Summary
Introduction
Protein Z is a vitamin K-dependent protein that serves as a cofactor for the inhibition of activated factor X by the serpin protein Z-dependent protease inhibitor (ZPI). Protein Z plasma levels have been shown to be reduced in patients with peripheral arterial disease (PAD), but ZPI levels have not yet been reported. The aim of this study was to more fully assess the protein Z–ZPI system in individuals with atherosclerosis selected by the presence of symptomatic PAD.
Materials and methods
Protein Z and ZPI levels were determined in 95 PAD patients (73 males; 22 females) [median age: 73 years (range, 50–86 years)] and in 190 controls comparable for age and gender. Protein Z was measured using a commercial immunoassay, and ZPI was measured with a homemade immunoassay and a functional assay.
Results
Protein Z antigen, ZPI antigen and ZPI function were found to be significantly lower in PAD patients with respect to controls [protein Z, median 72.5% (range: 3.4–123.7%) vs. 90.7% (range: 32.1–203.2%), P < 0.0001; ZPI antigen, 86.1% (range: 25.1–149.5%) vs. 93.2% (range: 48.9–171.3%), P = 0.004; ZPI function, 83.5% (range: 21.1–135.2%) vs. 97.2% (range: 50.5–175.5%), P < 0.0001]. The lowest tertiles of protein Z antigen [odds ratio (OR) 5.4, 95% confidence interval (CI) 2.2–13.5, P < 0.0001] and ZPI function (OR 2.4, 95% CI 1.1–5.5, P = 0.03) were associated with PAD on multivariate analysis after adjustment for age, gender, and traditional cardiovascular risk factors. A significant inverse relationship was also observed between protein Z and ZPI levels and the number of traditional cardiovascular risk factors and the clinical severity of disease (Fontaine stage).
Conclusions
Low levels of protein Z antigen and protein Z activity are significantly associated with the occurrence and severity of atherosclerotic PAD.
Keywords: atherosclerosis, peripheral arterial disease, protein Z, ZPI
Introduction
Protein Z is a single-chain, vitamin K-dependent glycoprotein identified in human plasma in 1984 [1]. Protein Z acts as a cofactor for the inactivation of activated factor X (FXa) by protein Z-dependent protease inhibitor (ZPI) [2,3]. ZPI, a member of the serpin superfamily of proteinase inhibitors, inhibits FXa in a protein Z-dependent, Ca2+-dependent and phospholipid-dependent fashion, and inhibits FXIa in the absence of cofactors [2,3]. Protein Z and ZPI circulate as a complex in plasma [4]. Under normal conditions, ZPI is in excess, and all of the protein Z is bound to ZPI.
Studies on the potential roles of protein Z and ZPI in different prothrombotic conditions have produced conflicting results [5–18]. We have reported an association of low levels of protein Z with coronary disease and peripheral arterial disease (PAD) [19–21]. Recently, Zhang et al. demonstrated that ZPI deficiency enhanced thrombosis in murine models, supporting a possible pathophysiologic role for ZPI in thromboembolic disease [22]. Up to now, however, the relationship of ZPI levels to atherothrombotic disease has been evaluated in only one study [14], and no information is available concerning the potential role of ZPI in PAD, a severe clinical manifestation of atherosclerotic disease. The aim of this study was to more fully assess the protein Z–ZPI system in PAD patients.
Materials and methods
Study population
The study population consisted of 95 patients [73 males, 22 females; median age, 73 years (range: 50–86 years)], with symptoms or signs suggestive of the presence of PAD, who were admitted to the Unit of Vascular Surgery of the University of Florence to be evaluated for possible surgical intervention. PAD was diagnosed when patients had typical symptoms of intermittent claudication, i.e. cramping pain of the calves or buttocks during exercise, and an ankle–brachial index at rest less than 0.90, calculated according to the recommendations of the American Heart Association [23].
None of the patients was undergoing anticoagulant treatment, and none reported a positive history for the FV Leiden mutation or antiphospholipid antibodies. All patients were also evaluated for atherosclerotic disease at other locations. In particular, a cardiologic evaluation, including electrocardiogram and echo-cardiogram, was performed in all patients, and, in patients with symptoms potentially related to ischemic heart disease, additional studies were performed (echocardiogram with drug-induced stress testing, myocardial scintigraphy, and/or coronary angiography). Moreover, carotid artery duplex scanning with color-coded echo flow imaging was also conducted.
The patients were compared with 190 clinical controls [median age,72 years(range:52–86 years);142 males;48 females]recruited from a population study conducted in Florence, Italy [24]. The control group was selected to be comparable for age and gender with the patient group. We used a structured questionnaire to identify disease-free controls and to exclude subjects who were suspected of having any form of vascular disease. The subjects were considered to have hypertension if they had been diagnosed as hypertensives according to the guidelines of the European Society of Hypertension/European Society of Cardiology [25] or were taking antihypertensive drugs. Dyslipidemia was defined according to the Third report of the National Cholesterol Education Program [26], and diabetes in agreement with the criteria of the American Diabetes Association [27]. A positive family history was defined as the presence of at least one first-degree relative who had developed cardiovascular disease before the age of 55 years for men and the age of 65 years for women. All subjects gave informed consent. The study complied with the Declaration of Helsinki and was approved by the local ethics committee.
Laboratory measurement
Blood samples were collected from the antecubital vein into evacuated plastic tubes (Vacutainer) containing 0.109 mol L−1 sodium citrate, in the morning after an overnight fast. Plasma samples, obtained by centrifugation at 3000 × g for 10 min at 4 °C, were stored in aliquots at − 80 °C until analysis. Protein Z antigen levels in plasma were measured using a commercial enzyme-linked immunosorbent assay (Zymutest Protein Z; Hyphen BioMed, Neuville-sur-Oise, France) by following the manufacturer’s instructions. ZPI immunoassays were performed as previously described [13].
The ZPI functional assay took advantage of the fact that ZPI is by far the most potent inhibitor of FXIa in plasma [28]. Fifty microliters of human FXIa (20 μg mL−1; Enzyme Research Laboratories, South Bend, IN, USA) in 0.1 mol L−1 NaCl and 0.02 mol L−1Hepes (pH 7.4)was incubated overnight at 4 °C in each well of a microtiter plate. Wells were washed with phosphate-buffered saline containing 0.05% Tween-20 (PBST), and 100-μL plasma samples diluted 1/50 in PBST were applied and incubated for 90 min at room temperature. After washing with PBST, 100 μL of biotin-conjugated anti-ZPI monoclonal antibody 4336 E5 (2 μg mL−1) was added to each well and incubated for 60 min at room temperature. After washing with PBST, 100 μL of streptavidin–horseradish peroxidase (1 μg mL−1; Thermo Scientific, Rockford, IL, USA)was added and incubated for 30 min at room temperature. After final washing with PBST, 200 μL of 3,3′,5,5′-tetramethylbenzidine (Sigma, St Louis, MO, USA) was added, and the reaction was stopped after 5 min by adding 100 μL of 0.5 mol L−1 H2SO4. Absorbance at 450 nm (A450 nm) was read in a microtiter plate reader, and compared with a standard curve produced with serial concentrations of purified ZPI (0–160 ng mL−1).
Protein Z antigen, ZPI antigen and ZPI functional assay results were normalized by assuming that the mean values for each in the control group represented 100%.
Statistical analysis
Statistical analysis was performed using SPSS (Statistical Package for Social Sciences Inc., Chicago, IL, USA) software for Windows (Version 13.0). Owing to their skewed distributions, protein Z antigen, ZPI antigen and ZPI function levels were log-analysed and back-transformed for data presentation. The Spearman correlation test for non-parametric data was used for correlation analyses. Results are expressed as median and range. The non-parametric Mann–Whitney test for unpaired data was used for comparison between single groups, and the chi-square test was used for test of proportions. To analyse the relationship between the parameters of the protein Z–ZPI system and PAD, the study population was divided into tertiles of the distribution of these parameters in the control group: protein Z antigen, first tertile < 86.5%, second tertile 86.5–100.4%, and third tertile > 100.4%; ZPI antigen, first tertile< 85.4%, second tertile 85.4–105.6%, and third tertile > 105.6%; ZPI function, first tertile < 85.2%, second tertile 85.2–106.7%, and third tertile > 106.7%. Univariate logistic regression analysis was performed with low levels defined as protein Z antigen, ZPI antigen and ZPI function in the first tertile of their distribution. Afterwards, a multivariate analysis was performed to evaluate the association between such levels and the disease after adjustment for traditional cardiovascular risk factors (smoking habit, hypertension, dyslipidemia, diabetes, and family history of cardiovascular diseases). To investigate the possible association between protein Z and ZPI levels and the number of traditional cardiovascular risk factors, as well as the clinical severity of the disease (Fontaine stage), a general linear model with Bonferroni adjustment for multiple confounders was used. Data were expressed as geometric means and 95% confidence interval (CIs). Odds ratios (ORs) with 95% CIs were calculated, and a P-value < 0.05 was considered to be statistically significant.
Results
Demographic and clinical characteristics of the study population are shown in Table 1. As expected, traditional cardiovascular risk factors (hypertension, diabetes, smoking habit, dyslipidemia, and family history of cardiovascular diseases) were significantly more prevalent in PAD patients than in controls. Atherosclerotic disease was detected at additional locations in 36 (37.9%) of the PAD patients: coronary artery disease, 22 (23.2%); carotid artery stenosis, eight (8.4%); and abdominal aortic aneurysm, six (6.3%).
Table 1.
Demographic and clinical characteristics of the study populations
| Variable | Patients (n = 95) | Controls (n = 190) | P-value |
|---|---|---|---|
| Age, years* | 73 (50–86) | 72 (52–86) | 0.7 |
| Male gender, n (%) | 73 (76.8) | 142 (74.7) | 0.9 |
| Hypertension, n (%) | 35 (36.8) | 38 (20) | 0.002 |
| Smoking habit, n (%) | 65 (68.4) | 41 (21.6) | < 0.0001 |
| Dyslipidemia, n (%) | 36 (37.9) | 38 (20) | 0.001 |
| Diabetes, n (%) | 24 (25.3) | 11 (5.8) | < 0.0001 |
| Family history of CVD, n (%) | 24 (25.3) | 23 (12.1) | 0.005 |
Median (range). CVD, cardiovascular disease.
Protein Zantigen levels, ZPI antigen levels and ZPI functional levels were significantly (P < 0.005 for all) lower in PAD patients [protein Z antigen, 72.5% (3.8–123.7%); ZPI antigen, 86.1%(25.1–149.5%); ZPI function, 83.5%(21.1–135.2%)] than in controls [protein Z antigen, 90.7% (32.1–203.2%); ZPI antigen, 93.2% (48.9–171.3%); ZPI function, 97.2% (50.5–175.5%)]. Significant correlations among the three different parameters in the whole study population were found (protein Z antigen–ZPI antigen, R = 0.55, P <0.0001; protein Zantigen–ZPI function, R = 0.52, P <0.0001; ZPI antigen–ZPI function, R = 0.68, P < 0.0001) (Fig. 1).
Fig. 1.
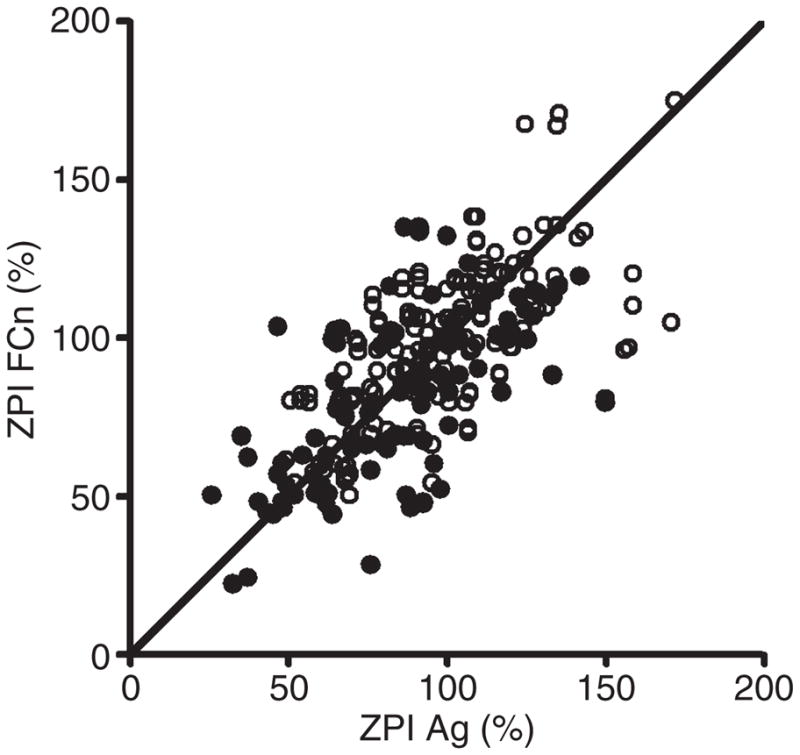
Correlation analysis between protein Z-dependent protease inhibitor (ZPI) antigen (Ag) and ZPI function (FCn).
To test the association between protein Z and ZPI levels and the presence of PAD, the study population was divided into tertiles according to the distribution of these parameters within the control group, and evaluated by logistic regression analysis. On univariate analysis, an increased risk of PAD was found for patients with protein Z antigen, ZPI antigen and ZPI function in the lowest tertile of their distribution (protein Z antigen, OR 5.9, 95% CI 2.8–12.2, P < 0.0001; ZPI antigen, OR 2.2 95% CI 1.2–4.1, P < 0.01; ZPI function, OR 2.8, 95% CI 1.5–5.3, P < 0.0001). After adjustment for age, gender, and traditional cardiovascular risk factors, the association between low levels of protein Z antigen and ZPI function and PAD was confirmed (protein Z antigen, OR 5.4, 95% CI 2.2–13.5, P < 0.0001; ZPI function, OR 2.4, 95% CI 1.1–5.5, P = 0.03), but ZPI antigen was no longer an independent risk factor for PAD (OR 1.6, 95% CI 0.7–3.6, P = 0.23).
The potential relationship between protein Z–ZPI parameters and the extent of atherosclerotic disease was also evaluated. The sample population was grouped on the basis of the number of traditional cardiovascular risk factors and analyzed using a general linear model with adjustment for age and gender. A significant relationship between decreasing levels of protein Z antigen, ZPI antigen and ZPI function and the number of traditional cardiovascular risk factors was observed (Table 2). Finally, a general linear model after adjustment for age, gender and traditional cardiovascular risk factors was used to evaluate the potential association between protein Z–ZPI levels and the clinical progression of PAD (defined by Fontaine’s clinical stages). Significant trends for decreasing protein Z antigen, ZPI antigen and ZPI function with increasing clinical stage of the disease were observed (Table 3).
Table 2.
Protein Z antigen, protein Z-dependent protease inhibitor (ZPI) antigen and function according to the number of traditional cardiovascular risk factors in the whole study population
| Protein Z antigen | ZPI antigen | ZPI function | |
|---|---|---|---|
| 0 (n = 84) | 91 (86–96) | 96 (90–101) | 97 (92–103) |
| 1 (n = 98) | 91 (86–96) | 97 (92–102) | 94 (89–99) |
| 2 (n = 74) | 76 (71–82) | 85 (79–91) | 89 (83–95) |
| 3 (n = 27) | 75 (66–84) | 86 (76–95) | 81 (72–90) |
| 4 (n = 2) | 26 (16–59) | 38 (22–73) | 33 (23–68) |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 |
Data are presented as geometric mean (95% confidence interval). General linear model adjusted for age and gender.
Table 3.
Protein Z antigen, protein Z-dependent protease inhibitor (ZPI) antigen and function according to the clinical extent (Fontaine stage) of the peripheral arterial disease
| Protein Z antigen | ZPI antigen | ZPI function | |
|---|---|---|---|
| Controls (n = 190) | 90 (87–94) | 93 (89–97) | 96 (92–100) |
| Stage IIb (n = 74) | 75 (70–81) | 92 (86–98) | 88 (82–94) |
| Stage III (n = 14) | 60 (47–73) | 68 (54–81) | 68 (54–82) |
| Stage IV (n = 7) | 58 (39–77) | 43 (23–62) | 50 (29–70) |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 |
Data are presented as geometric mean (95% confidence interval). General linear model adjusted for age, gender, smoking habit, hypertension, diabetes, dyslipidemia, and family history of cardiovascular diseases.
Discussion
Protein Z and ZPI null mice are born in lower than anticipated numbers (20% and 35% reduced, respectively) from heterozygous matings, and have enhanced thrombotic responses in mouse models of carotid artery occlusion and pulmonary thromboembolism [22]. When combined with the FV Leiden genotype, protein Z and ZPI deficiencies cause intrauterine and perinatal thrombosis and an apparent consumptive coagulopathy, with ZPI deficiency producing a significantly more severe phenotype than protein Z deficiency. These results strongly suggest that protein Z and ZPI deficiency are prothrombotic traits. Clinical studies exploring the association between protein Z levels and venous and arterial thrombotic disease, however, have produced both positive [5–9,13] and negative results [10–12,14]. More limited studies of ZPI and thrombosis have also produced conflicting results [13–18].
We have previously documented an association between low protein Z levels and atherosclerosis, in particular symptomatic coronary disease and PAD [19–21]. In PAD, protein Z levels were shown to be related to traditional atherosclerotic risk factors and disease severity [21]. The current study, conducted on a new cohort of patients and controls, confirms these results for protein Z, and demonstrates a similar relationship of reduced antigenic and functional ZPI levels with cardiovascular risk factors and the extent of PAD. The mechanism(s) underlying the apparent association of the protein Z–ZPI system and atherosclerosis is not known, and cannot be determined by the case–control design of this study. It is conceivable that low levels of protein Z and/or ZPI could accelerate the progression of vascular disease, and it is of interest in this regard that protein Z has been detected in atherosclerotic plaques [29]. Prospective studies investigating the relationship between reduced protein Z and/or ZPI and the thrombotic complications of atherosclerosis, however, have failed to demonstrate statistical significance [12,14]. Alternatively, the reductions in the levels of protein Z and ZPI in atherosclerosis could be acquired abnormalities, perhaps related to their consumption through the persistent activation of coagulation that occurs in patients with widespread vascular disease [30,31]. In this regard, it is notable that ZPI, but not protein Z, is consumed during coagulation in vitro [32]. Endothelial dysfunction also accompanies PAD [33], and the expression of protein Z, but not of ZPI, by endothelial cells has been detected [34]. The major source of protein Z in vivo, however, is the liver, and the low level of endothelial protein Z synthesis that was reported is unlikely to affect plasma protein Z concentrations. Inflammation is an integral part of the atherosclerotic process, and some studies have suggested a positive relationship between certain inflammatory cytokines and protein Z [19,35,36]. Furthermore, increased levels of protein Z have been found during the acute phase of stroke that resolve during convalescence [11,37]. A similar effect of inflammation in PAD, however, would tend to raise, rather than lower, protein Z and ZPI levels. Finally, protein Z and ZPI circulate as a complex [4], and it has been shown that the plasma concentration of one partner affects the concentration of the other [13,22]. Thus, a primary reduction in protein Z would be anticipated to reduce the level of ZPI and vice versa. A significant association of ZPI function with PAD was detected on both univariate and multivariate analysis, whereas the association of ZPI antigen was only noted on univariate analysis. The most likely reason for this discrepancy is the relatively small sample investigated in this study. It is conceivable, however, that disparities between ZPI function and ZPI antigen measurements may also have contributed. In the plot of ZPI function vs. ZPI antigen (Fig. 1), a number of points stray from the line of identity. Differences between ZPI functional and antigenic results could be due to ZPI polymorphisms that are recognized differentially in the monoclonal antibody sandwich antigen assay cleaved forms of ZPI that are detected in the antigen assay but lack functional activity [32], or altered forms of ZPI with a gain or loss of function. Further studies will be required to distinguish between these possibilities.
The limitations of this study include the relatively small number of PAD patients evaluated and the fact that we were not able to perform ankle-brachial index measurements and other diagnostic procedures in the control population. Therefore, individuals in the control group could have asymptomatic atherosclerotic disease.
In conclusion, low levels of protein Z antigen and ZPI antigen and function appear to be associated with the occurrence and severity of atherosclerotic PAD. The pathophysiologic process(es) responsible for this observation remains to be determined. Whether pre-existing deficiencies of protein Z and ZPI affect the development and progression of atherosclerosis will be addressed in ongoing studies using gene-deleted mice and murine models of atherosclerosis. If the reductions in protein Z and ZPI are acquired abnormalities, it is conceivable that their serial measurement in an individual patient may provide a means of following the progression of atherosclerosis and the effect of (e.g. statin) treatment.
Footnotes
Addendum
Study design: F. Sofi, R. Abbate, S. Fedi, and G. J. Broze Jr. Clinical evaluation of patients: F. Sofi, G. Pratesi, R. Pulli, and C. Pratesi. Laboratory investigation: F. Cesari, S. Fedi, and Y. Tu. Statistical analysis: F. Sofi, F. Cesari, and G. J. Broze Jr. Writing of the article: F. Sofi, G. Pratesi, C. Pratesi, R. Abbate, G. F. Gensini, S. Fedi, and G. J. Broze Jr. Critical revision of the draft: R. Pulli, C. Pratesi, G. F. Gensini, R. Abbate, S. Fedi, and G. J. Broze Jr.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Broze GJ, Jr, Miletich JP. Human protein Z. J Clin Invest. 1984;73:933–8. doi: 10.1172/JCI111317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han X, Fiehler R, Broze GJ., Jr Isolation of a protein Z-dependent plasma protease inhibitor. Proc Natl Acad Sci USA. 1998;95:9250–5. doi: 10.1073/pnas.95.16.9250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang X, Swanson R, Broze GJ, Jr, Olson ST. Kinetic characterization of the protein Z-dependent protease inhibitor (ZPI) reaction with blood coagulation factor Xa. J Biol Chem. 2008;283:29770–83. doi: 10.1074/jbc.M805214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabatabai A, Fiehler R, Broze GJ., Jr Protein Z circulates in plasma in a complex with protein Z-dependent protease inhibitor. Thromb Haemost. 2001;85:655–60. [PubMed] [Google Scholar]
- 5.Martinelli I, Razzari E, Bucciarelli P, Mannucci PM. Low levels of protein Z and the risk of venous thromboembolism. J Thromb Haemost. 2005;3:2817–19. doi: 10.1111/j.1538-7836.2005.01664.x. [DOI] [PubMed] [Google Scholar]
- 6.Kemkes-Matthes B, Nees M, Kuhnel G, Matzdorff A, Matthes KJ. Protein Z influences the prothrombotic phenotype of factor V Leiden in humans. Thromb Res. 2002;106:183–5. doi: 10.1016/s0049-3848(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 7.Vasse M, Guegan-Massardier E, Borg J-Y, Woimant F, Soria C. High frequency of protein Z deficiency in patients with ischemic stroke. Lancet. 2001;357:933–4. doi: 10.1016/S0140-6736(00)04218-5. [DOI] [PubMed] [Google Scholar]
- 8.Heeb M, Paganini-Hill A, Griffin J, Fisher M. Low protein Z levels and risk of ischemic stroke; differences by diabetic status and gender. Blood Cells Mol Dis. 2002;29:139–44. doi: 10.1006/bcmd.2002.0549. [DOI] [PubMed] [Google Scholar]
- 9.Santacroce R, Sarno M, Cappucci F, Sessa F, Colaizzo D, Brancaccio V, Grandone E, Margaglione M. Low protein Z levels and risk of occurrence of deep vein thrombosis. J Thromb Haemost. 2006;4:2417–22. doi: 10.1111/j.1538-7836.2006.02186.x. [DOI] [PubMed] [Google Scholar]
- 10.Lopaciuk S, Bykowska K, Kwiecinski H, Czlonkowska A, Kuczynska- Zardzewialy A. Protein Z in young survivors of ischemic stroke. Thromb Haemost. 2002;88:536. [PubMed] [Google Scholar]
- 11.McQuillan A, Eikelboom J, Hankey G, Baker R, Thom J, Staton J, Yi Q, Cole V. Protein Z in ischemic stroke and its etiologic subtypes. Stroke. 2003;34:2415–19. doi: 10.1161/01.STR.0000092124.52084.4B. [DOI] [PubMed] [Google Scholar]
- 12.Morange PE, Juhan-Vague I the PRIME study group. Protein Z plasma levels are not associated with the risk of coronary heart disease: the PRIME study. J Thromb Haemost. 2004;2:2050–1. doi: 10.1111/j.1538-7836.2004.00981.x. [DOI] [PubMed] [Google Scholar]
- 13.Al-Shanqeeti A, van Hycklama A, Berntorp E, Rosendaal FR, Broze GJ., Jr Protein Z and protein Z-dependent protease inhibitor: determinants of levels and risk of venous thrombosis. Thromb Haemost. 2006;93:411–13. doi: 10.1160/TH04-11-0715. [DOI] [PubMed] [Google Scholar]
- 14.Refaai MA, Ahn C, Lu L, Wu K, Broze GJ., Jr Protein Z and ZPI levels and cardiovascular events. J Thromb Haemost. 2006;4:1628–9. doi: 10.1111/j.1538-7836.2006.02012.x. [DOI] [PubMed] [Google Scholar]
- 15.van de Water N, Tan T, Ashton F, O’Grady A, Day T, Browett P, Ockelford P, Harper P. Mutations within the protein Z-dependent protease inhibitor gene are associated with venous thromboembolic disease: a new form of thrombophilia. Br J Haematol. 2004;127:190–4. doi: 10.1111/j.1365-2141.2004.05189.x. [DOI] [PubMed] [Google Scholar]
- 16.Corral J, Gonzalez-Conejero R, Soria JM, Gonzalez-Porras JR, Perez- Ceballos E, Lecumberri R, Roldan V, Souto JC, Minano A, Hernandez- Espinosa D, Alberca I, Fontcuberta J, Vicente V. A nonsense polymorphism in the protein Z-dependent protease inhibitor increases the risk for venous thromboembolism. Blood. 2006;108:177–83. doi: 10.1182/blood-2005-08-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razzari C, Marinelli I, Bucciarelli P, Viscardi Y, Biguzzi E. Polymorphisms of the protein Z-dependent protease inhibitor (ZPI) gene and the risk of venous thromboembolism. Thromb Haemost. 2006;95:909–10. [PubMed] [Google Scholar]
- 18.Folsom AR, Cushman M, Rasmussen-Torvik LJ, Heckbert SR, Tsai MY. Prospective study of polymorphisms of the protein Z-dependent protease inhibitor and risk of venous thromboembolism. Thromb Haemost. 2007;97:493–4. [PubMed] [Google Scholar]
- 19.Fedi S, Sofi F, Brogi D, Tellini I, Cesari F, Sestini I, Gazzini A, Comeglio M, Abbate R, Gensini GF. Low protein Z plasma levels are independently associated with acute coronary syndromes. Thromb Haemost. 2003;90:1173–8. doi: 10.1160/TH03-04-0237. [DOI] [PubMed] [Google Scholar]
- 20.Sofi F, Cesari F, Vigiani S, Fatini C, Marcucci R, Giglioli C, Valente S, Abbate R, Gensini GF, Fedi S. Protein Z plasma levels in different phases of activity of coronary atherosclerosis. J Thromb Haemost. 2005;3:2254–8. doi: 10.1111/j.1538-7836.2005.01536.x. [DOI] [PubMed] [Google Scholar]
- 21.Sofi F, Cesari F, Pratesi G, Cellai AP, Pulli R, Pratesi C, Gensini GF, Abbate R, Fedi S. Low protein Z levels in patients with peripheral arterial disease. Thromb Haemost. 2007;98:1114–17. [PubMed] [Google Scholar]
- 22.Zhang J, Tu Y, Lu L, Lasky N, Broze GJ., Jr Protein Z-dependent protease inhibitor deficiency produces amore severemurine phenotype than protein Z deficiency. Blood. 2008;111:4973–8. doi: 10.1182/blood-2007-12-126391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenland P, Abrams J, Aurigemma GP, Bond MG, Clark LT, Criqui MH, Crouse JR, 3rd, Friedman L, Fuster V, Herrington DM, Kuller LH, Ridker PM, Roberts WC, Stanford W, Stone N, Swan HJ, Taubert KA, Wexler L. Prevention Conference V: beyond secondary prevention: identifying the high-risk patient for primary prevention: non-invasive tests of atherosclerosis burden. Writing Group III. Circulation. 2000;101:E16–22. doi: 10.1161/01.cir.101.1.e16. [DOI] [PubMed] [Google Scholar]
- 24.Sofi F, Vecchio S, Giuliani G, Martinelli F, Marcucci R, Gori AM, Fedi S, Casini A, Surrenti C, Abbate R, Gensini GF. Dietary habits, lifestyle and cardiovascular risk factors in a clinically healthy Italian population: the ‘Florence’ diet is not Mediterranean. Eur J Clin Nutr. 2005;59:584–91. doi: 10.1038/sj.ejcn.1602112. [DOI] [PubMed] [Google Scholar]
- 25.Cifokova R, Erdine S, Fagard R, Farsang C, Heagerty AM, Kiowski W, Kjeldsen S, Luscher T, Mallion JM, Mancia G, Poulter N, Rahn KH, Rodicio JL, Ruilope LM, van Zwieten P, Waeber B, Williams B, Zanchetti A. ESH/ESC Hypertension Guidelines Committee. J Hypertension. 2003;21:1779–86. doi: 10.1097/01.hjh.0000084773.37215.1b. [DOI] [PubMed] [Google Scholar]
- 26.Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adult (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 27.Expert committee on the diagnosis and classification of diabetes mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26:S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 28.Rezaie AR, Sun M, Gailani D. Contributions of basic amino acids in the autolysis loop of factor XIa to serpin specificity. Biochemistry. 2006;45:9427–33. doi: 10.1021/bi060820+. [DOI] [PubMed] [Google Scholar]
- 29.Greten J, Kreis I, Liliensiek B, Allenberg J, Amiral J, Ziegler R, Nawroth PP. Localisation of protein Z in vascular lesions of patients with atherosclerosis. Vasa. 1998;27:144–8. [PubMed] [Google Scholar]
- 30.De Buyzere M, Philippe J, Duprez D, Baele G, Clement DL. Coagulation system activation and increase of D-dimer levels in peripheral arterial occlusive disease. Am J Hematol. 1993;43:91–4. doi: 10.1002/ajh.2830430204. [DOI] [PubMed] [Google Scholar]
- 31.Reininger CB, Graf J, Reininger A, Spannagi M, Steckmeier B, Schweiberer L. Increased platelet and coagulatory activity indicate ongoing thrombogenesis in peripheral arterial disease. Thromb Res. 1996;82:523–32. doi: 10.1016/0049-3848(96)00102-8. [DOI] [PubMed] [Google Scholar]
- 32.Han X, Fiehler R, Broze GJ., Jr Characterization of the protein Z-dependent protease inhibitor. Blood. 2000;96:3049–55. [PubMed] [Google Scholar]
- 33.Brevetti G, Schiano V, Chiariello M. Endothelial dysfunction: a key to the pathophysiology and natural history of peripheral arterial disease. Atherosclerosis. 2008;197:1–11. doi: 10.1016/j.atherosclerosis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Vasse M, Denoyelle C, Corbiere C, Litzler PY, Legrand E, Vannier JP. Human endothelial cells synthesize protein Z, but not the protein Z dependent inhibitor. Thromb Haemost. 2006;95:519–23. doi: 10.1160/TH05-11-0723. [DOI] [PubMed] [Google Scholar]
- 35.Cesari F, Gori AM, Fedi S, Abbate R, Gensini GF, So F. Modifications of protein Z and interleukin-6 during the acute phase of coronary artery disease. Blood Coagul Fibrinolysis. 2007;18:85–6. doi: 10.1097/MBC.0b013e3280124f2c. [DOI] [PubMed] [Google Scholar]
- 36.Ramsay JE, Stewart F, Friel H, Walker ID, Greer A, McColl MD. Protein Z in pregnancy: exaggerated rise in obese women. J Thromb Haemost. 2005;3:2584–6. doi: 10.1111/j.1538-7836.2005.01607.x. [DOI] [PubMed] [Google Scholar]
- 37.van Goor MPJ, Dippel DWJ, Jie KS-G, de Maat MPM, Koudstaal PJ, Leebeek FWG. Low protein Z levels, but not the protein Z gene G79A polymorphism are a risk factor for ischemic stroke. Thromb Res. 2008;123:213–18. doi: 10.1016/j.thromres.2008.02.006. [DOI] [PubMed] [Google Scholar]


