Abstract
Background and objectives: N-terminal probrain type natriuretic peptide (NTproBNP) has been proven to be a valuable biomarker for predicting cardiac events and mortality in the hemodialysis population. However recent reports have suggested that NTproBNP is a marker of volume overload rather than one of cardiac dysfunction. Therefore this study investigated the effect of fluid volume status on NTproBNP.
Design, setting, participants, & measurements: Volume status was determined pre- and postdialysis in 72 stable hemodialysis outpatients by multifrequency bioimpedance, and the relationship to NTproBNP values was examined.
Results: The mean and median NTproBNP values were 931.9 ± 230 and 242 (90 to 688) pmol/L, respectively. On simple correlation, NTproBNP was associated with markers of volume overload and cardiac dysfunction. However, on logistical regression analysis, the strongest association was with the predialysis ratio of extracellular water/total body water (β 26.6, F29.6, P = 0.000), followed by postdialysis mean arterial blood pressure (β 0.14, F17.1, P = 0.000), dialysate calcium concentration (β −1.19, F14.1, P = 0.002), and change in extracellular fluid volume with dialysis (β 0.27, F7.4, P = 0.009)
Conclusions: In this study, NTproBNP was not associated with cardiac dysfunction as assessed by transthoracic echo or nuclear medicine scintigraphy but was dependent on factors associated with volume overload. However, because bioimpedance results can also be affected by malnutrition with loss of cell mass, NTproBNP may be elevated not only in patients with volume overload, but also those with malnutrition.
Cardiac disease is prevalent in patients with chronic kidney disease (CKD), particularly those treated by hemodialysis, and is the most common cause of death (1). As patients progress through the stages of CKD, sodium retention typically occurs, leading to expansion of the extracellular fluid volume with the compensatory release of natriuretic peptides due to cardiac wall stretch. In addition to increased secretion, these peptides increase with CKD because they are naturally degraded by renal tubular neutral endopeptidases. As such, cardiac natriuretic peptides are often increased in hemodialysis patients and those with CKD (2). There is a series of natriuretic peptides, and these have been shown to be valuable prognostic biomarkers for cardiac outcomes in patients without kidney failure. Atrial natriuretic peptide (ANP) and its cleavage product N-terminal pro-ANP were the first natriuretic peptides to be studied, but more recently focus has shifted to brain natriuretic peptide (BNP), which is released by the ventricle rather than the atrium. In patients with ESRD on hemodialysis, ANP has been reported to be more responsive to changes in intravascular volume than BNP, whereas BNP appears more reflective of cardiac dysfunction (3). This may be due to the different sizes and half-lives of the peptides, because ANP is cleared during high-flux hemodialysis, with a post dialysis rebound taking some 80 to 100 minutes to re-equilibrate (Mathavakkannan, unpublished data). However, others have shown higher BNP values in volume-overloaded hemodialysis patients without overt cardiac dysfunction (4). Because BNP can also be cleared by high-flux dialysis and has been shown to sequentially fall during the course of a typical dialysis week (5), there has been debate as to whether these cardiac biomarkers are more reflective of fluid volume overload or intrinsic cardiac dysfunction in hemodialysis patients.
The situation is somewhat more confusing in that some studies have not specified when blood sampling has been taken, because BNP values will be greatest at the start of the dialysis week after the 72-hour interdialytic interval, and least after the third dialysis session of the week (5), or the time of sampling has varied between study subjects and then compounded by using different methods of assessing fluid volume status. Hence, although BNP appears to be a valuable prognostic biomarker for increased risk of mortality in hemodialysis patients, it is unclear as to whether this is related to volume overload or underlying cardiac dysfunction (6,7).
Because previous studies reported that BNP was relatively constant in hemodialysis patients after the midweek dialysis session (5), we introduced post-midweek measurement into clinical practice as a means of standardizing results. To investigate the relationship between N-terminal pro-BNP (NTproBNP), volume status, and cardiac dysfunction, we audited post-midweek dialysis NTproBNP values in a cohort of stable adult hemodialysis patients who had corresponding pre- and postdialysis multifrequency bioimpedance (8) measurements to assess volume status.
Methods and Patients
Seventy-two adult patients [50% male, median age 55 (41.5 to 70) years, 36.1% diabetic with 20.8% prescribed insulin] attending routine outpatient dialysis were studied during a routine midweek outpatient hemodialysis session. Median dialysis vintage was 28 (10 to 79) months. All patients were dialyzed with high-flux polysulphone dialyzers (Fresenius, Bad Homberg, Germany) using bicarbonate dialysate (35 mmol/L) containing 1 g/L glucose with a median dialysate sodium of 137 mmol/L (interquartile range 136 to 138 mmol/L), mean calcium of 1.29 ± 0.02 mmol/L, and dialysate temperature of 35 to 36°C. The mean dialysis session time was 3.76 ± 0.07 hours with a median on-line Kt/V of 1.34 (1.2 to 1.59). Relative blood volume monitoring and integrated blood pressure (BP) were recorded (Fresenius 4008, Fresenius, Bad Homberg, Germany). Constant ultrafiltration rate profiles were used in all patients, with a mean rate of 7.5 ± 0.4 ml/kg per h. Multifrequency bioimpedance was performed before and after dialysis (Biospace in body 720, Seoul, South Korea); therefore, patients with pacemakers/implantable defibrillators and amputees were excluded from the study. Serum biochemistry samples were analyzed with a standard multichannel biochemical analyzer (Roche Integra, Roche Diagnostics, Lewes, United Kingdom) using the bromocresol green method for albumin determination, and hemoglobin samples were analyzed by the sodium lauryl sulfate-Hb method (XE-2100 Sysmex Corporation, Kobe, Japan). NT-proBNP was measured by immunoassay (ECLIA Roche Diagnostics, GMBH, Mannhein, Germany), and postdialysis 24-hour urine collections were analyzed to determine urine volume and sodium content. Standard two-dimensional echocardiograms and ATP myocardial perfusion scintography (9) were performed on a nondialysis day.
Ethical approval was granted by the local ethical committee as audit and clinical service development.
Statistical Analyses
Results are expressed as mean ± SD, median and interquartile range, or percentage, as appropriate. Statistical analysis was by paired t test for parametric and the Wilcoxon rank sum pair test for nonparametric data, with Bonferroni correction for multiple analyses when appropriate (Graph Pad Prism version 3.0, Graph Pad, San Diego, CA). Simple regression analysis was performed with Pearson's rank correlation, and then logistical linear regression analysis was undertaken with SPSS version 15.0 (SPSS, University of Chicago, IL). Statistical significance was taken at or below the 5% level.
Results
The percent of patients prescribed antihypertensive medications was 76.4%. The median number of antihypertensives was 1 (1 to 2), with 23.6% receiving angiotensin converting enzyme inhibitors, 25% angiotensin receptor blockers, 33.3% beta-blockers, 15.3% calcium channel blockers, and 12.5% alpha blockers. The percent of patients with a past medical history of myocardial infarction was 8.3%, 2.8% had previous coronary artery bypass surgery, 13.9% had coronary artery stenting, and 63.9% had a history of hypertension.
BP was recorded before, during, and after dialysis. The predialysis systolic BP was 147.2 ± 3.3 mmHg, postdialysis BP was 138 ± 3.0 mmHg, and BP was lowest during dialysis at 119.7 ± 2.8 mmHg (Table 1). The median percentage weight loss during hemodialysis was 2.89% (1.9% to 3.6%) (Table 1), with a change in intracellular water of 3.2% (1.4% to 5.2%), extracellular water of 7.1% (4.8% to 9.2%), and a postdialysis relative blood volume of 92% (88.9% to 95%). The median C-reactive protein (CRP) was 5 (2 to 13) g/L, median parathyroid hormone was 20.2 (8.8 to 46.6) pmol/L, and median urine volume was 43 (0 to 1020) ml/d.
Table 1.
Change in weight, BP, and intracellular and extracellular fluid volumes following hemodialysis (mean ± SEM)
| Variable | Predialysis | Postdialysis |
|---|---|---|
| Weight, kg | 68.8 ± 1.74 | 66.8 ± 1.7a |
| Mean arterial pressure, mmHg | 102 ± 2.4 | 96.4 ± 2.1a |
| Hematocrit, % | 35.7 ± 0.6 | 37.8 ± 0.68a |
| Serum albumin, g/L | 40.5 ± 0.5 | 42.3 ± 0.7a |
| Serum sodium, mmol/L | 139.8 ± 0.4 | 140.7 ± 0.2 |
| Serum potassium, mmol/L | 5.06 ± 0.07 | 3.48 ± 0.05a |
| Serum glucose, mmol/L | 7.6 ± 1.0 | 6.8 ± 0.2 |
| Intracellular water, L | 21.9 ± 0.6 | 21.1 ± 0.6a |
| Extracellular water, L | 14.2 ± 0.4 | 13.2 ± 0.3a |
P < 0.001
Left ventricular end diastole internal diameter on standard two-dimensional transthoracic echocardiography was 4.53 ± 0.08 cm (normal U.K. reference range 3.9 to 5.3 for women and 4.2 to 5.9 for men), and left ventricular end systolic internal diameter was 2.99 ± 0.09 cm, giving a median cardiac ejection fraction of 59% (55.3% to 63%) (normal ≥55%), which was similar to that assessed by ATP myocardial perfusion scintography (9), median 62% (58.5% to 65%). Intraventricular wall thickness in diastole was 1.24 ± 0.03 cm (normal U.K. reference range 0.6 to 1.2 cm). Left atrial superior- to-inferior axis end systolic diameter was 3.85 ± 0.1 cm (normal U.K. reference range 2.7 to 3.8 cm). Cardiothoracic ratio measured on posterior-anterior chest x-ray was 0.49 ± 0.07.
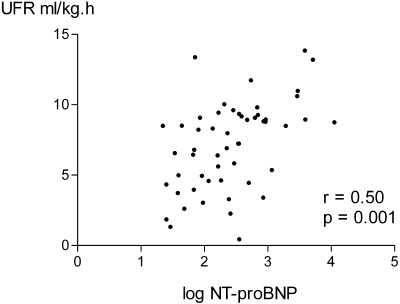
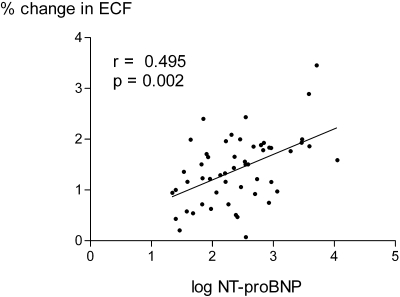
The mean and median NTproBNP were 931.9 ± 230 and 242 (90 to 688) pmol/L, respectively. Because NTproBNP was not normally distributed, values were log-transformed to produce a normal distribution for statistical analysis. There were simple correlations between log NTproBNP and the BP before, after, and lowest recorded during dialysis, whether measured as systolic, diastolic, or mean arterial pressure (Table 2). The strongest correlation was with postdialysis values; therefore, we used postdialysis mean arterial pressure for further analysis. There were no statistically significant correlations between log NTproBNP and cardiac ejection fractions, whether measured by echocardiography or nuclear medicine isotope scanning. Similarly there were no correlations with other measurements derived from echocardiography (e.g., left ventricular end diastolic and systolic dimensions, intraventricular septal and posterior ventricular wall thickness, and left atrial diameter and estimated right ventricular pressure) although there was a correlation with the grade of left ventricular diastolic dysfunction and the cardiothoracic ratio from chest x-rays. However, measurement of cardiac dimensions was only possible in approximately 88% of patients because of difficulty in obtaining reliable images. Simple correlations were noted between log NTproBNP and a positive history of hypertension with concurrent prescription of beta-blockers but not other antihypertensive medications. There were negative correlations with hematocrit and plasma albumin and postdialysis serum potassium and dialysate calcium concentration (Table 2). There were also correlations between log NTproBNP and patient weight, the change in extracellular fluid volume corrected for body surface area (Figure 1), extracellular fluid to total body water ratio, and the ultrafiltration rate (Figure 2).
Table 2.
Simple correlation analysis for log NTproBNP measured at the end of dialysis
| Variable | r | P |
|---|---|---|
| Percent weight loss | 0.50 | 0.0002 |
| Post MAP | 0.439 | 0.001 |
| Pre hematocrit | −0.386 | 0.0047 |
| Pre albumin | −0.33 | 0.015 |
| Post potassium | −0.28 | 0.045 |
| CTR | 0.44 | 0.009 |
| Prescribed beta-blocker | 0.42 | 0.0017 |
| History of hypertension | 0.39 | 0.0033 |
| Ultrafiltration rate | 0.50 | 0.001 |
| Dialysate calcium | −0.33 | 0.0156 |
| LV diastolic function | 0.358 | 0.0199 |
| ECW/TBWpre | 0.45 | 0.009 |
| ΔECFBSA | 0.495 | 0.0002 |
MAP, mean arterial blood pressure; CTR, cardiothoracic ratio on chest x-ray; LV, left ventricular; ECW/TBW, extracellular water to total body water ratio; ΔECFBSA, change in extracellular fluid volume corrected for body surface area; Pre, prehemodialysis; Post, posthemodialysis.
Figure 1.
Correlation between postdialysis log NTproBNP and ultrafiltrate rate.
Figure 2.
Correlation between postdialysis log NTproBNP and predialysis extracellular water volume corrected for body surface area.
To further explore factors affecting log NTproBNP, a series of step-backward logistical regression models were created using those variables with a statistically significant two-tailed Pearson correlation coefficient (P < 0.05). Five factors were found to remain significant with this analysis: predialysis ratio of extracellular water to total body water, postdialysis mean arterial BP, change in extracellular fluid volume with dialysis corrected for body surface area, dialysate calcium concentration, and postdialysis weight (Table 3).
Table 3.
Step-backward logistical regression model of log BNP (r2 = 0.69)
| Variable | β | F | SEM | 95% Confidence Limit | P |
|---|---|---|---|---|---|
| ECW/TBWPre | 26.6 | 29.6 | 4.88 | 16.8 to 36.4 | 0.000 |
| Post MAP | 0.14 | 17.09 | 0.003 | 0.007 to 0.21 | 0.000 |
| [Ca]dialysate | −1.119 | 11.1 | 0.336 | −1.79 to −0.44 | 0.002 |
| ΔECFBSA | 0.269 | 7.37 | 0.099 | 0.07 to 0.468 | 0.009 |
| Post weight | −0.009 | 4.73 | 0.035 | −0.17 to −0.001 | 0.035 |
[Ca], dialysis calcium concentration.
Discussion
Previous reports of NT-proBNP measurements in hemodialysis patients have shown that this is a highly predictive biomarker for subsequent cardiac events and mortality in dialysis patients (10,11). However, there has been debate as to whether NT-proBNP is a marker of volume overload (7,12) or a marker of left ventricular dysfunction (2,13), and also studies that have reported no association with changes in left ventricular mass index or volume status (14). We therefore audited NT-proBNP in a cohort of stable adult hemodialysis outpatients who had contemporaneous volume assessment with pre- and postdialysis multifrequency bioimpedance. As with other reports, we found that NT-proBNP values were not normally distributed (2–7); therefore, we logarithmically transformed data to allow standard statistical analysis (12).
There was no association with sex, age, or dialysis vintage. On simple correlation analysis and in keeping with other reports, we found that NT-proBNP was associated with markers suggestive of volume overload: ultrafiltration rate, cardiothoracic ratio, predialysis ratio of extracellular water to total body water, and change in extracellular fluid volume with dialysis and postdialysis BP (4,7,12). However, there were also correlations with left ventricular diastolic dysfunction as assessed by standard two-dimensional transthoracic echocardiograms and previous history of hypertension with current prescription of beta-blockers, suggestive of an association with cardiac function and supporting previous observations (2,13).
Interestingly there was a negative correlation between the dialysate calcium concentration and log NT-proBNP. Previous studies have shown the importance of dialysate calcium concentration in maintaining cardiovascular stability during dialysis (15). Although a recent trial again observed improved cardiovascular hemodynamics during hemodialysis with a higher calcium dialysate, this was due to an increased stroke volume with no significant change in BNP reported (16). In addition, we also noted negative correlations with predialysis hematocrit and albumin and the postdialysis potassium concentration. Although these could be a dilutional effect due to fluid overload, these could also reflect poor nutrition and be part of the malnutrition inflammation syndrome. Similarly, malnutrition may also contribute to an elevated ratio of extracellular water to total body water as a result of loss of fat weight (17). These findings are in keeping with recent reports of raised BNP being associated with inflammation (7,12) and overhydration (18). However, we did not find any association between CRP and log NT-proBNP, in keeping with other reports (18). One previous report found a correlation between log NT-proBNP and CRP, but interestingly in that study a raised CRP did not predict a raised log NT-proBNP, and their median CRP values were higher than in our cohort (12).
To investigate these associations further, logistical regression analysis was undertaken and five factors remained significant: postdialysis weight, BP, predialysis ratio of extracellular water to total body water, change in extracellular fluid volume with dialysis, and the dialysate calcium. Thus, our study did not show any sustained association between log NT-proBNP and cardiac function as assessed by transthoracic echocardiogram or ATP myocardial perfusion scintography. Other studies have reported an association between BNP and markers of left ventricular hypertrophy rather than volume status (19,20); however, in one of these studies, BNP values were lower in those hemodialysis patients with greater residual renal function and thereby potentially less fluid-overloaded (20). In both of these studies, the median BNP values were substantially greater than those in our study (19,20), as were the levels of overhydration (20).
Although the relationships with predialysis ratio of extracellular water to total body water and change in extracellular fluid volume with dialysis could suggest that log NT-proBNP is predominantly determined by volume overload, these factors can also be associated with the malnutrition inflammation syndrome. A potential link between NT-proBNP and malnutrition inflammation syndrome would also be supported by the observed association with lower postdialysis weights and the use of a higher calcium dialysate, because in our center the number of dialysate concentrates is limited and the only high-calcium dialysate (1.5 mmol/L) available has a potassium concentration of 3.0 mmol/L and is therefore restricted to hypokalemic patients, who are typically malnourished.
As with previous studies, although an increased extracellular water to total body water ratio determined by multifrequency bioimpedance and NT-proBNP are suggestive of overhydration in hemodialysis patients, single absolute values should always be interpreted within the clinical context (21). Raised BNP with normal hydration status should prompt cardiac investigation, whereas increased values of both parameters warrants reappraisal of postdialysis dry weight, and if overhydrated then targeted weight reduction can be monitored by serial reductions in bioimpedance and BNP measurements. Similarly very low BNP values with high normal or overhydrated bioimpedance values should trigger consideration of whether an increase in target weight is appropriate.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “BNP in Hemodialysis Patients,” on pages 954–955.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1.Foley RN, Parfrey PS: Cardiovascular disease and mortality in ESRD. J Nephrol 11: 239–245, 1998 [PubMed] [Google Scholar]
- 2.David S, Kümpers P, Seidler V, Biertz F, Haller H, Fliser D: Diagnostic value of N-terminal pro-B-type natriuretic peptide (NT-proBNP) for left ventricular dysfunction in patients with chronic kidney disease stage 5 on haemodialysis Nephrol Dial Transplant 23: 1370–1377, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Metry G, Hall C, Wilkstrom B, Kallskog V, Hansell P, Danielson B: Fluid balance in patients with chronic renal failure assessed with N-terminal proatrial natriuretic peptide, atrial natriuretic peptide and ultrasonography. Acta Physiol 171: 117–122, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Fagugli RM, Pasini P, Quintaliani G, Pasticici F, Cia G, Cicconi B, Ricciardi D, Santirosi PV, Buoncristiani E, Timio F, Valente F, Buoncristiani U: Association between brain natriuretic peptide and extracellular water in haemodialysis patients. Nephron Clin Pract 95: c60–c66, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Sheen V, Bhalla V, Tulua-Tata A, Bhalla MA, Weiss D, Chiu A, Abdeen O, Mullaney S, Maisel A. The use of B-type natriuretic peptide to assess volume status in patients with end-stage renal disease. Am Heart J 153: 244.e1–e5, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Sun L, Sun Y, Zhao X, Xu C, Chen D, Li L, Ma Y, Rong S, Mei C: Predictive role of BNP and N-proBNP in haemodialysis patients. Nephron Clin Pract 110: c178–c184, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Paniagua R, Ventura MD, Avila-Diaz M, Hinojosa-Heredia H, Mendez-Duran A, Cueto-Manzano A, Cisneros A, Ramos A, Mandonia-Juseino C, Belio-Caro F, Garcia-Contreras F, Trinidad-Ramos P, Vazquez R, Ilabaca B, Alcantara G, Amato D: NT-proBNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol Dial Transplant 25: 551–557, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Davenport A, Willicombe MK: V Comparison of fluid status in patients treated by different modalities of peritoneal dialysis using multi-frequency bio-impedance. Int J Artif Organs 32: 779–786, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Moran N, Buscombe J, Davenport A: Predicting cardiac events using adenosine triphosphate myocardial perfusion scintography in renal transplant candidates. J Nephrol Renal Transplant 1: 41–52, 2008 [Google Scholar]
- 10.Goto T, Takase H, Toriyama T, Sugimura T, Kurita Y, Masuda H, Hayashi K, Ueda R, Dohi Y: Increased circulating levels of natriuretic peptides predict future cardiac events in patients with chronic haemodialysis. Nephron 92: 610–615, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Naganuma T, Sugimura K, Wada S, Yasumoto R, Sugimura T, Masuda C, Uchida J, Nakatani T: The prognostic role of brain natriuretic peptides in haemodialysis patients. Am J Nephrol 22: 437–444, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Jacobs LH, van der Kerkhof JJ, Mingels AM, Passos VL, Kleijnen VW, Mazairac AH, van der Sande FM, Wodzig WK, Konings VW, Leunissen KM, van Dieijen-Visser MP, Kooman JP: Inflammation overhydration and cardiac biomarkers in haemodialysis patients: A longitudinal study. Nephrol Dial Transplant 25: 243–248, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Choi SY, Lee JE, Jang EH, Kim MO, Baek H, Ki CS, Park SW, Kim DJ, Huh WS, O HY, Kim YG: Association between changes in N-terminal pro-brain natriuretic peptide levels and changes in left ventricular mass index in stable haemodialysis patients. Nephron Clin Pract 110: c93–c100, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Goldfarb-Rumyantzev AS, Chelamcharla M, Bray BE, Leypoldt JK, Lavasani I, Nelson N, Lavasani T, Baird B, Cheung AK: Volume indicators and left ventricular mass during aggressive volume management in patients on thrice weekly haemodialysis. Nephron Clin Pract 113: c270–c280, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Leunissen KML, van den Berg BW, van Hoof JP: Ionized calcium plays a pivotal role in controlling blood pressure during haemodialysis. Blood Purif 7: 233–239, 1989 [DOI] [PubMed] [Google Scholar]
- 16.Gabutti L, Bianchi G, Soldini D, Marone C, Burnier M: Haemodynamic consequences of changing bicarbonate and calcium concentrations in haemodialysis fluids. Nephrol Dial Transplant 24: 973–981, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davenport A, Willicombe MK: Hydration status does not influence peritoneal equilibration test ultrafiltration volumes. Clin J Am Soc Nephrol 4: 1207–1212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangji AS, Brimble KS, Margetts PJ. Association between markers of inflammation, fibrosis and hypervolaemia in peritoneal dialysis patients. Blood Purif 28; 354–358, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Lee JA, Kim DH, Yoo SJ, Oh DJ, Yu SH, Kong ET: Association between serum N terminal natriuretic peptide concentration and left ventricular dysfunction and extracellular water in continuous ambulatory peritoneal dialysis patients. Perit Dial Int 26: 360–365, 2006 [PubMed] [Google Scholar]
- 20.Madsen LH, Ladefoged S, Corell P, Schou M, Hildebrandt PR, Atar D. N-terminal pro-brain natriuretic peptide predicts mortality in patients with end-stage renal disease in haemodialysis. Kidney Int 71: 548–554, 2007 [DOI] [PubMed] [Google Scholar]
- 21.van den Kerkhof JJ, Van der Sande FM, Leunissen K, Kooman JP: Are natriuretic peptides useful biomarkers in dialysis patients? Perit Dial Int 27: 636–640, 2007 [PubMed] [Google Scholar]