Abstract
Background and objectives: Clinical trials of the intensity of renal replacement therapy (RRT) for people with acute kidney injury (AKI) have produced conflicting results. A systematic review and meta-analysis was undertaken to assess the effect of different intensities of RRT on all-cause mortality and renal recovery in AKI patients.
Design, setting, participants, & measurements: MEDLINE, EMBASE, and the Cochrane Library database were systematically searched for trials published between 1950 and 2009. Inclusion criteria were completed, prospective, adult-population, randomized controlled studies. Relative risks (RRs) and 95% confidence intervals (CIs) were calculated. Summary estimates of RR were obtained using a random effects model. Heterogeneity, metaregression, publication bias, and subgroup analyses were conducted.
Results: Eight trials were identified that provided data on 3841 patients and 1808 deaths. More intense RRT (35 to 48 ml/kg per h or equivalent) had no overall effect on the risk of death (RR 0.89, 95% CI 0.76 to 1.04, P = 0.143) or recovery of renal function (RR 1.12, 95% CI 0.95 to 1.31, P = 0.181) compared with less-intensive regimens (20 to 25 ml/kg per h or equivalent). Significant heterogeneity was identified with contributing factors including publication year (P = 0.004) and Jadad score (P = 0.048).
Conclusions: Within the intensity ranges studied, higher intensity RRT does not reduce mortality rates or improve renal recovery among patients with AKI. The results do not negate the importance of RRT intensity in the treatment of AKI patients but rather reinforce the need to better understand the effects of treatment modalities, doses, and timing in this varied, high-risk population.
Acute kidney injury (AKI) is an increasingly common condition associated with significant morbidity and mortality. In its most severe form, necessitating renal replacement therapy (RRT), it is associated with up to 60% in-hospital mortality (1). Two large, multicenter randomized controlled trials assessing the effect of different intensities of RRT on mortality in severe AKI have been recently published (2,3). Neither study demonstrated a survival benefit for more intensive compared with less intensive RRT, nor a significant difference in rates of recovery of renal function. This contrasts the findings of earlier studies that reported a significant mortality benefit associated with intensive treatment (4,5). Thus we undertook a systematic review and meta-analysis to assess the totality of the currently available evidence regarding the effect of different intensities of RRT on mortality and renal recovery in people with AKI in the intensive care unit (ICU).
Materials and Methods
Data Sources and Searches
We performed a systematic review according to the PRISMA guidelines for the conduct of meta-analyses of intervention studies (6). Relevant studies were identified by searching the following data sources: MEDLINE via Ovid (from 1950 through July 2009), EMBASE (from 1966 through July 2009), and the Cochrane Library database (Cochrane Central Register of Controlled Trials; no date restriction). Searches were conducted using relevant text words and medical subject headings that included all spellings of hemodialysis, hemofiltration, hemodiafiltration, renal insufficiency, kidney failure, and mortality (see Appendix). The search was limited to randomized controlled trials without language restriction. Reference lists from identified trials and review articles were manually scanned to identify any other relevant studies. Trials of dialysis dose among end-stage kidney disease patients were excluded. The clinicaltrials.gov website was also searched for randomized trials that were registered as completed but not yet published.
Study Selection
The literature search, data extraction, and quality assessment were conducted independently by two authors using a standardized approach (M.J. and H.J.L.H.). All completed randomized controlled trials assessing the effects of different intensities of RRT in patients with AKI were eligible for inclusion. Outcomes analyzed were “all-cause mortality” and “renal recovery”, with the latter defined as patient survival without the need for ongoing dialysis. In calculating risk ratios, the total number of patients in each group was used as the denominator.
Data Extraction and Quality Assessment
Data extracted included patient characteristics (age, gender, primary cause of AKI, weight, and serum creatinine), follow-up duration, inclusion and exclusion criteria, outcome events, and dose of RRT. The Jadad score was used to quantify study quality (7) judged by the proper conduct of randomization, concealment of treatment allocation, similarity of treatment groups at baseline, the provision of a description of the eligibility criteria, completeness of follow-up, and use of intention-to-treat analysis (8). A third reviewer (V.P.) adjudicated any disagreement in abstracted data.
Data Synthesis and Analysis
In studies assessing the effects of dose of continuous renal replacement therapy (CRRT), lower intensity (usually a prescribed dose of 20 to 25 ml/kg per h) and higher intensity (a prescribed dose of 35 to 48 ml/kg per h) were defined as published in the original publications. In studies assessing the effects of dialysis dose using intermittent hemodialysis (IHD), higher and lower intensity were also defined as published (Table 1).
Table 1.
Characteristics of studies reporting the effects of RRT dose in patients with AKI
| Study | Inclusion Criteria | Treatment Group 1 | Treatment Group 2 | Control | Design | Number of Patients | Mean Age (years) | Male, n (%) | Primary Cause of AKI | Mean Creatinine (mg/dl) | Number of Mortality Events |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ronco et al. (4) | AKI: abnormal concentration of serum BUN and creatinine; oliguria (despite fluid resuscitation and diuretics) | CVVH (45 ml/kg per h) | CVVH (35 ml/kg per h) | CVVH (20 ml/kg per h) | Randomized single-center | 425 | 61 | 238 (56%) | Surgery (89%) | 3.6 | 205 (48%) |
| Bouman et al. (11) | 18 to 90 years (inclusive); AKI: oliguria (despite fluid resuscitation); hemodynamic optimization with dopamine or dobutamine; use of phosphodiesterase inhibitors or norepinephrine, use of diuretics; creatinine clearance of <20 ml/min; mechanical ventilation | CVVH early-high-volume (48 ml/kg per h) | CVVH early-low-volume (25 ml/kg per h) | CVVH late-low-volume (25 ml/kg per h) | Randomized two-center | 106 | 68 | 63 (59%) | Cardio-surgery (58%) | NR | 29 (27%) |
| Schiffl et al. (5) | ≥18 years; AKI severe acute tubular necrosis caused by recent ischemic or nephrotoxic injury | Daily IHD (weekly Kt/V = 8.4) | – | Alternate-daily IHD (weekly Kt/V = 4.2) | Quasi-randomized single-center | 160 | 60 | 80 (55%)a | Hypotension (54%) | 4.7 | 59 (37%) |
| Saudan et al. (12) | AKI: oliguria (despite fluid resuscitation and diuretics) and/or azotemia with urine output <1500 ml/12 h | CVVHDF (42 ml/kg per h) | – | CVVH (25 ml/kg per h) | Randomized single-center | 206 | 63 | 125 (61%) | Sepsis (60%) | 5.6 | 110 (53%) |
| Tolwani et al. (13) | AKI: volume overload/oliguria (despite diuretics/fluid resuscitation); anuria; azotemia; hyperkalemia and/or increase in serum creatinine | CVVHDF (35 ml/kg per h) | – | CVVHDF (20 ml/kg per h) | Randomized single-center | 200 | 60 | 116 (58%) | Sepsis (54%) | 4.25 | 95 (48%) |
| ATN study (2) | ≥18 years; AKI: severe acute tubular necrosis and requiring RRT; failure of ≥1 nonrenal organ systems (defined by SOFA) or sepsis | IHD/CVVHDF or SLED 6×/wk (36.2 ml/kg per h) | – | IHD/CVVHDF or SLED 3×/wk (21.5 ml/kg per h) | Randomized multicenter | 1124 | 60 | 793 (71%) | Ischemia (81%) | NR | 591 (53%) |
| Faulhaber-Walter et al. (14) | Non-post-renal AKI with RRT dependence: >30% loss of kidney function; oliguria, anuria, hyperkalemia, or severe acidosis | Dose to maintain plasma urea levels 120 to 150 mg/dl (IHD) | – | Dose to maintain plasma urea levels <90 mg/dl (IHD) | Randomized single-center | 156 | 51 | 99 (64%) | SIRS/sepsis (72%)b | 3.1 | 65 (42%) |
| RENAL (3) | Non-post-renal AKI with RRT dependence: >30% loss of kidney function; oliguria, anuria, hyperkalemia, or severe acidosis | CVVHDF (40 ml/kg per h) | – | CVVHDF (25 ml/kg per h) | Randomized multicenter | 1464 | 65 | 946 (65%) | Sepsis (48%) | 3.8 | 654 (45%) |
BUN, blood urea nitrogen; CVVH, continuous venovenous hemofiltration; CVVHDF, continuous venovenous hemodiafiltration; SLED, sustained low-efficiency dialysis; NR, not reported.
Fourteen patients withdrew from the study; study has only reported baseline patient characteristics on 146 patients.
Study has reported “comorbidity at RRT initiation”; value represents the highest comorbidity; all dose values indicate prescribed doses.
Individual study relative risks (RRs) and 95% confidence intervals (CIs) were calculated before data pooling. Summary estimates of RR ratios were obtained using a random effects model. The percentage of variability across studies attributable to heterogeneity beyond chance was estimated using the I2 statistic (9). Potential publication bias was assessed using the Egger test and represented graphically using Begg funnel plots of the natural log of the RR versus its standard error (10). Potential heterogeneity in estimates of treatment effect was explored using univariate metaregression (9). In addition, we investigated possible sources of heterogeneity by comparing summary results obtained from subsets of studies grouped by number of patients, number of mortality events, publication year, weight of patients in studies using CRRT, baseline serum creatinine, proportion of patients with sepsis, number of centers involved in the studies, and study quality. To further investigate the effect of body weight at baseline on the treatment effect, we performed additional individual patient data analyses using data from the Acute Renal Failure Trial Network (ATN) trial and the Randomized Evaluation of Normal versus Augmented Level RRT (RENAL) trial. The ATN and RENAL trials are the two largest trials to date assessing the effects of dialysis intensity in patients with AKI. A two-sided P value <0.05 was considered statistically significant for all analyses. All statistical analyses were performed with STATA, version 9.2 (Stata, College Station, TX).
Results
Literature Search and Characteristics of Studies
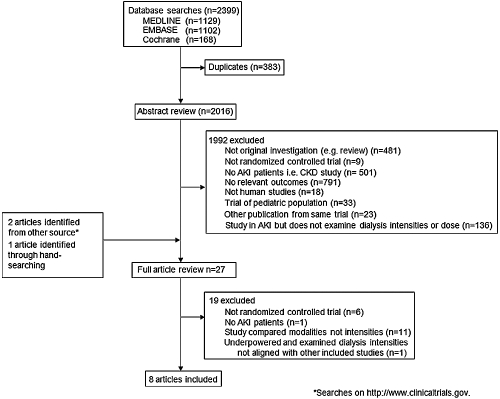
The literature search yielded 2399 articles, of which 27 were reviewed in full text (Figure 1). Of these, seven randomized clinical trials and one quasi-randomized trial met the inclusion criteria. These trials included a total of 3841 patients among whom there were 1808 deaths over a median follow-up of 29 days (range: 14 to 90 days) (2–5,11–14). Most other studies identified by our search were randomized clinical trials conducted in patients with end-stage kidney disease or that examined the effects of different RRT modalities rather than different intensities.
Figure 1.
Diagram showing the identification process for eligible studies.
Table 1 summarizes the characteristics of the included studies. They were reported between 2000 and 2009, with a sample size that ranged from 106 to 1464 participants and mortality rates that varied from 27% to 53%. Five single-center (4,5,12–14) and three multicenter studies (2,3,11) were identified. Seven studies assessed the effects of two RRT intensities (2,3,5,11–14), whereas one assessed the effects of three RRT intensities (4). In the study by Ronco et al. (4), the intermediate dose was similar to the more intensive RRT doses in other studies. For the purpose of analysis, we therefore combined the intermediate and higher treatment arms of this study. In the study by Bouman et al. (11), two arms received the same less-intensive RRT dose but differed only in the timing of initiation of RRT. For the purpose of analysis, we combined these two treatment groups. Overall, the prescribed dose of RRT in the lower intensity arms of studies using CRRT ranged between 20 and 25 ml/kg per h (2–4,11–13). The higher intensity arms ranged between 35 and 48 ml/kg per h. In the three studies utilizing IHD (2,5,14), higher intensity of therapy was achieved by increasing the frequency of treatment. The mean age of the study participants ranged between 51 and 68 years and the proportion of men ranged from 55% to 71%. All but two studies provided information regarding pretreatment mean serum creatinine concentration (range: 3.1 to 5.6 mg/dl). Four studies reported sepsis to be the primary cause of AKI (3,12–14).
Quality assessment revealed that six studies described concealment of allocation (Supplementary Table 1). Earlier studies (4,11) lacked detailed information regarding the process of randomization. All trials were open-label because double blinding is not feasible for these types of studies. Therefore, the maximum Jadad score that could be achieved was 3. Five trials had a Jadad score of 3, two trials scored 2, and one trial scored 0.
Effect of Different RRT Intensities on Mortality
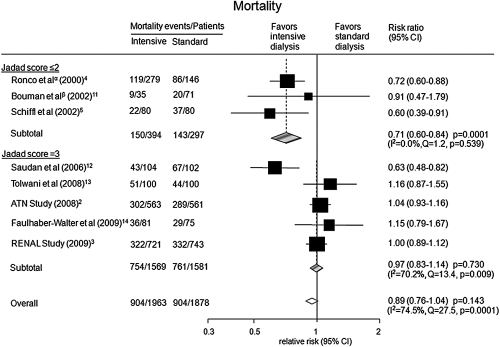
Overall, higher intensity RRT did not reduce the risk of death (RR 0.89; 95% CI: 0.76 to 1.04, P = 0.143) compared with lower intensity regimes (Figure 2). Formal statistical testing did not identify the presence of publication bias (Egger's test P = 0.113; Supplementary Figure 1).
Figure 2.
Risk of mortality for more intensive versus less intensive RRT regimes stratified by Jadad scores. αTwo treatment groups (35 and 45 ml/kg per h) were combined for the purpose of analysis. βTwo treatment groups (early-low volume and late-low volume) were combined for the purpose of analysis.
Effect of Sources of Heterogeneity
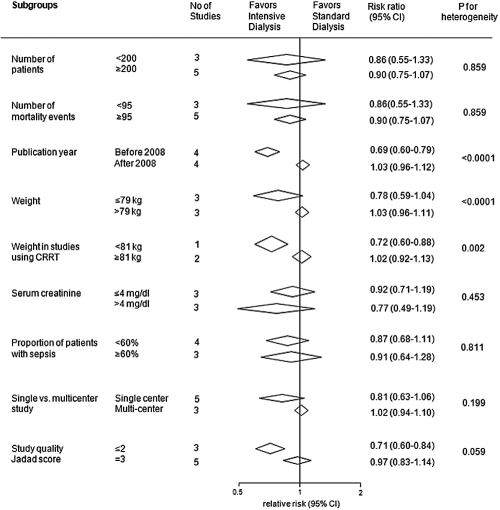
There was evidence of significant heterogeneity in the magnitude of the effect across the included studies (I2 = 74.5%; P value for heterogeneity = 0.0001; Figure 2). Univariate metaregression analysis suggested the heterogeneity was explained by year of publication (P = 0.004), Jadad score (P = 0.048), and the mean weight of study participants (P = 0.0001). Similarly, in subgroup analyses when studies were divided into groups defined by publication year (P < 0.0001) and mean weight (P < 0.0001), evidence of heterogeneity of effect was identified (Figure 3). The effect of the severity of disease at baseline was considered using the proportion of patients with sepsis and the APACHE scores. Neither parameter was found to be a significant source of heterogeneity (P = 0.157 for sepsis; P = 0.889 for APACHE score).
Figure 3.
Subgroup analyses for the effects of RRT intensity on mortality.
Effect of Higher Intensity RRT on Renal Recovery
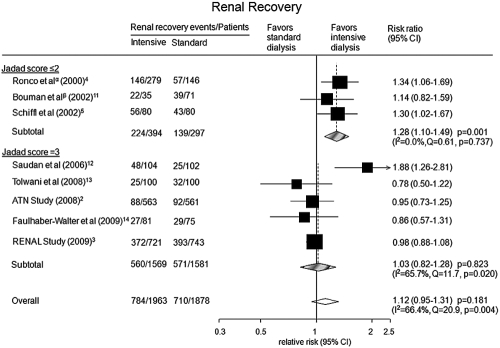
All eight studies reported information on renal recovery among survivors. Higher intensity RRT did not increase the probability of renal recovery (RR 1.12; 95% CI: 0.95 to 1.31, P = 0.181; Figure 4). However, there was evidence for heterogeneity among studies (I2 = 66.4%; P value for heterogeneity = 0.004). Univariate metaregression analysis suggested study size (P = 0.006), publication year (P = 0.001), proportion of males (P = 0.003), and mean weight of trial participants (P = 0.001) as significant sources of heterogeneity. Additional analyses assessing the proportion of survivors still requiring dialysis also showed that higher intensity RRT was not associated with an increased probability of renal recovery (RR 1.05; 95% CI: 0.90 to 1.22, P = 0.551; Supplementary Figure 2).
Figure 4.
Probability of renal recovery for more intensive versus less intensive RRT regimes. αTwo treatment groups (35 and 45 ml/kg per h) were combined for the purpose of analysis. βTwo treatment groups (early-low volume and late-low volume) were combined for the purpose of analysis.
Exploration of Weight as a Source of Heterogeneity
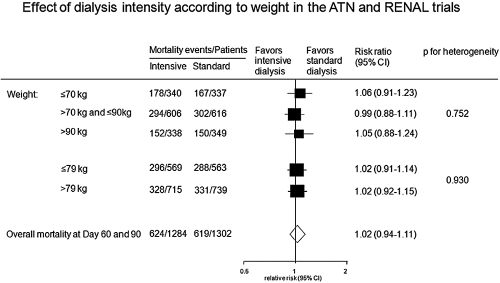
Baseline mean body weight was identified as a source of heterogeneity in the magnitude of the effect across the included eight studies, with those studies including participants of lower weight demonstrating reduced mortality with intensive RRT. We sought to explore this finding further using individual patient data from the ATN and RENAL trials. Analyses showed no heterogeneity in the treatment effect of allocation to more or less intensive RRT when individuals were classified according to the median weight of the meta-analysis (79 kg) or according to different weight categories (Figure 5).
Figure 5.
Risk of mortality for more intensive versus less intensive RRT regimes according to weight in the ATN and RENAL trials.
Discussion
The incidence of AKI is believed to be rising, with estimates of population incidence of between 1800 and 2800 per million (15–17). Although studies assessing the effects of RRT intensity on mortality in patients with AKI have been conducted since 1975 (18), there remains a lack of consensus regarding the optimal intensity of therapy to address the high mortality rate. In this systematic review and meta-analysis of randomized controlled trials, we demonstrated that within the range of dose of RRT studied, higher intensity therapy did not reduce mortality or increase the rate of renal recovery compared with lower intensity therapy.
Trials published before 2008 (4,5,11,12) clearly favored more intensive therapy, whereas trials published after 2008 (2,3,13,14) showed more intensive RRT to be no more effective than less intensive regimes. The source of this difference is not certain and is likely to reflect a range of study parameters rather than a single factor. Potentially important parameters may include differences in patient characteristics, the number of centers involved in the study, and the actual RRT dose delivered. An important possible explanation for the separation of effect is the quality of reporting. Adequate reporting of quality indicators, including the method of randomization and allocation concealment, is a validated measure of the quality of trial conduct and is strongly related to the internal validity of randomized controlled trials (19,20). Detailed assessment of the trials included in this analysis revealed that earlier positive studies tended to be single-center and to lack detailed descriptions of randomization and allocation concealment methodology. These studies therefore had comparatively lower Jadad scores.
One of the challenges in examining the evidence around dialysis intensity in patients with AKI is the numerous treatment modalities and the comparability of delivered dose. This is of particular concern when comparing IHD to CRRT modalities. Unfortunately, there was difficulty in distinguishing the exact number of patients receiving IHD or CRRT in those studies using a combination of both therapies (2,3,12). In addition, in many of the studies evaluating CRRT intensity, in which patients remained dialysis-dependent after ICU discharge, many are likely to have transitioned to IHD without regard to the original study-assigned dose of CRRT. In view of this, we chose to perform the analysis using data from all studies, irrespective of treatment modality. Although we do acknowledge that the doses for IHD and CRRT cannot be completely compared, this approach minimizes some of the complexity in the field and provides a conservative summary estimate of effect. Understanding the effects of these complexities of dialysis treatment is fundamental to improving AKI patient outcomes.
The apparent relationship between body weight and outcome raised the possibility that smaller patients may be more likely to benefit from more intense RRT, however, additional pooled analyses using individual patient data from the ATN and RENAL trials did not identify any relationship between participant weight and treatment effect (2,3). Observational data (21) suggest that changes in weight, as a measure of fluid status, may influence outcome in patients with AKI. However, it was not possible to further explore the relative effect of these factors in this analysis. Pooled analyses of individual participant data from all completed trials would allow these issues to be explored in more detail.
Higher intensity of RRT did not increase the probability of renal function recovery. This analysis was based on the calculation of the number of patients who regained renal function as a proportion of all patients assigned to each treatment regimen. Most studies have reported renal recovery using surviving patients as the denominator. However, reporting renal recovery among survivors alone does not preserve the previously achieved randomization, and the interpretation of such results may be misleading given that mortality is a competing end point with recovery of kidney function. We believe reporting the number of patients who have survived and recovered renal function, as a proportion of all patients treated, might convey a more clinically relevant message. In this review, neither analysis identified an overall benefit for intensive RRT with regards to the likelihood of renal function recovery.
In assessing renal recovery in AKI patients requiring RRT, it has become evident that the long-term renal outcomes beyond hospital discharge among survivors of AKI remain poorly characterized. A recent publication linking hospital admissions data with the U.S. Renal Data System Registry suggests that patients with AKI had a 13-fold increase in the risk of developing end-stage kidney disease over the following 2 years (22) and this increased to more than 40-fold if the patient had an episode of AKI on the background of pre-existing chronic kidney disease. Such studies raise critical concerns regarding the long-term outcomes of AKI and highlight the need for extended follow-up of patients and studies in AKI.
The strengths of this systematic review and meta-analysis include the rigorous methodology used and the importance of the clinical questions. The limitations include the use of aggregate published data, the limited statistical power to investigate the sources of heterogeneity, and the inability to extrapolate findings to patients with pre-existing moderate to severe CKD.
In conclusion, within the range of treatment intensity studied in randomized trials among patients with AKI in the ICU, higher intensity RRT does not decrease mortality or improve renal recovery compared with less intensive therapy. The results do not negate the importance of RRT intensity in the treatment of patients with AKI but rather reinforce the need to better understand the effects of treatment modalities, doses, and timing of initiation in this varied, high-risk patient population. With persistent high mortality for patients with AKI, the imperative remains to develop interventions that improve outcomes.
Disclosures
None.
Acknowledgments
M.J. was supported by a postgraduate scholarship from the Australasian Kidney Trials Network (AKTN). H.J.L.H. was supported by a fellowship from the Dutch Kidney Foundation and International Society of Hypertension Visiting Postdoctoral Fellowship awarded by the Foundation of High Blood Pressure Research Council of Australia. A.C. was supported by a Senior Research Fellowship from the Australian National Health and Medical Research Council. M.J., H.J.L.H., T.N., M.G., V.P., and A.C. were responsible for data collection, analysis, interpretation, and manuscript preparation. R.B. and J.M. contributed to data interpretation. A.C., V.P., H.J.L.H., M.G., R.B., J.M., S.F., J.A.K., and P.M.P. contributed to critical revision of the publication. The corresponding author had full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the analysis.
Appendix
Appendix 1.
MEDLINE (via Ovid)
|
Cochrane controlled trials
|
EMBASE
|
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C: Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P: Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuinness S, Myburgh J, Norton R, Scheinkestal C, Su S: Intensity of continuous renal replacement therapy in critically ill patients. N Engl J Med 361: 1627–1638, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G: Effect of different doses in continuous venovenous haemofiltration on outcomes of acute renal failure: A prospective randomised trial. Lancet 356: 26–30, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Schiffl H, Lang SM, Fischer R: Daily hemodialysis and the outcome of acute renal failure. N Engl J Med 346: 305–310, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG: Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Open Med 3: 123–130, 2009 [PMC free article] [PubMed] [Google Scholar]
- 7.Jadad AR, Carroll D, Moore A, McQuay H: Developing a database of published reports of randomised clinical trials in pain research. Pain 66: 239–246, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, Knipschild PG: The Delphi list: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 51: 1235–1241, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Woodward M: Epidemiology: Design and Data Analysis, 2nd Ed, Boca Raton, FL, Chapman and Hall/CRC Press, 2005 [Google Scholar]
- 10.Egger M, Davey Smith G, Schneider M, Minder C: Bias in meta-analysis detected by a simple graphical test. BMJ 315: 629–634, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J: Effect of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: A prospective, randomized trial. Crit Care Med 30: 2205–2211, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Saudan P, Niederberger M, De Seigneux S, Romand J, Pugin J, Perneger T, Martin PY: Adding a dialysis dose to continuous hemofiltration increases survival in patients with acute renal failure. Kidney Int 70: 1312–1317, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM: Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol 19: 1233–1238, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faulhaber-Walter R, Hafer C, Jahr N, Vahlbruch J, Hoy L, Haller H, Fliser D, Kielstein JT: The Hannover Dialysis Outcome study comparison of standard versus intensified extended dialysis for treatment of patients with acute kidney injury in the intensive care unit. Nephrol Dial Transplant 24: 2179–2186, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A: Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol 18: 1292–1298, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM: Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Bagshaw SM, George C, Bellomo R: Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care 11: R68, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conger JD: A controlled evaluation of prophylactic dialysis in post-traumatic acute renal failure. J Trauma 15: 1056–1063, 1975 [DOI] [PubMed] [Google Scholar]
- 19.Schulz KF, Chalmers I, Hayes RJ, Altman DG: Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273: 408–412, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Huwiler-Müntener K, Jüni P, Junker C, Egger M: Quality of reporting of randomized trials as a measure of methodologic quality. JAMA 287: 2801–2804, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL: Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 76: 422–427, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]