Abstract
Background and objectives: Hepatocyte nuclear factor 1β (HNF1β) is a transcription factor that is critical for the development of kidney and pancreas. In humans, mutations in HNF1B lead to congenital anomalies of the kidney and urinary tract, pancreas atrophy, and maturity-onset diabetes of the young type 5 and genital malformations.
Design, setting, participants, & measurements: We report HNF1B screening in a cohort of 377 unrelated cases with various kidney phenotypes (hyperechogenic kidneys with size not more than +3 SD, multicystic kidney disease, renal agenesis, renal hypoplasia, cystic dysplasia, or hyperuricemic tubulointerstitial nephropathy not associated with UMOD mutation).
Results: We found a heterozygous mutation in 75 (19.9%) index cases, consisting of a deletion of the whole gene in 42, deletion of one exon in one, and small mutations in 32. Eighteen mutations were novel. De novo mutations accounted for 66% of deletions and 40% of small mutations. In patients who carried HNF1B mutation and for whom we were able to study prenatal ultrasonography (56 probands), isolated hyperechogenic kidneys with normal or slightly enhanced size were the more frequent (34 of 56) phenotype before birth. Various other prenatal renal phenotypes were associated with HNF1B mutations, at a lesser frequency. Diabetes developed in four probands. Hyperuricemia and hypomagnesemia, although not systematically investigated, were frequently associated.
Conclusions: This large series showed that the severity of the renal disease associated with HNF1B mutations was extremely variable (from prenatal renal failure to normal renal function in adulthood) and was not correlated with the genotype.
Hepatocyte nuclear factor 1β gene (HNF1B) encodes a transcription factor that binds DNA as homodimer or as heterodimer with the related factor HNF1α. Heterozygous mutations of HNF1B were first described in maturity-onset diabetes of the young type 5 (1). Renal manifestations are frequently observed in patients with maturity-onset diabetes of the young type 5 and include a wide spectrum of phenotypes (2). More recently, HNF1B mutations were found to be associated with a subset of fetal bilateral hyperechogenic kidneys (3) and other kidney diseases diagnosed before birth (4). Besides diabetes, nonrenal anomalies involving Mullerian and Wolffian derivatives, liver and pancreas abnormalities, hyperuricemia with or without gout (5), and hypomagnesemia (6) have been reported.
HNF1B plays a crucial role in early development (7) and thereafter is involved in the organogenesis of several tissues, such as gut, pancreas, liver, lung, and kidney. The gene is also transiently expressed in the neural tube and in the epididymis, vas deferens, seminal vesicle, prostate, uterus, and oviduct (7,8). During kidney development, the gene is expressed in the ureteric bud, in the comma- and S-shaped bodies, and then in the proximal and distal tubules but not in the glomerulus (9). Kidney-specific inactivation of Hnf1B in the mouse leads to cystic disease, and HNF1β was shown to bind directly DNA elements that regulate the expression of genes whose mutations are responsible for cystic kidney diseases (Nphp1, polaris, Umod, Pkhd1, and Pkd2) (10) or of a gene identified as a candidate modifier in a mouse model of cystic kidney disease (Kif12) (11). Here we report on HNF1B mutation screening in a series of 377 unrelated patients who presented with various kidney phenotypes, giving special attention to the prenatal renal phenotypes.
Materials and Methods
Patients
This is a retrospective study in which we included all cases that were not previously reported and were tested for HNF1B mutations in two reference centers for rare kidney diseases in France. Criteria for inclusion were hyperechogenic kidneys (but with size not more than +3 SD), uni- or bilateral multicystic kidney disease (MCD), renal agenesis, renal hypoplasia, cystic dysplasia, or hyperuricemic tubulointerstitial nephropathy not associated with UMOD mutation. Patients' samples, medical records, genealogy, and written informed consent from patient and/or parents were sent from Pediatric, Pediatric Nephrology, Nephrology, or Obstetric Departments. Genomic DNA was extracted from venous blood or tissues collected from 377 unrelated cases (271 children, 57 adults, and 49 fetuses), 221 male and 156 female.
Prenatal ultrasonographs were available for 245 probands (usually performed at 12, 22, and 32 weeks of amenorrhea) and had been considered as normal in only 11 cases. Renal phenotypes before birth were isolated hyperechogenic kidneys (not larger than +3 SD in size) in 55 cases, bilateral MCD (13 cases), unilateral MCD (74 cases), unilateral agenesis (34 cases), bilateral agenesis (13 cases), renal hypoplasia (25 cases), urinary tract dilation (11 cases), and cystic disease (nine cases). In 132 patients, either the result of the prenatal ultrasound was not known or ultrasound was not performed (patients born before 1980). Renal phenotypes after birth were hyperechogenic kidneys (23 cases), unilateral MCD (12 cases), unilateral agenesis (8 cases), renal hypoplasia (33 cases), urinary tract dilation (2 cases), hyperuricemic tubulointerstitial nephritis (18 cases), unclassified cystic disease (35 cases), and only extrarenal symptoms (diabetes and uterine abnormalities; one case).
Patients with renal cavity dilation and/or recurrent acute pyelonephritis had voiding cystourethrogram. GFR was estimated by the Modification of Diet in Renal Disease (MDRD) formula for adults and by the Schwartz formula for children who were younger than 16.
Molecular Analysis
Quantitative multiplex PCR amplification of short fluorescence fragments (12) was performed as described previously (13) for the search of deletion. When deletion was not found, the nine exons and the exon–intron boundaries of the gene were screened for mutations by direct sequencing as described previously (1).
Statistical Analysis
Testing for difference in proportions was performed using the χ2. All tests were two sided. P < 0.05 was considered significant.
Results
Mutations
Heterozygous HNF1B alterations, which are thought to be pathogenic, were found in 75 probands (41 male and 34 female), leading to a mutation detection rate of 19.9% of tested index cases. They consisted of a heterozygous deletion of the entire gene in 42 cases (Table 1). Parent status was studied for 21 probands: deletions were de novo in 14 of 21 cases and inherited in seven of 21. Mutations that were not deletions of the entire gene are shown in Table 2. One patient was carrying a de novo heterozygous deletion of exon 4, which was previously reported (3,13). Twenty-four different heterozygous small mutations (11 missense, five nonsense, five frameshift, and three splice site mutations) were found in 32 probands. Parent status was studied for 20 of them. Mutation were shown to be de novo in eight of 20 cases and to be inherited in 12 of 20 cases; 18 were novel. Except for the mutation affecting the initiator codon, all missense mutations were localized in the DNA binding domain (Figure 1), were modifying a conserved amino acid, and were predicted to be probably damaging by the Polyphen program (14). In some families, there was a father-to-son transmission, in agreement with an autosomal dominant mode of inheritance (see proband 64 as an example).
Table 1.
Phenotypes in probands with complete HNF1B deletions
| Probands | Prenatal Renal Phenotype | Postnatal Renal Phenotype | Deletion Inheritance |
|---|---|---|---|
| 1 | Normal ultrasound | Large kidneys with numerous bilateral cysts, preterminal renal failure at 8 months; father with renal hypoplasia, GFR unknown | ND (parents not tested) |
| 2 | Normal ultrasound | Hyperechogenic and cystic kidneys, normal GFR at 6 years | De novo |
| 3 | Bilateral hyperechogenic kidneys | Hyperechogenic, normal-sized kidneys, normal GFR at 10 months | ND (parents not tested) |
| 4 | Bilateral hyperechogenic kidneys | Hyperechogenic kidneys, multiple microcysts, CRF (GFR 29 at 6 years); mother with renal cysts. | Deletion in the mother |
| 5 | Bilateral hyperechogenic kidneys | Hyperechogenic, normal-sized kidneys, microcysts, normal GFR at 5 years | ND (parents not tested) |
| 6 | Unilateral hypoplasia | Unilateral hypoplasia with cysts, normal GFR at 2 years | De novo |
| Father and paternal grandmother with renal cysts | |||
| 7 | Bilateral hyperechogenic kidneys | Small cystic kidneys, normal GFR at 3 years | ND (parents not tested) |
| 8 | Bilateral hyperechogenic kidneys | Normal-sized kidney with cortical cysts + left PUJO hyperuricemia, normal GFR at 5 years | ND (parents not tested) |
| 9 | Unilateral MCD, contralateral cysts | Unilateral MCD, cortical cysts on contralateral kidney, hyperuricemia, elevated liver enzymes, normal GFR at 10 years | ND (parents not tested) |
| 10 | Bilateral hyperechogenic kidneys | Bilateral cortical cysts, normal-sized kidney, normal GFR at 17 months; father with renal hypodysplasia, GFR unknown; paternal grandfather has CRF; previous TOP for MCD and anamnios in the mother | ND (parents not tested) |
| 11 | ND | Diabetes, bicornuate uterus TOP in the past because of anamnios, normal GFR at adult age; diabetes in sisters and father | Deletion in the father |
| 12 | Bilateral hyperechogenic kidneys | Few cysts, unknown GFR | De novo |
| 13 | Unilateral MCD, contralateral hyperechogenic kidney | Cysts in the single kidney, normal GFR at 9 years | ND (parents not tested) |
| 14 | Bilateral hyperechogenic kidneys, one cortical cyst | Hyperechogenic large (+2 SD) kidneys, CRF (unknown GFR) at 1 month | De novo |
| 15 | ND | Cystic kidney disease, uterine agenesis, imperforated vagina, mental retardation, normal GFR at 29 years | ND (parents not tested) |
| 16 | Bilateral pelvic dilation | Bilateral PUJO, unilateral small cortical cysts, normal GFR at 14 months | De novo |
| 17 | Bilateral hyperechogenic kidneys | Bilateral cortical cysts, normal GFR at 3 years; brother with pelvic kidney and PUJO; mother with normal kidneys and normal GFR, left hepatic agenesis, pancreas head hypoplasia, bicornuate uterus | Deletion in the mother |
| 18 | Bilateral hyperechogenic kidneys | Few cysts, normal-sized hyperechogenic kidneys, neonatal renal failure, normal GFR at 20 months; mother with cysts and gestational diabetes | Deletion in the mother |
| 19 | Bilateral hyperechogenic kidneys | Bilateral cortical cysts, normal GFR at 5 years; mother with renal cysts and severe cholestasis | Deletion in the mother |
| 20 | ND | Bilateral cysts, normal GFR at 11 years | ND (parents not tested) |
| 21 | Bilateral hyperechogenic kidneys, cortical cysts (MRI), diaphragmatic hernia | Dedifferentiated kidneys (54 and 58 mm) with cysts, acute renal failure at birth, GFR 40 ml/min per 1.73 m2 at 2 months | De novo |
| 22 | Bilateral hyperechogenic kidneys, cortical cysts, oligoamnios | TOP; renal histology showed cystic dilation of nearly all glomeruli with collapsed floculus, glomerular cysts were lined by fibrosis, interstitial fibrosis with rarefied tubules | De novo |
| 23 | ND | Bilateral hyperechogenic kidneys, cortical microcysts, normal GFR at 17 years | De novo |
| 24 | ND | Bilateral cortical microcysts, bicornuate uterus, diabetes, normal GFR at 20 years | ND (parents not tested) |
| 25 | ND | Bilateral hyperechogenic kidneys, cortical microcysts, normal GFR at 3 years | ND (parents not tested) |
| 26 | Bilateral hyperechogenic kidneys, pelvic dilation | Bilateral hyperechogenic hypoplastic kidneys, unknown GFR, microcysts in mother | Deletion in the mother |
| 27 | ND | Bilateral hyperechogenic kidneys, cortical microcysts, CRF (GFR 65 at 30 years) | ND (parents not tested) |
| 28 | Bilateral hyperechogenic kidneys | Bilateral hyperechogenic kidneys CRF (GFR 40 at 3 years) | ND (parents not tested) |
| 29 | ND | Bilateral hyperechogenic kidneys cortical microcysts, normal GFR at 35 years; mother with type 2 diabetes | ND (parents not tested) |
| 30 | ND | Bilateral hyperechogenic kidneys, cortical microcysts, normal GFR at 6 years; microcystic sole kidney in mother | ND (parents not tested) |
| 31 | Bilateral hyperechogenic kidneys | Bilateral hyperechogenic kidneys, CRF (GFR 35 at 1 year) | ND (parents not tested) |
| 32 | Bilateral hyperechogenic kidneys | Bilateral hyperechogenic kidneys, diabetes at 17 years, normal GFR at 20 years | ND (parents not tested) |
| 33 | Bilateral hyperechogenic kidneys | Bilateral hyperechogenic kidneys, normal GFR at 1 year | De novo |
| 34 | Unilateral MCD, other kidney hyperechogenic | Unilateral MCD, other kidney hyperechogenic with pelvic dilation, normal GFR at 3 years | De novo |
| 35 | Bilateral hyperechogenic kidneys | Bilateral hyperechogenic kidneys, normal GFR at 15 years | De novo |
| 36 | Bilateral hyperechogenic kidneys + unilateral macrocysts | Bilateral hyperechogenic kidneys + unilateral macrocysts, CRF (GFR 55 at 3 years) | De novo |
| 37 | Unilateral MCD, other kidney hyperechogenic | Unilateral MCD, hyperechogenic kidney, normal GFR at 6 years | De novo |
| 38 | Bilateral hyperechogenic kidneys, cortical microcysts | Bilateral hyperechogenic kidneys, cortical microcysts, normal GFR at 6 years | De novo |
| 39 | Unilateral agenesis | Single hyperechogenic kidney, cortical microcysts, normal GFR at 10 years | ND (parents not tested) |
| 40 | Unilateral agenesis, hyperechogenic kidney with microcysts | Single hyperechogenic kidney, microcysts, CRF (GFR 23 at 1 year); single kidney with cysts in the mother (GFR 75 at 30 years) | Deletion in the mother |
| 41 | Bilateral hyperechogenic kidneys | Bilateral hyperechogenic kidneys, unilateral VUR, unknown GFR | ND (parents not tested) |
| 42 | ND | Bilateral hyperechogenic kidneys, cortical microcysts, normal GFR at 3 years | ND (parents not tested) |
CRF, chronic renal failure; MRI, magnetic resonance imaging; ND, not done; PUJO, pelvi-ureteric junction obstruction; VUR, vesicoureteral reflux.
Table 2.
Mutations and phenotypes in patients with HNF1B mutations that are not complete deletions
| Probands | Nucleotide Change | Protein Change | Exon (Intron) | Reference | Prenatal Renal Phenotype | Postnatal Renal Phenotype | Mutation Inheritance |
|---|---|---|---|---|---|---|---|
| 43 | c.3G→A | p.Met1Ile | 1 | This study | Bilateral cortical cysts | Bilateral cortical microcysts, normal GFR at 7 years; father with renal cysts | Mutation in the father |
| 44 | c.3G→A | p.Met1Ile | Bilateral cortical cysts | Bilateral cortical microcysts, normal GFR at 7 years; father with diabetes and renal cysts | Mutation in the father | ||
| 45 | c.211 delAAGGGCC | p.Lys71fs | 1 | This study | ND | Hypodysplastic kidneys with microcysts, GFR 45 at 28 years; father with hyperuricemic nephropathy (GFR unknown) | Mutation in the father |
| 46 | c.232G→T | p.Glu78X | 1 | This study | Normal ultrasound | Bilateral cortical cysts, ESRF at 3 months | De novo |
| 47 | c.232G→T | p.Glu78X | 1 | MCD ×2 | TOP; septated uterus | ND | |
| 48 | c.322 delG | p.Ala108fs | 1 | This study | ND | Hyperuricemic nephropathy, ESRF at 33 years; father with hyperuricemic nephropathy (with kidney graft) and diabetes | Mutation in the father |
| 49 | IVS1 345–1G→A | (1) | This study | Bilateral hyperechogenic kidneys | Bilateral cortical cysts, neonatal renal failure, normal GFR at 8 years | ND | |
| 50 | IVS1 345–1G→A | (1) | Unilateral agenesis + hyperechogenic kidney | Single hyperechogenic kidney + CRF (GFR 25 at 17 years) | ND (parents not tested) | ||
| 51 | c.452C→G | p.Ser151Cys | 2 | This study | Unilateral MCD | Unilateral MCD and cortical cysts on the other kidney, normal GFR at 6 years | ND |
| 52 | c.476C→T | p.Pro159Leu | 2 | This study | Bilateral hyperechogenic kidneys | Isolated hyperechogenic kidneys, ultrasound normalized (size and echogenicity) at 10 months, normal GFR at 10 months | ND |
| 53 | c.494 G→A | p.Arg165His | 2 | (20) | ND | Small hyperechogenic kidneys, CRF (GFR 16 at 4 years); father with renal failure and diabetes | ND |
| 54 | c.494G→C | p.Arg165Pro | 2 | This study | Bilateral hyperechogenic kidneys | Bilateral hyperechogenic hypoplastic kidneys CRF (GFR 32 at 10 years) + pancreatic hypoplasia | De novo |
| 55 | c.513G→A | p.Trp171X | 2 | This study | Bilateral hyperechogenic kidneys | Bilateral cortical cysts, normal GFR at 11 months | ND |
| 56 | IVS2 544 + 3delAAGT | (2) | (6) | ND | Renal cysts and VUR, normal GFR at 6 years; mother with unilateral cysts and gestational diabetes, normal GFR at 30 years | Mutation in the mother | |
| 57 | IVS2 544 + 3delAAGT | (2) | ND | Single kidney, gout, CRF (GFR 25 at 65 years); mother and maternal cousin with renal failure; daughter with single kidney | ND | ||
| 58 | IVS2 544 + 3delAAGT | (2) | ND | Cysts, CRF (GFR 60 at 33 years), diabetes, hyperuricemia, elevated liver enzymes, hypomagnesaemia; father with ESRF | ND | ||
| 59 | c.544C→T | p.Gln182X | 2 | (20) | ND | Hyperechogenic kidneys, CRF (but unknown GFR); diabetes in the mother. | Mutation in the mother |
| 60 | c.544C→T | p.Gln182X | 2 | Unilateral MCD, other kidney with cysts | Unilateral MCD, other kidney with cortical cysts, normal GFR at 3 months | ND | |
| 61 | c.544C→T | p.Gln182X | 2 | Unilateral agenesis | Single hypoplastic hyperechogenic kidney, CRF (GFR 55 at 7 years) | ND | |
| 62 | c.758A→C | p.Gln253Pro | 3 | (3) | Bilateral MCD | TOP cysts in mother (GFR 62 at 25 years) and grandmother | Mutation in the mother |
| 63 | c.717delG | p.Ser242fs | 3 | This study | Bilateral hyperechogenic kidneys | Hyperechogenic kidneys, cortical microcysts, normal GFR at 1.5 years; father with renal cysts and diabetes | Mutation in the father |
| 64 | c.766C→T | p.Pro256Ser | 3 | This study | Bilateral hyperechogenic kidneys | Bilateral cortical cysts, normal-sized kidneys, normal GFR at 8 years; phenotype in the father unknown | Mutation in the father |
| 65 | IVS3 809 + 1G→A | (3) | This study | Bilateral hyperechogenic kidneys, bilateral cysts | Bilateral cortical cysts, unilateral UPJ, neonatal renal failure, normal GFR at 3 years; family history of diabetes; phenotype in the father unknown | Mutation in the father | |
| 66 | c.840delC | p.Pro280fs | 4 | This study | Enlarged kidneys, large cysts, pyelic dilation, duplicity, pancreas hypoplasia | TOP | ND |
| 67 | c.854G→A | p.Gly285Asp | (17) | Bilateral hyperechogenic kidneys | Bilateral hyperechogenic kidneys + unilateral cortical microcysts, normal GFR at 3 years; mother with renal cysts, GFR 55 at 35 years | Mutation in the mother | |
| 68 | c.883C→T | p.Arg295Cys | 4 | (17) | ND | Small and cystic kidneys (unknown GFR); mother with renal cysts and CRF (precise GFR unknown) | Mutation in the mother |
| 69 | c.883C→T | p.Arg295Cys | 4 | Bilateral hyperechogenic kidneys, bilateral cortical cysts | Cortical cysts, hyperuricemia, neonatal renal failure, normal GFR at 8 years | De novo | |
| 70 | c.895T→G | pTrp299Gly | 4 | This study | Bilateral hyperechogenic kidneys, bilateral cortical microcysts | Bilateral hyperechogenic kidneys, cortical microcysts CRF (GFR 51 at 3 years) | ND |
| 71 | c.766C→T | p.Asn302Lys | 4 | This study | Bilateral hyperechogenic kidneys | Bilateral cortical cysts, CRF (GFR 60 at 7 years) | ND |
| 72 | Exon 4 deletion c.810_1045 del236 | p.Arg270fs | 4 | (3,13) | Unilateral MCD | Absence of hypertrophy of the contralateral kidney, VUR, CRF (GFR 65 at 4 years) | De novo |
| 73 | c.1136C→A | p.Ser379X | 5 | This study | ND | Hyperechogenic kidneys, cortical microcysts, CRF (GFR 61 at 15 years), didelphic uterus + pancreatic hypoplasia | De novo |
| 74 | c.1360C→T | p.Gln454X | 7 | This study | Unilateral MCD, other kidney hyperechogenic | Unilateral MCD, other kidney hyperechogenic with cortical microcysts, normal GFR at 2 years | De novo |
| 75 | c.delAG1363–1364 | p.Ser455fs | 7 | This study | Bilateral hyperechogenic kidneys | Bilateral cortical cysts, left hypoplastic kidney CRF (GFR 80 at 14 years) | ND |
CRF, chronic renal failure; ESRF, end-stage renal failure; UPJ, ureteropelvic junction.
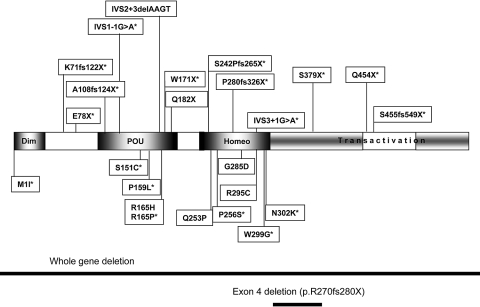
Figure 1.
HNF1β protein and localization of the various mutations identified in this study. The N-terminal portion of the protein consists of a short dimerization domain (dim). The DNA-binding domain is characterized by a region distantly related to the POU box-specific domain and an atypical homeodomain structure. The residues required for HNF1β transactivation have been mapped to the carboxy-terminal region. Deletions are indicated by a solid line. *Novel mutation.
Renal and Extrarenal Phenotype
Patients for Whom Prenatal Ultrasound Was Available
In 245 cases tested for HNF1B mutation, we were able to go back to the prenatal ultrasound. Mutations were identified in 56 of them.
Prenatal phenotype in patients with HNF1B mutation was isolated bilateral hyperechogenic kidneys with normal or moderately enlarged size in 34 cases, including one termination of pregnancy (TOP) because of an associated oligo-anamnios. Evaluation of these patients at last follow-up showed renal failure with GFR <80 ml/min per 1.73 m2 (range 32 to 61 ml/min per 1.73 m2) in eight patients (1 months to 14 years old), GFR >80 ml/min per 1.73 m2 in 20 patients (1 to 17 years old), and unknown in five patients. Five patients experienced transitory renal failure at birth, and one developed diabetes at the age of 17.
Other prenatal phenotypes in patients with HNF1B mutation were bilateral MCD (leading to TOP) in two patients, unilateral MCD in eight patients, unilateral renal agenesis (with hypoplasia and/or cysts on the single kidney) in four patients, unilateral renal hypoplasia in one patient, renal macrocysts in three patients (with urinary tract dilation, pancreas hypoplasia, and TOP in one patient), and isolated upper urinary tract dilation in one patient (who developed small cortical cysts after birth). In three patients who presented with severe cystic dysplasia on early ultrasound, the prenatal ultrasounds were considered as normal. In all cases with unilateral MCD, patients developed postnatal anomalies on the contralateral kidney. In the case with unilateral hypoplasia, cysts developed on the hypoplastic kidney after birth
Patients for Whom Prenatal Ultrasound Was not Available
In 132 patients who were tested for HNF1B mutation, we were not able to go back to prenatal ultrasound (either the result of it was not known, or ultrasound was not performed). We found an HNF1B mutation in 19 of them, including 10 who were tested during adulthood, six of whom had a family history of renal diseases. Four adult probands had cystic renal hypoplasia (associated with hypomagnesemia, gout, and a diabetes that occurred at 42 years in one and with gestational diabetes in another). Two had hyperechogenic kidneys with microcysts. One had solitary kidney and early gout, and another one had hyperuricemic interstitial nephropathy. One female born from consanguineous parents developed unclassified renal cystic dysplasia with uterus agenesis, imperforated vagina, cleft palate, and mental retardation. One presented with diabetes at the age of 31 and bicornuate uterus. Four adults (aged 29 to 35 years) had normal renal function and five (aged 28 to 33 years) had reduced GFR (65 ml/min per 1.73 m2 to end-stage renal failure), and renal function was unknown for one.
We found an HNF1B mutation in nine patients who were tested during childhood, two of whom had a family history of renal disease. Eight probands had hyperechogenic kidney and cysts and one hypoplastic kidney and/or uterus anomalies (n = 2) and/or pancreatic hypoplasia (n = 1). One developed diabetes at the age of 20 years. Renal function was normal in six patients (aged 3 to 20 years) and altered three times (aged 4 to 15 years).
Genotype–Phenotype Correlation
The severity of the renal disease that is associated with HNF1B mutation was extremely variable (from prenatal severe renal failure to normal renal function in adulthood). The type of mutation (deletion of the whole gene; missense mutation; or truncating mutation because of nonsense, frameshift, or splice mutation) was analyzed according to the renal phenotype for the 75 probands who carried an HNF1B mutation, as well as for other affected family members when their kidney phenotype was known (Figure 2). The percentage of each type of mutation was not statistically different when the group of patients who had prenatal hyperechogenic kidneys was compared with a group that included all other patients. We also looked for a relation between the type of mutation and the severity of the disease in terms of renal failure, independent of the type of renal disease. The patients with severe and early renal failure (six patients with TOP for oligohydramnios and six patients with terminal or preterminal renal failure that occurred before the age of 4 years) were associated either with deletions (seven patients), truncating mutation (three patients), or missense mutations (two patients), a figure that is not different from the proportion of each type of mutation in all patients. Figure 3 shows the number of patients with and without renal failure for each type of mutation. The proportion of patients with renal failure at last follow-up was significantly (P = 0.012) higher in patients who carried a truncating mutation than in patients who carried an HNF1B deletion; however, for unknown reasons, patients with truncating mutation were older than patients with gene deletion at last follow-up. This age difference may account, at least in part, for the different severity of the renal failure.
Figure 2.
Type of HNF1B mutation (□, deletion of the entire gene;  , missense mutations; ■, truncating mutations) according to the renal phenotype in patients and affected relatives. 1, prenatal hyperechogenic kidneys; 2, hyperechogenic kidney diagnosed after birth; 3, MCD; 4, unilateral renal agenesis; 5, cystic disease; 6, renal hypoplasia; 7, tubulointerstitial nephritis; 8, pyeloureteral junction; 9, pelvic kidney; 10, lack of renal anomaly.
, missense mutations; ■, truncating mutations) according to the renal phenotype in patients and affected relatives. 1, prenatal hyperechogenic kidneys; 2, hyperechogenic kidney diagnosed after birth; 3, MCD; 4, unilateral renal agenesis; 5, cystic disease; 6, renal hypoplasia; 7, tubulointerstitial nephritis; 8, pyeloureteral junction; 9, pelvic kidney; 10, lack of renal anomaly.
Figure 3.
Type of HNF1B mutation (deletion, missense mutations, truncating mutations) according to the GFR at last follow-up (■, GFR >80 ml/min per 1.73 m2; □, GFR <80 ml/min per 1.73 m2) and age at last follow-up (▧).
Discussion
To our knowledge, we report here the largest series of phenotypic and genetic analysis of patients who harbor renal diseases that are associated with HNF1B mutations. We screened 377 unrelated patients and identified an HNF1B mutation or deletion in 75 unrelated cases: 10 adults and 65 children or fetuses. This rate of mutation (19.9%) is not significantly different from that (23%) recently reported in a smaller cohort of children with renal malformation (6). Going back to the prenatal ultrasound when available, we report the renal phenotypes before birth in patients with HNF1B mutation and analyzed the evolution of their renal function.
We had information regarding prenatal ultrasound for 245 patients, and this study confirms our previous finding that isolated bilateral hyperechogenic fetal kidneys with normal or slightly enlarged (≤+3 SD) size were the most frequent phenotype observed before birth in patients who carried an HNF1B mutation (3); however, one limit of our study is that our population represents patients who had congenital anomalies of the kidney and urinary tract and whose samples were received for HNF1B testing in France during a certain period of time. Thus, it will be of interest to perform a prospective study that includes all hyperechogenic kidneys with normal or slightly enlarged size diagnosed before birth and test them for HNF1B mutation. Almost all patients with HNF1B mutation and moderately enlarged hyperechogenic kidneys before birth displayed normal-sized or small kidneys with hyperechogenicity and/or cortical cysts in the postnatal period, suggesting a slow-down in kidney growth after birth.
Besides hyperechogenic kidneys, HNF1B mutations were associated with several other prenatal renal abnormalities but far less frequently: bilateral or unilateral MCD, unilateral renal agenesis, kidney hypoplasia, isolated pyelic dilation, or kidneys with individualized cysts. Because unilateral renal agenesis has been reported in association with HNF1B abnormalities only in adults so far (5), it had been suggested that these cases may be due to involution of overlooked MCD (13). Our study shows that genuine renal unilateral agenesis can be associated with HNF1B mutation. The absence of cases of bilateral agenesis may be due to the small number of patients tested. In all cases of renal unilateral agenesis associated with HNF1B mutation, the single kidney was abnormal. More generally, except for one patient with unilateral hypoplasia and normal contralateral kidney, all probands who carried HNF1B mutation displayed bilateral kidney abnormalities. Regarding extrarenal symptoms, no patient with HNF1B mutation developed diabetes during early childhood. Only four presented diabetes at 17, 20, 31, and 42 years, respectively, and one developed gestational diabetes. Six other probands had family history of diabetes, but the type of diabetes in relatives was not always known.
Twelve patients with HNF1B mutation had early gout and/or hyperuricemia, a feature that has been reported in patients with HNF1B mutations (15), but this frequency must be underestimated because the uricemia dosage was not available for many patients in our cohort. Only one adult proband who presented with tubulointerstitial nephropathy and early hyperuricemia that was previously shown not to be associated with UMOD mutation was carrying an HNF1B mutation. The association of familial hyperuricemic nephropathy with HNF1B mutation has been reported previously (5,15), but the mechanisms responsible for the reduced fractional excretion of uric acid are not well understood. HNF1α/HNF1β heterodimers have been shown to bind and positively regulate the proximal promoter region of SLC22A12, encoding a transporter that is responsible for the resorption of urate in the apical membrane of the renal proximal tubule (16); therefore, loss of function would be expected to lead to hypouricemia. The overlap between phenotypes associated with HNF1B loss of function and familial UMOD hyperuricemic nephropathy may not seem surprising, because UMOD was shown to be a target of HNF1β (10); however, familial hyperuricemic nephropathy associated with UMOD mutations is thought to be due to a defect in uromodulin transport, associated with a dominant effect, rather than to haploinsufficiency. Thus, the development of the same phenotype associated with HNF1B haploinsufficiency is not fully understood. Nevertheless, the finding of hyperuricemia and/or of low uric acid excretion fraction should be an additional argument to screen for HNF1B mutation in patients who present with congenital anomalies of the kidney and urinary tract.
Hypomagnesemia was also found in several individuals with HNF1B mutation, although blood magnesium dosage was not always performed. Low plasma magnesium level was recently reported by another group and may be related to the transcriptional regulation of FXYD2 by HNF1β (6). In addition to the frequent and moderate elevation of liver enzymes that was previously reported (17), we observed a severe cholestasis with pruritus in the affected mother of one patient. Cholestasis associated with HNF1B mutation was previously reported (18) and is not unexpected given the known role of HNF1β in bile duct morphogenesis (19).
Both the type and the severity of the renal disease were variable in this series, and our data show that HNF1B mutations can be associated with very severe prenatal renal failure (in four probands, the pregnancy was terminated because of anamnios, and termination of previous pregnancy for severe renal disease with anamnios was reported in relatives in two additional families) as well as with normal renal function in adulthood. In our series, as in others, there was no obvious correlation between the type of mutation and the type and/or severity of renal disease. We observed both inter- and intrafamilial variability of the phenotype in patients who harbored the same mutation. The lack of genotype–phenotype correlation and the wide variability observed within a given family make the genetic counseling particularly difficult in these families.
Disclosures
None.
Acknowledgments
We thank the patients and their family for participation. We are grateful to the following physicians for contribution of material and clinical data from patients: Dr. Corinne Antoine (Assistance Publique–Hôpitaux de Paris, Hôpital Saint Louis, Paris, France); Dr. Danielle Bruno (Hôpital de la Timone, Marseille, France); Dr. Renato Demontis (Centre Hospitalier Laennec, Creil, France); Dr. Philippe Eckart (Centre Hospitalier Régional Universitaire de Caen, Caen France); Dr. Jérome Harambat (Centre Hospitalier Régional Universitaire de Bordeaux, Bordeaux, France); Dr. Anne Maisin (Assistance Publique–Hôpitaux de Paris, Hôpital Robert Debré, Paris, France); Dr. Jelena Martinovic (Assistance Publique–Hôpitaux de Paris, Hôpital Necker, Paris, France); Dr. Hubert Nivet (Centre Hospitalier Régional Universitaire de Tours, Tours, France); Dr. François Nobili (Centre Hospitalier Régional Universitaire de Besançon, Besançon, France); Dr. Jean Bernard Palcoux (Hôpital Hotel Dieu, Clermont Ferrand, France); Dr. Christine Piètrement (Centre Hospitalier Régional Universitaire de Reims, Reims, France); Dr. Natacha Raynaud (Centre Hospitalier Felix Guyon, La Réunion, France); and Dr. Christel Thauvin (Hôpital d'Enfants, Dijon, France). We are grateful to Nicolas Chassaing and Cathie Prouhèze (Service de Génétique, Hôpital Purpan, Toulouse, France) for help with molecular analysis. We thank Mario Tosi (INSERM U614, Rouen University Hospital, Rouen, France) for help with quantitative multiplex PCR amplification of short fluorescence fragments.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Horikawa Y, Iwasaki N, Hara M, Furuta H, Hinokio Y, Cockburn BN, Lindner T, Yamagata K, Ogata M, Tomonaga O, Kuroki H, Kasahara T, Iwamoto Y, Bell GI: Mutation in hepatocyte nuclear factor-1 beta gene (HNF1B) associated with MODY. Nat Genet 17: 384–385, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Zaffanello M, Brugnara M, Franchini M, Fanos V: HNF1B gene mutation leads to nephro-urological defects of unequal severity: an open question. Med Sci Monit 14: RA78–RA86, 2008 [PubMed] [Google Scholar]
- 3.Decramer S, Parant O, Beaufils S, Clauin S, Guillou C, Kessler S, Aziza J, Bandin F, Schanstra JP, Bellanné-Chantelot C: Anomalies of the HNF1B gene are the main cause of fetal bilateral hyperechogenic kidneys. J Am Soc Nephrol 18: 923–933, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Haumaitre C, Fabre M, Cormier S, Baumann C, Delezoide AL, Cereghini S: Severe pancreas hypoplasia and multicystic renal dysplasia in two human foetuses carrying novel HNF1beta/MODY5 mutations. Hum Mol Genet 15: 2363–2375, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Edghill EL, Bingham C, Ellard S, Hattersley AT: Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet 43: 84–90, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adalat S, Woolf AS, Johnstone KA, Wirsing A, Harries LA, Long DA, Hennekam RC, Ledermann SE, Rees L, van' Hoff W, Marks SD, Trompeter RS, Tullus K, Winyard PJ, Cansick J, Mushtaq I, Dhillon HK, Bingham C, Edghill EL, Shroff R, Stanescu H, Ryffel GU, Ellard S, Bockenhauer D: HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol 20: 1123–1131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbacci E, Reber M, Ott MO, Breillat C, Huetz F, Cereghini S: Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development 26: 4795–4805, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Reber M, Cereghini S: Variant hepatocyte nuclear factor 1 expression in the mouse genital tract. Mech Dev 100: 75–78, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Coffinier C, Barra J, Babinet C, Yaniv M: Expression of the vHNF1/HNF1beta homeoprotein gene during mouse organogenesis. Mech Dev 89: 211–213, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M: A transcriptional network in polycystic kidney disease. EMBO J 23: 1657–1668, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong Y, Ma Z, Patel V, Fischer E, Hiesberger T, Pontoglio M, Igarashi P: HNF-1beta regulates transcription of the PKD modifier gene Kif12. J Am Soc Nephrol 20: 41–47, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charbonnier F, Raux G, Wang Q: Detection of exon deletions and duplications of the mismatch repair genes in hereditary nonpolyposis colorectal cancer families using multiplex polymerase chain reaction of short fluorescent fragments. Cancer Res 60: 2760–2763, 2000 [PubMed] [Google Scholar]
- 13.Ulinski T, Lescure S, Beaufils S, Guigonis V, Decramer S, Morin D, Clauin S, Deschênes G, Bouissou F, Bensman A, Bellanné-Chantelot C: Renal phenotypes related to hepatocyte nuclear factor-1beta (HNF1B) mutations in a pediatric cohort. J Am Soc Nephrol 17: 497–503, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Sunyaev S, Ramensky V, Koch I, Lathe W, 3rd, Kondrashov AS, Bork P: Prediction of deleterious human alleles. Hum Mol Genet 10: 591–597, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Bingham C, Ellard S, van't Hoff WG, Simmonds HA, Marinaki AM, Badman MK, Winocour PH, Stride A, Lockwood CR, Nicholls AJ, Owen KR, Spyer G, Pearson ER, Hattersley AT: Atypical familial juvenile hyperuricaemic nephropathy associated with a hepatocyte nuclear factor-1beta gene mutation. Kidney Int 63: 1645–1651, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Kikuchi R, Kusuhara H, Hattori N, Kim I, Shiota K, Gonzalez FJ, Sugiyama Y: Regulation of tissue-specific expression of the human and mouse urate transporter 1 gene by hepatocyte nuclear factor 1 alpha/beta and DNA methylation. Mol Pharmacol 72: 1619–1625, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Bellanné-Chantelot C, Clauin S, Chauveau D, Collin P, Daumont M, Douillard C, Dubois-Laforgue D, Dusselier L, Gautier JF, Jadoul M, Laloi-Michelin M, Jacquesson L, Larger E, Louis J, Nicolino M, Subra JF, Wilhem JM, Young J, Velho G, Timsit J: Large genomic rearrangements in the hepatocyte nuclear factor-1beta (HNF1B) gene are the most frequent cause of maturity-onset diabetes of the young type 5. Diabetes 54: 3126–3132, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Beckers D, Bellanné-Chantelot C, Maes M: Neonatal cholestatic jaundice as the first symptom of a mutation in the hepatocyte nuclear factor-1beta gene (HNF-1beta). J Pediatr 150: 313–314, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Coffinier C, Gresh L, Fiette L, Tronche F, Schütz G, Babinet C, Pontoglio M, Yaniv M, Barra J: Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development 129: 1829–1838, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Bellanné-Chantelot C, Chauveau D, Gautier JF, Dubois-Laforgue D, Clauin S, Beaufils S, Wilhelm JM, Boitard C, Noël LH, Velho G, Timsit J: Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med 140: 510–517, 2004 [DOI] [PubMed] [Google Scholar]