Abstract
Background and objectives: The International Pediatric Peritonitis Registry (IPPR) was established to collect prospective data regarding peritoneal dialysis (PD)-associated peritonitis in children. In this report, we present the IPPR results that pertain to relapsing peritonitis (RP).
Design, setting, participants, & measurements: This was an online, prospective entry into the IPPR of data that pertain to peritonitis cases by participating centers.
Results: Of 490 episodes of nonfungal peritonitis, 52 (11%) were followed by a relapse. There was no significant difference between RP and non-RP in distribution of causative organisms and antibiotic sensitivities. Initial empiric therapy—ceftazidime with either first-generation cephalosporin or glycopeptide (vancomycin or teicoplanin)—was not associated with relapse. Switching to monotherapy with a first-generation cephalosporin on the basis of culture results was associated with higher relapse rate (23%) than other final antibiotic therapies (0 to 9%). Culture-negative RP was less likely to have a satisfactory early treatment response than non-RP (82 versus 98%). Young age, single-cuff catheter, downward-pointing exit site, and chronic systemic antibiotic prophylaxis were additional independent risk factors for RP in the multivariate analysis. Compared with non-RP, RP was associated with a lower rate of full functional recovery (73 versus 91%), higher ultrafiltration problems (14 versus 2%), and higher rate of permanent PD discontinuation (17 versus 7%).
Conclusions: This is the largest multicenter, prospective study to date to examine RP in children. In addition, this is the first report in the literature to examine specifically the relationship of postempiric antibiotic treatment regimens to the subsequent risk for relapse.
Relapsing peritonitis (RP), defined as the recurrence of peritonitis with the identical organism within 4 weeks of treatment of a primary episode of peritonitis, can be particularly difficult to treat and often leads to catheter removal, loss of peritoneal membrane function, and limitation of future dialysis options (1,2). Despite the critical importance of repeated peritonitis episodes for peritoneal dialysis (PD) outcomes, very few pediatric studies have addressed the clinical features, microbiology, predictive factors, and outcomes that are associated with RP in children who undergo PD (3).
The International Pediatric Peritonitis Registry (IPPR) is a global consortium that is composed of 47 pediatric centers from 14 countries (see Acknowledgments) (4–5). In this report, we present the IPPR results that pertain to RP.
Materials and Methods
Method of Data Collection
Anonymized data input was performed exclusively via an Internet-based web platform (http://www.peritonitis.org). Data that pertained to basic patient and PD modality characteristics, clinical presentation with peritonitis, microbiological results, empiric treatment and its subsequent modifications, clinical treatment response, and final outcomes were submitted sequentially along the course of a peritonitis episode. The data were automatically checked for accuracy and completeness as described in the previous reports of the IPPR (4). The registry protocol was approved by the ethical committees/institutional review boards at each participating center.
Definitions
Peritonitis.
Peritonitis was defined by the presence of (1) cloudy effluent, (2) an effluent cell count of ≥100 cells/μl, and (3) ≥50% polymorphonuclear cells in the differential cell count.
Catheter Exit-Site Appearance.
Catheter exit-site appearance was characterized according to a standardized scoring system on the basis of the presence and severity of swelling, crust, redness, pain on pressure, and discharge (6).
Treatment of Peritonitis.
Treatment of peritonitis was characterized as being conducted in accordance with the International Society for Peritoneal Dialysis (ISPD) guidelines (7) when (1) diagnostic criteria for peritonitis were fulfilled and/or an organism was grown on culture, (2) the patient was assessed for the presence of the peritonitis risk factors defined by the ISPD guidelines, and (3) empiric treatment was initiated according to the recommendations of the guidelines (i.e., with either a first-generation cephalosporin and ceftazidime or with a glycopeptide [vancomycin or teicoplanin] and ceftazidime). It was not necessary, however, to initiate treatment according to the recommended risk stratification of therapy described in the guidelines. Antibiotic therapy was intended to be modified in accordance with the results of the dialysate culture and sensitivity testing.
Disease Severity Score.
The patient's clinical status was evaluated by a disease severity score (DSS; 0 to 5), defined by the sum of points for pain (0, no pain; 1, moderate pain or nausea not requiring specific therapy; 2, severe pain, usually requiring analgesic therapy, or vomiting; or 3, peritonitic pain with tense abdomen and/or paralytic bowel) and fever (0, <37.5°C; 1, 37.5 to 38.9°C; or 2, >38.9°C). The body temperature at start of treatment and the maximum body temperature recorded at 48 to 60 hours and at 156 to 168 hours after start of treatment were used to calculate DSS on days 0, 3, and 7 of treatment, respectively (6).
Early Treatment Response.
Early treatment response was defined as the clinical response of the patient 72 hours after treatment initiation. The response was considered satisfactory when the DSS was ≤2 at 72 hours after the start of empiric antibiotic therapy and the effluent cloudiness had improved.
Late Treatment Response.
Late treatment response (final outcome) was characterized, 4 weeks after treatment initiation, by the presence or absence of ultrafiltration problems, adhesions, uncontrolled infection, secondary fungal peritonitis, need for catheter exchange, general therapy failure, and full functional recovery. Full functional recovery was assumed when PD was continued without functional impairment, irrespective of whether a relapse occurred or a catheter exchange was necessary.
Peritonitis Relapse.
Peritonitis relapse was defined as recurrence of peritonitis with the same organism (defined by biochemical differentiation and resistogram or the occurrence of two episodes remaining sterile) within 4 weeks after termination of antibiotic treatment. Antibiotic resistograms accompanied most but not all positive cultures. Some resistograms included equivalence assumptions (e.g., Gram-negative organisms regarded as resistant to glycopeptides, clindamycin, and rifampin; enterococci regarded as resistant to cephalosporins).
Statistical Analysis
Differences in group means were assessed by ANOVA followed by Student-Newman-Keuls tests. Differences in proportions were assessed using χ2 tests or z-test with Yates correction factor where appropriate. The potential effect of patient characteristics, initial presentation, culture results, and treatment modalities on the relative risk for adverse treatment outcomes (3-day treatment failure, incomplete 4-week functional recovery, catheter exchange) was assessed by univariate and multivariate logistic regression analysis, calculating odds ratios and 95% confidence intervals.
Results
Patients and Peritonitis Episodes
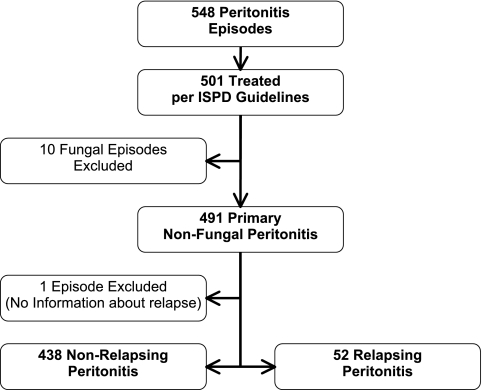
Between October 2001 and December 2004, data on 392 pediatric patients who were aged 1 month to 22 years (median 9.8 years), each of whom experienced one or more episodes of peritonitis while receiving long-term PD, were entered into the database. Data from both incident and prevalent patients were included. For these patients, a total of 548 episodes of peritonitis were recorded (mean 1.4 ± 0.8 episodes per patient; 54% male). Of the 548 peritonitis episodes, 501 (91%) met the criteria for an episode that was treated according to the ISPD guidelines and were included in the analyses. Ten (2%) of the 501 qualifying episodes were cases of fungal peritonitis and were excluded from further analyses that are presented in this article unless stated otherwise. One additional episode of peritonitis was excluded from this analysis because information regarding relapse was not available. Of the remaining 490 episodes of peritonitis, 52 (11%) were followed by one or several RP episodes (Figure 1).
Figure 1.
Registry profile. Selection of cases for analysis and breakdown of relapsing and non-relapsing cases.
Patient characteristics in RP and non-RP episodes are listed in Table 1. Patients with RP were significantly older than those who did not experience RP and had a higher frequency of single-cuff catheters. Forty-two patients who experienced RP did so one time only, whereas seven patients had two RP episodes after the primary infection, two patients had three subsequent episodes, and one patient experienced four episodes of RP after the primary infection.
Table 1.
Patient characteristics
| Characteristic | RP | Non-RP | P |
|---|---|---|---|
| Male (%) | 50 | 56 | 0.42 |
| Age (years; mean ± SD) | 12.5 ± 6.0 | 9.5 ± 5.8 | 0.0004 |
| Duration of dialysis (years; mean ± SD) | 1.7 ± 1.8 | 1.7 ± 1.5 | 0.99 |
| CAPD/CCPD/NIPD (%) | 18/64/18 | 26/47/27 | 0.08 |
| Nasal S. aureus carrier (%) | 25 | 15 | 0.11 |
| One/two-cuff catheters (%) | 25/75 | 13/87 | 0.03 |
| Exit-site orientation (%) | 0.07 | ||
| up | 15 | 22 | |
| down | 62 | 43 | |
| lateral | 23 | 34 | |
| other | 0 | 2 | |
| Exit-site granuloma (%) | 4 | 8 | 0.26 |
| Same organism grown at exit site (%) | 32 | 25 | 0.38 |
| Exit-site S. aureus (%) | 4 | 9 | 0.21 |
| Gastrostomy present (%) | 2 | 8 | 0.10 |
| Any ostomy present (%) | 12 | 12 | 0.91 |
CAPD, continuous ambulatory PD; CCPD, continuous cycling PD; NIPD, nocturnal intermittent PD.
Bacterial Cause and Clinical Features
RP was observed in 24 (11%) of 219 Gram-positive, 11 (9.2%) of 120 Gram-negative, and 17 (11.3%) of 151 culture-negative episodes. As in the total registry population, Staphylococcus organisms accounted for the greatest number of positive cultures, with S. aureus accounting for 21% and coagulase-negative Staphylococcus organisms accounting for 13% of episodes of RP. Overall, RP episodes consisted of 46% Gram-positive organisms, 21% Gram-negative organisms, and 33% culture-negative cases. There was no significant difference in the distribution of causative organisms in cases of RP compared with non-RP episodes (Table 2).
Table 2.
Microbiology of peritonitis episodes
| Culture Result | Non-RP (% [n]) | RP (% [n]) |
|---|---|---|
| S. aureus | 15.1 (66) | 21.1 (11) |
| Coagulase-negative Staphylococcus | 16.4 (73) | 11.5 (6) |
| Streptococcus | 6.2 (27) | 3.9 (2) |
| Enterococcus | 3.4 (17) | 3.9 (2) |
| Other Gram-positive | 3.4 (15) | 5.8 (3) |
| Pseudomonas species | 5.4 (24) | 5.8 (3) |
| Klebsiella species | 4.0 (18) | 3.8 (2) |
| Escherichia coli | 4.7 (21) | 1.9 (1) |
| Acinetobacter species | 2.7 (11) | 3.8 (2) |
| Other Gram-negative | 7.3 (32) | 5.8 (3) |
| Culture-negative | 30.6 (134) | 32.7 (17) |
| Total | 100.0 (438) | 100.0 (52) |
There were no significant differences in clinical features (severe abdominal pain, temperature >38°C, marked effluent cloudiness, effluent cell count, percentage of effluent neutrophils, and DSS) at presentation among the peritonitis cause groups (Gram-positive, Gram-negative, and culture-negative) for RP compared with non-RP. For the RP only, temperature >38°C was significantly less common with culture-negative (P = 0.03) and effluent cell count was higher with Gram-negative episodes (P = 0.01).
Empiric and Final Antibiotic Therapy of Initial Peritonitis Episode
The initial choice of antibiotics (ceftazidime and first-generation cephalosporin, or ceftazidime and a glycopeptide) did not differ significantly between RP and non-RP episodes (P = 0.94). The two recommended empiric regimens were used with equal frequency in both RP and non-RP episodes. RP and non-RP episodes did not differ with respect to the in vitro sensitivities of their causative organisms to the antibiotics and their combinations used for empiric treatment (P > 0.05).
There was no significant difference in satisfactory early treatment response rate (DSS ≤2) between RP and non-RP Gram-positive and Gram-negative cases for each of the two empiric antibiotic treatment regimens (P > 0.05); however, among culture-negative cases, a significantly higher rate of early treatment response failure (DSS >2) was noted for RP episodes (18%) than for non-RP episodes (2.2%; P = 0.002).
Antibiotic therapy was modified after 3 to 4 days as indicated on the basis of the early clinical response and culture results. The analysis of the final therapies that were administered in RP and non-RP allowed calculation of the percentage of episodes followed by a relapse upon completion of various postempiric antibiotic therapies (Table 3). The relapse rate was highest with borderline significance (P = 0.07) for episodes in which antibiotic therapy was switched to monotherapy with a first-generation cephalosporin. When all episodes in which patients were switched to antibiotic monotherapies on the basis of in vitro efficacy results were compared, peritonitis relapsed significantly more frequently after final monotherapy with a first-generation cephalosporin (23%) than in patients who received a glycopeptide, ceftazidime, or an aminoglycoside as monotherapy (0 to 9%; P = 0.02; Table 4).
Table 3.
Fractional incidence of RP by most common postempiric antibiotic therapies
| Final Antibiotic Therapy | No. of Episodes Treated | Episodes Followed by Relapse (% [P])a | Duration of Treatment (Days; Mean ± SD [P]) |
|
|---|---|---|---|---|
| Non-RP Episodes | RP Episodes | |||
| Glycopeptide + ceftazidime | 134 | 13 (0.36) | 14.2 ± 3.4 | 14.2 ± 3.4 (0.92b) |
| First-generation cephalosporin + ceftazidime | 62 | 15 (0.28) | 13.9 ± 2.9 | 15.4 ± 3.2 (0.15b) |
| Ceftazidime | 62 | 8 (0.48) | 13.3 ± 5.0 | 14.2 ± 0.4 (0.70b) |
| First-generation cephalosporin | 57 | 18 (0.07) | 14.9 ± 6.5 | 17.0 ± 7.3 (0.37b) |
| Glycopeptide | 51 | 8 (0.50) | 15.7 ± 4.9 | 13.0 ± 2.0 (0.29b) |
| Ceftazidime + aminoglycoside | 26 | 12 (0.87) | 19.4 ± 9.1 | 27.3 ± 3.1 (0.15b) |
| Aminoglycoside | 5 | 0 (0.44) | 19.0 ± 3.4 | NA |
Antibiotic therapies with no significant frequency of use not shown.
Statistical significance indicates difference compared with all other postempiric antibiotic therapies.
Versus duration of treatment in non-RP episodes.
Table 4.
Use and duration of monotherapies with in vitro efficacy and risk for relapse
| Administered Antibiotic with Documented In Vitro Efficacy | No. of Episodes | Total Duration of Administration (Days; Mean ± SD [P]) | % (P) Followed by Relapsea |
|---|---|---|---|
| First-generation cephalosporin | 26 | 15 ± 7 (0.47) | 23 (0.02) |
| Glycopeptide | 44 | 16 ± 5 (0.25) | 9 (0.77) |
| Ceftazidime | 45 | 14 ± 5 (0.78) | 4 (0.73) |
| Aminoglycoside | 5 | 19 ± 3 | 0 (0.44) |
Antibiotic therapies with no significant frequency of use not shown.
Statistical significance indicates difference compared with all other monotherapies.
Additional Factors that Affect the Risk for RP
By univariate analysis, increasing patient age (P = 0.0004); the use of systemic antibiotic prophylaxis, usually administered to prevent urinary tract infections (P = 0.007); nonsterile exit-site care (P = 0.009); a downward-pointing exit site (P = 0.011); and the presence of a single-cuff Tenckhoff catheter (P = 0.026) were associated with an increased relapse risk. In the multivariate analysis, the effect of the exit-site care technique was not confirmed, whereas the single-cuff catheter (P = 0.0003), the downward-pointing exit site (P = 0.001), and the use of systemic antibiotic prophylaxis (P = 0.028) remained significant risk factors. The multivariate correction for these conditions reversed older patient age from a risk factor into a highly significant protective factor (P < 0.0001).
Factors that were found to have no predictive value in development of RP included concomitant colonization of the exit site with the organism that caused the peritonitis, the exit-site score, the presence or absence of exit-site granuloma, the DSS at day 0 or 3, the use of catheters with curled versus straight tips, and individual center effects. The mean number of patients per center did not differ significantly between RP and non-RP (P = 0.3062) and was not a significant predictor of RP (P = 0.3066).
Final Outcome
The final outcomes of the 52 episodes of RP are compared with those of the non-RP episodes in Table 5. In RP, full functional recovery of PD was achieved significantly less frequently and ultrafiltration problems and permanent PD discontinuation occurred more commonly than in non-RP episodes. There were no significant differences between RP and non-RP cases regarding abdominal adhesions, persistent infection, secondary fungal infection, or general therapy failure. There were two (4%) patient deaths in the RP group and three (0.7%) deaths in the non-RP group (P = 0.17).
Table 5.
Final outcome of peritonitis in children with RP versus non-RP
| Outcome | RP |
Non-RP |
||||||
|---|---|---|---|---|---|---|---|---|
| PD Continued | PD Discontinued |
Total RP (n [%]) | PD Continued | PD Discontinued |
Total Non-RP (n [%])a | |||
| Temporary | Permanent | Temporary | Permanent | |||||
| Full functional recovery | 35 | 3 | 0 | 38 (73.1) | 385 | 6 | 0 | 391 (90.9)b |
| Ultrafiltration problems | 3 | 0 | 4 | 7 (13.5) | 5 | 1 | 3 | 9 (2.1)c |
| Adhesions | 1 | 1 | 2 | 4 (7.7) | 2 | 0 | 9 | 11 (2.6) |
| Uncontrolled infection | 0 | 0 | 1 | 1 (1.9) | 0 | 1 | 10 | 11 (2.6) |
| Secondary fungal peritonitis | 0 | 0 | 0 | 0 (0.0) | 0 | 0 | 4 | 4 (0.9) |
| General therapy failure | 0 | 0 | 2 | 2 (3.9) | 0 | 0 | 4 | 4 (0.9) |
| Total (n [%]) | 39 (75.0) | 4 (7.7) | 9 (17.3)d | 52 (100.0) | 392 (91.2) | 8 (1.9) | 30 (6.9) | 430 (100.0) |
Follow-up not available for eight patients with non-RP because of kidney transplantation within 4 weeks of onset of peritonitis.
P < 0.0001, RP versus non-RP.
P = 0.0084, RP versus non-RP.
P < 0.05, RP versus. non-RP.
Discussion
The rate of relapse in our study is consistent with previously reported rates of RP in the range of 8 to 21% of primary cases (6,8,9). We found no significant difference in the spectrum of causative organisms in RP and non-RP episodes. In RP, S. aureus occurred more frequently (21%) than coagulase-negative Staphylococcus (11.5%). This is at variance with previous studies, which reported a higher rate of coagulase-negative Staphylococcus than S. aureus (1,3,4,10). Whereas the rate of S. aureus carriage was 25% in RP compared with 15% in non-RP cases, this difference did not reach statistical significance (P = 0.11; Table 1). Our percentages of Gram-negative (21%) and culture-negative (33%) RP cases also were higher than those reported in the few studies that listed all bacterial causes of RP compared with non-RP or primary cases (1,6,8). It is possible that some of the culture-negative cases represent failure in specimen acquisition or laboratory culture techniques, which also might account for the differences in bacterial causes between our study and previous reports (4).
Follow-up peritoneal fluid cell counts and cultures were not required at the end of treatment of peritonitis episodes. We cannot demonstrate, by culture, resolution or persistence of infection before episodes defined as RP cases; however, it is unlikely that there would be clinical resolution of peritonitis 4 weeks after diagnosis if infection persisted.
Notably, culture-negative peritonitis episodes followed by RP were less likely to have a satisfactory initial clinical response than non-RP episodes (82 versus 97.8%; P = 0.002). It is possible that infections that arise from loci that are poorly accessible to antibiotics, such as the catheter tunnel, fibrin clots, or biofilm in the catheter surface, may predispose to a poor initial treatment response and an increased likelihood of recurrence despite a low yield of organisms from bacterial culture.
The choice of initial empiric therapy did not affect the likelihood of a relapse; however, a significantly higher relapse rate (23%) occurred when patients were switched to monotherapy with a first-generation cephalosporin on the basis of the culture and in vitro sensitivity results when compared with other final mono- or combination therapies. It is possible that organisms that are responsible for recurrence of peritonitis have different virulence factors or perhaps generate a biofilm that is less responsive to first-generation cephalosporins compared with glycopeptides or combinations of antibiotics. In addition, it is possible the bacterial organisms in recurrent peritonitis, although qualitatively sensitive to first-generation cephalosporins in culture, are quantitatively more resistant to first-generation cephalosporins than glycopeptides or other antibiotic regimens when considering minimum inhibitory concentration (MIC); however, data on minimum inhibitory concentration were not available for analysis in this study. In contrast, the duration of antibiotic therapy, which was 2 weeks in most episodes, in line with the clinical practice guidelines, did not affect the risk for relapse. In view of the fact that this is the first report in the literature to address specifically the antibiotic choice after empiric treatment and subsequent risk for relapse, these findings will have a major bearing on the formulation of revised clinical practice treatment guidelines.
Single-cuff catheters, systemic antibiotic prophylaxis, and downward-pointing exit sites were independently associated with an increased relapse risk. Whereas patient age seemed to increase the risk for relapse by univariate analysis, age was assigned a significant protective effect when correcting for the higher prevalence of downward-pointing exit sites in young infants by multivariate analysis. Hence, our data suggest that young children are at increased risk not only for peritonitis (11) but also for relapsing infections. It is possible that the immunologic immaturity of infants might contribute to their less efficient coping with PD-associated infections. Other factors that were not assessed by the IPPR, such as location of exit sites relative to gastrostomy sites and diaper areas, might also play a role in the increased risk for RP in younger patients and need to be addressed in future studies. Although we included a large number of variables in the multivariate regression analysis, because of the complicated nature of RP, we cannot exclude the possibility of residual confounding.
There was a six-fold elevated adjusted relapse risk with single-cuff compared with double-cuff catheters. The observed increase of the relapse risk associated with downward-pointing exit sites is not readily explained and is surprising because previous studies reported a decreased risk for peritonitis with a downward-pointing exit-site configuration (12–14). This is a finding that will require confirmation in future studies and will be monitored by the recently formed International Pediatric Peritoneal Dialysis Network.
A Cochrane review reported the lack of efficacy of oral antibiotic prophylaxis in preventing peritonitis (15). In our analysis, the prophylactic administration of systemic antibiotics, which was frequently performed for reasons other than peritonitis prevention, was even associated with a significantly increased risk for RP.
Finally, our outcome analysis highlights the marked clinical relevance of RP. Relative to sporadic peritonitis episodes, relapsing infections were associated with a three-fold risk for incomplete functional recovery, mainly as a result of an increased incidence of postinfection ultrafiltration problems. As a result of these complications, 17% of RP cases led to permanent PD technique failure, a highly significant increase compared with isolated episodes (7%).
Disclosures
The IPPR was funded by grants provided by the International Society for Peritoneal Dialysis and Baxter Health Care.
Acknowledgments
This study was presented in abstract form at the annual meeting of the Pediatric Academic Societies; May 2 through 5, 2009; Baltimore, MD.
We appreciate the continued support of the staff in the dialysis units that contributed data to the IPPR.
Participants of the IPPR: N. Aksu, S. Mir, N. Ozkayin, Izmir, Turkey; A. Sirin, B. Sadikoglu, L. Sever, Istanbul, Turkey; M. Zimmering, Berlin, Germany; F. Schaefer, R. Feneberg, Heidelberg, Germany; R. Munoz, Mexico City, Mexico; E. Sojos, Buenos Aires, Argentina; O. Donmez, Bursa, Turkey; K.E. Bonzel, Essen, Germany; N. Majkowski, J.C. Lane, Chicago, Illinois; S. Testa, G. Ardissino, Milano, Italy; D. Drozdz, Krakov, Poland; S.R. Alexander, Stanford, California; D.E. Müller-Wiefel, Hamburg, Germany; I. Mader, Wroclaw, Poland; I. Salusky, Los Angeles, California; Il Soo Ha, Seoul; J. Flynn, New York, New York; K. Rönnholm, Helsinki, Finland; A. Zurowska, Gdansk, Poland; M. Ekim, N. Besbas, S. Bakkaloglu, Ankara, Turkey; E. Verrina, Genoa, Italy; G. Offner, Hannover, Germany; B.A. Warady, Kansas City, Missouri; K. Arbeiter, Vienna, Austria; M. Fischbach, Strasbourg, France; C.K. Blaszak, Little Rock, Arkansas; E. Simkova, Prague, Czech Republic; G. Klaus, Marburg, Germany; P. Waber, M. Seikaly, Dallas, Texas; M. Sharbono, Birmingham, Alabama; A.R. Watson, Nottingham, United Kingdom; A. Gür Güven, Antalya, Turkey; J. Symon, Seattle, Washington; C. Stefanidis, Athens, Greece; A. Al-Uzri, Portland, Oregon; P. Kingwatanakul, Bangkok, Thailand; U. John, Jena, Germany; L. Stapenhorst, Cologne, Germany; K. Haluany, Leipzig, Germany; D. Gipson, Chapel Hill, North Carolina.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Klaus G, Schaefer F, Querfeld U, Soergel M, Wolf S, Mehls A: Treatment of relapsing peritonitis in pediatric patients on peritoneal dialysis. Adv Perit Dial 8: 302–305, 1992 [PubMed] [Google Scholar]
- 2.Andreoli SP, Leiser J, Warady BA, Schlichting L, Brewer ED: Adverse effect of peritonitis on peritoneal membrane function in children on dialysis. Pediatr Nephrol 13: 1–6, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Mujais S: Microbiology and outcomes of peritonitis in North America. Kidney Int Suppl 70: S55–S62, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Warady BA, Feneberg R, Verrina E, Flynn JT, Müller-Wiefel DE, Besbas N, Zurowska A, Aksu N, Fischbach M, Sojo E, Donmez O, Sever L, Sirin A, Alexander SR, Schaefer F, International Pediatric Peritonitis Registry (IPPR): Peritonitis in children who receive long-term peritoneal dialysis: A prospective evaluation of therapeutic guidelines. J Am Soc Nephrol 18: 2172–2179, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Schaefer F, Feneberg R, Aksu N, Donmez O, Sadikoglu B, Alexander SR, Mir S, Ha IS, Fischbach M, Simkova E, Watson AR, Möller K, von Baum H, Warady BA: Worldwide variation of dialysis-associated peritonitis in children. Kidney Int 72: 1374–1379, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Schaefer F, Klaus G, Müeller-Wiefel DE, Mehls O: Mid-European Pediatric Peritoneal Dialysis Study Group (MEPPS): Intermittent versus continuous intraperitoneal glycopeptide/ceftazidime treatment in children with peritoneal dialysis-associated peritonitis. J Am Soc Nephrol 10: 136–145, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Warady BA, Schaefer F, Holloway M, Alexander S, Kandert M, Piraino B, Salusky I, Tranaeus A, Divino J, Honda M, Mujais S, Verrina E, International Society for Peritoneal Dialysis (ISPD) Advisory Committee on Peritonitis Management in Pediatric Patients: Consensus guidelines for the treatment of peritonitis in pediatric patients receiving peritoneal dialysis. Perit Dial Int 20: 610–624, 2000 [PubMed] [Google Scholar]
- 8.Kavanagh D, Prescott GJ, Mactier RA, Scottish Renal Registry: Peritoneal dialysis-associated peritonitis in Scotland (1999–2002). Nephrol Dial Transplant 19: 2584–2591, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Schaefer F: Management of peritonitis in children receiving chronic peritoneal dialysis. Pediatr Drugs 5: 315–325, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Piraino B: Peritonitis as a complication of peritoneal dialysis. J Am Soc Nephrol 9: 1956–1964, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Howard RL, Millspaugh J, Teitelbaum I: Adult and pediatric peritonitis rates in a home dialysis program: Comparison of continuous ambulatory and continuous cycling peritoneal dialysis. Am J Kidney Dis 16: 469–472, 1990 [DOI] [PubMed] [Google Scholar]
- 12.Golper TA: Risk factors for peritonitis in long-term peritoneal dialysis: The Network 9 peritonitis and catheter survival studies. Academic subcommittee of the steering committee of the Network 9 peritonitis and catheter survival studies. Am J Kidney Dis 28: 428–436, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Furth SL, Donaldson LA, Sullivan EK, Watkins SL, North American Pediatric Renal Transplant Cooperative Study: Peritoneal dialysis catheter infections and peritonitis in children: A report of the North America Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 15: 179–182, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Neu AM, Ho PL, McDonald RA, Warady BA: Chronic dialysis in children and adolescents.: The 2001 NAPRTCS annual report. Pediatr Nephrol 17: 656–663, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Strippoli GF, Tong A, Johnson D, Schena FP, Craig JC: Antimicrobial agents for preventing peritonitis in peritoneal dialysis patients. Cochrane Database Syst Rev (4): CD004679, 2004 [DOI] [PubMed] [Google Scholar]