Abstract
Background and objectives: Because thrombosis and stenosis are the most frequent causes of arteriovenous graft (AVG) failure, several externally supported grafts were designed to enhance incompressibility and kinking resistance to improve the patency. However, consensus on advantage of these modifications having still not been reached might relate to the previous limited patient numbers and small studies. This study analyzed the longevity of externally supported and nonsupported AVG on the basis of the nationwide database to understand whether the externally supported design could improve the duration of patency of AVG.
Design, setting, participants, & measurements: Adult incident patients (n = 4402) were retrospectively enrolled who had began hemodialysis between January 1, 2002 and December 31, 2005. All incident patients with AVG, before or after beginning regular hemodialysis, between January 1, 2000 and December 31, 2006 were identified. The Cox proportional hazard model was used to compare the longevity of externally supported and nonsupported AVG by controlling other covariates.
Results: There were 990 patients with externally supported AVG and 3412 patients with nonsupported AVG. The patency rates of externally supported AVG were 49.4%, 31.6%, and 20.2% at 1, 2 and 3 years, respectively; those of nonsupported AVG were 31.9%, 17.4%, and 10.8%. The survival of nonsupported AVG was significantly inferior to that of externally supported AVG.
Conclusions: Externally supported AVG are superior to nonsupported AVG for long-term patency. The incompressibility and kinking resistance of ringed grafts may shed light on a direction for the future development of dialysis graft design.
Vascular access remains one of the most costly sources of morbidity and hospitalization in dialysis patients (1,2). Although there is less morbidity and longer duration of patency of autogenous arteriovenous fistulas (AVF) (3,4), there are still many ESRD patients who need arteriovenous graft (AVG) for dialysis access because of their smaller-caliber vessels or technical problems with AVF. AVG thrombosis and stenosis have always been the most frequent causes of graft failure (5–7); however, treatment with dipyridamole plus aspirin for the patients with newly created AVG could not reduce the cumulative graft failure and the composite of graft failure (8). Therefore, several externally supported grafts with additional plastic rings (in spiral or circular form) encircling the outer surface of the graft to enhance incompressibility and kinking resistance help to improve the patency of AVG and reduce the number of costly procedural interventions to restore patency (9–14). However, consensus on the advantage of these externally supported grafts having still not been reached might relate to the previously limited patient numbers and small studies (12,13). In this study, we analyzed the longevity between externally supported and nonsupported AVG via the nationwide database of the National Health Insurance (NHI) in Taiwan to understand whether the externally supported AVG could improve the duration of primary unassisted graft patency of incident patients with regular hemodialysis (HD).
Materials and Methods
Healthcare System and Data Source
As in other industrialized nations, the demand in Taiwan for universal healthcare led to the creation of a NHI program in 1995. All medical institutions must submit standard claim documents for medical expenses on a computerized form that includes admission and discharge dates, patient identification number, sex, date of birth, and International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes for the admission and outpatient clinic visit. These codes, which consist of the principal and up to four secondary diagnoses for inpatient care and two secondary diagnoses for outpatient care, facilitate healthcare pattern analyses. The database also includes codes for treatment procedures and materials, such as AVF (code 69032C) and AVG (code 69032C plus the material codes for graft). In 2007, Taiwan's NHI program included 22 million enrollees, accounting for 99% of the population targeted to receive program benefits (15). Because the NHI program is available to all residents regardless of age or income, its scope is extensive, and detailed analyses of particular patterns in healthcare are possible. Thus, in this retrospective cohort study, all of the patients' data needed were retrieved from the database of the Bureau of NHI with scrambled personal identification numbers.
Patient Population
Incident patients (n = 4402) aged ≥18 years who began HD (procedure codes 58001C, 58019C to 58025C) between January 1, 2002 and December 31, 2005 were included in this study. These patients had received regular HD for >3 months (16). All incident patients with first payment and operation code for AVG before or after beginning regular HD (indicative of having undergone AVG) between January 1, 2000, and December 31, 2006 were identified to analyze the longevity of externally supported and nonexternally supported AVG.
Dependent Variable
Duration of primary unassisted graft patency was defined as the time from graft placement to the time of any intervention designed to maintain or re-establish patency or to access thrombosis. Patients who died or whose vascular access remained patent after December 31, 2006 were censored. Graft dysfunction was identified based on the diagnostic code for vascular thrombosis (ICD-9-CM 996.73, other complications due to renal dialysis device implant and graft) and operative procedures for vascular thrombotic occlusion (thrombectomy, procedure code 38.0; reconstruction of access, procedure code, 69032C; embolectomy, arterial, 69001B; embolectomy, arterial catheter, 69002B; thrombectomy, venous, 69003B) at a subsequent admission or outpatient visit. Graft infection and aneurysm were identified by disease code. (infection: ICD-9-CM 996.62, 996.1; aneurysm: ICD-9-CM 442.0). Graft related intervention (percutaneous transluminal angiography (PTA), revision or removal of AVG) were identify either by NHI' s procedure code or by disease code.
Independent Variable
Externally Supported and Nonsupported AVG.
Because the claim documents from all medical institutions include codes of special medical materials and the fee schedule for medical services, 990 incident patients who received AVG with material codes of graft with external support regardless of manufacturer were grouped as “externally supported graft.” The manufacturers and trade names of externally supported grafts are listed in Appendix 1. The other 3412 incident patients with AVG were in the nonsupported graft group (Table 1). The choice of type of the graft was totally at the discretion of the implanting surgeon at the time.
Table 1.
Patient characteristics for externally supported and nonsupported AVG
| Total Patients |
Externally Supported |
Nonsupported |
P | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| n | 4402 | 100.0 | 990 | 22.5 | 3412 | 77.5 | |
| Gender | 0.4731 | ||||||
| female | 2763 | 62.8 | 631 | 63.7 | 2132 | 62.5 | |
| male | 1639 | 37.2 | 359 | 36.3 | 1280 | 37.5 | |
| Age group (years) | 0.8523 | ||||||
| 18 to 44 | 249 | 5.7 | 51 | 5.2 | 198 | 5.8 | |
| 45 to 64 | 1517 | 34.5 | 348 | 35.2 | 1169 | 34.3 | |
| 65 to 74 | 1569 | 35.6 | 350 | 35.4 | 1219 | 35.7 | |
| ≥75 | 1067 | 24.2 | 241 | 24.3 | 826 | 24.2 | |
| Time of vascular access creation | 0.3376 | ||||||
| before initiating dialysis | 1399 | 31.8 | 327 | 33.0 | 1072 | 31.4 | |
| after initiating dialysis | 3003 | 68.2 | 663 | 67.0 | 2340 | 68.6 | |
| Comorbiditiesa | |||||||
| myocardial infarct | 119 | 2.7 | 26 | 2.6 | 93 | 2.7 | 0.8652 |
| congestive heart failure | 1354 | 30.8 | 281 | 28.4 | 1073 | 31.4 | 0.0659 |
| peripheral vascular disease | 285 | 6.5 | 77 | 7.8 | 208 | 6.1 | 0.0583 |
| cerebrovascular disease | 756 | 17.2 | 197 | 19.9 | 559 | 16.4 | 0.0098 |
| dementia | 101 | 2.3 | 29 | 2.9 | 72 | 2.1 | 0.1297 |
| chronic pulmonary disease | 721 | 16.4 | 159 | 16.1 | 562 | 16.5 | 0.7585 |
| rheumatologic disease | 105 | 2.4 | 19 | 1.9 | 86 | 2.5 | 0.2750 |
| ulcer disease | 1027 | 23.3 | 228 | 23.0 | 799 | 23.4 | 0.7999 |
| mild liver disease | 247 | 5.6 | 64 | 6.5 | 183 | 5.4 | 0.1850 |
| mild diabetes mellitus | 2191 | 49.8 | 500 | 50.5 | 1,691 | 49.6 | 0.6007 |
| severe diabetes mellitus | 2208 | 59.2 | 481 | 48.6 | 1727 | 50.6 | 0.2608 |
| hemiplegia or paraplegia | 93 | 2.1 | 15 | 1.5 | 78 | 2.3 | 0.1376 |
| malignancy | 411 | 9.3 | 104 | 10.5 | 307 | 9.0 | 0.1512 |
| moderate or severe liver disease | 48 | 1.1 | 9 | 0.9 | 39 | 1.1 | 0.5326 |
| metastatic solid tumor | 40 | 0.9 | 15 | 1.5 | 25 | 0.7 | 0.0224 |
| Antiplatelet and anticoagulant usageb (%) | |||||||
| aspirin | 6.6 ± 18.0 | 5.6 ± 15.5 | 6.9 ± 18.6 | 0.0685 | |||
| clopidogrel | 2.1 ± 10.5 | 1.8 ± 9.3 | 2.2 ± 10.8 | 0.4180 | |||
| warfarin | 0.3 ± 2.6 | 0.4 ± 4.2 | 0.2 ± 1.9 | 0.0332 | |||
No one had AIDS comorbidity.
Data indicate the usage of antiplatelet and anticoagulatnt medication during the patency days (%) (mean ± SD) defined by (days of anticoagulant use/patency days) × 100%.
Covariates.
Patient information included age (18 to 44, 45 to 64, 65 to 74, and ≥75 years), sex (male and female), time of vascular access creation (before or after HD initiation), and medical comorbidities in the 1 year before initiating HD. According to the Romano–Charlson comorbidity index method (17,18), 16 potentially important comorbidities (excluding renal disease) were identified from the primary and secondary diagnosis codes in claim data. Instead of using a single comorbidity index in this analysis, each comorbidity was identified as a separate variable in this study. Antiplatelet and anticoagulant medications (aspirin, clopidogresl, and warfarin) used during graft patency periods were identified by the codes specified for those drugs.
Statistical Analyses
Statistical software SAS 9.1.3 for Windows (SAS Institute Inc., Cary, NC) was used to perform data analysis and SPSS 15.0 (SPSS Inc., Chicago, IL) was used to draw the covariate-adjusted survival curve by graft type. The association between variables and graft types was examined by the χ2 test. The stepwise Cox proportional hazard model was used to show the long-term graft patency of externally supported and nonsupported graft after adjusting for sex, age, time of vascular access creation, comorbidities, and anticoagulants.
Results
Patient Characteristics
Of 4402 patients, 990 (22.5%) and 3412 (77.5%) patients' AVG were externally supported with and without rings, respectively. There were no significant differences in sex, age, or time of vascular access creation between these two groups (Table 1). Patients with cerebrovascular disease had significantly more cases with externally supported AVG. In contrast, fewer externally supported AVG were used in patients with metastatic solid tumor. The frequencies of other comorbidities showed no difference between these two groups.
Patency, Infection, Aneurysm Rate, Number of Interventions, and Survival Curve for Long-Term Patency
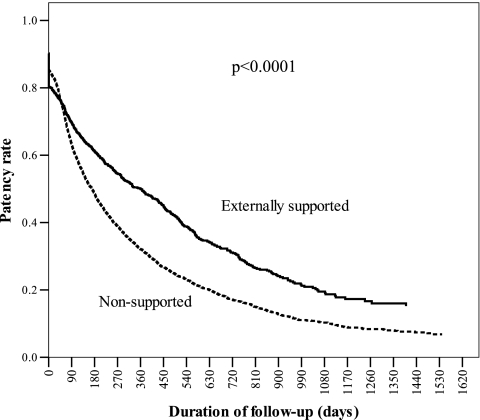
The patency rate of externally supported AVG was higher than that of nonsupported AVG at 1, 2, and 3 years. The patency rates of externally supported AVG were 49.4%, 31.6%, and 20.2% at 1, 2, and 3 years, respectively; those of nonsupported AVG were 31.8%, 17.4%, and 10.8%, respectively. The adjusted survival curve for graft patency showed externally supported AVG were significantly better than that for the nonsupported AVG (P < 0.0001, Figure 1). The infection rate at 1, 2, 3 years of externally supported AVG were significant lower than nonsupported AVG (P < 0.05). Although aneurysm rates of externally supported AVG were 0.5%, 0.6%, and 0.6% at 1, 2, and 3 years, respectively, and those of nonsupported AVG were 0.9%, 1.2%, and 1.6%, respectively, these were not significantly different in the two groups (P > 0.05). The number of percutaneous transluminal angioplasty procedures was significantly lower (P < 0.0001) in supported AVG in the first year. The number of revisions or removals of AVG were not significantly different in both groups (P > 0.05; Table 2).
Figure 1.
Graphs of the covariate-adjusted estimated survivorship function by graft type. The survival of externally supported AVG was significantly better than that for the nonsupported AVG (P < 0.0001). Covariates include sex, age, time of vascular access creation, and congestive heart failure disease.
Table 2.
Patency, infection, aneurysm, and intervention rate by graft type
| Period | Total Patients (n = 4402) |
Externally Supported (n = 990) |
Nonsupported (n = 3412) |
P | |||
|---|---|---|---|---|---|---|---|
| Casesa | % | Casesa | % | Casesa | % | ||
| Primary patency rate | |||||||
| 1 year | 4402 | 35.8 | 990 | 49.4 | 3412 | 31.9 | <0.0001 |
| 2 years | 1398 | 20.6 | 442 | 31.6 | 956 | 17.4 | <0.0001 |
| 3 years | 471 | 12.9 | 157 | 20.2 | 314 | 10.8 | <0.0001 |
| Infection rate | |||||||
| 1 year | 4402 | 17.3 | 990 | 13.6 | 3412 | 18.3 | 0.0006 |
| 2 years | 3630 | 26.9 | 853 | 22.8 | 2777 | 28.1 | 0.0009 |
| 3 years | 2488 | 33.7 | 588 | 28.7 | 1900 | 35.2 | 0.0003 |
| Aneurysm rate | |||||||
| 1 year | 4402 | 0.8 | 990 | 0.5 | 3412 | 0.9 | 0.2493 |
| 2 years | 4341 | 1.1 | 978 | 0.6 | 3363 | 1.2 | 0.1318 |
| 3 years | 3281 | 1.3 | 740 | 0.6 | 2541 | 1.6 | 0.0592 |
| Intervention in first year (times) | |||||||
| PTA | <0.0001 | ||||||
| 0 | 3748 | 85.1 | 887 | 89.6 | 2861 | 83.9 | |
| 1 | 443 | 10.1 | 82 | 8.3 | 361 | 10.6 | |
| 2 | 116 | 2.6 | 15 | 1.5 | 101 | 3.0 | |
| ≥ 3 | 95 | 2.2 | 6 | 0.6 | 89 | 2.6 | |
| Revision of AVG | 0.0770 | ||||||
| 0 | 4095 | 93.0 | 936 | 94.6 | 3159 | 92.6 | |
| 1 | 268 | 6.1 | 49 | 5.0 | 219 | 6.4 | |
| ≥ 2 | 39 | 0.9 | 5 | 0.5 | 34 | 1.0 | |
| Removal of AVG | 0.3780 | ||||||
| 0 | 4286 | 97.4 | 960 | 97.0 | 3326 | 97.5 | |
| ≥1 | 116 | 2.6 | 30 | 3.0 | 86 | 2.5 | |
PTA, percutaneous transluminal angioplasty.
The case number and percentage in each year are calculated using the life table analysis.
Bivariate and Multivariate Cox Regressions for Graft Patency
Bivariate Cox regression demonstrated that nonsupported AVG had higher a hazard ratio (HR) of thrombosis [HR 1.45, 95% confidence interval (CI) 1.34 to 1.58; Table 3]. Patients >75 years old had a higher HR than those 18 to 44 years old (HR 1.18, 95% CI 1.01 to 1.37). Graft creation before initiating dialysis had a lower hazard rate than graft creation after dialysis (HR 0.76, 95% CI 0.71 to 0.82). Congestive heart failure and chronic pulmonary disease instead of cerebrovascular disease and metastatic solid tumor were the 2 of 15 comorbid conditions that affected graft patency.
Table 3.
Stepwise Cox regression analysis of patency for externally supported AVG and other confounding factors (n = 4402)
| Variable | Crude |
Adjusted |
|||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Graft type (base = externally supported AVG) | 1.45 | 1.34 to 1.58 | <0.0001 | 1.46 | 1.34 to 1.59 | <0.0001 | |
| Gender (base = female) | 1.11 | 1.03 to 1.19 | 0.0045 | 1.12 | 1.04 to 1.20 | 0.0021 | |
| Age (base = 18 to 44 years) | |||||||
| 45 to 64 | 0.93 | 0.80 to 1.08 | 0.3634 | 0.96 | 0.82 to 1.12 | 0.5875 | |
| 65 to 74 | 1.07 | 0.92 to 1.25 | 0.3522 | 1.12 | 0.97 to 1.31 | 0.1307 | |
| ≥75 | 1.18 | 1.01 to 1.37 | 0.0421 | 1.21 | 1.04 to 1.41 | 0.0168 | |
| Time of vascular access creation (base = after initiating dialysis) | |||||||
| before initiating dialysis | 0.76 | 0.71 to 0.82 | <0.0001 | 0.776 | 0.71 to 0.82 | <0.0001 | |
| Comorbidities | |||||||
| myocardial infarct (base = no) | 1.03 | 0.84 to 1.26 | 0.8016 | ||||
| congestive heart failure (base = no) | 1.13 | 1.06 to 1.22 | 0.0006 | 1.15 | 1.07 to 1.24 | 0.0001 | |
| peripheral vascular disease (base = no) | 1.03 | 0.90 to 1.18 | 0.7033 | ||||
| cerebrovascular disease (base = no) | 1.00 | 0.92 to 1.10 | 0.9547 | ||||
| dementia (base = no) | 1.00 | 0.80 to 1.27 | 0.9698 | ||||
| chronic pulmonary disease (base = no) | 1.10 | 1.01 to 1.21 | 0.0330 | ||||
| rheumatologic disease (base = no) | 0.93 | 0.74 to 1.15 | 0.4894 | ||||
| ulcer disease (base = no) | 1.05 | 0.97 to 1.14 | 0.2205 | ||||
| mild liver disease (base = no) | 1.04 | 0.90 to 1.21 | 0.5754 | ||||
| mild diabetes mellitus (base = no) | 1.01 | 0.95 to 1.08 | 0.3608 | ||||
| severe diabetes mellitus (base = no) | 1.03 | 0.97 to 1.10 | 0.7417 | ||||
| hemiplegia or paraplegia (base = no) | 1.04 | 0.83 to 1.31 | 0.7353 | ||||
| malignancy (base = no) | 1.05 | 0.94 to 1.18 | 0.3931 | ||||
| moderate or severe liver disease (base = no) | 0.81 | 0.57 to 1.15 | 0.2319 | ||||
| metastatic solid tumor (base = no) | 1.17 | 0.80 to 1.71 | 0.4076 | ||||
| Antiplatelet and anticoagulant medication (%) | |||||||
| aspirin | 0.99 | 0.99 to 0.99 | <0.0001 | 0.99 | 0.99 to 1.00 | <0.0001 | |
| clopidogrel | 0.99 | 0.99 to 1.00 | 0.0542 | 1.00 | 0.99 to 1.00 | 0.0220 | |
| warfarin | 1.00 | 0.99 to 1.02 | 0.6367 | ||||
After stepwise selection strategy, graft type, gender, age, time of vascular access creation, congestive heart failure, and anticoagulant usage were selected in a multivariate Cox proportional hazard model. The HR of graft dysfunction for nonsupported graft was 1.46 (95% CI 1.34 to 1.59) versus externally supported graft after adjustment for the confounding factors (Table 3).
Discussion
In this study, there were only 990 of 4402 (22.5%) incident patients with externally supported AVG (Table 1). The low implantation rate of externally supported AVG might relate to the possible difficulties in graft puncture and previous limited small studies supporting the longer patency (11–14).
Although there was a significantly higher proportion of cerebrovascular disease in the externally supported AVG group than in the nonsupported AVG group, and a lesser proportion of metastatic solid tumor in the externally supported AVG group than in the nonsupported AVG group (Table 1), these two comorbid conditions were NS independent predictors for improved AVG survival after adjustment for other confounding factors (Table 3).
The feasibility of externally supported grafts for dialysis access has been demonstrated in this study of a nationwide database. The applications of externally supported grafts did not increase the infection rate and graft aneurysm occurrence. Conversely, the infection rate in the externally supported graft group was significantly lower than in the nonsupported group (Table 2). The externally supported grafts may possibly be helpful to hemostasis after dialysis sessions, which in turn decreases puncture-site-related infections and aneurysm formation. Moreover, the externally supported graft may be more resistant to excessive compression and further development of puncture-site stenoses. This may explain the fewer interventions (balloon angioplasties) in externally supported grafts than nonsupported grafts in our investigation. After variables were controlled, the duration patency was significantly longer for patients with externally supported AVG.
The longer duration patency of externally supported AVG in incident HD patients further supported the previous literature on the superiority of externally supported graft to nonsupported graft in peripheral artery reconstruction procedures such as femorofemoral, femoropopliteal, and axillofemoral bypass (19). Therefore, the significantly longer duration of externally supported AVG patency compared with nonsupported AVG in this study might further support that mishandling or excessive compression of dialysis grafts by patients may be one of the important risk factors leading to graft thrombosis and failure. Externally supported AVG could reduce the incidence of dialysis access failure caused by inappropriate external compression after dialysis treatment (Table 2 and Figure 1). However, the supported graft still could not solve the major problem of AVG in general—intimal hyperplasia—which presumably is no different between these two AVG formats. Thus the external rings do not overcome the major problem of AVG.
In addition to patient education to prevent excessive compression of access, usage of externally supported AVG may somehow prevent compressive occlusion and enhance the longevity of AVG. Although the causes of AVG failure are so versatile and a perfect AVG is still lacking, externally supported AVG may have some benefits.
In this study, the shorter patency of AVG in the patients with congestive heart failure might relate to its substantial morbidity and mortality in ESRD subjects treated by HD (20–23). The finding of lower HR of AVG creation before initiating dialysis also agreed with that of our previous report (4).
In conclusion, the feasibility and superiority of externally supported AVG in comparison to nonsupported AVG for long-term patency in dialysis access has been shown by this study. The incompressibility and kinking resistance of ringed grafts offer a better long-term patency and shed light on one of the directions in the future development of dialysis graft design.
This study has several limitations that should be noted. First, there was no definitive criterion for choosing externally supported or nonsupported AVG for dialysis access in this study as in previous literature. Second, the choosing of AVG types (supported or nonsupported) may not have been randomized. Many surgeons might have tended to implant externally supported grafts in patients who were sicker because of concern about unintended compression of the AVG by patients themselves. Therefore, the effect of externally supported graft might be underestimated. Finally, because our analysis depended on claims data, this could not explain why the AVG malfunctioned.
Notwithstanding these possible limitations, this study has several strengths. First, this is the first population-based study using a large sample population to provide important information on the relative longevity between externally supported AVG and nonsupported AVG. Second, claims data from universal health coverage in Taiwan allow identification of samples free from selection bias and of sufficient size to offset the aforementioned limitations and may document outcomes in reality. Third, insurance records can be used to unambiguously analyze the timing of AVG placement and failure. Finally, in our large patient population of incident HD patients, we found a strong graded association between externally supported AVG and patency.
Disclosures
None.
Acknowledgments
This study was supported by a grant from the National Science Council and Taipei Veterans General Hospital, Republic of China (NSC 96-2314-B-010-028-MY2, NSC 96-2314-B-075-020-MY3, NSC 98-2314-B-010-007-MY2, and VGH98ER2-005).
Appendix
Appendix 1.
Manufacturers and trade names of externally supported grafts used in Taiwan
| Manufacturer | Location | Trade Name |
|---|---|---|
| Atrium | Manchester, United Kingdom | Center helix support straight graft standard wall; Helix support straight graft thin wall; Helix support straight graft standard wall |
| Bard (Impra) | Covington, Georgia, United States | "BARD" distaflo bypass grafts with flex small beading, Center flex graft, End flex graft, Flex thin wall small beading graft, Flex thin wall graft, Carboflo graft (centerflex), Venaflo graft (center flex), Flex small beading graft Flex graft, Carboflo graft, flex thin wall small beading, Carboflo graft (straight flex), Carboflo graft, flex thin wall small beading, Carboflo graft, straight flex, Carboflo graft, flex thin wall small beading, Carboflo graft, straight flex, Carboflo graft, short tapered center flex, Flex tapered small beading graft, Carboflo graft, flex thin wall tapered small beading, Carboflo graft, stepped center flex |
| Baxter | Deerfield, Illinois, United States | Spiral externally supported straight graft, Spiral externally supported thin straight graft, center spiral straight ePTFE graft, center spiral stepped straight ePTFE graft |
| Boston Scientific | Natick, Massachusetts, United States | Exxcel ePTFE graft vascular graft externally supported, Exxcel soft ePTFE graft straight externally supported, Exxcel soft ePTFE graft straight centrally supported, Exxcel soft ePTFE graft thin wall straight externally supported |
| Edwards | Bolingbrook, Illinois, United States | Spiral externally supported straight graft, Center spiral straight ePTFE graft, Center spiral stepped straight ePTFE graft |
| Gore | Flagstaff, Arizona, United States | Ringed graft, Ringed thin walled graft, Removable ringed thin walled graft, Tapered graft with removal ringed graft |
| Meadox Medicals | Oakland, New Jersey, United States | Exxcel ePTFE vascular graft externally supported, Cooley low porosity graft, Exxcel ePTFE vascular graft externally supported (thin wall) |
| Vascutek | Renfrewshire, Scotland | PTFE thin wall full support straight graft, PTFE full support straight graft, PTFE central support straight graft, PTFE end support straight graft, PTFE short taper support graft, PTFE central support straight graft, PTFE stepped taper central support graft, Externally reinforced angle, gelsoft, Externally reinforced angle, gelsoft |
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Megerman J, Levin NW, Ing TS, Dubrow AJ, Prosl FR: Development of a new approach to vascular access. Artif Organs 23: 10–14, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Arora P, Kausz AT, Obrador GT, Ruthazer R, Khan S, Jenuleson CS, Meyer KB, Pereira BJ: Hospital utilization among chronic dialysis patients. J Am Soc Nephrol 11: 740–746, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Besarab A, Detroit MI: Clinical practice guidelines for vascular access, update 2006. Am J Kidney Dis 48: S176–S247, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Ng YY, Wu SC, Hung YN, Ko PJ: Effect of demographic characteristics and timing of vascular access maturation on patency in Chinese incident hemodialysis patients. Nephrol Dial Transpl 24: 3447–3453, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Liu YH, Hung YN, Hsieh HC, Ko PJ: Surgical thrombectomy for thrombosed dialysis grafts: Comparison of adjunctive treatments. World J Surg 32: 241–245, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Maya ID, Weatherspoon J, Young CJ, Barker J, Allon M: Increased risk of infection associated with polyurethane dialysis grafts. Semin Dialysis 20: 616–620, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Delorme JM, Guidoin R, Canizales S, Charara J, How T, Marois Y, Batt M, Hallade P, Ricci M, Picetti C, Contard S: Vascular access for hemodialysis: Pathologic features of surgically excised ePTFE grafts. Ann Vasc Surg 6: 517–524, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, Dember LM, Himmelfarb J, Gassman JJ, Greene T, Radeva MK, Davidson IJ, Ikizler TA, Braden GL, Fenves AZ, Kaufman JS, Cotton JR, Jr., Martin KJ, McNeil JW, Rahman A, Lawson JH, Whiting JF, Hu B, Meyers CM, Kusek JW, Feldman HI: Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med 360: 2191–2201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scott EC, Glickman MH: Conduits for hemodialysis access. Semin Vasc Surg 20: 158–163, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Berardinelli L: Grafts and graft materials as vascular substitutes for hemodialysis access construction. Eur J Vasc Endovasc 32: 203–211, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Jimenez Almonacid P, Criado Pallares E, Quintans Rodriguez A, Sanabria Valdes J, Rueda Orgaz JA, Polo JR: Comparative study of use of Diastat versus standard wall PTFE grafts in upper arm hemodialysis access. Ann Vasc Surg 14: 659–662, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Lenz BJ, Veldenz HC, Dennis JW, Khansarinia S, Atteberry LR, Goldman MH: A three-year follow-up on standard versus thin wall ePTFE grafts for hemodialysis. Discussion. J Vasc Surg 28: 464–470, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Kao CL, Chang JP: Fully ringed polytetrafluoroethylene graft for vascular access in hemodialysis. Asian Soc Cardio Surg 11: 171–173, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Kao CL, Chang JP: The reverse upper arm curved graft with ringed PTFE graft as an alternative vascular access procedure for hemodialysis. J Cardiovasc Surg 45: 55–57, 2004 [PubMed] [Google Scholar]
- 15.National Health Insurance Annual Statistical Report Taipei, Taiwan, Bureau of National Health Insurance, 2007 [Google Scholar]
- 16.Wu SC, Haung LG, Lei HL, Ng YY: Definition and analysis the patients with chronic dialysis from the database of National Health Insurance. Taiwan J Pub Health 23: 419–427, 2004 [Google Scholar]
- 17.Romano PS, Roos LL, Jollis JG: Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol 46: 1075, 1993 [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Mingoli A, Sapienza P, Feldhaus RJ, di Marzo L, Burchi C, Cavallaro A: Femorofemoral bypass grafts: Factors influencing long-term patency rate and outcome. Surgery 129: 451–458, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Trespalacios FC, Taylor AJ, Agodoa LY, Bakris GL, Abbott KC: Heart failure as a cause for hospitalization in chronic dialysis patients. Am J Kidney Dis 41: 1267–1277, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Stack AG, Bloembergen WE: A cross-sectional study of the prevalence and clinical correlates of congestive heart failure among incident US dialysis patients. Am J Kidney Dis 38: 992–1000, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Collins AJ: Cardiovascular mortality in end-stage renal disease. Am J Med Sci 325: 163–167, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS: Congestive heart failure in dialysis patients: Prevalence, incidence, prognosis and risk factors. Kidney Int 47: 884–890, 1995 [DOI] [PubMed] [Google Scholar]