Abstract
Anti-angiogenic therapies currently revolve around targeting vascular endothelial growth factor-A (VEGF-A) or its receptors. These therapies are effective to some degree, but have low response rates and poor side-effect profiles. Part of these problems is likely to be due to their lack of specificity between pro- and anti-angiogenic isoforms, and their nonspecific effects on proactive, pleiotropic survival and maintenance roles of VEGF-A in endothelial and other cell types. An alternative approach, and one which has recently been shown to be effective in animal models of neovascularization in the eye, is to target the mechanisms by which the cell generates pro-angiogenic splice forms of VEGF-A, its receptors and, co-incidentally, by targeting the upstream processes, other oncogenes that have antagonistic splice isoforms. The concept here is to target the splicing mechanisms that control splice site choice in the VEGF-A mRNA. Recent evidence on the pharmacological possibilities of such splice factors is described.
Keywords: angiogenesis, anti-angiogenic, cancer, eye disease, splicing, VEGF, VEGF165b
The good: anti-VEGF agents target VEGF-mediated tumor angiogenesis
In the 1970s, the late Judah Folkman postulated that tumors were dependent in blood vessels for their growth and expansion [1]. This concept of angiogenesis is the formation of new blood vessels from pre-existing vessels. The 1980s saw the advent in several laboratories of a tumor-derived protein inducing angiogenesis, denoted vascular endothelial growth factor-A (VEGF-A) [2-5], and vascular permeability factor (VPF), which increased permeability [6,7]. It was later revealed that these two proteins were one and the same, and was upregulated in all solid tumors acting via its receptors found mainly on endothelial cells in blood vessels (recently reviewed in [8,9].
In the 1990s, a series of landmark papers demonstrated that VEGF-A was upregulated in tumors, and was required for tumor growth in animal models and in other angiogenic conditions such as age-related macular degeneration (AMD) (reviewed in [10]). This validated it as a target for novel oncological therapeutics in preclinical models.
The 2000s were characterized by the translational leap from preclinical to clinical studies, and in 2004 after several failed preclinical trials of putative anti-angiogenic agents, bevacizumab, a humanized antibody to VEGF-A, was shown to increase the mean survivl length in colon cancer patients by 4.7 months [11]. To date, it has been licensed in combination with conventional chemotherapy to treat metastatic colon carcinoma, metastatic nonsquamous non-small-cell lung cancer and metastatic HER2-negative breast cancer [12]. At present, there are over 400 ongoing clinical trials with bevacizumab in more than 30 different tumors. Bevacizumab doesn’t come without side effects, and the most severe and common are hypertension, gastrointestinal perforations, wound healing complications and hemorrhage (reviewed in [13]).
VEGF-A levels are also increased in some ocular pathologies characterized by abnormal vessel growth. Ranibizumab, a licensed therapy for AMD, is an antibody fragment of bevacizumab and is in a Phase III clinical trial for the treatment of diabetic macular edema and retinal vein occlusion [14]. VEGF-Trap, a fusion between Ig loop 2 from VEGF receptor (VEGFR)-1 and loop 3 from VEGFR-2, blocks VEGF-A and PIGF [15], and is in Phase III clinical trials in combination with chemotherapy. VEGF-Trap-Eye is an Phase III to treat wet AMD. Successful data were shown with pegaptanib, which is a short modified RNA aptamer that specifically binds VEGF165, thought to be the most abundant VEGF-A [16] in the treatment of wet AMD [17].
An alternative strategy is to hit a broader spectrum of growth factor receptors using small molecular inhibitors. Sorafenib inhibits a wide range of targets such as Raf serine/threonine kinases, VEGFR-1, VEGFR-2, VEGFR-3 and PDGF receptor (PDGFR)-β, and is licensed for advanced renal cancer [18]. Similarly, sunitinib, which inhibts VEGFR and PDGFR, is now licensed for advanced renal cell carcinoma [19,20] and the rarer gastrointestinal stromal tumor [21].
Targeting angiogenesis using single agents or a combination of other angiogenesis inhibitors or chemotherapy against VEGF-A or its receptors has therefore been shown to have some efficacy, and is a promising way of reducing tumor growth and increasing survival rates. However, their efficacy is limited to a small population of patients, and they come with significant side effects. Moreover, anti-angiogenic treatment may accelerate invasion and metastasis in some tumors, as has been seen in animal models [22,23]. It is now becoming clear that alternative molecular targets for anti-angiogenic therapy may also be useful, for example, the Delta–Notch pathway (recently reviewed in [24,25], as VEGF is not the only protein involved in angiogenesis. Therefore, inhibiting two or more angiogenic molecules could be more beneficial.
The bad: anti-VEGF agents target VEGF, the endogenous pleiotropic growth & survival factor
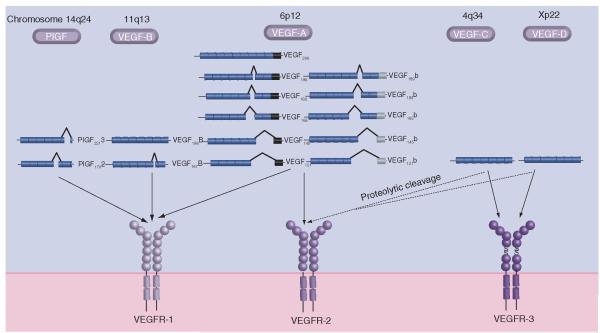
The VEGF gene superfamily consists of at least five ligands summarized in Figure 1: PIGF, VEGF-A to -D, parapoxvirus-derived VEGF-E and snake venom-derived VEGF-F, the latter two showing a lower degree of homology (reviewed in [9]). VEGF-A, hereafter denoted VEGF, is vital for normal vessel development, and knockout of one allele leads to mice that do not live beyond embryonic day 12 owing to a defective vascular development [26,27]. The different ligands bind specifically to either one or two of the three VEGF receptors (VEGFRs). VEGF binds VEGFR-1 and -2, which are found mainly on endothelial cells.
Figure 1. The VEGF superfamily and its receptors in humans.
The VEGF superfamily members are expressed by genes found on different chromosomes, and many of them are spliced to generate a multitude of ligands. PIGF is spliced into at least two isoforms and binds VEGFR-1. VEGF-B is also spliced into two isoforms that are VEGFR-1-specific ligands. VEGF-A mRNA can be spliced into two families of isoforms where the family members only differ in the last six amino acids able to bind VEGFR-1 and VEGF-C and VEGF-D bind VEGFR-3, but proteolytic cleavage can generate ligands that can bind VEGFR-2 as well.
VEGFR-1 has a modulator role during embryogenesis, as knockout leads to overgrowth of endothelial cells [28,29] but a positive role in inflammation [30,31] and cancer growth [32], as it appears to have a more widespread expression that initially described. Soluble VEGFR-1 has a possible decoy effect [33, 34] and is increased in serum from pregnant women suffering from pre-eclampsia, manifesting itself with hypertension and proteinuria [35,36].
VEGFR-2 is the main transducing receptor for VEGF, and VEGFF-2 knockout mice show defective vasculogenesis and die during embryo day E8–8.5 [37]. Expression has also been found on some hematopoetic cells [30, 38], neuronal cells [39-41], osteoblasts [42], retinal progenitor celles [43] and megakaryocytes [44], indicating that VEGF is not solely an endothelial factor acting in a paracrine fashion.
Indeed, although the evidence that VEGF is expressed in vivo by endothelial cells is modest, a recent study has shown that autocrine endothelial cell VEGF is required for the homeostasis of blood vessels in the adult animal, since genetic deletion of VEGF specifically in the endothelial lineage lead to progressive endothelial degeneration and sudden death in half of the animals at 6 months [45]. Furthermore, homozygous cell-specific VEGF knockout in visceral glomerular epithelial cells, for example, results in perinatal mortality, and heterozygous podocyte VEGF knockout resulted in renal disease characterized by proteinuria and endotheliosis [46]. The above studies suggest that endogenous VEGF expression has some crucial physiological role in the normal state. Paradoxically, this may be to inhibit angiogenesis (through VEGFxxxb isoforms, see below), and in effect to maintain the status quo, that is, a state of cell survival in the absence of new vessel formation, since VEGF isoforms of both families have been shown to be cytoprotective. VEGF165 is a survival factor for podocytes [47], and work in these laboratories has also investigated the effect of VEGF165b on epithelial cells, in particular, conditionally immortalized visceral glomerular epithelial cells (podocytes), retinal pigmented epithelial cells and colonic adenoma cells, as well as endothelial cells [Magnussen A, Rennel ES, Hua J et al. VEGF165b is anti-angiogenic but cytoprotective in the retina – potential in diabetic retinopathy. Manuscript submitted]. In all four of these primary or minimally transformed noninvasive cell types, VEGF165b acts as a survival factor, decreasing cytotoxicity and reducing apoptosis, indicating that VEGF165b exerts powerful prosurvival signals in multiple cell types [48, 49].
The complicated: splicing of VEGF & contrasting effects
In 2002, another subfamily of VEGF protein was identified, which was generated by exon 8 C-terminal distal splicing, leading to a six amino acid substitution (CDKPRR to SLTRKD). The first family member to be verified and studied was VEGF165b [50], and with the recent finding that VEGF121b exists, there is an indication that there is a whole sister family of VEGF isoforms [Rennel ES, Varey AHR, Churchill AJ et al. VEGF121b, a new member of the VEGFxxxb family of VEGF-A splice isoforms inhibits neovascularisation and tumour growth in vivo. Manuscript submitted].
VEGF165b shows a 96% homology with VEGF165 and binds VEGFR-1 and -2 with similar affinity, but it has a fundamentally different effect. By studying the two amino acid sequences and the crystal structures of VEGF165 fragments, three structural changes have been identified that can impact on function. Firstly, VEGF165b has an odd number of cysteine residues, leading to reduced C–C bonding. Secondly, a lack of an arginine residue leads to an overall reduced positive charge in VEGF165b. Thirdly, there is a different shape to the backbone of the C terminus in VEGF165b, as it lacks a proline residue. The C-terminal six amino acids are also important for heparin sulfate proteoglycan (HSPG) and Nrpl binding. VEGF165b is unable to bind to heparin and similar HSPGs, even though it contains the HSPG-binding exon 7, probably due to the altered 3D structure [51,52]. The coreceptor Nrp1 is implicated for full activation of VEGFR-2, and VEGF165b does not bind Nrp1.
These data together indicate that VEGF165b cannot fully assembly the VEGFR-2/Nrp1 complex [51,52], leading to a partial rotation of the intracellular domain of VEGFR-2 [53]. This results in reduced phosphorylation of intracellular tyrosine residue 1054 on VEGFR-2 [52] and a weaker and transient phosphorylation of downstream ERK1/2 [51].
Of interest is that VEGF159, which is engineered to lack both sets of the last six amino acids, is neither pro- nor anti-angiogenic [51], and a peptide of the terminal six amino acids of VEGF165b is unable to inhibit VEGF165-induced endothelial migration [Rennel ES et al. Manuscript Submitted]. This indicates that exon 8a, the common exons 1–5 and the 3-D structure are all vital for the angiogenic function of VEGF.
This partial activation of VEGFR-2 leads to a competition whereby VEGF165b inhibits VEGF165-induced processes such as migration, proliferation in endothelial cells in vitro [50,54,55] and vasodilation ex vivo [50], but is still able to stimulate survival signaling, In vivo, VEGF165b counteracts VEGF165 by inhibiting angiogenesis in the rat mesentery [55], physiological angiogenesis in mammary tissue in transgenic mice [56], vessel in-growth into implanted chambers in mice [51] and angiogenesis in the rabbit corneal eye pocket model [55]. VEGF165b is anti-angiogenic in embryonic stem cell systems [52] – implanted Matrigel™ plugs in mice [52] or chick chorioallantoic membrane assay [51]. In addition, VEGF165b does not increase chronic microvascular permeability [57], and induces reduced glomerular endothelial cell monolayer permeability in vitro [48]. This indicates that VEGF165b acts as a partial activator – it is an antagonist of the angiogenic processes stimulated by VEGF165, but it has similar cytoprotective functions to VEGF165.
Overexpression of VEGF165b in tumor cells delays the growth of melanoma [55], Ewing sarcoma [58], prostate [58], colon [49] and kidney cancers [58]. Administration of recombinant VEGF165b also inhibits the development of established colon tumors when administrated either as a subcutaneous or intraperitoneal injection [54]. Recombinant VEGF165b and VEGF121b inhibits hypoxia-induced retinal angiogenesis in mouse models of retinopathy of permaturity by reducing the proliferative neovascularization. [59; Rennel ES et al. Manuscript Submitted].
Splicing, tumor prognosis & disease
Approximately three out of four human genes are spliced to generate two or several proteins, sometimes with different, even antagonistic properties, cellular localization and degradation potentials [60,61]. Splicing is a highly regulated process and can be regulated by external stimuli, hormones, immune response and cellular stress, but the detailed mechanisms of splicing regulation are still in the process of being elucidated for most genes. The spliceosome is a macromolecular complex made up of several hundred components, and it recognizes specific splice sites, facilitates protein interactions and splicing (reviewed in [62,63]). Splicing dysregulation has been implicated as a cancer-causing (oncogenic), cancer-progressing and cancer-invasive process (reviewed in [64,65]), and splice variants of several genes have been identified in large screens involved in breast and ovarian cancer [66,67]. These include VEGFR-3 splice variants found in metastatic prostate tumors and lymph node metastases [68], and several splice variants of the commonly mutated p53 [69].
VEGF expression is induced by hypoxia, growth factors/cytokines and oncogenes, but how VEGF splicing is controlled is not yet fully understood. In human normal colon tissue, approximately 90% of the total VEGF is VEGFxxxb, and upregulation of VEGFxxx was seen in colorectal carcinoma compared with adjacent normal colon [49]. VEGF165 is known to be cytoprotective, and the same has been seen for VEGF165b. Moreover, overexpression of VEGF165b in tumor cells slowed tumor growth, but also inhibited the effect of the anti-VEGF antibody bevacizumab, suggesting that the outcome of VEGF therapies and side effects may be dependent on the VEGFxxx:VEGFxxxb ratios [49]. In another set of colon cancer patients, an association was observed between low VEGF165b levels and later stage cancers with vascular invasion and lympth node metastasis [70].
A similar switch towards VEGFxxx by tumors to increase angiogenesis has been observed at the mRNA level in prostate [58], renal [50] and bladder cancer, and at the protein level in bladder cancer [Harper SJ, Bates DO. Unpublished Data]. Immunohistochemical analysis of primary melanoma revealed a lower expression of VEGFxxxb in patients with distant metastases compared with melanomas that did not metastasize [71]. Moreover, increased VEGF189b expression was seen after treatment with chemotherapeutic agents, raising the possibility that resistance to therapy may also be associated with altered VEGF splicing [72].
An altered VEGFxxx:VEGfxxxb ratio has also been seen in other disease states dependent on increased angiogenesis. A reduction in VEGFxxxb ratios was seen in patients suffering from diabetic retinopathy compared with nondiabetics [73]. Reduced levels of VEGFxxxb were found in pre-eclamptic placenta compared with normal placenta [74], and at 12 weeks gestation VEGFxxxb levels were reduced in women who later developed pre-eclampsia, indicating that plasma levels of VEGFxxxb can be used as a clinical marker for increased risk of pre-eclampsia [75]. Denys–Drash syndrome is mainly caused by mutation in the Wilms’ tumor-1 (WT-1) gene, and is a rare syndrome leading to renal failure and increased risk for Wilm’s tumor. Podocytes from these patients expressed VEGF165, but lacked VEGF165b expression [76].
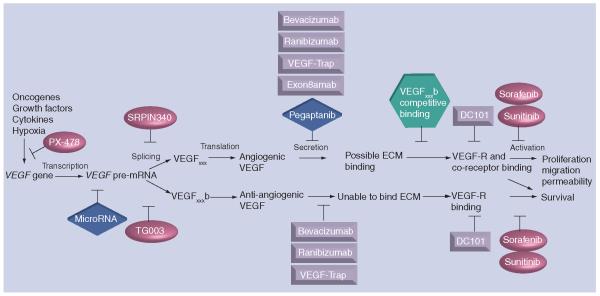
These data indicate that there can be a splice switch towards VEGFxxx in angiogenic-dependent disease states. With this comes two possibilities for novel therapies. The first is to use instead of bevacizumab an antibody that specifically binds exon 8a and would therefore only inhibit the angiogenic isoforms (exon8amab). This refinement in the binding specificities would perhaps to some degree circumvent the side effects seen with bevacizumab, as the cytoprotective VEGF165b would still be intact as only the angiogenic isoforms would be bound. The second possibility is to develop small molicular inhibitors that could change splicing towards VEGFxxxb in vivo and thereby reduce abgiogenesis. This would result in a change in the disease phenotype or reduction of tumor growth and progression. Figure 2 illustrates VEGF from gene to function, and the possibilities in regulating all or specific VEGF isoforms.
Figure 2. VEGF:from gene to function and the possible inhibition at the different levels.
VEGF is upregulated by a variety of processes and growth factors. Hypoxia stabilizes HIF1α, enabling it to bind to the HIF1β subunit and induce VEGF transcription. The inhibitor PX-478 inhibits HIF1α protein levels and activation, thereby reducing VEGF transcription and tumor growth in mouse models [96]. The recent focus has been on naturally occurring microRNA that can bind to mRNA, leading to degradation. Some putative miRNA have been found regulating VEGF mRNA [97], and future studies might reveal specific miRNA influencing the expression of angiogenic and anti-angiogenic isoforms. The splicing machinery involves several components and a subset of splice factors that can alter VEGF splicing [77]. SRPK1/2 kinase inhibitors [81] reduce VEGFxxx mRNA and protein production [Nowak DG, Amin EM, Rennel ES et al.: Regulation of VEGF splicing from pro-angiogenic to anti-angiogenic isoforms - a novel therapeutic strategy for angiogenesis. Manuscript Submitted], whereas Clk SR kinase inhibitors block phosphorylation of splice factors SRp55 and SRp75 [98] and can reduce VEGFxxxb expression [77]. Splicing of the pre-mRNA leads to splicing in the C-terminal, generating two distinct isoform families, VEGFxxx or VEGFxxxb. Most anti-VEGF treatments against the VEGF protein are unable to distinguish between the VEGF isoforms, but the generation of a VEGFxxx isoform-specific antibody, Exon8a-mAb, would remove the angiogenic effects, but possibly maintain the cytoprotective effects of VEGFxxxb. VEGF165b protein has been shown to compete with VEGF165 for binding to its receptors [54,55]. The VEGR-2-specific antibody DC101 inhibits VEGF binding to VEGFR-2 [99], and inhibits downstream signaling and effects. An alternative way to inhibit VEGFR function is by small molecular inhibitors such as sorafenib and sunitinib, but these tyrosine kinase inhibitors do not only inhibit VEGR function, but also similar tyrosine kinase receptors. Ovals represent pharmacological inhibitors, boxes represent antibody or modified antibody fragments, diamonds represent RNA-based constructions, and hexagons represent proteins.
Can VEGF splicing be regulated?
If a balanced ratio of VEGFxxx:VEGFxxxb could be important, what external factors and splice factors affect splicing, and what happens if these are altered? The growth factors IGF-1 and TNF-α were shown to stimulate VEGFxxx mRNA and protein production, whereas TGF-β1, on the contrary, could stimulate VEGFxxxb expression [77]. The serine–arginine-rich (SR) protein kinase, SRPK 1/2, phosphorylates the splicesome component ASF/SF2 [78-80], and inhibition of SRPK 1/2 kinase activity using SRPIN 340 [81], inhibits ASF/SF2 nuclear localization and reduced IGF-1-induced VEGFxxx expression [Nowak DG et al. Manuscript Submitted]. Similarly, intraocular injection of the SRPK1/2 inhibitor SPRIN340 reduced neovascularization in the oxygen-induced retinopathy model [Nowak DG et al. Manuscript Submitted]. SRPIN 340 is an isonicotinamide compound originally found to show antiviral effect by inhibiting SRPK1/2 activity, and downregulating SRp75, leading to reduced HIV infection of cells [81].
TGF-β1-induced splicing towards VEGFxxxb isoforms is regulated by p38 MAPK and CDC-like kinase (Clk-1) splice factor family [77]. Overexpression of several splice factors revealed that SRp55 that can be phosphorylated and regulated by Clk-1, could increase VEGFxxxb protein expression. Furthermore, knockdown of SRp55 reduces VEGFxxxb expression [77]. This is some of the first evidence relating to how VEGFxxx/VEGFxxxb splicing is regulated, and how small molecular inhibitors can be used to change the splicing towards VEGFxxxb and thereby reduce neovascularization.
Future perspective
It is beginning to become clear that aberrant splicing is involved in cancer and cancer progression, but there are still major questions outstanding. An increase in SR proteins has been seen in breast [82], lung [83] and ovarian [84] cancers. More specifically, the splice factor ASF/SF2 is upregulated in various tumors, partly due to amplification of the gene [84-86]. SRp55 is involved in FGFR-1 splicing [87], and aberrant splicing of FGFR-1 is associated with pancreatic [88] and breast cancers [89] and glioblastoma [90]. Increased expression of SRPK1/2 has been observed in pancreas, breast and colon cancer, and knockdown of SRPK1 increased the response to chemotherapy [91,92], but the mechanism of action is unknown. This leads to the question of whether dysregulated splicing is a cause or an effect of cancer? How specific are different splice factors and splicesome components, and would multiple proteins be affected? Would inhibitors to specific splice events be specific for the cancer cells or would they also affect normal tissues and development, and would that lead to possible side effects? It is not yet clear whether splicing factors inhibitors, or modulators of their regulatory proteins (e.g., SR protein kinase inhibitors) can inhibit tumor growth, although recent data suggests that they may be potentially therapeutic in ocular angiogenesis [Nowak DG et al. Manuscript Submitted]. In addition, little is known about what effects this has on other molecules that can be spliced and involved in angiogenesis.
An alternative approach could be to use short morpholino antisense oligonucleotides to change VEGF splicing. This method has been used in a mouse model for myotonic dystrophy, where morpholino antisense oligonucleotides were able to restore the defective splicing of a chloride channel [93]. Similarly, alternative splicing of FGFR-1 is found in human glioblastoma, and this defective exon skipping was reduced by morpholino oligonucleotides [94].
Comparing anti-VEGF treatment with the possible use of recombinant VEGF165b or small molecular inhibitors that change VEGF splicing towards VEGFxxxb indicates that VEGF165b would have a more beneficial effect, as the cytoprotective benefits of VEGF would be maintained but the angiogenic potential is removed. In some instances, such as AMD or diabetic retinopathy, systemic delivery or topical administration of small molecules may be possible instead of intraocular injection.
The advantages of generating endogenous anti-angiogenic agents by altering splicing include:
Increased normal cell survival by increasing endogenous survival factors while inhibiting tumor angiogenesis;
Pleiotropic effects of other splicing pathways common to tumorigenesis, including altering VEGFR function by altering VEGFR splicing, hijacking the tumors own hypoxic response to be more anti-angiogenic;
The lack of a direct effect on transcription in nontumor tissues.
It has been suggested that, on the contrary, targeting splicing will be nonspecific, toxic and likely to affect normal physiological function. However, there appear to be a collection of splicing factors that are much more involved in cancer splicing, and recent data using systemic administration of high doses of splicing factor kinase inhibitors show that they have low toxicity [95].
In summary, whereas the anti-angiogenic therapies of the past have been nonspecific anti-VEGF agents, it is now clear that specifically targeting the pro-angiogenic signaling of VEGF and its receptors would be a better therapeutic approach than inhibition of both the benerficial and pathological pathways. A further development, and future oncological therapy, could be to target the splicing factors and their kinases that regulate VEGF and other cancer-involved splicing processes.
Executive summary.
VEGE-A:splicing of the gene generates proteins with pleiotropic functions
Alternative splicing in the C-terminal of vascular endothelial growth factor-A (VEGF-A) leads to a six amino acid substitution.
VEGFxxx induces angiogenesis, Whereas VEGExxxb is a partial agonist of VEGFR, unable to induce angiogenesis, but inhibits VEGF165-induced angiogenic processes.
VEGFxxxb is cytoprotective and the relative level of VEGFXXXb is high in normal tissues and is reduced in canacer.
VEGF splicing can be regulated by growth factors and alternative splice factors.
VEGF treatments today & in the future
Interrupting VEGF/VEGFR to reduce angiogenesis has moved into the clinic to treat a range to tumor types and eye disease, but not without varied response rates, side effects and resistance.
Focusing on splicing and how this can be altered in vivo is a promising treatment target. Intial experiments show that affecting VEGF splicing is able to reduce neovascularization in the eye.
Splicing control may be a novel anti-angiogenic strategy.
Acknowledgments
The authors wish to thank Dr Sebastian Oltean for reading and discussions on the review, and Dr Jeanette Woolard for critically reading the review.
Footnotes
Financial & competing interests disclosure Dr Steven J Harper and Dr David O Bates are co-inventors on a patent relating to control of splicing. Dr Emma S Rennel is funded by Fight for Sight, and Dr David O Bates by the British Heart Foundation. This work was also critically dependent upon funding from the Association for International Cancer Research. Dr Steven J Harper and Dr David O Bates have received financial support for their research, and acted as a consultant for Philogene, who hold the license for therapeutic development of VEGF-A165b. Dr Steven Harper and Dr David O Bates are inventors on the VEGF-A165b patent, and Dr David O Bates has received travel support from Philogene Inc. over the last 12 months. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Emma S Rennel, Microvascular Research Laboratories, Department of Physiology and Pharmacology, School of Veterinary Sciences, University of Bristol, Southwell Street, Bristol BS2 8EJ, UK Tel:.+44 117 982 8367 Fax: +44 117 982 8151 Emma.Rennel@bris.ac.uk.
Steven J Harper, Microvascular Research Laboratories, Department of Physiology and Pharmacology, School of Veterinary Sciences, University of Bristol, Southwell Street, Bristol BS2 8EJ, UK.
David O Bates, Microvascular Research Laboratories, Department of Physiology and Pharmacology, School of Veterinary Sciences, University of Bristol, Southwell Street, Bristol BS2 8EJ, UK Tel:. +44 117 982 8367 Fax: +44 117 982 8151 Dave.Bates@bris.ac.uk.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a noval heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 3.Gospodarowicz D, Abraham JA, Schilling J. Isolation and characterization of a vascular endothelial cell mitogen produced by pituitary-derived folliculo stellate cells. Proc. Natl Acad. Sci. USA. 1989;86:7311–7315. doi: 10.1073/pnas.86.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 5.Plouet J, Schilling J, Gospodarowicz D. Isolation and characterization for a newly identified endothelial cell mitogen produced by AtT-20 cells. Embo. J. 1989;8:3801–3806. doi: 10.1002/j.1460-2075.1989.tb08557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keck PJ, Hauser SD, Krivi G, et al. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989;246:1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- 7.Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cel lines. Cancer. Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- 8.Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis. 2008;11:109–119. doi: 10.1007/s10456-008-9099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling –in control of vascular function. Nat. Rev. Mol. Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 10.Penn JS, Madan A, Caldwell RB, et al. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. •• The initial study showing that anti-VEGF and anti-angiogenic treatment is effective in patients with colorectal cancers.
- 12.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 13.Lemmens L, Claes V, Uzzell M. Managing Patients with metastatic colorectal cancer on bevacizumab. Br. J. Nurs. 2008;17:944–949. doi: 10.12968/bjon.2008.17.15.30695. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 15.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc. Natl Acad. Sci. USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruckman J, Green LS, Beeson J, et al. 2′-fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J. Biol. Chem. 1998;273:20556–20567. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- 17.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 18.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 20.Moutzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 21.Goodman VL, Rock EP, Dagher R, et al. Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin. Cancer. Res. 2007;13:1367–1373. doi: 10.1158/1078-0432.CCR-06-2328. [DOI] [PubMed] [Google Scholar]
- 22.Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purow B. Notch inhibitors as a new tool in the war on cancer: a pathway to watch. Curr. Pharm. Biotechnol. 2009;10:154–160. doi: 10.2174/138920109787315060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thurston G, Kitajewski J. VEGF and Delta-Notch: interacting signalling pathways in tumour angiogenesis. Br. J. Cancer. 2008;99:1204–1209. doi: 10.1038/sj.bjc.6604484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carmeliet P, Ferreira V, Breier G, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 27.Ferrara N, Carver-Moore K, Chen H, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 28.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinasein regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 29.Fong GH, Zhang L, Bryce DM, Peng J. Increased hemangioblast commitment, not vascular disorganization, is the primary defet in flt-1 knock-out mice. Development. 1999;126:3015–3025. doi: 10.1242/dev.126.13.3015. [DOI] [PubMed] [Google Scholar]
- 30.Lyden D, Hattori K, Dias S, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 31.De Bandt M, Mahdi MH Ben, Ollivier V, et al. Blockade of vascular endothelial growth factor receptor I (VEGF-RI), but not VEGF-RII,suppresses joint destruction in the K/BxN model of rheumatoid arthritis. J. Immunol. 2003;171:4853–4859. doi: 10.4049/jimmunol.171.9.4853. [DOI] [PubMed] [Google Scholar]
- 32.Hiratsuka S, Maru Y, Okada A, et al. Involvement of Flt-1 tyrosine kinase (vascular endothelial growth factor receptor-1) in pathological angiogenesis. Cancer. Res. 2001;61:1207–1213. [PubMed] [Google Scholar]
- 33.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl Acad. Sci. USA. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka K, Yamaguchi S, Sawano A, Shibuya M. Characterization of the extracellular domain in vascular endothelial growth factor receptor-1 (Flt-1 tyrosine kinase) Jpn J. Cancer. Res. 1997;88:867–876. doi: 10.1111/j.1349-7006.1997.tb00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J. Clin. Endocrinol. Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 36.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 38.Asahara T, Takahashi T, Masuda H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. Embo. J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin K, Zhu Y, Sun Y, et al. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl Acad. Sci. USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lafuente JV, Argandona EG, Mitre B. VEGFR-2 expression in brain injury: its distribution related to brain–blood barrier markers. J. Neural Transm. 2006;113:487–496. doi: 10.1007/s00702-005-0407-0. [DOI] [PubMed] [Google Scholar]
- 41.Skold MK, Risling M, Holmin S. Inhibition of vascular endothelial growth factor receptor 2 activity in experimental brain contusions aggravates injury outcome and leads to early increased neuronal and glial degeneration. Eur. J. Neurosci. 2006;23:21–34. doi: 10.1111/j.1460-9568.2005.04527.x. [DOI] [PubMed] [Google Scholar]
- 42.Deckers MM, Karperien M, van der Bent C, et al. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667–1674. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- 43.Nishiguchi KM, Nakamura M, Kaneko H, Kachi S, Terasaki H. The role of VEGF and VEGFR2/Flk 1 in proliferation of retinal progenitor cells in murine retinal degeneration. Invest. Ophthalmol. Vis. Sci. 2007;48:4315–4320. doi: 10.1167/iovs.07-0354. [DOI] [PubMed] [Google Scholar]
- 44.Casella I, Feccia T, Chelucci C, et al. Autocrine-paracrine VEGF loops potentiate the maturation of megakaryocytic precursors through Flt1 receptor. Blood. 2003;101:1316–1323. doi: 10.1182/blood-2002-07-2184. [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eremina V, Sood M, Haigh J, et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foster RR, Hole R, Anderson K, et al. Functional evidence that vascular endothelial growth factor may act as an autocrine factor on human podocytes. Am. J. Physiol. Renal Physiol. 2003;284:F1263–F1273. doi: 10.1152/ajprenal.00276.2002. [DOI] [PubMed] [Google Scholar]
- 48.Bevan HS, van den Akker NM, Qiu Y, et al. The alternatively spliced anti-angiogenic family of VEGF isoforms VEGF XXXb in human kidney development. Nephron. Physiol. 2008;110:57–67. doi: 10.1159/000177614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varey AH, Rennel ES, Qiu Y, et al. VEGF 165 b, an antiangiogenic VEGF-A isoform, binds and inhibits bevacizumab treatment in experimental colorectal carcinoma: balance of pro- and antiangiogenic VEGF-A isoforms has implications for therapy. Br. J. Cancer. 2008;98:1366–1379. doi: 10.1038/sj.bjc.6604308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bates DO, Cui TG, Doughty JM, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer. Res. 2002;62:4123–4131. •• First evidence for a sister family of anti-angiogenic VEGF isoforms.
- 51.Cebe Suarez S, Pieren M, Cariolato L, et al. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell Mol. Life Sci. 2006;63:2067–2077. doi: 10.1007/s00018-006-6254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawamura H, Li X, Harper SJ, Bates DO, Claesson-Welsh L. Vascular endothelial growth factor (VEGF)-A165b is a weak in vitro agonist for VEGF receptor-2 due to lack of coreceptor binding and deficient regulation of kinase activity. Cancer. Res. 2008;68:4683–4692. doi: 10.1158/0008-5472.CAN-07-6577. •• Cell signaling study of VEGF165b/VEGF-2 altered interaction and signal transduction response, along with data showing that VEGF165b can not induce angiogenesis in vivo.
- 53.Ruch C, Skiniotis G, Steinmetz MO, Walz T, Ballmer-Hofer K. Structure of a VEGF-VEGF receptor complex determined by electron microscopy. Nat. Struct. Mol. Biol. 2007;14:249–250. doi: 10.1038/nsmb1202. [DOI] [PubMed] [Google Scholar]
- 54.Rennel ES, Hamdollah-Zadeh MA, Wheatley ER, et al. Recombinant human VEGF165b protein is an effective anti-cancer agent in mice. Eur. J. Cancer. 2008;44:1883–1894. doi: 10.1016/j.ejca.2008.05.027. • Experimental indications that administration of recombinant VEGF165b can reduce mouse tumor growth in vivo with no apparent adverse effects.
- 55.Woolard J, Wang WY, Bevan HS, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer. Res. 2004;64:7822–7835. doi: 10.1158/0008-5472.CAN-04-0934. [DOI] [PubMed] [Google Scholar]
- 56.Qiu Y, Bevan H, Weeraperuma S, et al. Mammary alveolar development during lactation is inhibited by the endogenous antiangiogenic growth factor isoform, VEGF165b. Faseb J. 2008;22:1104–1112. doi: 10.1096/fj.07-9718com. • Physiological angiogenesis in the female is disrupted by overexpression of VEGF165b. Indications that exact controlling of the splice variants is needed.
- 57.Glass CA, Harper SJ, Bates DO. The anti-angiogenic VEGF isoform VEGF165b transiently increases hydraulic conductivity, probably through VEGF receptor 1 in vivo. J. Physiol. 2006;572:243–257. doi: 10.1113/jphysiol.2005.103127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rennel E, Waine E, Guan H, et al. The endogenous anti-angiogenic VEGF isoform, VEGF165b inhibits human tumour growth in mice. Br. J. Cancer. 2008;98:1250–1257. doi: 10.1038/sj.bjc.6604309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konopatskaya O, Churchill AJ, Harper SJ, Bates DO, Gardiner TA. VEGF165b, an endogenous C-terminal splice variant of VEGF, inhibits retinal neovascularization in mice. Mol. Vis. 2006;12:626–632. [PubMed] [Google Scholar]
- 60.Johnson JM, Castle J, Garrett-Engele P, et al. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science. 2003;302:2141–2144. doi: 10.1126/science.1090100. [DOI] [PubMed] [Google Scholar]
- 61.Modrek B, Lee CJ. Alternative splicing in the human, mouse and rat genomes is associated with an increased frequency of exon creation and/or loss. Nat. Genet. 2003;34:177–180. doi: 10.1038/ng1159. [DOI] [PubMed] [Google Scholar]
- 62.Harper SJ, Bates DO. VEGF-A splicing: the key to anti-angiogenic therapeutics? Nat. Rev. Cancer. 2008 doi: 10.1038/nrc2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Long JC, Caceres JF. The SR protein family of splicing factors: master regulators of gene expression. Biochem. J. 2009;417:15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- 64.Venables JP. Unbalanced alternative splicing and its significance in cancer. Bioessays. 2006;28:378–386. doi: 10.1002/bies.20390. [DOI] [PubMed] [Google Scholar]
- 65.Venables JP. Aberrant and alternative splicing in cancer. Cancer. Res. 2004;64:7647–7654. doi: 10.1158/0008-5472.CAN-04-1910. [DOI] [PubMed] [Google Scholar]
- 66.Klinck R, Bramard A, Inkel L, et al. Multiple alternative splicing markers for ovarian cancer. Cancer. Res. 2008;68:657–663. doi: 10.1158/0008-5472.CAN-07-2580. [DOI] [PubMed] [Google Scholar]
- 67.Venables JP, Klinck R, Bramard A, et al. Identification of alternative splicing markers for breast cancer. Cancer. Res. 2008;68:9525–9531. doi: 10.1158/0008-5472.CAN-08-1769. [DOI] [PubMed] [Google Scholar]
- 68.Stearns ME, Wang M, Hu Y, Kim G, Garcia FU. Expression of a flt-4 (VEGFR3) splicing variant in primary human prostate tumors. VEGF D and flt-4t(Delta773–1081) overexpression is diagnostic for sentinel lymph node metastasis. Lab. Invest. 2004;84:785–795. doi: 10.1038/labinvest.3700075. [DOI] [PubMed] [Google Scholar]
- 69.Mills AA. p53: link to the past, bridge to the future. Genes Dev. 2005;19:2091–2099. doi: 10.1101/gad.1362905. [DOI] [PubMed] [Google Scholar]
- 70.Diaz R, Pena C, Silva J, et al. p73 Isoforms affect VEGF, VEGF165b and PEDF expression in human colorectal tumors: VEGF165b downregulation as a marker of poor prognosis. Int. J. Cancer. 2008;123:1060–1067. doi: 10.1002/ijc.23619. [DOI] [PubMed] [Google Scholar]
- 71.Pritchard-Jones RO, Dunn DB, Qiu Y, et al. Expression of VEGFxxxb, the inhibitory isoforms of VEGF, in malignant melanoma. Br. J. Cancer. 2007;97:223–230. doi: 10.1038/sj.bjc.6603839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller-Kasprzak E, Jagodzinski PP. 5-Aza-2′-deoxycytidine increases the expression of anti-angiogenic vascular endothelial growth factor 189b variant in human lung microvascular endothelial cells. Biomed. Pharmacother. 2008;62:158–163. doi: 10.1016/j.biopha.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 73.Perrin RM, Konopatskaya O, Qiu Y, Harper S, Bates DO, Churchill AJ. Diabetic retinopathy is associated with a switch in splicing from anti- to pro-angiogenic isoforms of vascular endothelial growth factor. Diabetologia. 2005;48:2422–2427. doi: 10.1007/s00125-005-1951-8. [DOI] [PubMed] [Google Scholar]
- 74.Bates DO, MacMillan PP, Manjaly JG, et al. The endogenous anti-angiogenic family of splice variants of VEGF, VEGFxxxb, are down-regulated in pre-eclamptic placentae at term. Clin. Sci. (Lond.) 2006;110:575–585. doi: 10.1042/CS20050292. [DOI] [PubMed] [Google Scholar]
- 75.Bills VL, Varet J, Millar AB, Harper SJ, Soothil PW, Bates D. Failure to upregulate VEGF165b in maternal plasma is a first trimester predictive marker for pre-eclampsia. Clin. Sci. (Lond.) 2009;116:265–272. doi: 10.1042/CS20080270. • VEGF165b as a possible early predictive marker for pre-eclampsia.
- 76.Schumacher VA, Jeruschke S, Eitner F, et al. Impaired glomerular maturation and lack of VEGF165b in Denys–Drash syndrome. J. Am. Soc. Nephrol. 2007;18:719–729. doi: 10.1681/ASN.2006020124. [DOI] [PubMed] [Google Scholar]
- 77.Nowak DG, Woolard J, Amin EM, et al. Expression of pro- and anti-angiogenic isoforms of VEGF is differentially regulated by splicing and grwoth factors. J. Cell Sci. 2008;121:3487–3495. doi: 10.1242/jcs.016410. •• First data on how VEGF splicing is regulated and what external factors, signaling pathways and splice factors can be involved.
- 78.Colwill K, Feng LL, Yeakley JM, et al. SRPK1 and Clk/Sty protein kinases show distinct substrate specificities for serine/arginine-rich splicing factors. J. Biol. Chem. 1996;271:24569–24575. doi: 10.1074/jbc.271.40.24569. [DOI] [PubMed] [Google Scholar]
- 79.Ma CT, Velazquez-Dones A, Hagopian JC, et al. Ordered multi-site phosphorylation of the splicing factor ASF/SF2 by SRPK1. J. Mol. Biol. 2008;376:55–68. doi: 10.1016/j.jmb.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 80.Wang HY, Lin W, Dyck JA, et al. SRPK2: a differentially expressed SR protein-specific kinase involved in mediating the interaction and localization of pre-mRNA splicing factors in mammalian cells. J. Cell Biol. 1998;140:737–750. doi: 10.1083/jcb.140.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fukuhara T, Hosoya T, Shimizu S, et al. Utilization of host SR protein kinases and RNA-splicing machinery during viral replication. Proc. Natal Acad. Sci. USA. 2006;103:11329–11333. doi: 10.1073/pnas.0604616103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stickeler E, Kittrell F, Medina D, Berget SM. Stage-specific changes in SR splicing factors and alternative splicing in mammary tumorigenesis. Oncogene. 1999;18:3574–3582. doi: 10.1038/sj.onc.1202671. [DOI] [PubMed] [Google Scholar]
- 83.Zerbe LK, Pino I, Pio R, et al. Relative amounts of antagonistic splicing factors, hnRNP A1 and ASF/SF2, change during neoplastic lung growth: implications for pre-mRNA processing. Mol. Carcinog. 2004;41:187–196. doi: 10.1002/mc.20053. [DOI] [PubMed] [Google Scholar]
- 84.Fischer DC, Noack K, Runnebaum IB, et al. Expression of splicing factors in human ovarian cancer. Oncol. Rep. 2004;11:1085–1090. [PubMed] [Google Scholar]
- 85.Karni R, de Stanchina E, Lowe SW, et al. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat. Struct. Mol. Biol. 2007;14:185–193. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sinclair CS, Rowley M, Naderi A, Couch FJ. The 17q23 amplicon and breast cancer. Breast Cancer. Res. Treat. 2003;78:313–322. doi: 10.1023/a:1023081624133. [DOI] [PubMed] [Google Scholar]
- 87.Wang F, Kan M, Yan G, Xu J, McKeehan WL. Alternately spliced NH2-terminal immunoglobulin-like Loop I in the ectodomain of the fibroblast growth factor (FGF) receptor 1 lowers affinity for both heparin and FGF-1. J. Biol. Chem. 1995;270:10231–10235. doi: 10.1074/jbc.270.17.10231. [DOI] [PubMed] [Google Scholar]
- 88.Vickers SM, Huang ZQ, MacMillan-Crow L, Greendorfer JS, Thompson JA. Ligand activation of alternatively spliced fibroblast growth factor receptor-1 modulates pancreatic adenocarcinoma cell malignancy. J. Gastrointest. Surg. 2002;6:546–553. doi: 10.1016/s1091-255x(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 89.Luqmani YA, Mortimer C, Yiangou C, et al. Expression of 2 variant forms of fibroblast growth factor receptor 1 in human breast. Int. J. Cancer. 1995;64:274–279. doi: 10.1002/ijc.2910640411. [DOI] [PubMed] [Google Scholar]
- 90.Yamaguchi F, Saya H, Bruner JM, Morrison RS. Differential expression of two fibroblast growth factor-receptor genes is associated with malignant progression in human astrocytomas. Proc. Natl Acad. Sci. USA. 1994;91:484–488. doi: 10.1073/pnas.91.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hayes GM, Carrigan PE, Beck AM, Miller LJ. Targeting the RNA splicing machinery as a novel treatment strategy for pancreatic carcinoma. Cancer. Res. 2006;66:3819–3827. doi: 10.1158/0008-5472.CAN-05-4065. [DOI] [PubMed] [Google Scholar]
- 92.Hayes GM, Carrigan PE, Miller LJ. Serine-arginine protein kinase 1 overexpression is associated with tumorigenic imbalance in mitogen-activated protein kinase pathways in breast, colonic, and pancreatic carcinomas. Cancer. Res. 2007;67:2072–2080. doi: 10.1158/0008-5472.CAN-06-2969. [DOI] [PubMed] [Google Scholar]
- 93.Wheeler TM, Lueck JD, Swanson MS, Dirksen RT, Thornton CA. Correction of ClC-1 splicing eliminates chloride channelopathy and myotonia in mouse models of myotonic dystrophy. J. Clin. Invest. 2007;117:3952–3957. doi: 10.1172/JCI33355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bruno IG, Jin W, Cote GJ. Correction of aberrant FGFR1 alternative RNA splicing through targeting of intronic regulatory elements. Hum. Mol. Genet. 2004;13:2409–2420. doi: 10.1093/hmg/ddh272. [DOI] [PubMed] [Google Scholar]
- 95.Hagiwara M. Alternative splicing: a new drug target of the post-genome era. Biochim. Biophys. Acta. 2005;1754:324–331. doi: 10.1016/j.bbapap.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 96.Koh MY, Spivak-Kroizman T, Venturini S, et al. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1α. Mol. Cancer Ther. 2008;7:90–100. doi: 10.1158/1535-7163.MCT-07-0463. [DOI] [PubMed] [Google Scholar]
- 97.Hua Z, Lv Q, Ye W, et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS ONE. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yomoda J, Muraki M, Kataoka N, et al. Combination of Clk family kinase and SRp75 modulates alternative splicing of adenovirus E1A. Genes Cells. 2008;13:233–244. doi: 10.1111/j.1365-2443.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 99.Witte L, Hicklin DJ, Zhu Z, et al. Monoclonal antibodies trageting the VEGF receptor-2 (Flk1/KDR) as an anti-angiogenic therapeutic strategy. Cancer Metastasis. Rev. 1998;17:155–161. doi: 10.1023/a:1006094117427. [DOI] [PubMed] [Google Scholar]