- 42.Casanova MF, van, I K, Switala AE, van EH, Heinsen H, Steinbusch HW, Hof PR, Trippe J, Stone J, Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- 43.Mountcastle VB. The columnar organization of the neocortex. Brain. 1997;120(Pt 4):701–722. doi: 10.1093/brain/120.4.701. [DOI] [PubMed] [Google Scholar]
- 44.Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10:303–317. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- 45.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gillberg C, Billstedt E. Autism and Asperger syndrome: coexistence with other clinical disorders. Acta Psychiatr. Scand. 2000;102:321–330. doi: 10.1034/j.1600-0447.2000.102005321.x. [DOI] [PubMed] [Google Scholar]
- 47.Hughes JR, Melyn M. EEG and seizures in autistic children and adolescents: further findings with therapeutic implications. Clin. EEG. Neurosci. 2005;36:15–20. doi: 10.1177/155005940503600105. [DOI] [PubMed] [Google Scholar]
- 48.Buldyrev SV, Cruz L, Gomez-Isla T, Gomez-Tortosa E, Havlin S, Le R, Stanley HE, Urbanc B, Hyman BT. Description of microcolumnar ensembles in association cortex and their disruption in Alzheimer and Lewy body dementias. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5039–5043. doi: 10.1073/pnas.060009897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clusters of significant group differences in ReHo (non-normalized KCC). Positive values (yellow clusters) represent greater ReHo in the ASD than in the TD group; negative values (blue clusters) indicate inverse effects (TD>ASD; all clusters p<0.05, corrected for multiple comparisons). Color scale reflects T-scores.