Abstract
We examined the somatodendritic compartment of nigral dopaminergic neurons by immunocytochemistry and confocal microscopy, with the aim of identifying proteins that participate in dopamine packaging and release. Nigral dopaminergic neurons were identified by location, cellular features and tyrosine hydroxylase immunoreactivity. Immunoreactive puncta of vesicular monoamine transporter type 2 and proton ATPase, both involved in the packaging of dopamine for release, were located primarily in dopaminergic cell bodies, but were absent in distal dopaminergic dendrites. Many presynaptic proteins associated with transmitter release at fast synapses were absent in nigral dopaminergic neurons, including synaptotagmin 1, syntaxin1, synaptic vesicle proteins 2a and 2b, synaptophysin and synaptobrevin 1 (VAMP 1). On the other hand, syntaxin 3, synaptobrevin 2 (VAMP 2) and SNAP-25-immunoreactivities were found in dopaminergic somata and dendrites Our data imply that the storage and exocytosis of dopamine from the somatodendritic compartment of nigral dopaminergic neurons is mechanistically distinct from transmitter release at axon terminals utilizing amino acid neurotransmitters.
Keywords: dopamine, substantia nigra, somatodendritic, exocytosis, dopamine release proteins
Parkinson’s disease, a movement disorder involving the basal ganglia, results primarily from degeneration of the dopaminergic (DAergic) projection from substantia nigra (SN) to striatum (Birkmayer and Hornykiewicz, 1976). The underlying nigrostriatal pathway arises from large midbrain DAergic neurons situated in the SN pars compacta (SNc). Laterally extending dendrites emitted by the DAergic perikarya intermingle in the SNc and vertical dendrites extend ventrally into the adjacent pars reticulata (SNr) (Dahlström and Fuxe, 1964; Juraska et al., 1977; Wassef et al., 1981, Tepper et al., 1987).
Previous studies have shown that dopamine (DA) is released both by the nigrostriatal axonal terminals within striatum and by DAergic somata and dendrites in the midbrain (Björkland and Lindvall, 1975; Groves et al., 1975; Geffen et al., 1976; Nieoullon et al., 1977; Rice et al., 1994, Jaffe et al., 1998; Chen and Rice, 2001). The requisite conditions for DA release at these two sites differ, however. Striatal DA release shows a steep dependence on extracellular calcium (Ca2+) concentration ([Ca2+]o) and is undetectable when [Ca2+]o falls below 1.0 mM, whereas somatodendritic release persists in 0.5 mM [Ca2+]o and plateaus above 1.5 mM (Chen and Rice, 2001). However, somatodendritic release is prevented by extracellular cadmium (Cd2+), a non-specific blocker of voltage-gated Ca2+ channels and attenuated by a Ca2+ chelator (BAPTA-AM) (Jaffe et al., 1998; Patel et al., 2009), indicating that Ca2+ entry and an increase in intracellular Ca2+ are required. Furthermore we have recently shown that somatodendritic DA release is facilitated by mobilization of Ca2+ from intracellular stores (Patel et al., 2009). Another important distinction is that DAergic terminals in striatum show typical presynaptic aggregates of agranular vesicles (Pickel et al., 1996; Nirenberg et al., 1997), whereas DAergic somata in SNc lack these aggregates (Nirenberg et al., 1996), as do DAergic dendrites within SNr (Wassef et al., 1981, Groves and Linder, 1983 but cf. Wilson et al., 1977). Nonetheless, there is evidence for quantal DA release from DAergic somata in the SNc (Jaffe et al., 1998). These findings suggest that, although mechanistically distinct, both terminal and perikaryal DA release involve Ca2+-dependent exocytosis.
It is well known that neurotransmitter release by vesicle exocytosis depends on a multistage process (reviewed in Südhof, 2004), each step of which is dependent on an interlocking network of presynaptic proteins distributed among different sites, including the synaptic vesicle and at the active zones of the plasma membrane where vesicle exocytosis occurs. Parenthetically, axonal DAergic release sites may be differently organized than the vast majority of CNS synapses, in that at least some DAergic junctions in the striatum lack an apposed post-synaptic ending (Beaudet and Descarries, 1978). Moreover, DAergic axonal synapses are completely absent in the SNc (Juraska et al., 1977; Wassef et al., 1981), and only sparse DAergic dendrodendritic synapses have been reported there (Wassef et al., 1981; Groves and Linder, 1983). Dopamine thus reaches its targets by diffusion, a process called volume transmission (Agnati et al., 1986; Rice, 2000). Even though DA release sites in the somatodendritic compartment lack a synaptic structure they nevertheless resemble classical synapses in utilizing a Ca2+-dependent exocytotic mechanism for DA release. We will therefore use the neutral term ‘DA release protein’ to refer to the proteins involved in DA exocytosis.
The identities of canonical presynaptic proteins have been established primarily from studies of fast amino acid transmitter release. Whether the same or different proteins are found within DAergic somata and dendrites remains largely unknown and is the main experimental question of the present study. Previous studies (Bergquist et al., 2002; Fortin et al., 2006) showed, by pharmacology and immunocytochemistry, that the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) protein, SNAP-25, was present in the somatodendritic compartment of nigral DAergic neurons and the presence of a synaptobrevin was inferred, suggesting the presence of exocytotic machinery in DAeregic perikarya and dendrites. In the present study we used immunocytochemistry to identify proteins involved in Ca2+-dependent exocytosis of DA in SN DAergic somata and dendrites. Our goal was to test the hypothesis that differences exist in the molecular organization of DAergic somatodendritic release sites relative to those of fast synapses. We found that although many vesicular and plasma membrane proteins commonly encountered in synaptic endings of fast synapses were absent, certain isoforms of these proteins were present in DAergic somata and dendrites. Our findings thus contribute to a growing understanding of the unique characteristics of DA release from the somatodendritic compartment.
Experimental Procedures
Animals
Young adult guinea pigs (male, Hartley, 150–250g) were obtained from Charles River Laboratories (Wilmington, MA). All animal handling procedures were in accordance with National Institutes of Health guidelines and were approved by the New York University School of Medicine Animal Care and Use Committee.
Guinea pigs were deeply anesthetized with an intraperitoneal injection of Nembutal (sodium pentobarbital at 50 mg/kg), then perfused transcardially with phosphate-buffered saline (PBS: 9 g NaCl/L in 10 mM phosphate buffer, pH 7.3) followed by freshly prepared paraformaldehyde (4%) in PBS. The brain was then removed and immersed in the same fixative for 1 h. A coronal block of midbrain was dissected free and washed in PBS for 30 min before being placed for 16–24 h at 4°C in 30% sucrose in PBS. Frozen coronal sections through the midbrain were cut (at 20 µm), mounted on slides, dried for 1 h at 37 °C, then stored frozen until used for immunocytochemistry.
Immunocytochemistry
Slides were washed 3 times for 7 min each (3 × 7 min) in PBS and 30 min in blocking solution (10 ml PBS, containing 0.1 g bovine serum albumin, 30 µL Triton-X 100 and 100 µL 10% (w/v) Na azide), then incubated in a mixture of primary antibodies for 16–20 h at room temperature. Following further 3 × 7 min washes in PBS, sections were exposed to secondary antibodies, Alexa 488 (Invitrogen, Carlsbad, CA) and Cy3 (Jackson Immunoresearch, West Grove, PA) for 2 h. After a final 3 × 7 min washes in PBS, sections were coverslipped inVectaShield (Vector Labs, Burlingame, CA).
Antibodies and their specificity
A list of the antibodies used, including their sources and effective dilutions, is provided in Table I. [Table I about here] The numbers that sometimes follow a name (e.g., synaptotagmin 1,2) indicate the isoforms recognized by the antibody we tested. We found that omission of the primary antibodies resulted in no staining. We tested all the commercially available immunogenic peptides, and found invariably that preadsorption of the primary antibody with its immunogenic peptide abolished immunostaining; these findings are documented in the Figures. For the remaining antibodies utilized, we relied on published studies and/or the manufacturer’s descriptions of specificity, based either on abolition of immunostaining following immunogen preadsorption, or Western blots showing that the antibody recognized a band of appropriate molecular size.
TABLE I.
Primary Antibodies
| Antibody | Animal | Source | Catalog no. | Dilution |
|---|---|---|---|---|
| Proton ATPase | Rabbit | Synaptic Systems | 111 011 | 1:500 |
| SNAP-25 | Mouse | Synaptic Systems | 119 002 | 1:1000 |
| SV2a | Rabbit | Synaptic Systems | 119 002 | 1:800–2000 |
| SV2b | Rabbit | Synaptic Systems | 119–102 | 1:500–2000 |
| Synaptophysin 1 | Mouse | Synaptic Systems | 101 011 | 1:500 |
| Synaptotagmin 1,2 | Rabbit | Synaptic Systems | 105 002 | 1:1000 |
| Syntaxin 1 | Mouse | Sigma | S0064 | 1:5000–10000 |
| Syntaxin 3 | Rabbit | Abcam | ab4113 | 1:1000 |
| Synaptobrevin 1 | Rabbit | Synaptic Systems | 104 002 | 1:200–1000 |
| Synaptobrevin 2 | Rabbit | Synaptic Systems | 104 202 | 1:1000 |
| VMAT2 | Rabbit | PhosphoSolutions | 2200- VMAT2C |
1:3000 |
| TH | Mouse | Chemicon | MAB 318 | 1:500 |
| TH | Rabbit | Chemicon | AB 152 | 1:800 |
Data Acquisition and Analysis
Fluorescent images were obtained with a Nikon PM 800 confocal microscope equipped with a digital camera controlled by the Spot software program (Diagnostic Instruments, Sterling Heights, MI). Digital images were acquired separately from each laser channel, then recombined. Digital files were processed with deconvolution software (AutoQuant Imaging, Watervliet, NY). Adobe Photoshop 7.0 was used for further processing of digital images. Any adjustments to brightness and contrast were made uniformly to all parts of the image.
We assessed the distribution of immunoreactivity (ir) within SN DAergic perikarya and dendrites for each antibody tested by obtaining z-axis confocal scans through the tissue. For DAergic perikarya the scans were at 2.0 µm intervals and for dendrites, at 1.0 µm intervals, using a 100x objective throughout, for which the depth of field is estimated to be 0.41 µm. This estimate is derived from the formula dtot = (λo n/NA2) + (n/M × NA)e, where dtot is the total depth of field, λo is the wavelength of illuminating light (540 nm), n is the refractive index of the medium (immersion oil, 1.479), NA is the numerical aperture (1.4) and e is the smallest distance (0.5 µm) that can be resolved by a detector splaced in the image plane of the microscope objective where lateral magnification is M (100) (cf. Nikon at http://www.microscopyu.com/tutorials/java/depthoffield/index.html)
Results
A. General features of SNc and SNr
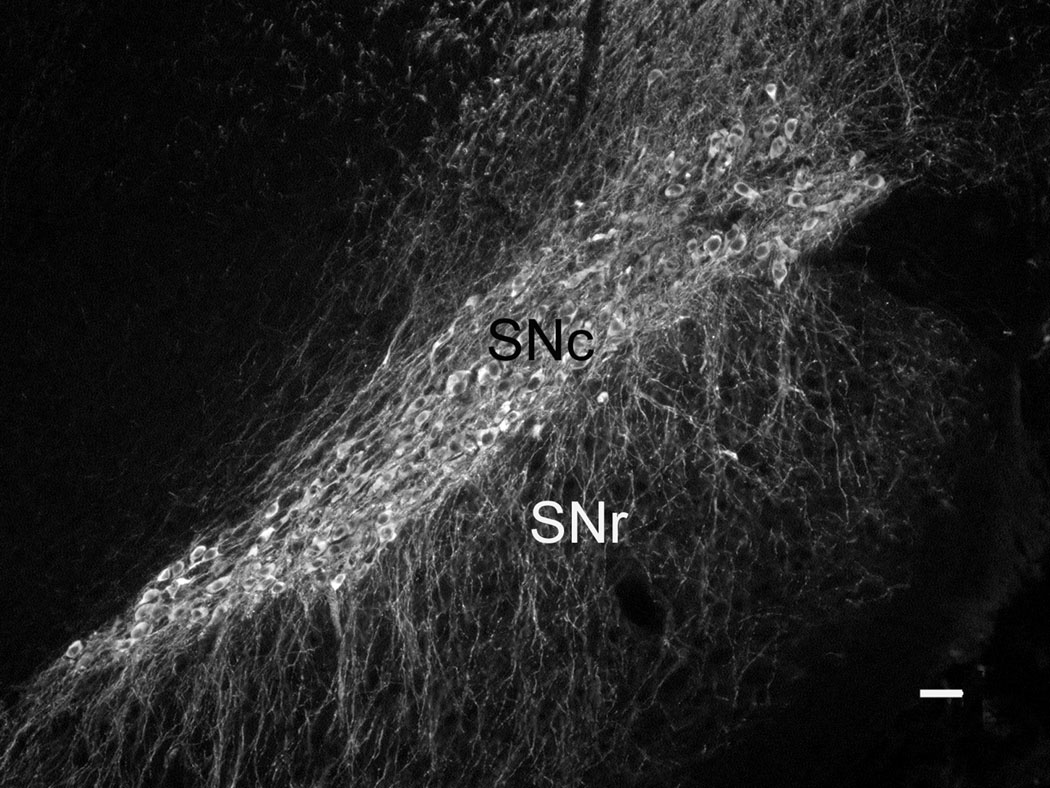
The SNc is characterized by a high density of DAergic somata as well as a dense network of overlapping DAergic dendrites, as seen when the midbrain is immunostained for tyrosine hydroxylase (TH), the rate-limiting enzyme for DA synthesis (Fig. 1). The DAergic perikarya in SNc tend to be ovoid, with a long axis of 20–30 µm. Each cell body emits multiple dendrites that extend either laterally within the SNc or ventrally into SNr. Within SNr, DAergic perikarya are sparse (Wassef et al., 1981), but DAergic dendrites are prominent, sometimes running in bundles towards the ventral surface of the midbrain (Fig. 1).
Fig. 1. TH immunostaining in Substantia Nigra.
A low magnification view of substantia nigra as revealed by TH-ir. The main subdivisions are pars compacta (SNc) and pars reticulata (SNr). The former consists of a mixture of large perikarya and dendrites, whereas SNr is largely free of DAergic cell bodies but contains abundant TH-ir dendrites with a primarily vertical orientation. Scale bar is 50 µm.
Because SNc and SNr also contain abundant non-DAergic cells and processes (Nair-Roberts et al., 2008), our sole criterion for associating a particular protein with a DAergic perikaryon or process was the colocalization of its immunostaining with TH-ir. The use of TH-ir is advantageous in that it extends throughout the DAergic cell, filling the cell body, dendrites and axons, including the finest processes. In contrast, the immunostaining of the proteins we examined was punctate, except for syntaxin 1, whose immunostaining pattern is a mixture of puncta embedded in a continuous matrix (cf. Fig. 3). The general distribution we obtained with an anti-syntaxin antibody is in agreement with earlier studies (Ruiz-Montasell et al., 1996; Goutan et al., 1999), which documented that syntaxin 1-ir is diffusely distributed throughout the neuropil of many rat brain regions, including SNr.
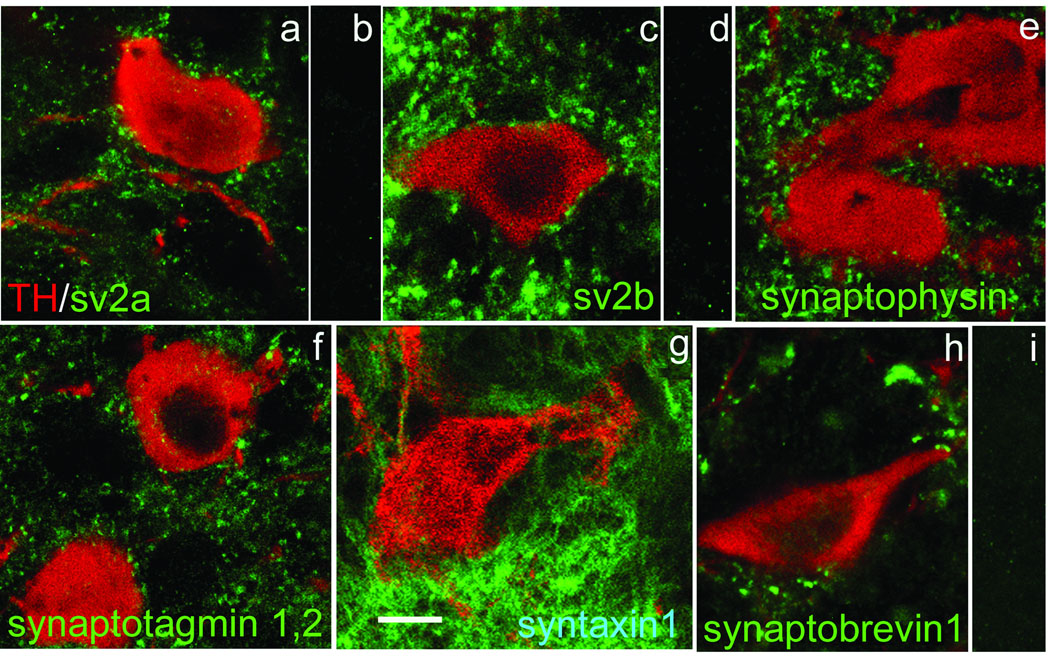
Fig. 3. Presynaptic proteins which do not colocalize with TH in nigral DAergic neurons.
Each of panels a,c,e-h shows an overlapped image of TH-ir (red) and the indicated presynaptic protein in green. In every case the presynaptic protein-ir is present in the neuropil and abuts the TH-ir DAergic soma, but is absent within it. Panels b,d, and i show that immunoreactivity was lost for, respectively, sv2a, sv2b and synaptobrevin 1 following preadsorption of the antibody with its immunogenic peptide. Scale bar in (g) is 10 µm and applies to all panels.
B. Vesicular monoamine transporter 2 in SN DAergic somata
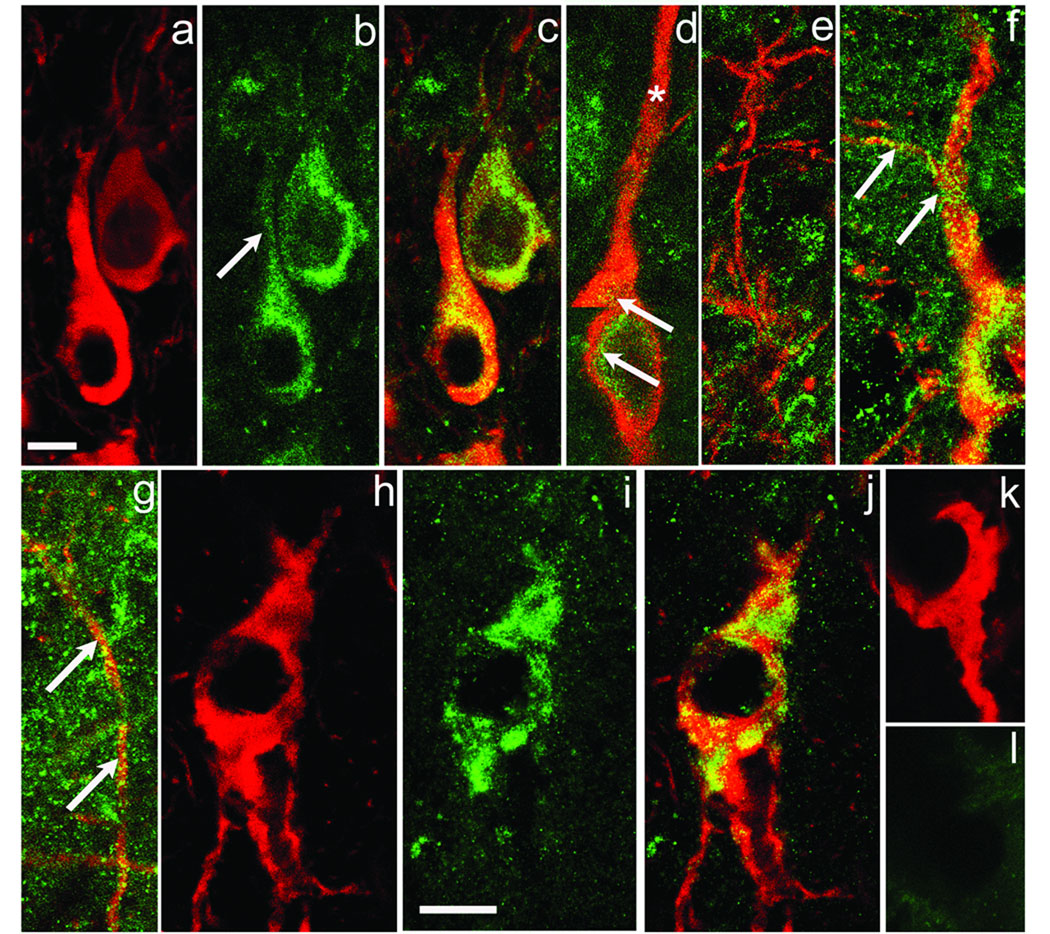
The vesicular monoamine transporter type 2 (VMAT2) transports DA into synaptic vesicles or other intracellular compartments participating in DA release. In the SN, somatodendritic DA release is minimal after VMAT2 inhibition (Elverfors and Nissbrandt, 1991; Rice et al., 1994; Heeringa and Abercrombie, 1995). Prior EM studies showed that SNc DAergic perikarya contain VMAT2, associated primarily with the smooth endoplasmic reticulum, but not organized into vesicle clusters (Nirenberg et al., 1996). We utilized an anti-VMAT2 antibody to gain an impression of the general distribution of VMAT2 in SNc and SNr. VMAT2-ir is distributed primarily as puncta in the perikaryal cytoplasm (Fig. 2a–c), with the highest density observed in a ring surrounding the nucleus. Additional VMAT2-ir puncta are located closer to the perikaryal surface but their density adjacent to the plasma membrane is low (Fig. 2c,d) With one exception, VMAT2-ir was noted only at the initial portions of DAergic dendrites, falling to a very low density at more distal locations (Fig. 2d), including throughout the SNr (Fig 2e). The exception is the DAergic dendrite from which the axon branches (Tepper et al., 1987). VMAT2-ir extends along this process (Fig. 2 f, g), reflecting the fact that VMAT2 is synthesized in the cell body and transported to the axon terminals in striatum where it is reported to be associated with DAergic axons and their synaptic terminals (Nirenberg et al., 1997).
Fig. 2. VMAT2- and v-ATPase-ir colocalize with DAergic perikarya.
General Comment: In the color figures, TH-ir is invariably shown in red, the other antibody being tested in green, and yellow indicates colocalization. Panels a,b illustrate, respectively, TH-ir and VMAT2-ir in two DAergic somata. Panel c shows the overlapped images, documenting that VMAT2-ir is located primarily around the DAergic cells’ nucleus, extending more sparsely into proximal portions of primary dendrites (b, arrow). Panel d is a montage from a z-stack through a DAergic perikaryon and one primary dendrite. Note abundant VMAT2-ir (yellow green) around the cell’s nucleus and initial portion of the dendrite (arrows), but disappearing in the dendrite’s more distal portion (*). VMAT2-ir is very sparse in DAergic dendrites within SNr (panel e). However, the dendrite from which the axon branches has abundant VMAT2-ir (arrows, panel f). Panel g shows the continuation of the axon branch (arrows) illustrated in panel f, but turned 90° to fit the figure. Panels h–j illustrate, respectively, TH-ir, v-ATPase-ir and the overlapped images. Similar to VMAT2-ir, v-ATPase-ir is confined almost entirely to the DAergic perikaryon. Panels k and l show, respectively TH-ir and the loss of v-ATPase-ir following preadsorption of the v-ATPase antibody by its immunogenic peptide. Scale bar in (a) is 10 µm and applies to panels a-g; the scale bar in (i) is 10 µm and applies to panels h-l.
The finding that VMAT2-ir is essentially absent in distal DAergic dendrites was tested further by examining the distribution of proton (vacuolar) ATPase (v-ATPase) within DAergic neurons (Fig. 2h–l). This proton pump is intrinsic to catecholaminergic vesicles and is responsible for establishing the proton gradient that energizes VMAT2-dependent transport (Eiden et al., 2004). Our results are illustrated in Fig. 2h–l. Similar to VMAT2-ir, v-ATPase-ir was seen as regions of patchy staining within DAergic perikarya (Fig. 2h–j), but was absent from DAergic dendrites. Preadsorption of the v-ATPase antibody with its immunogenic peptide resulted in a complete loss of v-ATPase-ir (Fig. 2k–l). Together these data suggest that the packaging and storage of DA for release differs in dendrites within SNr from that observed in DAergic somata and striatal axon terminals.
C. Presynaptic proteins not associated with SN DAergic somata
Synaptic vesicles contain a large number of intrinsic proteins (reviewed in Südhof, 2004) among which either synaptic vesicle proteins 2a or 2b (sv2a, sv2b) isoforms are invariably present. However, we found that both sv2a- and sv2b-ir were absent in DAergic somata (Fig. 3a,c), although present throughout the surrounding midbrain tissue, including in clusters immediately adjacent to the DAergic perikarya. Sv2a- and sv2b-ir were abolished following preadsorption of these primary antibodies by their respective immunogenic peptides (Fig. 3b,d). These data are consistent with previous studies indicating that the SN DAergic somatodendritic compartment lacks conventional synaptic vesicles (Nirenberg et al., 1996).
DAergic perikarya also were negative for staining with antibodies against three additional synaptic vesicle proteins: synaptophysin (Fig. 3e), the Ca2+ sensor, synaptotagmin 1,2 (Fig. 3f), and synaptobrevin 1, also called vesicle-associated membrane protein 1 (VAMP1) (Fig. 3h). We similarly found no immunostaining for the plasma membrane protein, syntaxin 1 (Fig. 3g), a so-called SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) involved in the fusion of synaptic vesicles with the plasma membrane. It is noteworthy that all of the presynaptic proteins we examined (Fig. 3) were found in the neuropil surrounding the DAergic somata, presumably indicating their association with some or all of the diverse terminals making synapses onto the DAergic somata (Nair-Roberts et al., 2008). The majority of these inputs arise in other basal ganglia nuclei and are GABAergic or glutamatergic (Lee and Tepper, in press).
Given that agranular vesicle accumulations were reported in presumed DAergic dendrites within SNc (Wilson et al., 1977) we extended our search for proteins involved in vesicle exocytosis to DAergic dendrites, even for those proteins not found in DAergic perikarya (see above and Fig. 3). We examined dendritic profiles in both SNc and SNr and our results were uniformly negative (not illustrated), i.e., we found no evidence for the presence of any of the above mentioned presynaptic proteins in DAergic dendrites.
D. DA release proteins
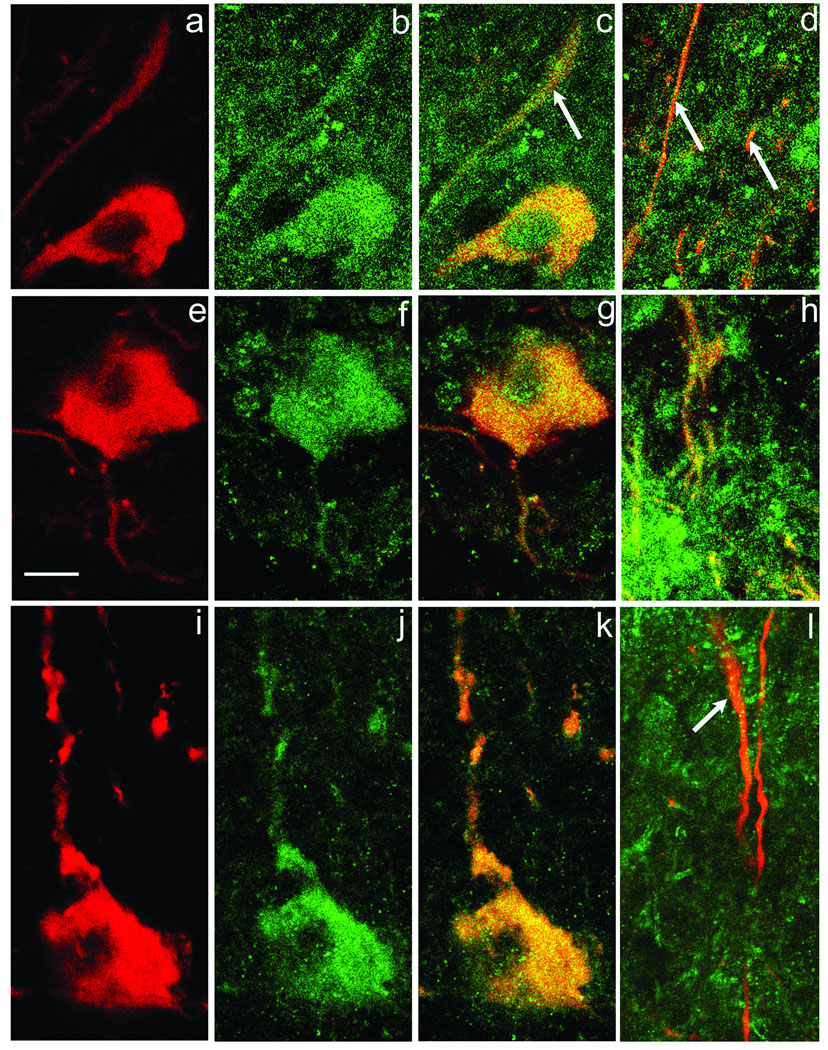
DAergic neurons of the SNc are not completely devoid of release proteins, however. Indeed, three were found to be associated with DAergic somata: syntaxin 3, SNAP-25 and synaptobrevin 2 (VAMP2) (Fig. 4). Syntaxin 3 and SNAP-25 are SNARE proteins localized in the plasma membrane, typically at the presynaptic ending, whereas synaptobrevin 2 is an intrinsic vesicle protein. We found that syntaxin 3-ir is punctate and is distributed throughout the perikaryon of a DAergic neuron, extending to the cell surface (Fig. 4a–c). Additionally, syntaxin 3-ir is observed within DAergic dendrites in the SNr (Fig. 4d). A similar distribution of SNAP 25-ir within TH-ir somata is shown in Fig. 4 (e–g) and within DAergic dendrites in SNr (Fig. 4h). The presence of synaptobrevin 2-ir within a DAergic perikaryon is illustrated in Fig. 4i–k and in a DAergic dendrite in (Fig. 4l). The observation that within SNr, synaptobrevin 2-ir is much denser in the space surrounding the DAergic profiles than in SNc, results in some chance overlap of synaptobrevin 2-ir with TH-ir processes. Nevertheless, as illustrated in Fig. 4l, there is a low density of synaptobrevin 2-ir puncta consistently associated with the DAergic dendritic profile. Thus our data clearly indicate that both SN DAergic somata and dendrites have a subset of DA release proteins required for exocytosis of synaptic vesicles or some comparable intracellular membrane-bound storage compartment.
Fig. 4. Colocalization of release proteins with DAergic perikarya and dendrites.
Panels a–c illustrate, respectively TH-ir, syntaxin 3-ir and the overlapped image in a DAergic perikaryon. Additionally, colocalization of TH and syntaxin 3-ir is noted in dendrites within SNc (arrow in c) and also in SNr (panel d, arrows). Panels e–h are identically organized to a-d, except that the dopamine release protein is SNAP-25 (green). Panels i–l illustrate the same sequence for TH-ir (red) and synaptobrevin 2 (green). In every case, colocalization is observed both within DAergic perikarya and dendrites, although the density of synaptobrevin 2-ir puncta in dendrites is lower (arrow in l) than that for either SNAP-25 or syntaxin 3-ir puncta. Scale bar in (e) is 10 µm and applies to all panels.
Discussion
Our examination of DAergic neurons of the SNc resulted in two main experimental findings. First, two proteins responsible for packaging DA into exocytotic compartments, VMAT2 and v-ATPase, are confined to the DAergic perikaryon and the most proximal portion of its dendrites, but are absent in the more distal regions of the dendrites. The second main finding is that although SNc DAergic somata manifest a subset of proteins generally associated with vesicular release, except for SNAP-25, this group of DA release proteins differs from the subset commonly encountered in presynaptic terminals of fast synapses utilizing amino acid transmitters such as glutamate (reviewed in Südhof, 2004).
A. Proteins involved in DA release by SN DAergic neurons
Prior studies have established that a protein subset consisting of syntaxin, SNAP and synaptobrevin isoforms is required for vesicle fusion (Söllner et al., 1993). Our data reveal a difference in the particular isoforms utilized by the SN DAergic somatodendritic compartment compared to fast synapses mediated by amino acid neurotransmitters. At fast synapses, a commonly encountered group is syntaxin 1a or 1b, SNAP-25 and synaptobrevin 1 or 2 (Söllner et al., 1993; Südhof, 2004). In cells for which neurotransmitter release is relatively slow, other pairings have been identified. For example, at photoreceptor ribbon synapses, Curtis et al. (2008) identified syntaxin 3b, SNAP-25 and synaptobrevin 2, the same subset we found in SN DAergic somata and dendrites. Curtis et al. (2008) also demonstrated that populations of vesicles artificially loaded with either SNAP-25/syntaxin 3b or synaptobrevin 2 bound to each other, indicating their probable association in vivo. Although syntaxin 3 is relatively less abundant than syntaxin 1 in mammalian brain, cDNA clones for all four syntaxin 3 isoforms have been found in mouse brain (Ibaraki et al., 1995).
An analogous situation occurs for isomers of the putative Ca2+-sensing protein, synaptotagmin, an intrinsic protein of the synaptic vesicle. Synaptotagmin 1 and 2 are involved in neurotransmitter release at fast synapses (Xu et al., 2007), but we found them to be absent in SN DAergic perikarya and dendrites. This implies that DAergic cells employ different isomers for somatodendritic release. Indeed, recent evidence suggests that synaptotagmin 7 and possibly synaptotagmin 4 are involved in somatodendritic DA release from mesencephalic cultures (Mendez et al., 2008). Moreover, synaptotagmin 7 is involved in slow exocytosis from chromaffin cells (Schonn et al., 2008). Collectively these findings provide evidence that the molecular components mediating DA release from DAergic somata are unique.
Botulinum toxin A, which cleaves SNAP-25, impairs DA release from SN DAergic perikarya (Bergquist et al., 2002; Fortin et al., 2006), demonstrating a functional role for SNAP-25 (Fig. 4e–h) and supporting the idea that somatodendritic DA release occurs by exocytosis. Other functional studies demonstrated that DA release from the somatodendritic compartment is abolished when a non-selective Ca2+ channel blocker such as Cd2+ is added to the bathing solution (Jaffe et al., 1998; Patel et al., 2009), or when intracellular Ca2+ is reduced by a fast-acting buffer such as BAPTA-AM (Patel et al., 2009). Thus there is good presumptive evidence that the triad of syntaxin 3/SNAP-25/VAMP 2 participates in the exocytosis of a DA-filled organelle. Fortin et al. (2006) found that botulinum B toxin significantly decreased DA release from cultured DAergic somata, consistent with our finding of abundant VAMP 2-ir in DAergic cell bodies. Conversely, the report that neither tetanus toxin nor botulinum toxin B (which cleave VAMP2) altered DA release in SNr (Bergqvist et al., 2002). accords with the relatively low density of VAMP2 we observed in DAergic dendritic profiles of SNr.
B. Somatodendritic DA Storage
The nature of the exocytotic compartment(s) in DAergic somata and dendrites remains to be established. Nirenberg et al. (1996) failed to find aggregates of synaptic vesicles in SN DAergic perikarya, although they are evident in DAergic terminals within the striatum (Pickel et al., 1996). Moreover, VMAT2 is associated with the smooth endoplasmic reticulum in DAergic somata (Nirenberg et al., 1996). Our finding that SN DAergic perikarya lack many proteins typically associated with synaptic vesicles, e.g., sv2a and sv2b, tends to support the idea that DAergic neurons utilize some unconventional exocytotic organelle.
Although there are reports of DA release from SNr (Nieoullon et al., 1977; Rice et al., 1994; Heeringa and Abercrombie, 1995) the mechanism of DA release from DAergic dendrites, whether within SNc or SNr, remains to be clarified. Wilson et al., 1977 reported that synaptic vesicle clusters were found within monoaminergic dendrites, based on their uptake of the false neurotransmitter, 5-hydroxydopamine. However, Wassef et al. (1981) found that only 0.2% of identified DAergic dendrites in SNr had synaptic vesicles, concluding that the processes described previously may not have been DAergic. Groves and Linder (1983), moreover, reported that dendrodendritic contacts between DAergic neurons were absent in SNr. Our data, particularly the absence of sv2a and sv2b in nigral DAergic somata and dendrites, are in agreement with the earlier study by Nirenberg et al. (1996), indicating that clusters of conventional small agranular vesicles are absent.
Whatever the nature of the exocytosing organelle, it must be filled with DA through a combined action of VMAT2 and v-ATPase. The apparent absence of VMAT2 and v-ATPase in distal DAergic dendrites, despite the presence of the SNARE proteins, syntaxin 3b, SNAP-25 and synaptobrevin 2, may indicate differences in the release process between DAergic somata and dendrites. Indeed, at least one form of syntaxin (1a) has been shown to associate with the DA transporter and promote DA release by amphetamine (Binda et al., 2008). Moreover there is as yet no description of putative vesicle exocytosis in distal DAergic dendrites, nor is it known whether or through what mechanism the DAergic exocytotic organelle is recaptured and recycled in the DAergic somatodendritic compartment. The data of the present report are in agreement with a study of retinal DAergic neurons (Witkovsky et al., 2004) in which it was found that VMAT2-ir was confined to the perikaryal region of the soma and the axon terminals, but was absent in dendrites.
Clearly much remains to be determined about the process of DA release from the somatodendritic compartment. Our data suggest that it may be a relatively slow process, consistent with the finding that repeated stimulation of DAergic neurons evokes a progressively diminishing DA release, as assessed by voltammetry (Rice et al., 1997).
Conclusions
Our data provide evidence that the molecular and cellular organization underlying DA release from SN DAergic somata differs substantially from the organization of presynaptic terminals at nerve endings utilizing amino acid neurotransmitters. Additionally our data suggest that DA release from DAergic dendrites in SNr may differ from that seen in the somata, i.e., the somatodendritic compartment may not be homogeneous with regard to the mechanism of DA release. The fate of the DA organelle subsequent to exocytosis is unknown – whether the organelle membrane fuses with the plasma membrane, is recaptured and the site and mechanism of refilling are all important topics for future study, since they will influence strongly the kinetics of dopamine release and recycling in the midbrain.
Acknowledgements
This work was supported by the Richard H. Chartrand Foundation (PW), National Institutes of Health-National Institute of Neurological Disorders and Stroke Grant R01NS036362 (MER), and the Edmond J. Safra Foundation (MER).
Abbreviations
- ATPase
adenosine triphosphatase
- BAPTA-AM
1,2-Bis(2-aminophenoxy)ethane-N,N,N’,N’’- tetraacetic acid tetrakis(acetoxymethyl ester)
- Ca2+
calcium ion
- Cd2+
cadmium ion
- CNS
central nervous system
- DA
dopamine
- DAergic
dopaminergic
- g
gram
- GABA
gamma-amino butyric acid
- ir
immunoreactivity
- kg
kilogram
- mg
milligram
- µm
micrometer
- NaCl
sodium chloride
- PBS
phosphate-buffered saline
- SNAP-25
synaptosome-associated protein-25
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SN
substantia nigra
- SNc
substantia nigra pars compacta
- SNr
substantia nigra pars reticulate
- sv2a,2b
synaptic vesicle protein 2a,2b
- TH
tyrosine hydroxylase
- VMAT2
vesicular monoamine transporter type 2
- VAMP
vesicle associated membrane protein
- w/v
weight per volume
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F. A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Progr. Brain Res. 1986;68:291–301. doi: 10.1111/j.1748-1716.1986.tb07967.x. [DOI] [PubMed] [Google Scholar]
- Beaudet A, Descarries L. The monoamine innervation of rat cerebral cortex: synaptic and nonsynaptic axon terminals. Neurosci. 1978;3:851–860. doi: 10.1016/0306-4522(78)90115-x. [DOI] [PubMed] [Google Scholar]
- Birkmayer W, Hornykiewicz O, editors. 5th Intl. Symp. Parkinson’s Disease. Vienna: Basel:Roche; 1976. Advances in Parkinsonism. Biochemistry, Physiology, Treatment. [Google Scholar]
- Bergquist F, Niazi HS, Nissbrandt H. Evidence for different exocytosis pathways in dendritic and terminal dopamine release in vivo. Brain Res. 2002;95:245–253. doi: 10.1016/s0006-8993(02)03047-0. [DOI] [PubMed] [Google Scholar]
- Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU, Zhang M, Sen N, Colbran RJ, Gnegy ME, Gether U, Javitch JA, Erreger K, Galli A. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Molec. Pharmacol. 2008;74:1101–1108. doi: 10.1124/mol.108.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. doi: 10.1016/0006-8993(75)90849-5. [DOI] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic release: substantia nigra versus striatum. J Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis LB, Doneske B, Liu X, Thaller C, McNedw JA, Janz R. Syntaxin 3b is a t-SNARE specific for ribbon synapses of the retina. J Comp Neurol. 2008;510:550–559. doi: 10.1002/cne.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system – I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand. 1964;62 suppl. 232:5–55. [PubMed] [Google Scholar]
- Eiden LE, Schäfer MK, Weihe E, Schütz B. The vesicular amine transport family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine. Pflüg Arch. 2004;447:636–640. doi: 10.1007/s00424-003-1100-5. [DOI] [PubMed] [Google Scholar]
- Fortin GD, Desrosiers CC, Yamaguchi N, Trudeau L-E. Basal somatodendritic dopamine release requires snare proteins. J Neurochem. 2006;96:1740–1749. doi: 10.1111/j.1471-4159.2006.03699.x. [DOI] [PubMed] [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- Goutan E, Marti E, Ferrer I. Expression of synaptic proteins in the developing rat cerebellum following ionizing radiation. Intl J Dev Neurosci. 1999;17:275–283. doi: 10.1016/s0736-5748(99)00044-1. [DOI] [PubMed] [Google Scholar]
- Groves PM, Linder JC. Dendro-dendritic synapses in substantia nigra: descriptions based on analysis of serial sections. Exp. Brain Res. 1983;49:209–217. doi: 10.1007/BF00238581. [DOI] [PubMed] [Google Scholar]
- Groves PM, Wilson CJ, Young SJ, Rebec GV. Self-inhibition by dopaminergic neurons. Science. 1975;190:522–529. doi: 10.1126/science.242074. [DOI] [PubMed] [Google Scholar]
- Heeringa MJ, Abercrombie ED. Biochemistry of somatodendritic dopamine release in substantia nigra: an in vivo comparison with striatal dopamine release. J Neurochem. 1995;65:192–200. doi: 10.1046/j.1471-4159.1995.65010192.x. [DOI] [PubMed] [Google Scholar]
- Ibaraki K, Horikawa HP, Morita T, Mori H, Sakimura K, Mishina M, Saisu H, Abe T. Identification of four different forms of syntaxin 3. Biochem Biophys Res Comm. 1995;211:997–1005. doi: 10.1006/bbrc.1995.1910. [DOI] [PubMed] [Google Scholar]
- Jaffe E, Marty A, Schulte A, Chow RH. Extrasynaptic vesicular transmitter release from the somata of substantia neurons in rat midbrain slices. J Neurosci. 1998;18:3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, Wilson CJ, Groves PM. The substantia nigra of the rat: a Golgi study. J Comp. Neurol. 1977;172:585–600. doi: 10.1002/cne.901720403. [DOI] [PubMed] [Google Scholar]
- Lee CR, Tepper JM. Basal ganglia control of substantia nigra dopaminergic neurons. J Neural Trans In Press. 2009 doi: 10.1007/978-3-211-92660-4_6. [DOI] [PubMed] [Google Scholar]
- Mendez J, Bourque MJ, Trudeau LE. Somatodendritic dopamine release requires synaptotagmin 4 and 7 and the participation of voltage-gated calcium channels. Soc Neurosci Abstr. 2008;34:11. doi: 10.1074/jbc.M111.218032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neurosci. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A, Chéramy A, Glowinski J. Release of dopamine in vivo from cat substantia nigra. Nature. 1977;266:375–377. doi: 10.1038/266375a0. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J Neurosci. 1996;16:4135–4145. doi: 10.1523/JNEUROSCI.16-13-04135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. Vesicular monoamine transporter-2: immunogold localization in striatal axons and terminals. Synapse. 1997;26:194–198. doi: 10.1002/(SICI)1098-2396(199706)26:2<194::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Patel JC, Witkovsky P, Avshalumov MV, Rice ME. Mobilization of calcium from intracellular stores facilitates somatodendritic dopamine release. J Neurosci. 2009;29:6568–6579. doi: 10.1523/JNEUROSCI.0181-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Nirenberg MJ, Milner TA. Ultrastructural view of central catecholaminergic transmission: immunocytochemical localization of synthesizing enzymes, transporters and receptors. J Neurocytol. 1996;25:843–856. doi: 10.1007/BF02284846. [DOI] [PubMed] [Google Scholar]
- Pyle JL, Kavalalai E, Piedras-Renteria ES, Tsien RW. Raid reuse of readily releasable pool vesicles at hippocampal synapses. Neuron. 2000;28:221–231. doi: 10.1016/s0896-6273(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Rice ME. Distinct regional differences in dopamine-mediated volume transmission. Prog. Brain Res. 2000;125:275–288. doi: 10.1016/S0079-6123(00)25017-6. [DOI] [PubMed] [Google Scholar]
- Rice ME, Richards CD, Nedergaard S, Hounsgaard J, Nicholson C, Greenfield SA. Direct monitoring of dopamine and 5-HT release in substantia nigra and ventral tegmental area in vitro. Exp. Brain Res. 1994;100:395–406. doi: 10.1007/BF02738400. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitgro. J Neurophysiol. 1997;77:853–862. doi: 10.1152/jn.1997.77.2.853. [DOI] [PubMed] [Google Scholar]
- Ruiz-Montasell B, Aguado F, Majó G, Chapman ER, Canals JM, Marsal J, Blasi J. Differential distribution of syntaxin isoforms 1A and 1B in the rat central nervous system. Eur J Neurosci. 1996;8:2544–2552. doi: 10.1111/j.1460-9568.1996.tb01548.x. [DOI] [PubMed] [Google Scholar]
- Söllner T, Bennet MK, Whiteart SW, Scheller RH, Rothman JE. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- Schonn JS, Maximov A, Lao Y, Südhof TC, Sørenson JB. Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for eocytosis in adrenal chromaffin cells. Proc Natl Acad Sci. 2008;105:3998–4003. doi: 10.1073/pnas.0712373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Sawyer SF, Groves PM. Electrophysiologically identified nigral dopaminergic neurons intracellularly labeled with HRP: light-microscopic analysis. J Neurosci. 1987;7:2794–2806. doi: 10.1523/JNEUROSCI.07-09-02794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef M, Berod A, Sotelo C. Dopaminergic dendrites in the pars reticulate of the rat substantia nigra and their striatal input. Combined immunocytochemical localization of tyrosine hydroxylase and anterograde degeneration. Neurosci. 1981;6:2125–2139. doi: 10.1016/0306-4522(81)90003-8. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM, Fifkova E. Monoaminergic synapses, including dendro-dendritic synapses in the rat substantia nigra. Exp Brain Res. 1977;30:161–174. doi: 10.1007/BF00237248. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Veisenberger E, Haycock JW, Akopian A, Garcia-Espana A, Meller E. Activity-dependent phosphorylation of tyrosine hydroxylase in dopaminergic neurons of the rat retina. J Neurosci. 2004;24:4242–4249. doi: 10.1523/JNEUROSCI.5436-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Südhof TC. Synaptotagmin-1,-2 and -9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]