Abstract
Context
Health risks associated with subclinical hypothyroidism in older adults are unclear.
Objective
To compare the functional mobility of seventy-year-olds by thyroid function categorized by thyroid stimulating hormone (TSH) level as euthyroid (0.4 mU/L< TSH <4.5 mU/L) or having mild (4.5 mU/L≥ TSH <7.0 mU/L) or moderate subclinical hypothyroidism (7.0 mU/L≤ TSH ≤20.0 mU/L with normal FT4) cross-sectionally and over two years.
Design, Setting, and Participants
2,290 community residents participating in the Year 2 clinic visit (July 1998-June 1999) of the Health, Aging and Body Composition study with measured TSH, capacity to walk 20 meters unaided and not taking thyroid medication or having TSH levels consistent with hyper- or hypothyroidism.
Main Outcome Measures
Self-reported and performance-based measures of mobility (usual and rapid gait speed and endurance walking ability) assessed at study baseline (Year 2) and two years later.
Results
In age- and sex-adjusted analyses the mild subclinical hypothyroid group demonstrated better mobility – faster usual and rapid gait speed (1.20 vs. 1.15 m/s and 1.65 vs. 1.56 m/s; p<.001) and higher percent with good cardiorespiratory fitness and reported walking ease (39.2 vs. 28.0 and 44.7 vs. 36.5; p<.001). After two years, persons with mild subclinical hypothyroidism experienced similar decline as the euthyroid, but maintained their mobility advantage. Persons with moderate subclinical hypothyroidism had similar mobility and decline as the euthyroid group.
Conclusions
Generally well-functioning seventy-year-olds with subclinical hypothyroidism do not demonstrate increased risk of mobility problems and those with mild elevations in TSH show a slight functional advantage.
INTRODUCTION
Prevalence of subclinical hypothyroidism, defined as elevated thyroid stimulating hormone (TSH) with free thyroxine (FT4) in the normal range, increases with age affecting about 6% of persons aged 70 to 79 years and 10% of those aged 80 or older.1 Although a chief concern with subclinical hypothyroidism has been risk of progression to overt hypothyroid disease,2, 3 elevated lipid levels,4–6 increased risk of cognitive impairment7 and cardio-vascular conditions, including myocardial infarction8 and mortality,9–11 research findings have not consistently supported these concerns,12–14 particularly in elderly individuals15–17 and those with only mild elevations of TSH.9, 14 In fact, findings in a cohort of persons aged 85 years suggest that subclinical hypothyroidism may be associated with a prolonged survival.18 Additionally, an analysis of age-specific distributions of serum thyrotropin and antithyroid antibodies in the U.S. population indicates a shift toward higher concentrations of TSH with increasing age1 suggesting a resultant upward shift in normal concentrations.
In this study we sought to evaluate the potential meaning and importance of various levels of TSH traditionally defined as in the normal to subclinical hypothyroidism range by examining associations with several dimensions of walking ability concurrently and prospectively over two years. We focused on functional mobility because it is widely considered a global marker of current health status and risk of future negative health events in older adults.19, 20.
The few studies of older adults that have distinguished levels of subclinical hypothyroidism have generally found the mild group to exhibit the most favorable profile.18 On the basis of studies restricted to older adults,16 we hypothesized that persons with moderate subclinical hypothyroidism would exhibit similar mobility status and rates of mobility decline as persons with traditionally defined normal thyroid function and those with mild subclinical hypothyroidism would demonstrate a slightly more favorable mobility profile and less decline.
METHODS
Study Population
The study population derives from the Health, Aging and Body Composition (Health ABC) study, a population-based biracial cohort study of 3075 men and women residing in the communities of Memphis, TN or Pittsburgh, PA aged 70 to 79 years at the baseline enrollment period (Year 1) extending from April 1997 through June 1998. Potential participants were identified from a random sample of white and all age-eligible black Medicare beneficiaries living in designated zip code areas surrounding the study sites who were screened to select well-functioning individuals – those reporting no difficulty walking one quarter mile, climbing one flight of stairs or performing basic activities of daily living. These individuals are representative of the approximately fifty percent of U.S. residents aged 70 to 79 years with no apparent mobility limitations. Persons participating in behavioral intervention studies, planning to leave the area within three years or having a diagnosis of a life-threatening cancer were excluded.
Participants eligible for the current study had a valid fasting blood draw and measurement of TSH level in Year 2 (n=2800). Persons taking either thyroid antagonist (n=2) or exogenous thyroid hormone (n=276) medications and those with evidence of overt hypothyroidism (TSH >20 mU/L; n=13 or TSH ≥7.0 mU/L and a thyroxine <0.8 6 dg/dL; n=7) or low TSH consistent with hyperthyroidism (TSH <0.45 mU/L; n=51) were excluded. Given the interest in mobility parameters, persons unable to walk 20 meters without a walking aid (n=88) or who had a home visit (n=73) and therefore were not administered the 20-m walk test were also excluded, yielding an analysis cohort of 2290. This group of 161 excluded individuals did not differ with respect to thyroid function from those included in the study (p=.70).
Measures
Thyroid Function
Thyrotropin levels were measured at a central laboratory (University of Vermont) in all participants who had a clinic or home visit and agreed to and had a blood draw (n=2,800) by immunoassay (ACS; Chiron Diagnostics Corp, Emeryville, CA). The normal range provided by the manufacturer for this assay is 0.35 to 5.5 mU/L, with a lower limit of detection of 0.03 mU/L and a coefficient of variation of 3.6% at a level of 1.26 mU/L. FT4 was assessed by competitive immunoassay (ACS; Diagnostics Corp, Emeryville, CA) on all participants with a TSH level ≤0.1 mU/L or ≥7.0 mU/L. The normal range provided by the manufacturer is 0.8 to 1.8 ng/dL. For the main analyses, three groups were distinguished – euthyroid (0.4 mU/L< TSH <4.5 mU/L), mild subclinical hypothyroid (4.5 mU/L≥ TSH <7.0 mU/L) and moderate subclinical hypothyroid (7.0 mU/L≤ TSH ≤20.0 mU/L with normal FT4), based on the definitions used by the U.S. Preventive Services Task Force.14 We selected a TSH level of 7.0 mU/L to distinguish mild from moderate subclinical hypothyroidism since it represents the 97.5 centile in a representative U.S. population aged 70 and older free of thyroid disease.1 As a secondary analysis to better understand the relationship between mobility and thyroid function over a continuum of TSH, we constructed a nine level variable in which the first 7 seven levels were defined in 1.0 mU/L increments and the last two encompass moderate subclinical hypothyroidism subdivided at 10.0 mU/L.
Mobility Parameters
The mobility parameters include both self-report and performance-based measures designed to tap the full continuum of mobility from perceived difficulty and ease walking and basic to advanced walking performance.
Reported mobility capacities and limitations were determined from responses to a series of questions beginning with, “Because of a health or physical problem, do you have any difficulty walking a quarter of a mile that is about 2 or 3 blocks, without stopping?” Those reporting difficulty were asked whether they had a little, some or a lot of difficulty or were unable to walk. Persons expressing no difficulty were asked how easy it is for them to walk a quarter of a mile – very, somewhat, or not so easy - followed by whether they have any difficulty walking one mile and the ease of walking one mile if no difficulty was reported.21 Responses to these questions were combined to create a walking ability index ranging from 0 to 9, where 0 represents unable to walk ¼ mile and 9 indicates walking one mile is very easy. We also examined separately prevalence of walking limitation (any difficulty walking ¼ mile) and good walking capability (reporting walking one mile is very easy).
Performance-based evaluations include usual and rapid gait speed, measures of basic and reserve capacity, assessed over a 20 meter course. Participants were asked first to walk at their “usual walking pace” and then “as fast as [they] can” for the return trip. Total time recorded to the hundredth of a second was divided into 20 to obtain respectively usual and rapid gait speed in meters per second. At follow-up, mean change and proportion experiencing meaningful decline defined as reduction in gait speed of 4% annually on average22 were evaluated. For persons alive at Year 4 who did not have a clinic but had a home visit, timed gait over 4 meters was used. Self-report of severe walking difficulty (a lot of difficulty or inability to walk ¼ mile) was considered indicative of decline among those with phone contact only. Anyone requiring a walking aid was also considered to have declined.
Cardiorespiratory fitness was determined from performance on the Long Distance Corridor Walk (LDCW), a two-stage, self-paced endurance walk test performed over a 20-m course.23, 24 Persons with any of the following electrocardiogram abnormalities: Wolff-Parkinson-White or ventricular pre-excitation, idioventricular rhythm, ventricular tachycardia, third degree or complete A–V block, evidence of acute injury or ischemia or marked T-wave abnormality; systolic or diastolic blood pressure exceeding 199 mmHg or 109 mmHg, respectively; or heart rate below 40 or above 110 beats per minute were excluded from testing. Participants reporting a myocardial infarction, angioplasty, or heart surgery in the prior 3 months or experiencing new or worsening symptoms of chest pain, shortness of breath, fainting or angina were also excluded.
Heart rate was monitored during testing (Polar Pacer, Model 61190, Polar Electro, Oy, Finland) and participants were stopped if their heart rate exceeded 135 beats per minute (85 to 95% of the age-predicted maximum heart rate25) or they experienced debilitating pain, shortness of breath, syncope or excessive fatigue. Meeting exclusion criteria, inability to complete the test, time needed to complete 400 meters and fitness categories were examined. At follow-up, only those persons who completed the LDCW in Year 2 were evaluated for decline defined as exclusion from or inability to complete the LDCW in Year 4 or needing 8% more time to walk 400 meters.
Covariates
Covariates encompass socio-demographic factors including age, self-designated white or black race, sex, and study site; self-reported physician diagnosed thyroid disease obtained from the Year 1 interview and behavioral factors known to impact walking ability in late life including walking related physical activity, measured weight, smoking status (current and late-life quitters versus never and former smokers who quit prior to age 50), and self-perceived health status. Use of thyroid hormone medications for study exclusion in Year 2 and to adjust for treatment in Year 3 was determined from an inventory of all medications taken within the past two weeks coded using the Iowa Drug Information System by trained interviews from the containers participants brought to their clinic visit.
Statistical Analyses
For analysis, the population was divided into three groups – euthyroid, mild subclinical hypothyroid and moderate subclinical hypothyroid – as described above. Participant characteristics within thyroid function group were compared using a chi-square or t test as appropriate. Baseline (Year 2) differences in the walking ability parameters across the three thyroid function categories were evaluated using least squares means adjusted initially for age and sex and then for race, study site, smoking, reported thyroid disease, body weight, self-rated health, walking behavior and any significant interactions between thyroid function category and sex and race. Differences in usual and rapid gait speed and walking index score over nine categories TSH were evaluated using least square means adjusted for age and sex. In the longitudinal analyses conducted two years later, for each mobility parameter we compared the mean change and percent decline between Year 2 and Year 4, and mean value in Year 4 across categories of thyroid function assessed in Year 2. In the analyses of mean change in addition to age and sex, Year 2 status of the outcome of interest was included in the modeling as was new use of thyroid agonists over the follow-up period using medication data obtained in Year 3. These longitudinal analyses were limited to individuals with complete follow-up data. All analyses used SAS version 9.1.3 (Cary, NC).
RESULTS
Concurrent Associations
The 2290 participants examined had a mean age of 74.6 years and 10.6% of men and 12.3% of women met criteria for subclinical hypothyroidism. With the exception of a higher percentage of blacks in the euthyroid group and greater walking activity in the mild subclinical hypothyroid group, participant characteristics did not differ across categories of thyroid function (Table 1). Mobility status, however, was somewhat better in the mild subclinical hypothyroid group, who in age- and sex-adjusted analyses demonstrated faster mean usual and rapid gait speed, better cardio-respiratory fitness as indicated by a higher percentage having good fitness and an overall faster mean 400-m walk time in persons completing the test, and better perceived walking ability, evidenced by a higher percentage who reported walking for 1 mile is very easy and a higher mean walking ability score (Table 2a). The extent of differences observed for usual and rapid gait speed of .05 m/s and .10 m/s are considered clinically meaningful.22 The mobility status of persons with moderate subclinical hypothyroidism was generally no worse than the euthyroid group with the exception of having higher rates of exclusion from the LDCW and reported walking difficulty.
Table 1.
Baseline Characteristics by Thyroid Status in the Health ABC Study
| TSH, mU/L | |||
|---|---|---|---|
| Characteristics | 0.45 – 4.49 | 4.50 – 6.99 | 7.00 – 20.0a |
| N = 2290, No. | 2028 | 191 | 71 |
| Age, mean (SD), y | 74.6 (2.9) | 75.0 (2.8) | 75.0 (2.5) |
| Female, % | 47.0 | 50.8 | 52.1 |
| Black, % | 41.5 | 23.6 | 22.5 |
| Memphis site, % | 50.1 | 52.4 | 45.1 |
| Current or recent smoker, % | 29.0 | 23.0 | 33.8 |
| Reported diagnosis of hypothyroidism | 2.5 | 4.7 | 2.8 |
| Reported diagnosis of hyperthyroidism | 2.5 | 2.1 | 4.2 |
| Very good to excellent self-rated health, % | 47.5 | 48.2 | 48.6 |
| Fair to poor self-rated health, % | 14.4 | 15.7 | 14.3 |
| Weight, mean (SD), kg | 75.7 (15.1) | 73.2 (14.6) | 75.2 (15.9) |
| Walks less than 30 minutes/week, % | 44.2 | 35.6 | 39.4 |
Abbreviations: Health ABC, Health, Aging and Body Composition; TSH, thyroid-stimulating hormone
With normal free thyroxine levels (0.8 to 1.8 ng/dL for assay used)
Table 2.
| Table 2a. Baseline Mobility Parametersa by Thyroid Status | |||
|---|---|---|---|
| TSH, mU/L | |||
| Walking Ability Parameters | 0.45 – 4.49 | 4.50 – 6.99 | 7.0 – 20.0b |
| N = 2290, No. | 2028 | 191 | 71 |
| Usual gait speed, mean (SD), m/s | 1.15 (.20) | 1.20 (.22)c | 1.17 (.23) |
| Rapid gait speed, mean (SD), m/s | 1.56 (.31) | 1.65 (.33)c | 1.59 (.32) |
| Excluded from LDCW, % | 13.3 | 10.8 | 22.3d |
| Incomplete LDCW, % | 17.0 | 14.4 | 14.5 |
| 400 meter time, mean (SD), s | 321.7 | 311.1d | 314.8 |
| Good cardiorespiratory fitness, % | 28.0 | 39.2c | 28.3 |
| Reports difficulty walking ¼ mile, % | 16.2 | 14.7 | 26.2d |
| Reports walking 1 mile is very easy, % | 36.5 | 44.7d | 36.1 |
| Walking ability index score, mean (SD) | 6.74 | 7.08 | 6.23 |
| Table 2b. Baseline Mobility Parameterse by Thyroid Status - Fully Adjusted | |||
|---|---|---|---|
| TSH, mU/L | |||
| Walking Ability Parameters | 0.45 – 4.49 | 4.50 – 6.99 | 7.0 – 20.0b |
| N = 2290, No. | 2028 | 191 | 71 |
| Usual gait speed, mean (SD), m/s | 1.15 (.20) | 1.18 (.22)d | 1.17 (.23) |
| Rapid gait speed, mean (SD), m/s | 1.56 (.31) | 1.62 (.33)f | 1.57 (.32) |
| Excluded from LDCW, % | 13.1 | 12.3 | 23.9d |
| Incomplete LDCW, % | 16.8 | 16.7 | 16.1 |
| 400 meter time, mean (SD), s | 321.6 | 312.5d | 314.6 |
| Good cardiorespiratory fitness, % | 28.3 | 36.2d | 28.6 |
| Reports difficulty walking ¼ mile, % | 16.0 | 17.0 | 26.8d |
| Reports walking 1 mile is very easy, % | 37.0 | 40.0 | 34.0 |
| Walking ability index score, mean (SD) | 6.76 | 6.87 | 6.16d |
Abbreviations: TSH, thyroid-stimulating hormone; LDCW, Long Distance Corridor Walk
Adjusted for age and sex
With normal free thyroxine levels (0.8 to 1.8 ng/dL for assay used)
P<.001 for comparison with euthyroid category
P<.05 for comparison with euthyroid category
Adjusted for age, sex, race, study site, smoking, reported thyroid disease, body weight, self-rated health, walking behavior, and the interaction between race and thyroid function
P<.01 for comparison with euthyroid category
The mobility advantage of the mild subclinical hypothyroid over the euthyroid group with respect to usual and rapid gait speed and cardiorespiratory fitness remained after adjustment for race, study site, smoking status, body weight, reported thyroid disease diagnosis, self-rated health, walking behavior and the interaction between race and thyroid function (Table 2b). After adjustment, persons with moderate subclinical hypothyroidism continued to show a higher prevalence of mobility deficits with respect to meeting exclusion criteria for endurance walk testing and reported walking difficulty. There was no interaction between sex and thyroid function group and any mobility parameter (p=.13 – .73), but significant interactions between race and thyroid function with respect to usual walking speed (p=.058) and 400-m walk time (p=.010).
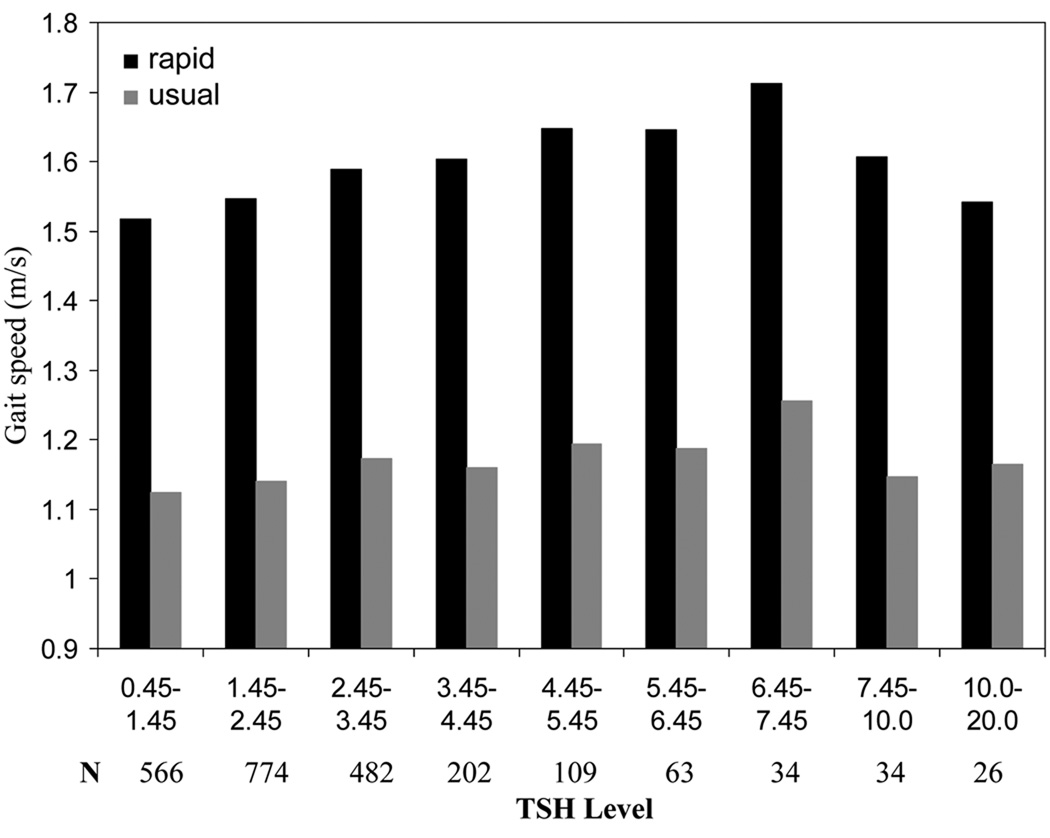
Figure 1 provides age and sex adjusted means for usual and rapid gait speed across nine categories of TSH. Both usual and rapid gait speed increased with TSH level up to approximately 7.45 mU/L with the zenith occurring at the TSH category ranging from 6.45 to 7.45 mU/L. Mean usual and rapid gait speed at this level were statistically different from the first four categories with TSH levels ranging from 0.45 to 4.45 mU/L (p<.001 to p=.044). For raid gait speed, categories 3 through 6 (2.45–6.45 mU/L) were also statistically different from categories 1 and 2 (0.45–2.45 mU/L; p<.001 to p=.016), supporting a mild protective association between mild subclinical hypothyroidism and mobility defined by gait speed. Analysis of walking index score showed no association.
Figure 1.
Mean usual and rapid gait speed (m/s) by TSH level
Prospective Associations
Two years later, at the Year 4 visit, of the 2290 individuals included in the concurrent analyses, 102 had died, 4 withdrew from study and 46 had a missed visit and therefore unknown mobility status leaving 2138 available for evaluation. Neither mortality nor visit status varied by thyroid function (p>.6). Decline in usual and rapid gait speed, defined as newly developed inability to walk at least 4 meters without a walking aid or a reduction of 8% or more in speed,22 occurred overall in 28.2% and 35.9%, respectively and did not vary by thyroid function (see Table 3a). Mean percent decline in the euthyroid and mild and moderate subclinical hypothyroid groups for usual gait speed was 3.9, 4.6 and 3.9 respectively and 7.3, 8.0 and 8.3 for rapid gait speed which also did not vary by thyroid function in adjusted analyses. Among the 1652 participants completing the LDCW in Year 2, 1444 (87.4%) had a Year 4 clinic visit, the likelihood of which did not vary by thyroid function (p>.8). Just over 25% evidenced decline in cardio-respiratory fitness (exclusion from or inability to complete the LDCW or 8% slower time to walk 400 meters) which also did not vary by thyroid function. Lastly, overall, 34% experienced at least a one point decrease in their walking ability score, which did not differ by thyroid status.
Table 3.
| Table 3a. Prevalence of Decline in Walking Ability after Two Yearsa by Baseline Thyroid Status | |||
|---|---|---|---|
| TSH, mU/L | |||
| Walking Ability Parameters | 0.45 – 4.49 | 4.50 – 6.99 | 7.0 – 20.0b |
| N = 2138, No. | 1897 | 176 | 65 |
| Declined in usual gait speed, % | 28.1 | 31.3 | 22.2 |
| Declined in rapid gait speed, % | 36.4 | 31.5 | 33.0 |
| Declined in cardiorespiratory fitnessc, % | 25.4 | 23.5 | 25.2 |
| Declined in walking ability score, % | 34.1 | 29.9 | 41.0 |
| Table 3b. Walking Ability after Two Yearsd by Baseline Thyroid Status | |||
|---|---|---|---|
| TSH, mU/L | |||
| Walking Ability Parameters | 0.45 – 4.49 | 4.50 – 6.99 | 7.0 – 20.0b |
| N = 2138, No. | 1897 | 176 | 65 |
| Usual gait speed in year 4, mean, s | 1.10 | 1.15e | 1.14 |
| Rapid gait speed in year 4, mean, s | 1.44 | 1.55f | 1.50 |
| Good cardiorespiratory fitnessg, % | 23.0 | 31.6e | 27.0 |
| 400 meter time in year 4, mean, s | 328.5 | 312.3f | 316.3 |
| Reports difficulty walking ¼ mile, % | 21.0 | 17.5 | 25.2 |
| Reports walking 1 mile is very easy, % | 36.9 | 46.4e | 28.9 |
| Walking ability score in year 4, mean | 6.46 | 6.90e | 6.10 |
Abbreviations: TSH, thyroid-stimulating hormone
Adjusted for age, sex, baseline walking ability and use of thyroid agonists in Year 3
With normal free thyroxine levels (0.8 to 1.8 ng/dL for assay used)
Among the 1444 participants completing the LDCW in Year 2 having a Year 4 clinic visit
Adjusted for age, sex and use of thyroid agonists in year 3.
P<.05 for comparison with euthyroid category
P<.01 for comparison with euthyroid category
Among the 1959 participants having a Year 4 clinic visit
Even though the likelihood of decline was uniform across categories of thyroid function, persons with mild subclinical hypothyroidism retained a slight functional advantage relative to the euthyroid group two years later with respect to usual and rapid gait speed, time to walk 400 meters among those completing the test and reported function – percent responding that walking 1 mile is very easy and mean walking ability score (see Table 3b).
DISCUSSION
Study findings indicate that generally well-functioning persons in their seventies with a TSH level falling in the mild subclinical hypothyroidism range (4.5–7.0 mU/L) do not have poorer functional mobility than their euthyroid counterparts. On the contrary, on the most demanding mobility parameters examined, these individuals appear to have a slight mobility advantage. Although persons in the moderate subclinical range (7.0–20.0 mU/L) had higher rates of perceived walking difficulty and contraindication to endurance activity in comparison to those in the euthyroid range, they demonstrated similar function on other mobility parameters. An examination of gait speed by finer categorizations of TSH level indicates that functional reserve increases with increasing TSH until 7.45 mU/L when it begins to decline. Remarkably, even in persons with TSH levels up to 10 mU/L rapid gait speed was statistically significantly faster than persons at the lowest level of TSH (.45–1.45 mU/L; all p<.001 except the second category where p=.011).
Few studies have examined functional status by thyroid function in older individuals. The Leiden 85-Plus Study18 found no association between TSH level and prevalence of disability in basic and instrument activities of daily living (ADL), however, those with higher TSH levels had less decline in instrumental ADL over four years. Even though measures of functional mobility are not directly comparable to ADL competence, findings from the current study are generally consistent with this work; in that, thyroid function was not found associated with functional deficits and when differences emerged persons with mildly elevated TSH levels demonstrated a functional advantage. The findings reported herein provide further support for a positive association between TSH level and functional independence and extends the association to a slightly younger group. Another study26 involving community-resident men aged 73 years and older in The Netherlands found greater grip strength and better lower-extremity performance in persons with higher FT4 levels. The association between TSH level and physical performance was not reported.
Even though findings indicate that older adults with subclinical hypothyroidism function as well as if not better than those with normal thyroid function, it is not known whether individuals with subclinical hypothyroidism who initiate treatment would experience improved or less decline in mobility. Based on medication data collected in Year 3, just under half (31 / 47.7%) of the 65 individuals with moderate subclinical hypothyroidism in Year 2 who were alive in Year 4 with complete follow-up data began new use of thyroid agonists. Examining gait speed decline, we found no difference between those who did and did not initiate treatment (27.7 vs. 18.9% p=.412 for usual and 44.4 vs. 24.2% p=.096 for rapid gait); however, the sample size is too small to make a definitive judgment. If anything, those initiating treatment tended to exhibit higher rates of decline.
It remains unclear if a mildly elevated TSH level directly contributes to better mobility status or reflects an underlying positive adaptation that fosters robust health. Recent observations of a right shift in the distribution of TSH with age1 and the apparent protective association with mortality in advanced age,18 have led to speculation that increased TSH secretion may be an adaptive response to an accumulation of thyroid antibodies that frequently occurs with age16 and thus may be a marker of pituitary resiliency and health. Others have suggested that a lower metabolic rate may protect against excessive catabolism.26
An important study limitation concerns incomplete assessment of thyroid function. As in most other observational cohort studies, 5, 8, 10 TSH level was measured on a single occasion. Given that acute stress, high physical activity and several pharmacologic agents can affect TSH level, a single high reading may represent a transient benign elevation.14, 16 FT4 was not measured in participants with a TSH between 4.5 and 6.9 mU/L, therefore some persons with overt hypothyroidism may have been included with the mild subclinical hypothyroidism group, which would tend to bias the findings towards poorer mobility function and underestimate any mobility advantage. In as much as casual assessment of TSH undertaken in a routine medical visit may serve as the basis for follow-up testing and possible treatment, the findings reported here suggest that for individuals in their seventies, a mildly elevated TSH level is no cause for concern. The small number of participants in the moderate subclinical hypothyroid group constitutes another limitation and thus findings of no difference between groups should be treated with caution.
Even though few studies have considered new treatment in examining differential outcomes by thyroid function over time,26 assessment of medication use over the follow-up period was limited to Year 3 only. Since participants received a report of their TSH levels after their Year 2 clinic visit, we would expect most new use to occur in Year 3 and as reported above new use was not found to impact rate of mobility decline. Therefore the lack of medication data from Year 4 does not appear to present a major shortcoming.
In summary, in generally well-functioning community resident persons in their seventies, mildly elevated TSH levels do not appear to indicate or confer health risks as reflected by multiple parameters of functional mobility. Despite the Institute of Medicine’s recommendation against routine TSH screening and thus treatment of asymptomatic subclinical hypothyroidism,26 controversy remains as to whether and when treatment should be initiated even in older adults.27 We believe the findings reported herein provide supportive evidence that mild to moderate elevations in TSH with normal FT4 pose little threat to the health and functioning of older adults. A better understanding of the meaning of the age-related increase in TSH level and its potential benefit warrants further study and consideration.
Acknowledgement
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging and contracts N01-AG-6-2101, N01-AG-2103 and N01-AG-6-2106. Dr, Eleanor M. Simonsick affirms that she had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the U.S. population: Implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92(12):4575–4582. doi: 10.1210/jc.2007-1499. [DOI] [PubMed] [Google Scholar]
- 2.Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol. 1995;43(1):55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga WM. Subclinical hypothyroidism and hyperthyroidism.I. Prevalence and clinical relevance. Netherlands J Med. 1995;46:197–204. doi: 10.1016/0300-2977(94)00089-r. [DOI] [PubMed] [Google Scholar]
- 4.Bauer DC, Ettinger B, Browner WS. Thyroid function and serum lipids in older women: A population-based study. Am J Med. 1998;104:546–551. doi: 10.1016/s0002-9343(98)00116-8. [DOI] [PubMed] [Google Scholar]
- 5.Walsh JP, Bremmer AP, Bulsara MK, et al. Thyroid dysfunction and serum lipids: a community-based study. Clin Endocrinol. 2005;63:670–675. doi: 10.1111/j.1365-2265.2005.02399.x. [DOI] [PubMed] [Google Scholar]
- 6.Asvold BO, Vatten LJ, Nilsen TIL, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT study. Eur J Endocrinol. 2007;156:181–186. doi: 10.1530/eje.1.02333. [DOI] [PubMed] [Google Scholar]
- 7.Hogervorst E, Huppert F, Mattews FE, Brayne C. Thyroid function and cognitive decline in the MRC cognitive function and ageing study. Psychoneuroendocrinology. 2008;33:1013–1022. doi: 10.1016/j.psyneuen.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Hak AE, Pols HAP, Visser TJ, Drexhage HA, Hofman A, Witteman JCM. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: The Rotterdam Study. Ann Intern Med. 2000;132:270–278. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- 9.Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events and death. Arch Intern Med. 2005;165:2460–2466. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 10.Cappola AR, Fried LP, Arnold AM, et al. Thyroid status, cardiovascular risk and mortality in older adults. JAMA. 2006;295(9):1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochs N, Auer R, Bauer DC, et al. Meta-analysis: Subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med. 2008;148:832–845. doi: 10.7326/0003-4819-148-11-200806030-00225. [DOI] [PubMed] [Google Scholar]
- 12.Hueston WJ, Pearson WS. Subclinical hypothyroidism and the risk of hypercholesterolemia. Ann Fam Med. 2004;2(4):351–355. doi: 10.1370/afm.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceresini G, Lauretani F, Maggio M, et al. Thyroid function abnormalities and cognitive impairment in elderly people: Results of the Invecchiare in Chianti study. J Am Geriatr Soc. 2009;57:89–93. doi: 10.1111/j.1532-5415.2008.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helfand M. Screening for subclinical thyroid dysfunction in nonpregnant adults: A summary of the evidence for the U.S. Preventive Services task Force. Ann Intern Med. 2004;140:128–141. doi: 10.7326/0003-4819-140-2-200401200-00015. [DOI] [PubMed] [Google Scholar]
- 15.Razvi S, Shakoor A, Vanderpump M, Weaver JU, Pearce SHS. The influence of age on the relationship between subclinical hypothyroidism and ischemic heart disease: A metaanalysis. J Clin Endocrinol Metab. 2008;93:2998–3007. doi: 10.1210/jc.2008-0167. [DOI] [PubMed] [Google Scholar]
- 16.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endrocr Rev. 2008;29(1):76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 17.Gussekloo J, van Exel E, de Craen AJM, Meinders AE, Frölich M, Westerndorp RGJ. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292(21):2591–2599. doi: 10.1001/jama.292.21.2591. [DOI] [PubMed] [Google Scholar]
- 18.Takashima N, Niwa Y, Mannami T, Tomoike H, Iwai N. Characterization of subclinical thyroid dysfunction from cardiovascular and metabolic viewpoints – The Suita Study. Circ J. 2007;71:191–195. doi: 10.1253/circj.71.191. [DOI] [PubMed] [Google Scholar]
- 19.Guralnik JM, Ferrucci L, Simonsick EM, Salive M, Wallace RB. Lower extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newman AB, Simonsick EM, Naydeck BL, et al. Association of Long-Distance Corridor Walk performance with mortality, cardiovascular disease, mobility limitation and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 21.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: Expanding familiar approaches in the Health ABC study. J Gerontol Med Sci. 2001;56A(10):M644–M649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 22.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 23.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: The Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49(11):1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 24.Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: Treadmill Validation of the Long Distance Corridor Walk. J Am Geriatr Soc. 2006;54(1):127–132. doi: 10.1111/j.1532-5415.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;137(1):153–156. doi: 10.1016/s0735-1097(00)01054-8. [DOI] [PubMed] [Google Scholar]
- 26.van den Beld AW, Visser TJ, Feelders RA, Grobbee DE, Lamberts SWJ. Thyroid hormone concentrations, disease, physical function, and mortality in elderly men. J Clin Endocrinol Metab. 2005;90(12):6403–6409. doi: 10.1210/jc.2005-0872. [DOI] [PubMed] [Google Scholar]
- 27.Cooper DS. Thyroid disease in the oldest old: the exception to the rule. JAMA. 2004;292(21):2651–2654. doi: 10.1001/jama.292.21.2651. [DOI] [PubMed] [Google Scholar]