Abstract
Background
A failure to develop normal language is one of the most common first signs that a toddler might be at risk for autism. Currently the neural bases underlying this failure to develop language are unknown.
Methods
In this study, functional magnetic resonance imaging (fMRI) was utilized to identify the brain regions involved in speech perception in 12 2–3 year-old children with autism spectrum disorder (ASD) during natural sleep. We also recorded fMRI data from two typically developing control groups: a mental age-matched (MA) (n=11) and a chronological age-matched (CA) (n=12) group. During fMRI data acquisition, forward and backward speech stimuli were presented with intervening periods of no sound presentation.
Results
Direct statistical comparison between groups revealed significant differences in regions recruited to process speech. In comparison to their MA-matched controls, the ASD group showed reduced activity in an extended network of brain regions, which are recruited in typical early language acquisition. In comparison to their CA-matched controls, ASD participants showed greater activation primarily within right and medial frontal regions. Laterality analyses revealed a trend towards greater recruitment of right hemisphere regions in the ASD group and left hemisphere regions in the CA group during the forward speech condition. Furthermore, correlation analyses revealed a significant positive relationship between right hemisphere frontal and temporal activity to forward speech and receptive language skill.
Conclusions
These findings suggest that at 2–3 years, children with ASD may be on a deviant developmental trajectory characterized by a greater recruitment of right hemisphere regions during speech perception.
Keywords: language, development, laterality, fMRI, pediatric, sleep
INTRODUCTION
A striking disparity in language development between autistic and typical children is seen by the second year of life (1). In fact, a delay in language is often one of the first warning signs to parents and clinicians that a child may be at risk for autism (2; 3). Language impairments in autism can be severe with approximately 50% percent of individuals never acquiring functional language (4). Those autistic children who do develop functional language commonly show impairments in semantic and pragmatic aspects of language, such as use of prosody, pronoun, or inferring intentions of the speaker, while structural aspects, such as syntax and grammar, more often appear relatively less impaired (5), although more recent evidence suggests some children with autism do show structural impairments as well (6). While much research has elucidated the behavioral characteristics of language impairments (for review see (7)), remarkably little is known about the neural bases of language abnormalities in autism, particularly at young ages.
The extant fMRI and PET (positron emission tomography) studies examining the neural bases of language processing in autism have all been conducted with relatively high-functioning older children and adults (8–13), except one in which sedation was used with children 4–10 years of age (14). In general, these studies reveal an abnormal frontal and/or temporal response during language processing in autism, and some show a pattern of reduced or reversed laterality in frontal cortex (8–11). While these studies have advanced our understanding of brain abnormalities underlying language processing at a middle or endpoint of development, the findings may not reflect the initial brain abnormalities at the time of the emergence of the disorder nor brain abnormalities of children on the lower-functioning end of the autism spectrum. Much evidence suggests deviant patterns of brain structure are not only greater, but may also be different, at younger than at older ages in autism (15–21). For example, while amygdala volume and neuron number is normal or reduced at older ages, amygdala volume is increased at younger ages (19; 22–26), suggesting neurobiological processes in the initial phase of autism may be unique.
Only one study has examined the neural correlates of linguistic processing in children as young as 3 years of age with ASD (27). In that study, presentation of deviant phonemes failed to elicit an event-related potential (ERP) index of sound discrimination, the mismatch negativity (MMN), in children with autism. Interestingly, studies of older children with autism revealed an intact MMN response to phonemes but reduced P3a amplitude as compared to typical children (28–30). In sum, limited functional evidence from very young children with ASD also suggests that the neural bases of autism need to be addressed from a developmental perspective as brain activation patterns from the older child and adult with autism may not necessarily reflect those of the younger child. These electrophysiological studies lack the whole brain resolution afforded by functional MRI methods. Thus, a critical question remains: what are the specific neural structures underlying language impairments in autism at the time the disorder is first reliably identified and diagnosed, namely at 2–3 years of age?
The paucity of functional neuroimaging from very young children with autism is likely due to the difficulty in acquiring such data. Previous work by our group (31; 32) and others (33–35) have identified reliable fMRI activation patterns during presentation of auditory stimuli during natural sleep in infants, toddlers, and very young children. In a previous fMRI study we identified age-related changes in the Blood Oxygenated Level Dependent (BOLD) response between typically developing toddlers and 3 year-old children during presentation of text passages in sleep (31). In that study, the 3 year-old group utilized primarily bilateral superior temporal and parietal regions to process forward speech as compared to no sound presentation, while the toddler group recruited a large number of cortical and subcortical brain regions. We suggest that these regions may be part of an extended network of brain activation here and in our previous paper (31) although the extent to which these regions are functioning as a network has not been directly tested. We raised the possibility that this hypothesized network of regions in toddlers may reflect the state of a young brain that has not yet become specialized for language, but rather is poised and ready to acquire language through social, attention, and other systems that are available.
In this study, we presented the same speech paradigm used in the Redcay et al. study (31) to 2–3 year-old children with a provisional diagnosis of autism spectrum disorder (ASD) during sleep. The design contained both a mental age-matched typically developing control group (MA) to control for the effects of language skill and a chronological age-matched (CA) typically developing control group to control for the effects of maturation. These control groups largely overlapped with the toddler and 3-year old groups in our previous paper (31). The goal of the study was to determine whether the ASD group would show an extended network pattern of brain activation like their MA-matched controls or whether activity would be specialized to superior temporal regions like that seen in the CA-matched controls.
METHODS AND MATERIALS
Participants
Twenty-three children between 2- and 3- years of age with a provisional diagnosis of autism spectrum disorder (ASD) participated in the current study. Children with ASD were recruited from the San Diego Regional Center, Rady Children’s Hospital toddler school, online advertisements and parent groups, the UCSD autism research program and flyers. For details on diagnostic information for the ASD group see Supplementary Information text and Table S1.
Eight of the twenty-three children were unable to fall or stay asleep in the scanner even after three separate nights of repeated attempts. Thus, functional and structural MRI data were acquired from 15 children with provisional ASD. Of these 15, one child’s data was discarded due to motion artifacts and 2 children did not meet criteria for AD or ASD on the ADI-R on follow-up assessments at 3 years of age. In sum, reliable fMRI data was acquired from a total of 12 participants with ASD (11 AD, 1 ASD) (Table 1).
Table 1.
Participant Information
| ID | Sex | Age (mo) | Receptive Language Age Equivalent | Visual Language Age Equivalent | Composite Score | Receptive Language T-score | Visual T-score |
|---|---|---|---|---|---|---|---|
|
Autism Spectrum Disorder (ASD) | |||||||
| ASD1 | M | 25.7 | 10 | 13 | 54 | 20 | 20 |
| ASD2 | M | 26.5 | 10 | 17 | 54 | 20 | 27 |
| ASD3 | M | 29.7 | 18 | 25 | 75 | 29 | 43 |
| ASD4 | M | 30.3 | 14 | 23 | 68 | 20 | 34 |
| ASD5 | M | 30.3 | 5 | 18 | 45 | <20 | 20 |
| ASD6 | M | 31.8 | 6 | 15 | 54 | <20 | 20 |
| ASD7 | M | 32.1 | 10 | 15 | 30 | <20 | 20 |
| ASD8 | M | 36.2 | 9 | 17 | 44 | <20 | 20 |
| ASD9 | M | 41.0 | 18 | 25 | 60 | <20 | 20 |
| ASD10 | M | 41.9 | 23 | 21 | 51 | 20 | 24 |
| ASD11 | M | 46.5 | 25 | 25 | 54 | <20 | 20 |
| ASD12 | M | 46.9 | 36 | 33 | 68 | 35 | 27 |
| n=12 | 12M | 34.9(7.4) | 15.3(9.1) | 20.6(5.8) | 54.8(12.2) | 24.0(6.5) | 24.6(7.3) |
|
Mental Age-Matched Controls (MA) | |||||||
| MA1 | M | 13.1 | 9 | 12 | 89 | 35 | 46 |
| MA2 | M | 14.5 | 8 | 12 | 80 | 31 | 46 |
| MA3 | M | 14.3 | 23 | 26 | 90 | 47 | 56 |
| MA4 | M | 16.3 | 24 | 18 | 134 | 72 | 57 |
| MA5 | F | 20.3 | 14 | 19 | 107 | 54 | 53 |
| MA6 | M | 21.2 | 23 | 16 | 87 | 56 | 36 |
| MA7 | M | 22.5 | 28 | 24 | 124 | 65 | 56 |
| MA8 | M | 22.9 | 25 | 21 | 105 | 58 | 47 |
| MA9 | F | 22.9 | 28 | 31 | 110 | 59 | 65 |
| MA10 | M | 23.8 | 18 | 27 | 85 | 32 | 55 |
| MA11 | M | 23.9 | 23 | 21 | 91 | 47 | 43 |
| n=11 | 9M 2F | 19.6(4.2) | 20.3(7.1) | 20.6(6.1) | 100.2(17.3) | 50.6(13.5) | 50.9(8.1) |
|
Chronological Age-Matched Controls (CA) | |||||||
| CA1 | F | 24.8 | 30 | 21 | 105 | 63 | 43 |
| CA2 | F | 30.5 | 30 | 26 | 92 | 49 | 42 |
| CA3 | M | 34.0 | 39 | 52 | 121 | 56 | 77 |
| CA4 | M | 35.2 | 39 | 41 | 111 | 56 | 60 |
| CA5 | M | 36.4 | 47 | 39 | 123 | 68 | 56 |
| CA6 | M | 36.4 | 39 | 46 | 118 | 56 | 67 |
| CA7 | M | 36.9 | 36 | 27 | 82 | 47 | 29 |
| CA8 | F | 38.0 | 44 | 43 | 121 | 58 | 58 |
| CA9 | M | 38.9 | 36 | 45 | 125 | 57 | 72 |
| CA10 | F | 44.7 | * | * | * | * | * |
| CA11 | M | 31.1 | 31 | 39 | 103 | 49 | 63 |
| CA12 | M | 41.5 | 62 | 50 | 132 | 76 | 63 |
| n=12 | 8M 4F | 35.7(5.3) | 39.4(9.3) | 39.0(10.2) | 112.1(15.2) | 57.3(14.2) | 55.3(13.3) |
Mean and standard deviation are given for each of the three groups separately as mean(stdev)
T scores and Age equivalent scores are taken from the Mullen Scales of Early Learning
Composite score reflects the composite of the 4 subtests of the Mullen: Visual Reception, Fine Motor, Receptive and Expressive Language. The standardized mean is 100
Two control groups of typically developing children were recruited: a chronological age-matched group (CA) and a mental age-matched group (MA). The chronological age-matched group was matched to the autism group based on mean chronological age. The mental age-matched group was much younger than the ASD group. In this way, the mean mental age, as determined by the receptive language (RL) age equivalent score from the Mullen Scales of Early Learning, was similar in both groups (Table 1). Mental age-matching was done as the best approximation of language level as the very low language skill of half the autism group (T score <20) makes the measure of receptive language age-equivalent less reliable. For both control groups, a portion of the subjects were included in our prior publication examining speech perception in typical development using the same paradigm and protocol as the current study (31). Data from six additional control participants were included in the present study in order to provide closer matching in chronological age and mental age for the CA and MA groups, respectively.
All participants received behavioral assessments including the Mullen Scales of Early Learning and the Vineland Adaptive Behavior Scales. Additionally, several parent-report questionnaires were obtained, including the MacArthur-Bates Communicative Developmental Inventory (CDI) and a family medical history questionnaire. The Institutional Review Board of Children’s Hospital and the University of California, San Diego approved this study. Informed written consent was obtained from the parents and they were compensated monetarily for participation.
Stimuli & Design
Participants were presented with the same auditory stimuli in the same design as in our previous study (31). These stimuli consisted of three stimulus conditions: 1) Forward speech, simple (F:s) 2) Forward speech, complex (F:c) 3) Backward speech (B). For details see Supplementary Information text. Stimuli were presented in a block design in which each condition was presented for 20 seconds and followed by 20 seconds of “rest” (no auditory stimulus presented).
Data Acquisition
Images were acquired on a 1.5 Siemens Symphony scanner at the UCSD Hillcrest Medical center. Whole brain axial slices were collected using a gradient recalled echo planar sequence (EPI) [TR = 2500 ms; TE = 35 ms; flip angle = 90 deg; field of view = 25.6 mm; 64×64 matrix (4×4 mm in-plane resolution), number of slices = 30; slice thickness = 4 mm; 154 volumes acquired]. A T1-weighted anatomical image in the coronal plane using an MPRAGE sequence was collected prior to fMRI scanning for co-registration with the functional images (FOV=22.8 mm; matrix = 256×256; 128 slices; .89 × .89 mm in-plane resolution; slice thickness = 1.5 mm)
Data Analyses
All analyses were performed with the Analysis of Functional NeuroImages (AFNI) software (36). Several pre-processing steps were performed prior to individual general linear model analyses. Each dataset was time shifted to account for slice time-offsets in volume acquisitions. Motion correction was performed with an automated volume alignment program which registered each volume to a specified volume in the time series using an iterative time series. The middle volume of the run was chosen as the reference volume unless signal outliers or motion were detected within the middle volume. Data points not correctable by head motion were censored from the analyses (See Supplementary Information). Images were spatially smoothed using a smoothing kernel of 6 mm at full width half maximum (fwhm).
A general linear model analyses was conducted to fit the individual time series to an ideal hemodynamic response function (gamma variate). The first two volumes in each data series were removed to compensate for T1 equilibration effects. Motion covariates were included in the general linear model to model noise due to movement in 3 rotational (x, y, z) and 3 translational (roll, pitch, yaw) planes. The mean and linear trend were included in the general linear model. A general linear test was included to obtain a main effect of forward speech (F) by modeling the amplitude response to both F:s and F:c. A general linear test was also included to contrast forward and backward speech. For this paper, discussion of forward speech will refer to the collapsed ‘F’ condition rather than the separate F:s and F:c analyses. The linear contrast coefficient for each condition was converted to percent signal change by calculating the percent difference from the baseline model.
Before group analyses, each individual’s data from the general linear model analyses were registered into standard Talairach space through a 6 parameter affine transformation based on landmarks identified from the high-resolution anatomical image. Because brain anatomy differs in young children from an adult template, we conducted pilot studies to determine the fidelity of anatomical co-registration for two anatomical landmarks in a group of typical toddlers, 3-year olds children, and adults as well as autistic 2–3 year old children. See Supplementary Information for discussion.
Group analyses were conducted both within group and between groups using repeated measures ANOVA analyses. For within-subject group analyses, a one-way ANOVA was run for each group separately with condition as the repeated measure. For the between-group analyses, a repeated-measure ANOVA was run using the ANOVA program in the AFNI matlab package. Contrasts were run within each group (ASD, CA, MA) to contrast percent signal change values between the forward (F) and backward (B) speech conditions. Additionally, contrasts were run to identify differences between the ASD group and the two control groups (MA and CA) separately for both the forward and backward speech conditions.
To directly test whether hemispheric asymmetries were present during processing of forward speech, a whole-brain, voxel-wise, paired t-test was performed within each group. The t-test compared percent signal value in the forward speech condition of each voxel in one hemisphere to that of the corresponding voxel in the contralateral hemisphere. This analysis revealed voxels in which the response to forward speech was significantly greater in one hemisphere than the other across the group. This analysis was conducted for the ASD and CA groups separately.
Due to the large variability in behavioral and clinical measures in the ASD group, exploratory correlations were run with the ASD group in order to determine whether the response to forward speech varied by behavioral and clinical measures. These measures included the receptive language age-equivalent (RL age) measures from the Mullen Scales of Early Learning and autism severity score from the CARS.
RESULTS
Response to forward speech
The group-averaged BOLD response to forward speech as compared to no sound presentation is shown in Figure 1 for each of the 3 groups at p<.01, corrected at 960 mm3. For details, see Table 2 and text in Supplementary Information.
Figure 1.
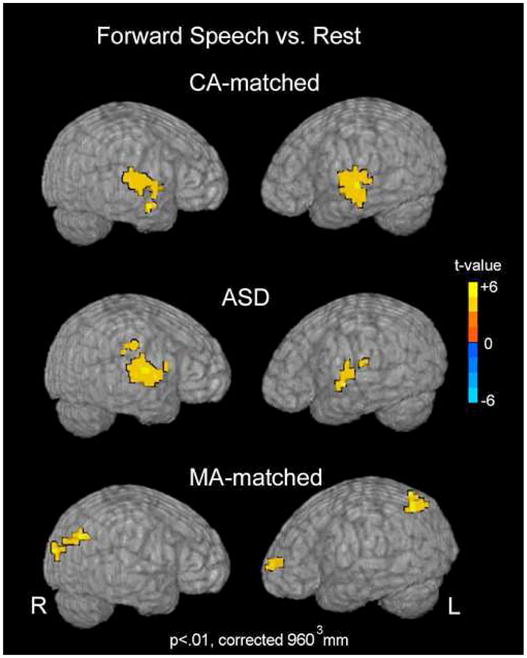
Forward Speech vs. Rest. Group activation maps for the forward speech as compared to rest conditions are shown for each group. Activation maps are projected onto the surface of a rendered brain from a single, representative subject. Data are presented at an intensity of p<.01 and voxel-wise cluster correction of 960 mm3.
Table 2.
Within Group Comparisons
| Region | Side | BA | Talairach Coords (x,y,z) | t-value | Region | Side | BA | Talairach Coords (x,y,z) | t-value | Region | Side | BA | Talairach Coords (x,y,z) | t-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASD Group | CA Group | MA Group | ||||||||||||
| Forward Speech > Rest | ||||||||||||||
| Temporal | Temporal | Frontal | ||||||||||||
| Superior Temporal Gyrus | L | 22 | (−54,−5,−2) | 4.61 | Superior Temporal Gyrus | L | 22 | (−63,−18,3) | 5.39 | Medial Frontal Cortex | L | 32 | (−10,46,6) | 4.19 |
| Superior Temporal Gyrus | R | 42/22 | (64,−21,7) | 5.72 | Superior Temporal Gyrus | L | 22 | (−46,−21,2) | 3.64 | Middle Frontal Gyrus | L | 10 | (−31,54,3) | 5.47 |
| Transverse Temporal Gyrus | R | 42 | (50,−6,6) | 5.79 | Middle Temporal Gyrus | L | 21 | (−62,−14,−6) | 4.27 | Parietal | ||||
| Superior Temporal Gyrus | R | 42 | (49,−21,7) | 4.51 | Angular Gyrus | L | 39 | (−44,−61,42) | 6.45 | |||||
| Superior Temporal Gyrus | R | 22 | (65,−16,6) | 3.87 | Occipital | |||||||||
| Parahippocampal Gyrus | L | 30 | (−26,−57,7) | 4.86 | Cuneus | L | 18 | (−14,−100,4) | 5.46 | |||||
| Cuneus | R | 19 | (19,−89,25) | 3.94 | ||||||||||
| Lingual Gyrus | R | 18 | (19,−78,09) | 5.04 | ||||||||||
| Rest > Forward Speech | ||||||||||||||
| Frontal | Frontal | Parietal | ||||||||||||
| Superior Frontal Gyrus | R | 9 | (−30,43,27) | −4.29 | Superior Frontal Gyrus | R | 6 | (13,7,42) | −5.39 | Postcentral Gyrus | L | 5 | (−30,−41,63) | −6.50 |
| Subcortical | ||||||||||||||
| Thalamus | L | (−10,−22,15) | −4.30 | |||||||||||
| Thalamus | R | (9,−22,16) | −5.47 | |||||||||||
| Backward Speech > Rest | ||||||||||||||
| None | Frontal | Frontal | ||||||||||||
| Insula | L | (−27,22,6) | 4.47 | Middle Frontal Gyrus | R | 46 | (30,10,22) | 5.21 | ||||||
| Middle Frontal Gyrus | L | 8 | (−42,2,31) | 4.72 | Inferior Frontal Gyrus | R | 44 | (57,5,22) | 4.35 | |||||
| Temporal | ||||||||||||||
| Superior Temporal Gyrus | R | 22 | (57,−18,2) | 4.89 | ||||||||||
| Superior Temporal Gyrus | R | 22 | (59,−5,−1) | 5.25 | ||||||||||
| Superior Temporal Gyrus | L | 22 | (−58,−21,2) | 6.49 | ||||||||||
| Transverse Temporal Gyrus | L | 41 | (−43,−14,7) | 4.58 | ||||||||||
| Middle Temporal Gyrus | L | 21 | (−58,−50,−5) | 6.40 | ||||||||||
| Rest > Backward Speech | ||||||||||||||
| None | None | Frontal | ||||||||||||
| Precentral Gyrus | L | 4 | (−17,−21,61) | 3.93 | ||||||||||
| Parietal | ||||||||||||||
| Postcentral Gyrus | R | 3 | (21,−33,59) | 4.11 | ||||||||||
| Paracentral Lobule | R | 4 | (2,−33,54) | 3.73 | ||||||||||
| Forward Speech > Backward Speech | ||||||||||||||
| Frontal | Temporal | Frontal | ||||||||||||
| Precentral Gyrus | R | 43 | (50,−4,13) | 3.61 | Superior Temporal Gyrus | R | 42 | (50,−20,11) | 3.47 | Insula | L | (−34,−1,6) | 4.05 | |
| Temporal | Superior Temporal Gyrus | R | 21 | (67,−18,4) | 3.44 | Middle Frontal Gyrus | L | 11 | (−22,44,−14) | 3.99 | ||||
| Superior Temporal Gyrus | R | 42 | (51,−28,11) | 3.17 | Middle Temporal Gyrus | R | 21 | (61,−10,−17) | 4.96 | Medial Frontal Gyrus | L | 32 | (−6,47,−9) | 4.25 |
| Superior Temporal Gyrus | L | 22 | (−50,−6,6) | 4.11 | Inferior Temporal Gyrus | R | 20 | (45,−1,−25) | 4.59 | Medial Frontal Gyrus | R | 32 | (10,45,−13) | 4.28 |
| Parahippocampal Gyrus | L | 30 | (−27,−57,7) | 3.45 | Anterior Cingulate Cortex | L | 32 | (−3,34,19) | 4.32 | |||||
| Parietal | Cingulate Gyrus | R | 32 | (1,6,34) | 4.33 | |||||||||
| Precuneus | L | 40 | (−22,−62,31) | 3.45 | Temporal | |||||||||
| Precuneus | R | 7 | (13,−66,35) | 3.97 | Superior Temporal Gyrus | L | 22 | (−47,−17,2) | 3.26 | |||||
| Paracentral Lobule | L | 7 | (−5,−32,46) | 3.93 | Parahippocampal Gyrus | R | 28 | (21,−13,−13) | 3.64 | |||||
| Precuneus | L | 7 | (−1,−57,46) | 4.10 | Parahippocampal Gyrus | R | 28 | (−14,−25,−9) | 3.24 | |||||
| Parietal | ||||||||||||||
| Precuneus | L | 7 | (−3,−41,43) | 4.21 | ||||||||||
| Angular Gyrus | L | 39 | (−42,−61,43) | 4.77 | ||||||||||
| Occipital | ||||||||||||||
| Cuneus | L | 18 | (−18,−88,27) | 4.78 | ||||||||||
| Cuneus | L | 17 | (−18,−97,3) | 4.00 | ||||||||||
| Cuneus | R | 18 | (10,−93,23) | 5.16 | ||||||||||
| Subcortical | ||||||||||||||
| Putamen | R | (18,−1,−5) | 3.72 | |||||||||||
| Putamen | L | (−18,10,10) | 3.25 | |||||||||||
| Backward Speech > Forward Speech | ||||||||||||||
| Frontal | Frontal | Frontal | ||||||||||||
| Middle Frontal Gyrus | L | 9 | (−29,43,27) | −3.69 | Precentral Gyrus | R | (50,3,35) | −3.74 | Postcentral Gyrus | R | 43 | (61,−6,15) | −4.11 | |
| Parietal | Temporal | |||||||||||||
| Subcortical | Postcentral Gyrus | L | (−15,−47,63) | −2.86 | Superior Temporal Gyrus | L | 21 | (−38,−9,−5) | −3.65 | |||||
| Cerebellum | R | (30,−48,−37) | −4.99 | Subcortical | ||||||||||
| Cerebellum | L | (−38,−68,−21) | −4.02 | Thalamus | L | (−7,−6,7) | −3.91 | |||||||
The peak t-value and Talairach coordinate is given for each region or Brodmann Area showing significant activity.
ASD vs. MA-matched response to forward speech
In direct statistical comparison to the MA group, the ASD group showed reduced activity within an extended number of brain regions, including regions within bilateral frontal, temporal, parietal, and occipital lobes, cerebellar cortex, and right caudate (Fig 2, Table 3). The only regions showing a greater response to forward speech in the ASD than the MA group were bilateral postcentral gyri.
Figure 2.
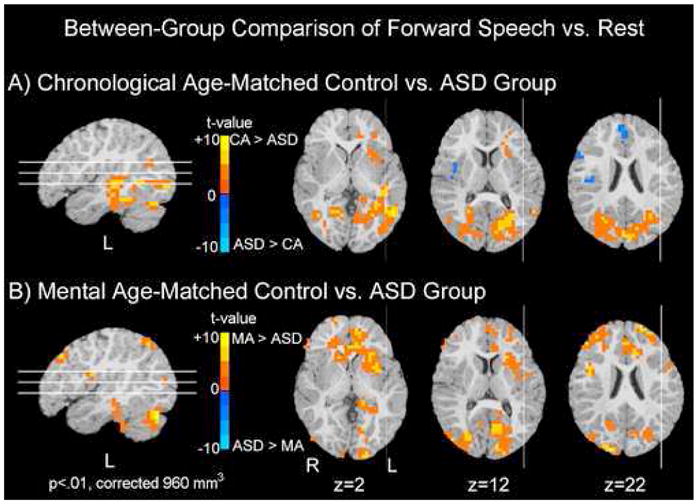
Between-group Comparison of Forward Speech vs. Rest. Significant differences in activation to forward speech vs. rest between the ASD group and the two control groups (MA-matched and CA-matched) are shown. Regions in blue depict regions in which the ASD group showed significantly greater activity than either the CA (top row) or MA (bottom row) control groups. Conversely, regions shown in red are ones in which either the MA or CA group showed greater activation than the ASD group. In comparison to the CA-matched group, the ASD group recruited medial and right frontal regions to a greater extent while the CA-matched group recruited greater left frontal, temporal, and bilateral posterior regions than the ASD group. In comparison to the MA-matched group, the ASD group showed reduced activity in a number of brain regions. Data are represented on a brain image from a single subject.
Table 3.
Between Group Comparisons
| Region | Side | BA | Talairach Coordinates (x,y,z) | t-value | Region | Side | BA | Talairach Coordinates (x,y,z) | t-value |
|---|---|---|---|---|---|---|---|---|---|
| MA > ASD |
CA > ASD |
||||||||
| Frontal | Frontal | ||||||||
| Anterior Cingulate Cortex | L | 24 | (−6,31,3) | 5.93 | Anterior Cingulate | L | 24 | (−10,28,−6) | 4.71 |
| Anterior Cingulate Cortex | R | 24 | (14,34,−1) | 4.40 | Middle Frontal Gyrus | L | 10 | (−29,39,3) | 3.52 |
| Medial Frontal Cortex | L | 32 | (−11,46,6) | 5.12 | Insula | L | (−30,12,0) | 4.57 | |
| Medial Frontal Cortex | R | 32/9 | (2,36,23) | 4.23 | Temporal | ||||
| Superior Frontal Gyrus | R | 9 | (29,38,26) | Superior Temporal Gyrus | L | 22 | (−43,−30,2) | 5.66 | |
| Superior Frontal Gyrus | L | 9 | (−28,43,26) | 5.07 | Middle Temporal Gyrus | L | 37 | (−50,−55,3) | 6.03 |
| Superior Frontal Gyrus | R | 6 | (18,−3,55) | 4.61 | Fusiform Gyrus | L | 37 | (−42,−38,−13) | 5.22 |
| Superior Frontal Gyrus | R | 10 | (18,62,14) | 4.29 | Parahippocampal Gyrus | L | 36 | (−28,−45,3) | 4.79 |
| Cingulate Gyrus | R/L | 31 | (2,7,31) | 4.61 | Inferior Temporal Gyrus | R | 20 | (48,−33,−12) | 4.70 |
| Orbitofrontal Gyrus | L | 11 | (−19,23,−16) | 5.55 | Inferior Temporal Gyrus | L | 37 | (−45,57,−16) | 4.25 |
| Orbitofrontal Gyrus | R | 11 | (22,27,−18) | 4.79 | Parietal | ||||
| Middle Frontal Gyrus | R | 10 | (38,47,15) | 3.74 | Superior Parietal Lobule | R | 7 | (25,−73,39) | 6.14 |
| Inferior Frontal Gyrus | R | 46 | (50,34,11) | 3.22 | Superior Parietal Lobule | L | 7 | (−26,−57,42) | 6.40 |
| Precentral Gyrus | R | 4 | (41,−1,27) | 4.98 | Posterior Cingulate | L | 30 | (−22,−65,11) | 5.68 |
| Precentral Gyrus | L | 4 | (−43,−6,19) | 4.19 | Inferior Parietal Lobule | R | 40 | (33,−47,43) | 4.47 |
| Insula | L | (−33,2,3) | 4.43 | Cingulate Gyrus | L | 31 | (−11,−29,44) | 5.29 | |
| Temporal | Occipital | ||||||||
| Parahippocampal Gyrus | R | 36 | (33,−24,−13) | 3.53 | Lingual Gyrus | R | 19 | (17,−54,−1) | 5.08 |
| Parahippocampal Gyrus | L | 30 | (−22,−42,−1) | 4.53 | Cuneus | L | 18 | (−10,−82,16) | 5.63 |
| Inferior Temporal Gyrus | L | 20 | (−46,−33,−20) | 4.39 | Cuneus | L | 19 | (−10,−81,35) | 5.68 |
| Parietal | Subcortical | ||||||||
| Superior Parietal Lobule | L | 7 | (−9,−42,43) | 3.55 | Cerebellum | R | (33,−54,−21) | 5.25 | |
| Superior Parietal Lobule | R | 7 | (9,−41,43) | 4.95 | Cerebellum | L | (−33,−65,−16) | 7.65 | |
| Angular Gyrus | L | 39 | (−35,−73,38) | 4.11 | |||||
| Precuneus | L | 7 | (−8,−62,35) | 4.04 | |||||
| Precuneus | R | 7 | (15,−68,31) | 3.51 | |||||
| Occipital | |||||||||
| Cuneus | R | 19 | (18,−89,26) | 6.27 | |||||
| Cuneus | L | 18 | (−5,−69,14) | 5.45 | |||||
| Lingual Gyrus | L | 18 | (−15,−86,−5) | 4.88 | |||||
| Subcortical | |||||||||
| Cerebellum | L | (−37,−70,−20) | 8.20 | ||||||
| Cerebellum | R | (34,−51,−29) | 3.99 | ||||||
| Caudate | R | (13,19,−1) | 4.45 | ||||||
| ASD > MA |
ASD > CA |
||||||||
| Parietal | Frontal | ||||||||
| Postcentral Gyrus | R | 3 | (38,−29,55) | −3.69 | Medial Frontal Gyrus | R | 32/9 | (2,45,19) | −4.67 |
| Postcentral Gyrus | L | 3 | (−38,−32,52) | −3.93 | Inferior Frontal Gyrus | R | 44/9 | (49,15,31) | −4.31 |
| Insula | R | (37,−9,15) | −4.50 | ||||||
| Parietal | |||||||||
| Postcentral Gyrus | R | 43 | (41,−18,28) | −3.88 | |||||
The peak t-value and Talairach coordinate is given for each region or Brodmann Area (BA) showing significant activity.
ASD vs. CA-matched response to forward speech
In comparison to the CA-matched group, the ASD group recruited a greater number of right hemisphere frontal and parietal regions [frontal: right medial frontal gyrus, t=4.67; right inferior frontal gyrus, t=4.31; right insula, t=−4.5; parietal: right postcentral gyrus, t=3.88]. In direct statistical comparison to the ASD group, the CA-matched control group recruited a greater number of both left hemisphere frontal and temporal regions [(frontal: left anterior cingulate, t=4.71; left middle frontal gyrus, t=3.52; temporal: left superior temporal gyrus (STG), t=5.66; left middle temporal gyrus (MTG), t=6.03; left fusiform gyrus, t=5.22)]. The CA-matched group additionally recruited greater bilateral posterior regions within parietal, extrastriate, and cerebellar cortices (Fig 2, Table 3) in comparison to the ASD group.
Laterality Analyses for ASD and CA Groups
To directly test whether differences in laterality are found within the ASD and CA groups, we ran a paired t-test between hemispheres within the ASD and CA groups for the response to forward speech as compared to rest. The results reported were significant at a trend level (p<.05, corrected at 384 mm3). For the ASD group, there was a trend towards greater RH than LH activation within a number of frontal, temporal, occipital, and parietal regions, as well as the caudate nucleus. There was a trend for the CA-group to show overall greater LH than RH activation within frontal, temporal, and parietal regions (Fig 3, Table 4).
Figure 3.
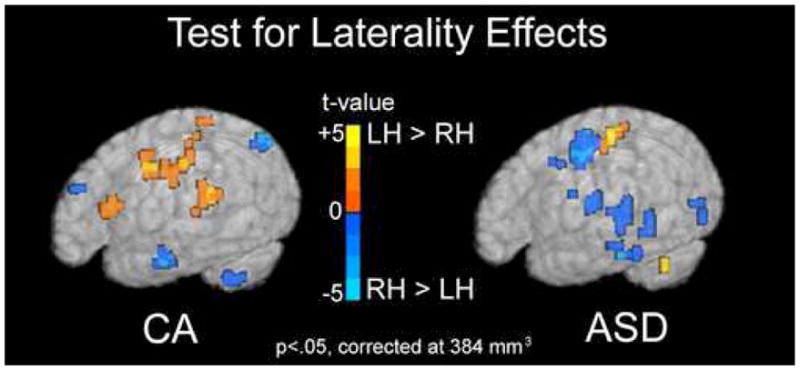
Test for Laterality Effects. Regions showing a trend towards hemispheric asymmetry in response to forward speech are shown for both the CA and ASD groups separately. Regions in red are those in which the left hemisphere voxels were significantly greater than the right. Regions in blue are those in which the right hemisphere was significantly greater than the left. In the ASD group, a number of regions show a trend toward greater right than left hemisphere activation (blue). The CA group shows a trend towards greater left than right hemisphere activation (red) in inferior frontal and superior temporal regions. Maps are show at an intensity threshold of p<.05 and a cluster threshold of 384 mm3 and displayed on a single subject’s rendered brain image.
Table 4.
Laterality Effects
| Region | BA | Talairach Coordinate (x,y,z) | t-value | Region | BA | Talairach Coordinate (x,y,z) | t-value |
|---|---|---|---|---|---|---|---|
| Left > Right (red) | Right > Left (blue) | ||||||
| CA | |||||||
| Frontal | Frontal | ||||||
| Inferior Frontal Gyrus | 44/45 | (−41,16,11) | 3.91 | Middle Frontal Gyrus | 9 | (−37,26,24) | 4.05 |
| Precentral Gyrus | 6 | (−56,−2,39) | 3.63 | Temporal | |||
| Middle Frontal Gyrus | 10 | (−30,44,−1) | 3.89 | Middle Temporal Gyrus | 21 | (−55,−8,−17) | 3.87 |
| Medial Frontal Cortex | 32 | (−4,39,14) | 2.54 | Parietal | |||
| Anterior Cingulate | 32 | (−4,22,−8) | 3.64 | Angular Gyrus | 39 | (−39,−65,38) | 3.84 |
| Superior Medial Frontal Gyrus | 8/9 | (−2,43,38) | Subcortical | ||||
| Temporal | Cerebellum | (−14,−66,−22) | 3.17 | ||||
| Transverse Temporal Gyrus | 41 | (−38,−25,7) | 3.60 | ||||
| Superior Temporal Gyrus | 42 | (−45,−37,11) | 3.05 | ||||
| Parhippocampal Gyrus | 18 | (−25,−56,3) | 2.97 | ||||
| Parietal | |||||||
| Postcentral Gyrus | 3 | (−59,−22,40) | 3.31 | ||||
| Cingulate Gyrus | 31 | (−18,−21,31) | 2.92 | ||||
| ASD | |||||||
| Temporal | Frontal | ||||||
| Superior Temporal Gyrus | 38 | (−38,−6,−9) | 2.88 | Superior Frontal Gyrus | 10 | (−26,43,19) | 3.90 |
| Parietal | Cingulate Gyrus | 31 | (−−10,−2,42) | 4.51 | |||
| Postcentral Gyrus | 1/2 | (−50,−26,51) | 5.62 | Inferior Frontal Gyrus | 47 | (−31,26,−13) | 4.13 |
| Subcortical | Precentral Gyrus | 6 | (−45,−14,10) | 3.24 | |||
| Cerebellum | (−31,−53,−29) | 3.93 | Insula | 13 | (−33,−9,18) | 2.78 | |
| Temporal | |||||||
| Superior Temporal Gyrus | 21/22 | (−43,−33,1) | 2.34 | ||||
| Middle Temporal Gyrus | 21 | (−43,−46,−1) | 2.73 | ||||
| Inferior Temporal Gyrus | 20 | (−46,−29,−22) | 3.12 | ||||
| Fusiform Gyrus | 20 | (−39,−25,−18) | 3.74 | ||||
| Occipital | |||||||
| Middle Occipital Gyrus | 19 | (−42,−74,3) | 2.97 | ||||
| Parietal | |||||||
| Inferior Parietal Lobule | 40 | (−35,−54,31) | 4.38 | ||||
| Parcentral Lobule | 4 | (−18,−33,55) | 3.46 | ||||
| Subcortical | |||||||
| Caudate | (−6,12,14) | 2.40 | |||||
The peak t-value and Talairach coordinate is given for each region or Brodmann Area (BA) showing significant effects of laterality.
Speech-specific response
The ASD group showed a greater response to forward as compared to backward speech within bilateral superior temporal gyri and right precentral gyrus (Fig 4 Table 2). The CA group also recruited superior temporal regions to a greater extent during forward speech presentation than backward speech (Fig 4 Table 2). However, activations in the left superior temporal regions did not reach significance at a cluster volume of 960mm3 in the CA group. The MA group recruited a number of regions throughout cortical and subcortical regions. For a full list see Table 2.
Figure 4.
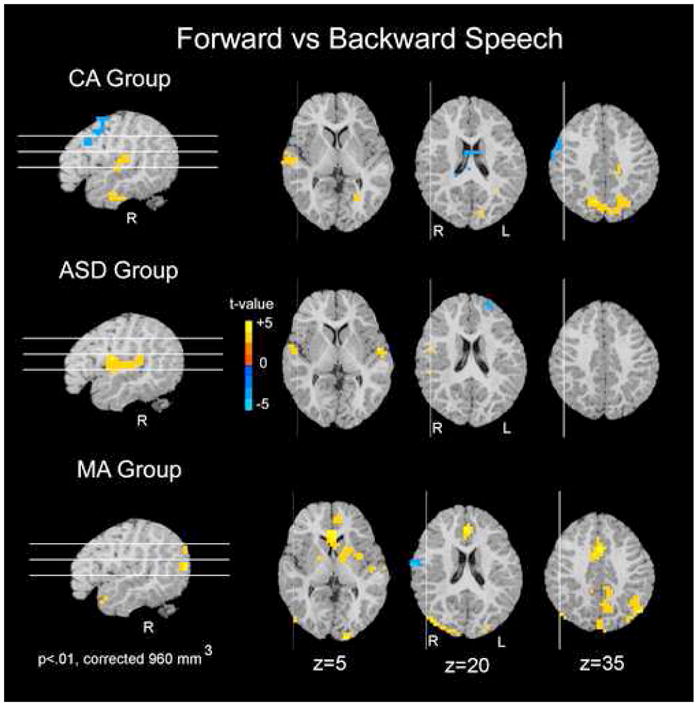
Speech-specific Response within each Group. For each of the three groups separately, regions in which forward speech elicited greater activation than backward speech are shown in red. Regions in which backward speech elicited greater activation than forward speech are shown in blue. All three groups showed a differential response between forward and backward speech; however, this difference is primarily within superior temporal and parietal regions for the CA and ASD groups. Data are represented on a brain image from a single subject.
It is interesting to note that while the CA group recruited robust bilateral superior temporal regions during presentation of backward speech as compared to rest, the MA and ASD groups did not (Table 2). This difference could account for the reduced discrimination between forward and backward speech in the CA group. See Supplementary Information text for further discussion.
Correlation Analyses
As the ASD group had a wide range of language skill and autism severity, whole-brain correlations with percent signal data from the forward speech condition and two clinical variables (RL age-equivalent score and CARS autism severity score) were run to examine individual differences in the response to forward speech in the ASD group. Correlation analyses revealed that as receptive language age increased, so did activity in right hemisphere frontal and temporal regions (i.e. medial and inferior frontal gyri and STS/MTG). Similarly, as autism severity decreased, increased activity was seen in right hemisphere inferior and medial frontal cortex as well as right STS and MTG. Left hemisphere frontal (superior and middle frontal) and temporal (STS/G and MTG) activity also showed a significant negative correlation with autism severity (Fig 5, Table S2).
Figure 5.
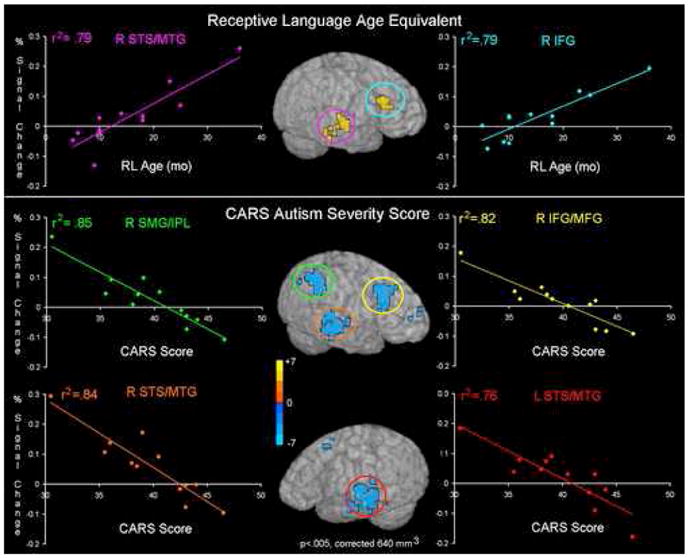
Individual Differences in the ASD Group. Regions showing a significant correlation between percent signal change in the forward speech condition and receptive language age (top row) or autism severity (bottom row) are shown on an single rendered brain. Individual data were extracted from these regions and plotted to obtain the R2 correlation coefficient. The color of the circled region corresponds to the color of the plot of the behavioral measure and the mean percent signal change for that region. Right superior temporal sulcus and right inferior temporal gyrus both show significant correlations with receptive language age and autism severity.
DISCUSSION
In this first fMRI study of 2–3 year-old children with ASD, we identified a pattern of neural response to speech that differed in children with ASD from both their chronological age- and mental age-matched typically developing controls. In comparison to MA-matched controls, the ASD group showed reduced activity in an extended number of brain regions in response to speech. In comparison to their CA-matched controls, the ASD group showed both a delayed and deviant pattern of brain response to speech, characterized by a greater recruitment of right hemisphere frontal regions.
A deviant pattern of laterality in ASD in response to speech vs. rest was identified in three separate analyses. First, in comparison to their CA-matched controls, the ASD group recruited greater right frontal regions (Fig 2). Second, in a paired hemispheric comparison, the ASD group showed a trend toward greater recruitment of right hemisphere frontal and temporal regions during the forward speech condition, while the CA group showed a trend towards greater left hemisphere recruitment in a number of brain regions (Fig 3). Third, correlations with receptive language age revealed a greater reliance on primarily right hemisphere frontal and temporal regions with increasing language abilities and decreasing autism severity (Fig 5). Autism severity, however, was also correlated with a similar pattern in the left hemisphere. These findings suggest that not only is the right hemisphere recruited to a greater extent in autism than in controls at 2–3 years of age, but also that early right hemisphere recruitment may be predictive of a better language outcome in autism.
In comparison to their MA-matched controls, the ASD group showed reduced activity in an extended network of brain regions. In a previous paper (31), we hypothesized that an extended network of brain regions including frontal, occipital, and cerebellar regions may be recruited at the cusp of the rapid burst in language skills seen in the second year of life. This hypothesis is additionally supported by ERP studies in which the response to known words progresses from widespread to more focal patterns with increasing language skill in typically developing 20 month-old children (37–39). In addition to reduced activity in a number of frontal, occipital, and cerebellar regions, the ASD group also showed deviant patterns of right and medial frontal activation in comparison to their CA-matched controls. Taken together, these findings reveal that the pattern of activity in ASD is both reduced and deviant as compared to the pattern recruited in the MA-matched controls. The reduced and deviant activation in the ASD group could reflect a failure to engage the full network of brain regions which may facilitate language learning. Studies of 1–2 year old children with provisional ASD will be needed to determine whether a typical extended network is ever recruited or whether evidence of a deviant developmental trajectory is already present at even younger ages.
This pattern of deviant lateralization and immature, frontal recruitment in autism as compared to controls suggests a possible lack of specialization for language systems in autism by 2–3 years of age. Previous studies of the older child and adult have identified patterns of reduced or reversed laterality in frontal and/or temporal cortex in structural studies (40–43) and functional studies using ERP (44; 45), PET (8; 10), fMRI (8–11), and MEG (46). However, this is the first study to suggest abnormal laterality in children with ASD as young as 2–3 years of age. Furthermore, the significant correlation of right hemisphere frontal and temporal activation to speech with receptive language skill in autism suggests that by 2–3 years of age, children with autism may already be on a deviant developmental trajectory characterized by right hemisphere recruitment for language.
The cause of this deviant developmental trajectory can only be speculated. Some evidence suggests recruitment of right hemisphere regions may be a compensatory mechanism to account for the more effortful processing required to process language in autism (9). However, the current study was conducted during natural sleep, suggesting cognitive strategies alone cannot account for the reversed asymmetries seen in the autism group. Structural MRI studies have revealed that a number of structures which showed evidence of deviance in functional laterality at 2–3 years of age in the current study (e.g. inferior frontal and posterior middle and inferior temporal regions) also show greater rightward asymmetry at 5–11 years of age in ASD (42; 43; 47), suggesting a possible structural bias underlying the deviant functional patterns. However, it is not possible to disentangle whether this structural asymmetry is a cause or consequence of aberrant functional patterns early in life. Some evidence suggests brain volume in right temporal and frontal regions is strongly dependent on genetic factors, whereas left hemisphere regions are more influenced by experience (48) and develop more slowly than the right (49). It is possible that a combination of both genetic and experiential factors very early in life results in a greater reliance on right hemisphere regions, and possible reduced development of left hemisphere regions for language processing. Given the differing rates of development within each hemisphere, the timing of brain insults could be particularly important in altering hemispheric asymmetries.
Three limitations of the current study warrant discussion as they have may have potential implications for the interpretation of the findings. First, while the ASD group contained only males, both control groups contained a small number of females (4 out of 12 in the CA group and 2 out of 11 in the MA group). This limitation is addressed in Supplementary Information through a post-hoc analysis with a male-only control sample and supplementary text (Figure S1, Table S4).
Second, while this study contained two typical control groups for the ASD sample (one chronological age- and one language-age matched group), it did not contain a contrast group of children with developmental language disorder (DLD), or specific language impairment (SLI). Evidence suggests some overlap in language profiles (6) and anatomical asymmetries (43; 47) between children with autism and those with language-impairments but not autism. Thus, the inclusion of this third contrast group could elucidate functional activation patterns that may be specific to autism and not due to language impairments alone.
A third limitation of the current study is that data were recorded during natural sleep without monitoring sleep stage. As discussed in our previous paper (31), REM onset latency differs between 1 and 3 year-old typical children; however, for both ages mean REM onset latency is reported to be approximately 60 minutes or greater (50; 51). These data were recorded approximately 45 minutes into sleep, and thus, a systematic difference in sleep stage is not expected between groups. In studies of older children and adolescents with autism, REM onset latency was not significantly different between autism and control groups (52; 53). Thus, while sleep stage could affect patterns of brain activation, between group differences are not expected to be due to sleep stage differences alone. However, further studies would benefit from polysomnographic recording as much variability is often seen in latency to REM onset (50; 51).
The use of fMRI during natural sleep poses a number of advantages. First, fMRI data can be acquired from infants, toddlers and young children with minimal motion artifact. Second, children across a broad range of cognitive and behavioral function can be studied. FMRI studies of awake and performing older children and adults inherently require high-functioning participants with ASD: a narrow subset of the autism population. Third, effects of arousal, anxiety, or attentional state that typically can confound fMRI studies of patient populations are not present. Thus, sleep fMRI may be a valuable tool in understanding the biological bases of autism. Identification of specific structures and networks showing functional abnormalities at the time of emergence of autism could give clues for where to look for microstructural differences or gene candidates (e.g. those involved in hemispheric patterning) in autism.
Supplementary Material
Acknowledgments
We are grateful to the parents and children who participated in this study. We are also grateful to members of our research team who performed clinical testing (Dr. Cindy Carter & Dr. Natacha Akshoomoff) and assisted with data collection (Anna Bajo, Anne Erickson, Vera Grindell, Cindy Hsu, Grace Kim, Doreen Nguyen, Lindsey Schubert, Randy Wu & Weifang Zhou). We also thank Dr. Ruth Carper, Graham Wideman, and Dr. Natalia Kleinhans for their contributions to the Talairach validation study discussed in Supplementary Information. This research was supported by the National Institute of Health MH-36840 grant and a gift from the Donald C. and Elizabeth M. Dickinson Foundation awarded to E.C.
Footnotes
Financial Disclosures: The authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Charman T, Drew A, Baird C, Baird G. Measuring early language development in preschool children with autism spectrum disorder using the MacArthur Communicative Development Inventory (Infant Form) J Child Lang. 2003;30:213–236. doi: 10.1017/s0305000902005482. [DOI] [PubMed] [Google Scholar]
- 2.Wetherby AM, Woods J, Allen L, Cleary J, Dickinson H, Lord C. Early indicators of autism spectrum disorders in the second year of life. J Autism Dev Disord. 2004;34:473–493. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]
- 3.Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. Int J Dev Neurosci. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Lord C, Paul R. Language and Communication in Autism. In: Cohen D, Volkmar F, editors. Handbook of autism spectrum and pervasive developmental disorders. New York: John Wiley & Sons; 1997. pp. 195–225. [Google Scholar]
- 5.Tager-Flusberg H. On the nature of linguistic functioning in early infantile autism. J Autism Dev Disord. 1981;11:45–56. doi: 10.1007/BF01531340. [DOI] [PubMed] [Google Scholar]
- 6.Tager-Flusberg H, Joseph RM. Identifying neurocognitive phenotypes in autism. Phil Trans R Soc Lond B. 2003;358:303–314. doi: 10.1098/rstb.2002.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tager-Flusberg H, Paul R, Lord C. Language and Communication in Autism. In: Volkmar FR, Paul R, Klin A, Cohen D, editors. Handbook of Autism and Pervasive Developmental Disorders Volume 1: Diagnosis, Development, Neurobiology, and Behavior. Vol. 1. New Jersey: John Wiley & Sons; 2005. pp. 335–364. [Google Scholar]
- 8.Boddaert N, Belin P, Chabane N, Poline JB, Barthelemy C, Mouren-Simeoni MC, et al. Perception of complex sounds: abnormal pattern of cortical activation in autism. Am J Psychiatry. 2003;160:2057–2060. doi: 10.1176/appi.ajp.160.11.2057. [DOI] [PubMed] [Google Scholar]
- 9.Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006;129:932–943. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller RA, Behen ME, Rothermel RD, Chugani DC, Muzik O, Mangner TJ, Chugani HT. Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. J Autism Dev Disord. 1999;29:19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi M, Harada M, Matsuzaki K, Nishitani H, Mori K. Difference of signal change by a language task on autistic patients using functional MRI. J Med Invest. 2004;51:59–62. doi: 10.2152/jmi.51.59. [DOI] [PubMed] [Google Scholar]
- 12.Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, et al. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006 doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Gaffrey MS, Kleinhans NM, Haist F, Akshoomoff N, Campbell A, Courchesne E, Muller RA. Atypical [corrected] participation of visual cortex during word processing in autism: an fMRI study of semantic decision. Neuropsychologia. 2007;45:1672–1684. doi: 10.1016/j.neuropsychologia.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boddaert N, Chabane N, Belin P, Bourgeois M, Royer V, Barthelemy C, et al. Perception of Complex Sounds in Autism: Abnormal Auditory Cortical Processing in Children. American Journal of Psychiatry. 2004;161:2117–2120. doi: 10.1176/appi.ajp.161.11.2117. [DOI] [PubMed] [Google Scholar]
- 15.Redcay E, Courchesne E. When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biol Psychiatry. 2005;58:1–9. doi: 10.1016/j.biopsych.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 16.Courchesne E, Karns C, Davis HR, Ziccardi R, Carper R, Tigue Z, et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology. 2001;57:245–254. doi: 10.1212/wnl.57.2.245. [DOI] [PubMed] [Google Scholar]
- 17.Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59:175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- 19.Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, et al. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. Journal of neuroscience. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. Journal of the American Medical Association. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 21.Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–1051. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- 22.Sparks BF, Friedman SD, Shaw DW, Aylward E, Echelard D, Artru AA, et al. Brain sructural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- 23.Pierce K, Müller R-A, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: Evidence from fMRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 24.Haznedar MM, Buchsbaum MS, Wei T-C, Hof PR, Cartwright C, Bienstock CA, Hollander E. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonace imaging. American Journal of Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- 25.Aylward E, Minshew N, Goldstein G, Honeycutt N, Augustine A, Yates K, et al. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- 26.Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhl PK, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: behavioral and electrophysiological measures. Dev Sci. 2005;8:F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 28.Ceponiene R, Lepisto T, Shestakova A, Vanhala R, Alku P, Naatanen R, Yaguchi K. Speech-sound-selective auditory impairment in children with autism: they can perceive but do not attend. Proc Natl Acad Sci U S A. 2003;100:5567–5572. doi: 10.1073/pnas.0835631100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lepisto T, Kujala T, Vanhala R, Alku P, Huotilainen M, Naatanen R. The discrimination of and orienting to speech and non-speech sounds in children with autism. Brain Res. 2005;1066:147–157. doi: 10.1016/j.brainres.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 30.Courchesne E, Kilman BA, Galambos R, Lincoln AJ. Autism: processing of novel auditory information assessed by event-related brain potentials. Electroencephalography and Clinical Neurophysiology. 1984;59:238–248. doi: 10.1016/0168-5597(84)90063-7. [DOI] [PubMed] [Google Scholar]
- 31.Redcay E, Haist F, Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Dev Sci. 2008;11:237–252. doi: 10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- 32.Redcay E, Kennedy DP, Courchesne E. fMRI during natural sleep as a method to study brain function during early childhood. Neuroimage. 2007;38:696–707. doi: 10.1016/j.neuroimage.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 34.Dehaene-Lambertz G, Hertz-Pannier L, Dubois J, Meriaux S, Roche A, Sigman M, Dehaene S. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc Natl Acad Sci U S A. 2006;103:14240–14245. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson AW, Marois R, Colson ER, Peterson BS, Duncan CC, Ehrenkranz RA, et al. Neonatal auditory activation detected by functional magnetic resonance imaging. Magn Reson Imaging. 2001;19:1–5. doi: 10.1016/s0730-725x(00)00231-9. [DOI] [PubMed] [Google Scholar]
- 36.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 37.Mills DL, Neville HJ. Electrophysiological studies of language and language impairment. Semin Pediatr Neurol. 1997;4:125–134. doi: 10.1016/s1071-9091(97)80029-0. [DOI] [PubMed] [Google Scholar]
- 38.Conboy BT, Mills DL. Two languages, one developing brain: event-related potentials to words in bilingual toddlers. Dev Sci. 2006;9:F1–12. doi: 10.1111/j.1467-7687.2005.00453.x. [DOI] [PubMed] [Google Scholar]
- 39.Mills DL, Plunkett K, Prat C, Schafer G. Watching the infant brain learn words: effects of vocabulary size and experience. Cognitive Development. 2005;20:19–31. [Google Scholar]
- 40.Rojas DC, Bawn SD, Benkers TL, Reite ML, Rogers SJ. Smaller left hemisphere planum temporale in adults with autistic disorder. Neurosci Lett. 2002;328:237–240. doi: 10.1016/s0304-3940(02)00521-9. [DOI] [PubMed] [Google Scholar]
- 41.Rojas DC, Camou SL, Reite ML, Rogers SJ. Planum temporale volume in children and adolescents with autism. J Autism Dev Disord. 2005;35:479–486. doi: 10.1007/s10803-005-5038-7. [DOI] [PubMed] [Google Scholar]
- 42.Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, et al. Abnormal asymmetry in language association cortex in autism. Ann Neurol. 2002;52:588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- 43.De Fosse L, Hodge SM, Makris N, Kennedy DN, Caviness VS, Jr, McGrath L, et al. Language-association cortex asymmetry in autism and specific language impairment. Ann Neurol. 2004;56:757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- 44.Dawson G, Finley C, Phillips S, Galpert L. Hemispheric specialization and the language abilities of autistic children. Child Development. 1986;57:1440–1453. [PubMed] [Google Scholar]
- 45.Dawson G, Finley C, Phillips S, Lewy A. A comparison of hemispheric asymmetries in speech-related brain potentials of autistic and dysphasic children. Brain and Language. 1989;37:26–41. doi: 10.1016/0093-934x(89)90099-0. [DOI] [PubMed] [Google Scholar]
- 46.Gage NM, Siegel B, Roberts TP. Cortical auditory system maturational abnormalities in children with autism disorder: an MEG investigation. Brain Res Dev Brain Res. 2003;144:201–209. doi: 10.1016/s0165-3806(03)00172-x. [DOI] [PubMed] [Google Scholar]
- 47.Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Kennedy DN, Filipek PA, et al. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128:213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- 48.Geschwind DH, Miller BL, DeCarli C, Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci U S A. 2002;99:3176–3181. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol. 1977;1:86–93. doi: 10.1002/ana.410010109. [DOI] [PubMed] [Google Scholar]
- 50.Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117:741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- 51.Louis J, Cannard C, Bastuji H, Challamel MJ. Sleep ontogenesis revisited: a longitudinal 24-hour home polygraphic study on 15 normal infants during the first two years of life. Sleep. 1997;20:323–333. doi: 10.1093/sleep/20.5.323. [DOI] [PubMed] [Google Scholar]
- 52.Elia M, Ferri R, Musumeci SA, Del Gracco S, Bottitta M, Scuderi C, et al. Sleep in subjects with autistic disorder: a neurophysiological and psychological study. Brain Dev. 2000;22:88–92. doi: 10.1016/s0387-7604(99)00119-9. [DOI] [PubMed] [Google Scholar]
- 53.Limoges E, Mottron L, Bolduc C, Berthiaume C, Godbout R. Atypical sleep architecture and the autism phenotype. Brain. 2005;128:1049–1061. doi: 10.1093/brain/awh425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


