Summary
New vessel formation during development and in the adult is triggered by concerted signals of largely endothelial-specific receptors for ligands of the VEGF, angiopoietin and ephrin families. The signals and genes induced by these receptors operate in the context of additional signals transduced by non-endothelial specific growth factor receptors, inflammatory cytokine receptors as well as adhesion molecules. We summarize here available data on characteristic signaling of the VEGF receptor-2 and the current state of knowledge regarding the additional different receptor tyrosine kinases of the VEGF, Tie and Ephrin receptor families. Furthermore, the potential cross-talk with signals induced by other growth factors and inflammatory cytokines as well as the modulation by VE-cadherin is discussed.
Keywords: Angiogenesis and inhibitors, signal transduction, endothelial cells
Introduction
Blood vessels are essential to carry oxygen, nutrients and hormones to distant organs, whereas lymphatic vessels function to return fluid and cells from the interstitial space. In the embryo the first blood vessels are generated from endothelial precursors, which have a common origin with hematopoietic progenitors, – a process known as vasculogenesis (1). The vasculature develops further by angiogenesis, the sprouting of new capillaries from existing vascular structures. Lymphatic endothelial cells are thought to arise by sprouting from embryonic veins (2). In the adult endothelial cells maintain the ability to rapidly grow and sprout in response to physiological stimuli such as hypoxia for blood vessels and inflammation for lymphatics. Blood and lymph vessel angiogenesis is normally reactivated mainly during wound healing and repair. However, angiogenesis can be switched on in many pathologies, such as malignant, ocular and inflammatory disorders, and then significantly contributes to the progress of the disease (3).
It has become clear over the last decade that vasculogenesis as well as angiogenesis are primarily regulated through several receptors, which are preferentially expressed in endothelial cells, and which belong to the VEGF, Tie and ephrin receptor families (4). Three VEGF receptors, two Tie receptors and one ephrin receptor have been shown to control different stages in endothelial cell differentiation and vessel formation. Whereas VEGF receptors regulate endothelial differentiation and the initiation of vasculogenesis and angiogenesis, the Tie receptors control later stages in vessel formation such as the stabilization of the initial endothelial sprout and its interaction with subendothelial cells (5). The EphB4 receptor interacting with its ligand ephrinB2 is thought to be involved in arterial-venous specification (6). All these receptors have intrinsic tyrosine kinase activity and multiple tyrosine residues in their cytoplasmic tails. It is currently not known to which extent they induce differential signals and gene repertoires based on a differential docking to signaling cascades in the endothelial cell and to which extent their distinct biological effects are caused by the differential spatial and temporal expression of the receptors. Generally, adult endothelium is in a quiescent state and has a very low turn over rate (7); however, upon wounding or in pathologic conditions connected with chronic inflammation or cancer endothelial sprouting, migration and proliferation can be initiated.
Further, endothelial cells express a number of receptors found on many different cell types, which can either synergize or interfere with signals of the endothelial-specific receptors. Among those is the bFGF receptor, which can transduce a strong angiogenic signal by itself and appears to be decisively involved in pathologic forms of angiogenesis, especially in tumor angiogenesis (8). For example, it has been shown in a transgenic mouse model of pancreatic beta cell carcinogenesis that angiogenesis could be inhibited synergistically by blocking bFGF and VEGF with soluble receptors (9). In this model soluble FGF receptor appeared to preferentially impair the maintenance of tumor angiogenesis, whereas soluble VEGF receptor-1 predominantly affected the initiation of tumor angiogenesis. Other growth factor receptors on endothelial cells include members of the PDGF and EGF receptor families (10, 11). PDGF receptor-beta expression on early endothelial precursors seems to accelerate the differentiation of endothelial cells and to regulate vascular/hematopoietic development (12). EGF receptor expression has been described in tumor endothelium and seems to contribute to endothelial cell proliferation (10). The TGF-β receptors are involved in later stages of vessel maturation (7). They can inhibit endothelial proliferation and induce extracellular matrix deposition, thus playing a role in the resolution phase of angiogenesis. In contrast to bFGF, PDGF and EGF receptors, which all are tyrosine kinase receptors, the TGF-β receptor has serine/threonine kinase activity (13). There is further considerable evidence that receptors for inflammatory cytokines play important roles and participate in the cross-regulation of inflammation and angiogenesis in many pathologies (14, 15).
The integrity of the endothelial cell layer depends on cell-to-cell and cell-to-extracellular-matrix contacts mediated by adhesion molecules such as VE-cadherin and specific forms of integrins. VE-cadherin forms adherens junctions, mediates contact inhibition and survival of endothelial cells and signals through its association with β-catenin (16). Integrins attach the cells to fibronectin and vitronectin, and this appears to modulate signals by growth factor receptors in endothelial cells (17).
A specific case of receptors, which are expressed in several cell types including endothelial and neuronal cells, are further the neuropilin receptors. They lack intrinsic catalytic activity but can function as co-receptors for VEGF and modulate signaling via VEGF receptors (18). For example, inclusion of neuropilin in the VEGF-A/VEGF receptor-2 signaling complex can enhance endothelial cell biological responses.
To date most data available concern the signaling of VEGF-A/VEGF receptor-2 in endothelial cells. We will therefore first discuss in more detail the VEGF receptor-2 signaling and how it is distinguished from signals induced by other growth factors and inflammatory cytokines. Then we will summarize the in part limited knowledge on the other receptors and the potential cross-talk in the promotion of endothelial differentiation and angiogenesis.
Signals induced by the VEGF receptors
The VEGF receptor family comprises three receptors known as VEGF receptor-1 (or Fms-like tyrosine kinase-1, Flt-1) (19), VEGF receptor-2 (also KDR in humans or Flk-1 in mice) (20-22) and VEGF receptor-3 (or Flt-4) (23). These are activated by a family of in part cross-reactive ligands, VEGF-A binds both VEGF receptor-1 and VEGF receptor-2, VEGF-B and P1GF (placenta growth factor) bind selectively to VEGF receptor-1 (24-26), whereas VEGF-C and -D are ligands of VEGF receptor-3 (27). The proteolytically processed VEGF-C/-D isoforms bind also to VEGF receptor-2, however, with a binding affinity an order of magnitude lower than VEGF-A (27). The complexity of the system increases by the recent findings of VEGFxxxb isoforms with anti-angiogenic properties (28), which are formed using an alternative 3′ splice site in exon 8 and differ from other VEGF forms by only six amino acids at the C-terminus. Finally, the endocrine-gland-derived vascular endothelial growth factor (EG-VEGF) is an example of a highly specific growth factor for capillary endothelial cells of endocrine glands with no significant activity on other endothelial cells (29). Furthermore, several parapox viruses encode VEGF-like proteins known as the VEGF-E family, which specifically bind and activate only VEGF receptor-2 (30, 31), whereas the recently discovered VEGF-F from snake venom binds VEGF receptor-1 and VEGF receptor-2 (32).
VEGF receptor-2 is expressed at a high level in vascular endothelial progenitors during embryogenesis (1). Expression then declines during development, but can be upregulated on endothelial cells in pathologic angiogenesis (33). In addition, VEGF receptor-2 expression has been described in hematopoietic stem cells and some non-endothelial cells such as neuronal cells and osteoblasts (34, 35). Besides its expression in endothelial cells, VEGF receptor-1 is also found in cells of the monocytic lineage and regulates migration of these cells (36). VEGF receptor-3 is primarily expressed in lymphatic endothelial cells (2).
The VEGF receptors can induce several cellular functions: similar to other growth factor receptors they provide signals important for survival, proliferation and migration. However, in addition they are capable of triggering endothelial specific functions such as sprouting and the formation of tubular structures, and they promote differentiation in progenitor cells (37). The basis to understanding many of the important functions of the VEGF receptors in regard to vasculogenesis and angiogenesis has been provided by gene knock-out experiments in mice (1). One further primary function of VEGF-A is the induction of blood vessel permeability (38, 39). VEGF receptors are also involved in the induction of nitric oxide (NO) via endothelial nitric oxide synthase (eNOS), which regulates vascular tone, cell adhesion to the endothelium, inhibition of platelet aggregation and smooth muscle cell proliferation (40).
All three VEGF receptors contain multiple tyrosine residues in their cytoplasmic domain, in the case of VEGF receptor-2 there are 19 tyrosines. Several of these have been been identified as autophosphorylation sites (residues 951, 1054, 1059, 1175, 1214) (41). Y1054 and Y1059 are implicated in positive regulation of the intrinsic receptor kinase (42), and others have been shown to serve as docking sites for adaptors and signaling molecules such as Y1175 for PLC-γ (43) and Y951 for TsAd (41). It is possible that differences in the recruitment of adaptors/signaling molecules contribute, in addition to differences in receptor expression, to specific biological functions exerted by the VEGF receptors in comparison to other growth factor receptors with tyrosine kinase activity.
In this respect we have investigated the downstream signaling of VEGF receptor-2 and the specific gene repertoire induced in comparison to another, non-endothelial specific growth factor receptor, the EGF receptor, and the inflammatory cytokines TNF-α and IL-1 [ref. (44-46) and B. Schweighofer and E. Hofer, in preparation] (see Fig. 1). The obtained data show that VEGF-A induces a large spectrum of more than 100 genes. It is a characteristic feature of the VEGF-A-induced gene repertoire that about 40% of these genes are also inducible by IL-1. In contrast, this fraction of genes cannot be upregulated by EGF. It seems that gene activation via PLC-γ, PKC and calcineurin provides VEGF-A with the competence to regulate many genes in common with IL-1, since the EGF receptor does not trigger PLC-γ and NFAT in endothelial cells, but rather induces the MAPK pathway predominantly via Ras (B. Schweighofer and E. Hofer, in preparation). Based on these data it appears that VEGF receptor-2 signaling and gene regulation includes an “inflammatory” component, which at least in part could be caused by the upregulation of a group of genes containing NFAT as well as NF-κB binding sites in their promoters. VEGF receptor-2 signals seem to induce NFAT for the upregulation of this group of genes, whereas the IL-1 receptor rather uses NF-κB. Furthermore, about 20% of the genes are specifically regulated by VEGF and not by EGF or IL-1, and we propose that these might be important for specific angiogenic properties of the factor.
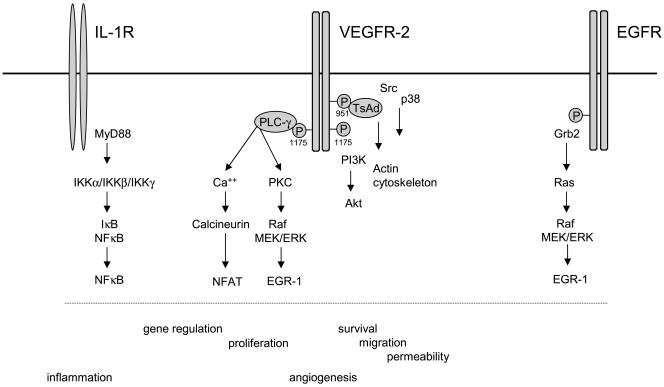
Figure 1. Signal transduction by VEGF-A/VEGF receptor-2.
Major characteristic pathways activated by VEGF-A/VEGF receptor-2 and in part distinct from pathways induced by the IL-1 and EGF receptors in endothelial cells are shown. VEGF-A couples via Tyr1175 to PLC-γ (43, 55) activating the Ca++/calcineurin and PKC/MAPK pathways. This leads to the induction of the transcription factors NFAT and EGR-1, which are important for part of the VEGF-induced gene repertoire and the angiogenic/proliferative response (45-47, 53) (G. Schabbauer and E. Hofer, in preparation). Other pathways activated lead via PI3K/Akt, TsAd, Src and p38 to the promotion of survival, migration and potentially permeability, respectively (41, 57, 112, 115). The IL-1 receptor induces genes to a large extent via NF-κB (103, 104). A significant fraction of the NF-κB-induced genes appears to be also upregulated by VEGF-A-induced NFAT. EGF seems to induce the MAPK pathway via Ras and does not activate NFAT (53) (Schweighofer B and Hofer E, in preparation). Neither VEGF-A nor EGF trigger NF-κB activation to a significant extent (45).
Additional data have shown that EGR-1 is an important transcription factor for VEGF-mediated gene induction in endothelial cells (46), since a specific suppressor of EGR-1, NAB2, inhibits part of VEGF-mediated gene induction and angiogenesis models in vitro and in vivo (47, 48). EGR-1 has been described to play similarly important roles during hypoxia and chronic inflammation, especially in the context of vascular pathologies (49, 50) and as a regulator of growth and differentiation in several cell types including nerve cells (51, 52). Thus it appears that EGR-1 functions as a necessary transcription factor for the regulation of multiple genes by different inducers in many cell types. It may be required in the cooperation with other potentially more inducer-specific transcription factors such as NFAT or NF-κB to achieve appropriate transcriptional activation. In endothelial cells we have further analyzed signals originating from the VEGF receptor-2 and leading to EGR-1. The upregulation of EGR-1 by VEGF could be blocked by a specific MEK inhibitor and by a PKC inhibitor suggesting the upregulation of EGR-1 via PKC and the MEK/ERK MAP-kinase cascade (45).
Another aspect of our work deals with the identification of natural feed-back inhibitors of endothelial cell activation, which could be used to inhibit angiogenic gene induction. As a matter of fact the groups of Aird and Gerber (53, 54) and our laboratory (Schweighofer, B. and Hofer, E., in preparation) find that among the genes most strongly upregulated by VEGF is the DSCR1 gene, which encodes an inhibitor of calcineurin. It is induced by NFAT likely to limit calcineurin and consecutive NFAT activation in a negative feed-back loop. Another example is NAB2, a specific co-repressor of EGR-1, which is induced by EGR-1 and limits EGR-1 effects (47). In this respect adenoviral overexpression of DSCR1 and NAB2 can be used to inhibit VEGF-induced expression of genes leading to reduced sprouting and tubule formation in angiogenesis models (47, 53).
A preferential activation of PKC and Ca++/calcineurin via PLC-γ by VEGF receptor-2 is further supported by data obtained using point mutations of single tyrosine residues in the VEGF receptor-1. When one of the major autophosphorylation sites in the VEGF receptor-2, Tyr1175, was substituted by phenylalanine, the receptor lost the ability to tyrosine phosphorylate PLC-γ and strongly reduced MAP-kinase phosphorylation and DNA synthesis in response to VEGF-A (55). This supports that phosphorylated Tyr1175 is a major docking site for PLC-γ and that VEGF receptor-2 uses this pathway to induce proliferation. Moreover, when knock-in mice were generated with a mutation of the corresponding residue in the murine receptor, Tyr1173, homozygous mice died at days 8.5 to 9.5 due to a failure to generate organized blood vessels. This is the most direct evidence for the importance of the VEGF receptor-2-induced PLC-γ pathway for blood vessel formation (43).
Furthermore, Tyr799 as well as Tyr1173 in the murine receptor seem to play a crucial role in PI-3-kinase activation via binding of p85, resulting in PIP3 production and subsequent activation of PKC-ζ, Akt/PKB and stimulation of p70 S6 kinase (56). In the human receptor Tyr1175 has been also described as a docking site for the adaptor Shb, which mediates PI-3-kinase stimulation, focal adhesion kinase activation and migration induced by VEGF-A (57). The PI-3-kinase induced Akt protein is an important mediator of anti-apoptotic signals but seems also to be implicated in cell migration and tumor progression (58, 59). Another important role of Akt is the regulation of the activity of endothelial eNOS via its phosphorylation at Ser1177. eNOS via the production of NO has significant effects on angiogenesis, blood vessel maturation as well as vasodilation (60, 61). For Tyr951 it has been shown that it binds the adaptor TSAd (T cell specific adaptor) and this appears to link to actin stress fiber induction and migration, but not mitogenesis (41).
Additionally, the focal adhesion kinase FAK is known to significantly contribute to endothelial cell migration via distinct pathways. One of them requires the activation of Src kinase, which is responsible for the phosphorylation dependent recruitment of vinculin to FAK. The other pathway requires HSP90 dependent activation of RhoA and RhoA kinase (ROCK), followed by recruitment of paxillin and vinculin to FAK, due to phosphorylation of Tyr407 within FAK (62). Another VEGF receptor-2 binding molecule implicated in cellular motility and morphogenesis is IQGAP1. It activates via Rac1 NAD(P)H oxidase, leading to a subsequent increase in reactive oxygen species (ROS) generation (63).
A number of further pathways and the binding of additional transcription factors have been described to be activated by VEGF-A/VEGF receptor-2 including factors of the STAT (64) and GATA families (53). However, their activation through specific tyrosines and their relevance for angiogenesis has not yet been elucidated in detail.
In contrast to the strong VEGF receptor-2 signaling VEGF receptor-1 transduces only weak intracellular signals. Although several autophosphorylation sites have been identified including the Tyr1169 as a potential binding site for PLC-γ, the extent of phosphorylation appears to be minor (65, 66). Current evidence suggests that VEGF receptor-1 as a decoy receptor is a negative regulator of vasculogenesis in the embryo, which is supported by the findings that VEGF receptor-1 ko mice die due to overgrowth of endothelial cells and disorganized blood vessels (67). In addition, mice expressing a VEGF receptor-1 lacking the kinase domain develop a normal vasculature (68), suggesting that the kinase domain is dispensable for development. However, the situation may be different for angiogenesis in the adult, since a synergy has been postulated between VEGF receptor-1 and VEGF receptor-2 in tumor angiogenesis (69).
The third isoform of VEGF receptors, VEGF receptor-3, is involved in the first stages of blood vascular development in the embryo, but later expression is largely restricted to lymphatic vessels, and the receptor specifically directs sprouting of lymphatic vessels (70). Although five tyrosines have been identified as phosphorylation sites in the VEGF receptor-3, little is known so far about transduced downstream signals. Tyr1337 has been shown to bind Shc and Grb2 and to initiate the MAP-kinase pathway (71). Furthermore, the transcription factor FOXC2 seems to act downstream of and to cooperate with VEGF receptor-3 in lymphatic vessel development (72). Additional evidence supports a role for Prox-1 as a master regulator of the lymphatic vasculature (73).
In regard to VEGF receptor-2 and VEGF receptor-3 it has been shown that the co-receptors neuropilin-1 and neuropilin-2 can modulate downstream signaling by the receptors. The interaction seems to enhance the VEGF receptor phosphorylation threshold (18). Neuropilins, although they lack intrinsic catalytic activity, appear to enhance signaling and biological responses to VEGF receptors and may be required for certain functions such as the organization of endothelial cells into 3-dimensional vessel structures (74).
Signals induced by the Tie and Eph receptors
Tie1 and Tie2 form a distinct family of tyrosine kinase receptors with preferential expression in endothelial cells (75, 76). Four ligands, angiopoietin 1–4, are known to bind to Tie2 (4). Ang1 has been shown to promote structural integrity of blood vessels, Ang2 appears to be a natural antagonist of Ang1-induced signaling. Ang 3 and 4 are less investigated, but seem to have functions similar to Ang2 and Ang1, respectively. In the absence of clear ligand binding to Tie1, a ligand-independent function has been postulated for this receptor (77), although recent findings suggest that Ang1 and Ang4 can activate Tie1 and its interaction with Tie2 (78).
Targeted mutation of angiopoietins and the Tie receptors in mice has shown that during vasculogenesis these receptors function primarily in remodeling and maturation of the vessels. It appears that Ang1-stimulated Tie2 signaling mediates cell-cell and cell-matrix interactions and the recruitment of peri-endothelial mesenchymal cells to the vessels (79, 80). For the vasculature in the adult the accumulated data suggest a model where constitutively produced paracrine Ang1 acts as a regulator of vessel maturation and vascular quiescence. In turn Ang2, which antagonizes Ang1 binding and signaling of Tie2, seems to function in an autocrine way after release from Weibel-Palade bodies to sensitize endothelial cells to inflammatory and angiogenic activation (81).
A significant amount of data is available on the signal transduction pathways triggered by Ang1/Tie2, which seem to be at least partially turned off by Ang2; however, the functional significance in vivo of most of these cascades is still largely unclear. For example, the p85 subunit of PI-3-kinase binds to Tie2 and signaling through PI-3-kinase has been described to be important for induction of survival, sprouting, migration and capillary tube formation (82, 83). Activated Tie2 was further found to associate with Dok-R/Nck/PAK (84), SHP2 and Grb2, which could activate the Ras-mitogen activated MAPK pathway (85, 86). Furthermore binding of Grb7, Grb14 and ShcA (86, 87) as well as a link to the NF-κB pathway via ABIN-2 was reported (88). Ang-1 is also able to activate STAT3 and 5 (89). Despite this information, it is largely unknown which of these pathways specifically mediate different aspects of the stable and quiescent phenotype characterized by anti-permeability, anti-inflammatory and anti-angiogenic properties. It is possible that part of these properties is caused by interference with and inhibition of signals generated from inflammatory cytokine and VEGF receptors.
Another receptor/ligand system involved in vascular development is the Eph receptor – ephrin ligand system. Eph receptors are the largest family of receptor tyrosine kinases with important roles in the establishment of neuronal and vascular networks (90). They generally have key roles in regulation of cellular migration and adhesion required to form patterns of cell organization (91). Activation of the Eph receptors or ephrin ligands can lead to cell repulsion or to cell adhesion and invasion. Due to their structural properties, Eph receptors and their corresponding ephrin ligands are classified into A and B subfamilies. For the vascular system, gene knock-out experiments in mice have identified EphB4 and ephrinB2 as important determinants of the arterio-venous differentiation (92, 93). Structural characteristics of EphB receptors are an extracellular ephrin ligand-binding domain, an EGF-like motif, and two fibronectin type III motifs. Intracellularly they exhibit a tyrosine kinase domain, followed by a SAM- and a PDZ-binding domain. Signaling of the Eph receptors is referred to as forward signaling, whereas ephrinB ligands, which are transmembrane proteins containing an extracellular Eph receptor-binding domain and a cytoplasmic PDZ-binding domain, confer a respective reverse signal (94). The reverse signal is transmitted via the recruitment of SH2 domain-containing adaptors and SH3-binding proteins to conserved tyrosine and serine phosphorylation sites as well as via the PDZ-domain (6). Expression of EphB4 versus ephrinB2 seems to be controlled by a large number of genes, among which are COUP-TFII, Notch 1/4, Delta4, Gr1 and Hey1/2 (6). Since both Eph receptors and ephrin ligands are membrane molecules, Eph receptor/ephrin signaling is dependent on the cell-cell contact of neighboring cells. Given a preferential angiogenic and arteriogenic expression of ephrin B2, the antagonistic function of EphB4 and ephrinB2 support an artery-to-vein push-and-pull model for invasive angiogenesis (95). It has been suggested that the repulsive Eph receptor signaling is influenced by a cross-talk phenomenon with integrins and results in cytoskeletal alterations involving Rho (96, 97). Furthermore, certain Eph receptors have been shown to directly interact with Ephexin (Eph-interacting exchange factor), a guanine nucleotide exchange factor for Rho family GTPases (98). However, no significant information is so far available on the molecular nature of additional intracellular signals generated in endothelial cells and how they interfere/synergize with signals of the other endothelial-specific tyrosine kinase receptors.
Signals induced by non-endothelial specific receptors
Besides those receptors expressed preferentially in endothelial cells and with largely specific functions for vasculogenesis and angiogenesis, a number of growth factor and cytokine receptors can be found on endothelial cells, which are expressed on a wide variety of tissues. Some of these are important stimulators of pathologic angiogenesis such as the bFGF receptor. Others are involved in later stages of vessel maturation, and some are necessary for additional functions of intercellular communication, monitoring the cells environment or recruitment of immune cells to locations of pathogen infection. Many of these receptors are likely involved in cross-talk with the endothelial cell-specific receptors, are important for the integration of angiogenic signals and their unbalanced stimulation can contribute to aberrant and pathologic angiogenesis. Among them are members of the bFGF-, TGF-β-, PDGF-β- and EGF-receptor families, inflammatory receptors such as the IL-1 receptor and potentially also tissue factor.
bFGF (basic FGF or FGF2) has been known for a long time as potent stimulator of angiogenesis in vitro and in vivo, although its exact role in physiological vessel formation has remained controversial. bFGF is a member of a family of 23 heparin-binding growth factors which can interact with four main types of tyrosine kinase receptors (99). FGF receptor-1 is the main FGF receptor expressed on endothelial cells and can induce proliferation, migration and tubular morphogenesis. A smaller amount of FGF receptor-2 is also detected, but activation of FGF receptor-2 only activates motility. Disruption of the FGF receptor-1 and FGF receptor-2 genes in mice yields embryos that are arrested in development before the onset of vascularization, demonstrating a decisive role in mesoderm formation. However, individual disruption of the bFGF and other FGF genes do not present a vascular phenotype arguing for non-essential or redundant roles of the various FGF isoforms in developmental vasculogenesis and angiogenesis (100). However, a large number of observations is in favor of a decisive role of FGFs/FGF receptors in repair-associated angiogenesis and especially in tumor angiogenesis, since blockade of the FGF pathway by various methods including antisense and neutralizing antibodies inhibits tumor angiogenesis and growth. It has been proposed that bFGF induces neovascularization indirectly since it was shown to up-regulate VEGF and VEGF receptors, and in certain systems bFGF effects could be inhibited by VEGF receptor antagonists. In other models, such as the RIP Tag transgenic mouse model, a synergistic activation of tumor angiogenesis by bFGF and VEGF is suggested (8).
Signaling of the FGF receptor-1 has been investigated in fibroblasts, but limited data are available for endothelial cells. The FGF receptor-1 has seven tyrosine residues in its cytoplasmic domain, two of which have been shown to be important for catalytic activity and signaling, Tyr653 and Tyr654. In addition, Tyr766 has been shown to bind and activate PLC-γ. Further activation of the Src signaling pathway, Crk-mediated signaling as well as signaling via SNT-1/FRS2 has been reported (99). There is evidence from endothelial cells that the gene repertoires induced by VEGF and bFGF are to some degree distinct. Our own data suggest that in endothelial cells bFGF is a much weaker inducer of genes upregulated via the PLC-γ/Ca++/NFAT pathway when compared to VEGF, and only part of the genes found to be induced specifically by VEGF, but e.g. not by EGF or IL-1, are also weakly regulated by bFGF (B. Schweighofer and E. Hofer, in preparation).
Furthermore, members of the TGF-β superfamily are of critical importance for normal vascular development and physiology. The genetic inactivation of the genes for TGF-β1 and of the corresponding type I (Alk1 and Alk5) and type II receptors cause embryonic lethality due to defects in the formation of the primitive vasculature (1). TGF-β signaling contributes in a pleiotropic manner to the resolution and maturation phase of angiogenesis. The available data suggest that TGF-β at low doses contributes to the angiogenic switch by upregulating angiogenic factors, whereas at high doses it inhibits endothelial cell growth (7). TGF-β family members stimulate the serine/threonine activity of type II receptors, which phosphorylate type I receptors and thus activate the downstream signaling Smads. In endothelial cells mainly two distinct TGF-β signaling cascades are activated one via the activin receptor-like kinase 5 (ALK5) and Smad2/3, the second via ALK1 and Smad1/5 (13). A proposed angiogenic switch model suggests that due to differences in affinity low concentrations of TGF-β would stimulate preferentially ALK1-Smad1/5 and migration/proliferation, whereas high TGF-β would induce mainly ALK5-Smad2/3, which leads to PAI-1 and fibronectin expression and inhibition of angiogenesis.
Members of the PDGF and PDGF receptor families play also important roles in vessel formation. Four PDGFs (PDGF-A, -B, -C, -D) and two PDGF receptor chains (PDGF receptor-β, PDGF receptor-β) can form either homo- or heterodimers. In the vascular system it is well established that PDGF-BB and its receptor PDGF receptor-β are essential for the stabilization of nascent blood vessels by recruiting PDF receptor-β positive mesenchymal progenitors (7). PDGF receptor-β has also been reported to be expressed on endothelium (11) and could therefore contribute to endothelial cell signaling. Furthermore, a recent report describes that PDGF receptor-β on early hematopoietic/endothelial (hemangio) precursors is involved in vascular/hematopoietic development and accelerates endothelial differentiation (12). It has been established in different cell types that signaling via PDGF receptors involves multiple signal transduction pathways including Ras/ERK, Src, PI3K and PLC-γ (101).
Another ubiquitously expressed family of growth factor receptors is the epidermal growth factor (EGF) receptor family. It consists of four tyrosine kinase receptors, EGF receptor/ErbB1/HER1, ErbB2/Neu/HER2, ErbB3/HER3, ErbB4/HER4. These receptors can be activated by 13 polypeptide ligands, with EGF, TGF-alpha, HB-EGF and the neuroregulins (NRGs) among them. ERBB1 (EGF receptor) binds to multiple ligands and can form homodimers and three functional heterodimers. EGF receptor, ErbB2 and ErbB4 expression has been described in tumor endothelial cells and seem to contribute to endothelial cell proliferation (10), whereas expression of an inhibitive form of the NRG receptor ErbB3 can be lost in tumor endothelium. It has been described for several cell types that following activation adaptor proteins like GRB2 and Shc are recruited to the phosphorylated form of EGF receptor, leading to recruitment of Ras and the activation of the MAPK cascades. STAT5 is another direct substrate of the EGF receptor and the receptor also couples via Ras to the PI3K-AKT pathway (102). Treatment of HUVEC with EGF results in a strong upregulation of the early response gene EGR-1, but in contrast to VEGF receptor-2 the EGF receptor in endothelial cells seems to be unable to trigger any Ca++/calcineurin-mediated NFAT activation (G. Schabbauer and E. Hofer, in preparation). This suggests that the EGF receptor triggers EGR-1 upregulation solely via Ras and does not activate PLC-γ, which results in the induction of a much smaller gene repertoire when compared to VEGF receptor-2 (B. Schweighofer and E. Hofer, in preparation).
Angiogenesis during wound healing and in tumors takes place in an inflammatory surrounding created by immune cells recruited to the damaged or malignant tissues. Inflammatory mediators such as TNF-α or IL-1 are activators of strong signals in endothelial cells leading to the expression of a large number of inflammatory genes involved in various aspects of immune defense and tissue repair. Inflammatory activation of endothelial cells is characterized by the strong upregulation of the NF-κB pathway, but there is also cross-talk to the MAPK pathways (45). The NF-κB pathway, however, seems to be a major determinant and essential for the upregulation of the majority of the inflammatory genes (103, 104). Details of the activation and inhibition of the inflammatory NF-κB pathway are summarized in the accompanying review by Winsauer and de Martin in this issue of Thrombosis and Haemostasis (see article beginning page 364). It is of interest that a substantial amount of observations suggest a cross-stimulation of inflammation and angiogenesis in many diseases (14). Given the finding that VEGF and IL-1 share an induced gene repertoire of about 40% (B. Schweighofer and E. Hofer, in preparation), it is conceivable that inflammatory signals may considerably lower the threshold for angiogenic stimulation and vice versa.
One of the genes induced by VEGF, but not by EGF, in endothelial cells is the tissue factor gene. Tissue factor is a distant member of the cytokine receptor family and functions on the one hand as high-affinity receptor and co-factor of coagulation factors VII/VIIa. As such it is the major initiator of the extrinsic coagulation pathway. However, it has been shown further that tissue factor on the other hand participates directly in signaling events via its cytoplasmic tail or indirectly by activating downstream coagulation proteases and the PAR-2 receptor (105). Although the biological relevance of tissue factor expression in the process of vessel formation is still under debate, some evidence suggests a role of tissue factor not only during inflammatory, but also during angiogenic response programs of endothelial cells (105-107). Significant tissue factor expression can also be observed in endothelial progenitors and tissue factor signaling could contribute to differentiation or migration of these cells (J. Pomyje and E. Hofer, unpublished observations).
Modulation of signals by adhesion molecules
VE-cadherin is specific for the endothelium and the major constituent of adherens junction (16). It regulates survival and contact inhibition of endothelial cells. Although differential signaling mediated through VE-cadherin is still incompletely understood it is clear that VE-cadherin can influence signaling by interaction with cell-specific growth factor receptors and by controlling β-catenin. It has been shown that VE-cadherin associates with VEGF receptor-2 and modulates its signaling pathways to promote survival and reduce proliferation (16). VE-cadherin expression and clustering reduced tyrosine phosphorylation of VEGF receptor-2, and prevented excessive cell growth and aberrant alterations in vascular development observed when VE-cadherin is absent (108). This action seemed to be connected to density-enhanced phosphatase (DEP1), which causes receptor dephosphorylation. Recent data provide additional insight into how interaction with VE-cadherin influences VEGF receptor-2 signaling (109). When VE-cadherin is absent or not engaged at junctions, the VEGF-induced and clathrin-dependent internalization of VEGF receptor-2 is faster, and the receptor remains longer in endosomal compartments. Importantly, the internalized VEGFR-2 seems to continue to signal, since it is phosphorylated, codistributes with active PLC-γ and activates p44/42 MAP kinases and proliferation.
Vice versa triggering of receptor tyrosine kinases such as VEGF and EGF receptors has been described to reduce VE-cadherin-mediated adhesion (110), whereas tyrosine phosphatases such as VE-PTP promote VE-cadherin-mediated adhesion (111). Also in this respect a recent report provides an explanation. It describes that VEGF stimulation of VEGF receptor-2 via activation of Src, Vav2 and Rac promotes p21-activated kinase (PAK)-mediated phosphorylation of the cytoplasmic tail of VE-cadherin. Consecutively, β-arrestin2 is recruited to the serine-phosphorylated VE-cadherin, and this is followed by rapid endocytosis of VE-cadherin into clathrin-coated vesicles resulting in the disassembly of intercellular junctions (112). This presumably constitutes a major pathway how VEGF induces vascular permeability and leakage.
Furthermore, another signaling function of VE-cadherin appears to be achieved through sequestering β-catenin at the membrane. In this respect tyrosine phosphorylation of beta-catenin seems to be an important regulatory mechanism, since phosphorylated β-catenin is less tightly bound to VE-cadherin (113). VE-cadherin normally retains β-catenin at the membrane and prevents its translocation to the nucleus, thus reducing catenin-mediated gene regulation in conjunction with the Wnt-signaling pathway involving the transcription factors Lef/TCF (114).
Concluding remarks
Enormous progress has been achieved over the recent years in identifying the relevant ligands and receptors functional in promoting vasculogenesis and angiogenesis. However, our understanding of how the different receptors involved achieve their distinct biological effects by their differential spatial and temporal expression and by the induction of differential signal transduction pathways and gene repertoires is still very incomplete. A significant amount of data is available for the VEGF receptor-2, displaying that this tyrosine kinase receptor besides its induction of the PI3-kinase pathway is distinct as a preferential and strong inducer of PLC-γ, which is essential for proliferation and likely is also important for the induction of a large gene repertoire and at least part of the strong angiogenic properties induced by VEGF-A. It will be important to derive a still more complete picture of the pathways induced by the different VEGF and Tie receptors and to understand how blood vessel versus lymph vessel angiogenesis is promoted through VEGF receptor-2 and VEGF receptor-3, respectively, and how the Tie receptors synergize or interfere with the VEGF signals. Furthermore, relevant data are available of how VE-cadherin/β-catenin can modulate angiogenesis signals by forming a complex with VEGF receptor-2. However, despite a significant knowledge of signaling details for the various individual receptors and adhesion molecules, in general the integration of signals of the different receptors, their cross-talk and their modulation by adhesion molecules in endothelial cells is not yet well understood. It is to be assumed that the rapid accumulation of additional signaling and microarray expression data in the near future will provide information on differential induction of genes by the different receptors and their cross-modulation and will aid in the understanding and the definition of key intracellular regulators, which conceivably could be used as alternative targets for modulation of angiogenesis.
Acknowledgements
The work of the authors is supported by grants of the Austrian Science Fund (NFN-S94–3) and the European Commission (LSHC-CT-2005–518178).
References
- 1.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet E. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 4.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 5.Eklund L, Olsen BR. Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp Cell Res. 2006;312:630–641. doi: 10.1016/j.yexcr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Heroult M, Schaffher F, Augustin HG. Eph receptor and ephrin ligand-mediated interactions during angiogenesis and tumor progression. Exp Cell Res. 2006;312:642–650. doi: 10.1016/j.yexcr.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 8.Presta M, Dell'Era P, Mitola S, et al. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Compagni A, Wilgenbus P, Impagnatiello MA, et al. Fibroblast growth factors are required for efficient tumor angiogenesis. Cancer Res. 2000;60:7163–7169. [PubMed] [Google Scholar]
- 10.Amin DN, Hida K, Bielenberg DR, et al. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66:2173–2180. doi: 10.1158/0008-5472.CAN-05-3387. [DOI] [PubMed] [Google Scholar]
- 11.Zeller PJ, Skalak TC, Ponce AM, et al. In vivo chemotactic properties and spatial expression of PDGF in developing mesenteric microvascular networks. Am J Physiol Heart Circ Physiol. 2001;280:H2116–2125. doi: 10.1152/ajpheart.2001.280.5.H2116. [DOI] [PubMed] [Google Scholar]
- 12.Rolny C, Nilsson I, Magnusson P, et al. Platelet-derived growth factor receptor-beta promotes early endothelial cell differentiation. Blood. 2006;108:1877–1886. doi: 10.1182/blood-2006-04-014894. [DOI] [PubMed] [Google Scholar]
- 13.Goumans MJ, Lebrin F, Valdimarsdottir G. Controlling the angiogenic switch: a balance between two distinct TGF-b receptor signaling pathways. Trends Cardiovasc Med. 2003;13:301–307. doi: 10.1016/s1050-1738(03)00142-7. [DOI] [PubMed] [Google Scholar]
- 14.Rajashekhar G, Willuweit A, Patterson CE, et al. Continuous endothelial cell activation increases angiogenesis: evidence for the direct role of endothelium linking angiogenesis and inflammation. J Vase Res. 2006;43:193–204. doi: 10.1159/000090949. [DOI] [PubMed] [Google Scholar]
- 15.Majno G. Chronic inflammation: links with angiogenesis and wound healing. Am J Pathol. 1998;153:1035–1039. doi: 10.1016/S0002-9440(10)65648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261–270. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 17.Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651–658. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 18.Favier B, Alam A, Barron P, et al. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243–1250. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- 19.Shibuya M, Yamaguchi S, Yamane A, et al. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990;5:519–524. [PubMed] [Google Scholar]
- 20.Millauer B, Wizigmann-Voos S, Schnurch H, et al. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- 21.Terman BI, Carrion ME, Kovacs E, et al. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene. 1991;6:1677–1683. [PubMed] [Google Scholar]
- 22.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 23.Pajusola K, Aprelikova O, Korhonen J, et al. FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res. 1992;52:5738–5743. [PubMed] [Google Scholar]
- 24.de Vries C, Escobedo JA, Ueno H, et al. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 25.Maglione D, Guerriero V, Viglietto G, et al. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci USA. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terman BI, Dougher-Vermazen M, Carrion ME, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187:1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 27.Joukov V, Pajusola K, Kaipainen A, et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. Embo J. 1996;15:1751. [PMC free article] [PubMed] [Google Scholar]
- 28.Ladomery MR, Harper SJ, Bates DO. Alternative splicing in angiogenesis: The vascular endothelial growth factor paradigm. Cancer Lett. 2006 doi: 10.1016/j.canlet.2006.08.015. e-pub. [DOI] [PubMed] [Google Scholar]
- 29.LeCouter J, Kowalski J, Foster J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 30.Lyttle DJ, Fraser KM, Fleming SB, et al. Homologs of vascular endothelial growth factor are encoded by the poxvirus orf virus. J Virol. 1994;68:84–92. doi: 10.1128/jvi.68.1.84-92.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer M, Clauss M, Lepple-Wienhues A, et al. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. Embo J. 1999;18:363–374. doi: 10.1093/emboj/18.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suto K, Yamazaki Y, Morita T, et al. Crystal structures of novel vascular endothelial growth factors (VEGF) from snake venoms: insight into selective VEGF binding to kinase insert domain-containing receptor but not to fms-like tyrosine kinase-1. J Biol Chem. 2005;280:2126–2131. doi: 10.1074/jbc.M411395200. [DOI] [PubMed] [Google Scholar]
- 33.Vajkoczy P, Farhadi M, Gaumann A, et al. Microtumor growth initiates angiogenic sprouting with simultaneous expression ofVEGF, VEGF receptor-2, and angiopoietin-2. J Clin Invest. 2002;109:777–785. doi: 10.1172/JCI14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438:954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 35.Deckers MM, Karperien M, van der Bent C, et al. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667–1674. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- 36.Clauss M, Weich H, Breier G, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 37.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 38.Clauss M, Gerlach M, Gerlach H, et al. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990;172:1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 40.Li XA, Everson W, Smart EX. Nitric oxide, caveolae, and vascular pathology. Cardiovasc Toxicol. 2006;6:1–13. doi: 10.1385/ct:6:1:1. [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto T, Bohman S, Dixelius J, et al. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. Embo J. 2005;24:2342–2353. doi: 10.1038/sj.emboj.7600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kendall RL, Rutledge RZ, Mao X, et al. Vascular endothelial growth factor receptor KDR tyrosine kinase activity is increased by autophosphorylation of two activation loop tyrosine residues. J Biol Chem. 1999;274:6453–6460. doi: 10.1074/jbc.274.10.6453. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai Y, Ohgimoto K, Kataoka Y, et al. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc Natl Acad Sci USA. 2005;102:1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mechtcheriakova D, Clauss M, Hofer E. Vascular Endothelium: Source and Target of Inflammatory mediators. IOS Press; Amsterdam: 2001. Specificity, diversity and convergence in angiogenic and inflammatory signaling in endothelial cells; pp. 211–226. [Google Scholar]
- 45.Mechtcheriakova D, Schabbauer G, Lucerna M, et al. Specificity, diversity, and convergence in VEGF and TNF-alpha signaling events leading to tissue factor up-regulation via EGR-1 in endothelial cells. Faseb J. 2001;15:230–242. doi: 10.1096/fj.00-0247com. [DOI] [PubMed] [Google Scholar]
- 46.Mechtcheriakova D, Wlachos A, Holzmuller H, et al. Vascular endothelial cell growth factor-induced tissue factor expression in endothelial cells is mediated by EGR-1. Blood. 1999;93:3811–3823. [PubMed] [Google Scholar]
- 47.Lucerna M, Mechtcheriakova D, Kadl A, et al. NAB2, a corepressor of EGR-1, inhibits vascular endothelial growth factor-mediated gene induction and angiogenic responses of endothelial cells. J Biol Chem. 2003;278:11433–11440. doi: 10.1074/jbc.M204937200. [DOI] [PubMed] [Google Scholar]
- 48.Fahmy RG, Dass CR, Sun LQ, et al. Transcription factor Egr-1 supports FGF-dependent angiogenesis during neovascularization and tumor growth. Nat Med. 2003;9:1026–1032. doi: 10.1038/nm905. [DOI] [PubMed] [Google Scholar]
- 49.Khachigian LM. Early growth response-1 in cardiovascular pathobiology. Circ Res. 2006;98:186–191. doi: 10.1161/01.RES.0000200177.53882.c3. [DOI] [PubMed] [Google Scholar]
- 50.Silverman ES, Collins T. Pathways of Egr-1-mediated gene transcription in vascular biology. Am J Pathol. 1999;154:665–670. doi: 10.1016/S0002-9440(10)65312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qu Z, Wolfraim LA, Svaren J, et al. The transcriptional corepressor NAB2 inhibits NGF-induced differentiation of PC12 cells. J Cell Biol. 1998;142:1075–1082. doi: 10.1083/jcb.142.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shafarenko M, Liebermann DA, Hoffman B. Egr-1 abrogates the block imparted by c-Myc on terminal M1 myeloid differentiation. Blood. 2005;106:871–878. doi: 10.1182/blood-2004-08-3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Minami T, Horiuchi K, Miura M, et al. Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis. J Biol Chem. 2004;279:50537–50554. doi: 10.1074/jbc.M406454200. [DOI] [PubMed] [Google Scholar]
- 54.Hesser BA, Liang XH, Camenisch G, et al. Down syndrome critical region protein 1 (DSCR1), a novel VEGF target gene that regulates expression of inflammatory markers on activated endothelial cells. Blood. 2004;104:149–158. doi: 10.1182/blood-2004-01-0273. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi T, Yamaguchi S, Chida K, et al. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. Embo J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dayanir V, Meyer RD, Lashkari K, et al. Identification of tyrosine residues in vascular endothelial growth factor receptor-2/FLK-1 involved in activation of phosphatidylinositol 3-kinase and cell proliferation. J Biol Chem. 2001;276:17686–17692. doi: 10.1074/jbc.M009128200. [DOI] [PubMed] [Google Scholar]
- 57.Holmqvist K, Cross MJ, Rolny C, et al. The adaptor protein shb binds to tyrosine 1175 in vascular endothelial growth factor (VEGF) receptor-2 and regulates VEGF-dependent cellular migration. J Biol Chem. 2004;279:22267–22275. doi: 10.1074/jbc.M312729200. [DOI] [PubMed] [Google Scholar]
- 58.Fujio Y, Walsh K. Akt mediates cytoprotection of endothelial cells by vascular endothelial growth factor in an anchorage-dependent manner. J Biol Chem. 1999;274:16349–16354. doi: 10.1074/jbc.274.23.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bellacosa A, Kumar CC, Di Cristofano A, et al. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 60.Lee PC, Salyapongse AN, Bragdon GA, et al. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am J Physiol. 1999;277:H1600–1608. doi: 10.1152/ajpheart.1999.277.4.H1600. [DOI] [PubMed] [Google Scholar]
- 61.Dimmeler S, Fleming I, Fisslthaler B, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 62.Le Boeuf F, Houle F, Huot J. Regulation of vascular endothelial growth factor receptor 2-mediated phosphorylation of focal adhesion kinase by heat shock protein 90 and Src kinase activities. J Biol Chem. 2004;279:39175–39185. doi: 10.1074/jbc.M405493200. [DOI] [PubMed] [Google Scholar]
- 63.Yamaoka-Tojo M, Ushio-Fukai M, Hilenski L, et al. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species--dependent endothelial migration and proliferation. Circ Res. 2004;95:276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- 64.Bartoli M, Gu X, Tsai NT, et al. Vascular endothelial growth factor activates STAT proteins in aortic endothelial cells. J Biol Chem. 2000;275:33189–33192. doi: 10.1074/jbc.C000318200. [DOI] [PubMed] [Google Scholar]
- 65.Landgren E, Schiller P, Cao Y, et al. Placenta growth factor stimulates MAP kinase and mitogenicity but not phospholipase C-gamma and migration of endothelial cells expressing Flt 1. Oncogene. 1998;16:359–367. doi: 10.1038/sj.onc.1201545. [DOI] [PubMed] [Google Scholar]
- 66.Sawano A, Takahashi T, Yamaguchi S, et al. The phosphorylated 1169-tyrosine containing region of flt-1 kinase (VEGFR-1) is a major binding site for PLCgamma. Biochem Biophys Res Commun. 1997;238:487–491. doi: 10.1006/bbrc.1997.7327. [DOI] [PubMed] [Google Scholar]
- 67.Fong GH, Rossant J, Gertsenstein M, et al. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 68.Hiratsuka S, Minowa O, Kuno J, et al. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Autiero M, Waltenberger J, Communi D, et al. Role of P1GF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 70.Oliver G, Detmar M. The rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculature. Genes Dev. 2002;16:773–783. doi: 10.1101/gad.975002. [DOI] [PubMed] [Google Scholar]
- 71.Dixelius J, Makinen T, Wirzenius M, et al. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J Biol Chem. 2003;278:40973–40979. doi: 10.1074/jbc.M304499200. [DOI] [PubMed] [Google Scholar]
- 72.Petrova TV, Karpanen T, Norrmen C, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- 73.Hong YK, Detmar M. Prox1, master regulator of the lymphatic vasculature phenotype. Cell Tissue Res. 2003;314:85–92. doi: 10.1007/s00441-003-0747-8. [DOI] [PubMed] [Google Scholar]
- 74.Olsson AK, Dimberg A, Kreuger J, et al. VEGF receptor signalling – in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 75.Dumont DJ, Yamaguchi TP, Conlon RA, et al. tek, a novel tyrosine kinase gene located on mouse chromosome 4, is expressed in endothelial cells and their presumptive precursors. Oncogene. 1992;7:1471–1480. [PubMed] [Google Scholar]
- 76.Partanen J, Armstrong E, Makela TP, et al. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol Cell Biol. 1992;12:1698–1707. doi: 10.1128/mcb.12.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yabkowitz R, Meyer S, Black T, et al. Inflammatory cytokines and vascular endothelial growth factor stimulate the release of soluble tie receptor from human endothelial cells via metalloprotease activation. Blood. 1999;93:1969–1979. [PubMed] [Google Scholar]
- 78.Saharinen P, Kerkela K, Ekman N, et al. Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2. J Cell Biol. 2005;169:239–243. doi: 10.1083/jcb.200411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 80.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 81.Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 82.DeBusk LM, Hallahan DE, Lin PC. Akt is a major angiogenic mediator downstream of the Ang1/Tie2 signaling pathway. Exp Cell Res. 2004;298:167–177. doi: 10.1016/j.yexcr.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 83.Kim I, Kim HG, So JN, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphati-dylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 84.Jones N, Dumont DJ. The Tek/Tie2 receptor signals through a novel Dok-related docking protein, Dok-R. Oncogene. 1998;17:1097–1108. doi: 10.1038/sj.onc.1202115. [DOI] [PubMed] [Google Scholar]
- 85.Huang L, Turck CW, Rao P, et al. GRB2 and SH-PTP2: potentially important endothelial signaling molecules downstream of the TEK/TIE2 receptor tyrosine kinase. Oncogene. 1995;11:2097–2103. [PubMed] [Google Scholar]
- 86.Jones N, Master Z, Jones J, et al. Identification of Tek/Tie2 binding partners. Binding to a multifunctional docking site mediates cell survival and migration. J Biol Chem. 1999;274:30896–30905. doi: 10.1074/jbc.274.43.30896. [DOI] [PubMed] [Google Scholar]
- 87.Audero E, Cascone I, Maniero F, et al. Adaptor ShcA protein binds tyrosine kinase Tie2 receptor and regulates migration and sprouting but not survival of endothelial cells. J Biol Chem. 2004;279:13224–13233. doi: 10.1074/jbc.M307456200. [DOI] [PubMed] [Google Scholar]
- 88.Hughes DP, Marron MB, Brindle NP. The anti-inflammatory endothelial tyrosine kinase Tie2 interacts with a novel nuclear factor-kappaB inhibitor ABIN-2. Circ Res. 2003;92:630–636. doi: 10.1161/01.RES.0000063422.38690.DC. [DOI] [PubMed] [Google Scholar]
- 89.Korpelainen EI, Karkkainen M, Gunji Y, et al. Endothelial receptor tyrosine kinases activate the STAT signaling pathway: mutant Tie-2 causing venous malformations signals a distinct STAT activation response. Oncogene. 1999;18:1–8. doi: 10.1038/sj.onc.1202288. [DOI] [PubMed] [Google Scholar]
- 90.Yamaguchi Y, Pasquale EB. Eph receptors in the adult brain. Curr Opin Neurobiol. 2004;14:288–296. doi: 10.1016/j.conb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 91.Poliakov A, Cotrina M, Wilkinson DG. Diverse roles of eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell. 2004;7:465–480. doi: 10.1016/j.devcel.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 92.Adams RH, Wilkinson GA, Weiss C, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 94.Adams RH, Diella F, Hennig S, et al. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 95.Fuller T, Korff T, Kilian A, et al. Forward EphB4 signaling in endothelial cells controls cellular repulsion and segregation from ephrinB2 positive cells. J Cell Sci. 2003;116:2461–2470. doi: 10.1242/jcs.00426. [DOI] [PubMed] [Google Scholar]
- 96.Carter N, Nakamoto T, Hirai H, et al. EphrinA1-induced cytoskeletal re-organization requires FAK and p130(cas) Nat Cell Biol. 2002;4:565–573. doi: 10.1038/ncb823. [DOI] [PubMed] [Google Scholar]
- 97.Huynh-Do U, Stein E, Lane AA, et al. Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through alphavbeta3 and alpha5betal integrins. Embo J. 1999;18:2165–2173. doi: 10.1093/emboj/18.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schmucker D, Zipursky SL. Signaling downstream of Eph receptors and ephrin ligands. Cell. 2001;105:701–704. doi: 10.1016/s0092-8674(01)00391-9. [DOI] [PubMed] [Google Scholar]
- 99.Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- 100.Auguste P, Javerzat S, Bikfalvi A. Regulation of vascular development by fibroblast growth factors. Cell Tissue Res. 2003;314:157–166. doi: 10.1007/s00441-003-0750-0. [DOI] [PubMed] [Google Scholar]
- 101.Tallquist M, Kazlauskas A. PDGF signaling in cells and mice. Cytokine Growth Factor Rev. 2004;15:205–213. doi: 10.1016/j.cytogfr.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 102.Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 103.Oitzinger W, Hofer-Warbinek R, Schmid JA, et al. Adenovirus-mediated expression of a mutant IkappaB kinase 2 inhibits the response of endothelial cells to inflammatory stimuli. Blood. 2001;97:1611–1617. doi: 10.1182/blood.v97.6.1611. [DOI] [PubMed] [Google Scholar]
- 104.Wrighton CJ, Hofer-Warbinek R, Moll T, et al. Inhibition of endothelial cell activation by adenovirus-mediated expression of I kappa B alpha, an inhibitor of the transcription factor NF-kappa B. J Exp Med. 1996;183:1013–1022. doi: 10.1084/jem.183.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Versteeg HH, Ruf W. Emerging insights in tissue factor-dependent signaling events. Semin Thromb Hemost. 2006;32:24–32. doi: 10.1055/s-2006-933337. [DOI] [PubMed] [Google Scholar]
- 106.Carmeliet P, Mackman N, Moons L, et al. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 107.Belting M, Dorrell MI, Sandgren S, et al. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nat Med. 2004;10:502–509. doi: 10.1038/nm1037. [DOI] [PubMed] [Google Scholar]
- 108.Grazia Lampugnani M, Zanetti A, Corada M, et al. Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148. J Cell Biol. 2003;161:793–804. doi: 10.1083/jcb.200209019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lampugnani MG, Orsenigo F, Gagliani MC, et al. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Esser S, Lampugnani MG, Corada M, et al. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111:1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 111.Nawroth R, Poell G, Ranft A, et al. VE-PTP and VE-cadherin ectodomains interact to facilitate regulation of phosphorylation and cell contacts. Embo J. 2002;21:4885–4895. doi: 10.1093/emboj/cdf497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8:1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 113.Huber AH, Weis WI. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell. 2001;105:391–402. doi: 10.1016/s0092-8674(01)00330-0. [DOI] [PubMed] [Google Scholar]
- 114.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 115.Issbrucker K, Marti HH, Hippenstiel S, et al. p38 MAP kinase--a molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. Faseb J. 2003;17:262–264. doi: 10.1096/fj.02-0329fje. [DOI] [PubMed] [Google Scholar]