Abstract
Introduction
Although studies have shown reductions in mortality from AIDS after the introduction of combination antiretroviral treatment (cART), little is known about cause-specific mortality in low income settings in the cART era. We explored predictors of AIDS and non-AIDS mortality and compared cause-specific mortality across high and low income settings in the Asia Pacific region.
Methods
We followed patients in the Asia Pacific HIV Observational Database from the date they started cART (or cohort enrolment if cART initiation was identified retrospectively), until the date of death or last follow-up visit. Competing risks methods were used to estimate the cumulative incidence, and to investigate predictors, of AIDS and non-AIDS mortality.
Results
Of 4252 patients, 215 died; 89 from AIDS, 97 from non-AIDS causes and 29 from unknown causes. Age >50 years (hazard ratio (HR), 4.29; 95%CI, 2.10-8.79) and CD4 counts ≤100 cells/μL (HR, 8.59; 95%CI, 5.66-13.03) were associated with an increased risk of non-AIDS mortality. Risk factors for AIDS mortality included CD4 counts ≤100 cells/μL (HR, 34.97; 95%CI, 18.01-67.90) and HIV RNA ≥10,001 (HR 4.21; 95%CI 2.07-8.55). There was some indication of a lower risk of non-AIDS mortality in Asian high, and possibly low, income countries compared to Australia.
Conclusions
Immune deficiency is associated with an increased risk of AIDS and non-AIDS mortality. Older age predicts non-AIDS mortality in the cART era. Less conclusive was the association between country-income level and cause-specific mortality because of the relatively high proportion of unknown causes of death in low income settings.
Keywords: HIV; cohort studies; AIDS; antiretroviral therapy, highly active; causes of death; survival analysis, competing-risks models
INTRODUCTION
In settings where antiretroviral drugs and health care are available, mortality has declined substantially among individuals with HIV infection since combination antiretroviral treatment (cART) was introduced [1-7]. The decline can largely be attributed to reduced AIDS deaths [8, 9]. Despite this, mortality rates remain higher in individuals with HIV infection than the general population [6, 10-13], reflecting an increasing proportion of deaths from non-AIDS diseases [2, 9, 14-17] and possibly toxicity from prolonged exposure to cART [18-21].
The shift in importance from AIDS to non-AIDS morbidity and mortality in HIV-infected populations has largely been documented in high income settings to date, although recent evidence suggests a similar transition is underway in middle income settings where cART is available [15]. In low income settings, mortality rates have been shown to be higher in the first months of cART compared to high income settings, with similar mortality rates by 6-12 months [22]. It is plausible, then, that mortality outcomes beyond one year of cART may be similar in low and high income settings where cART is available. There are, however, other factors that may influence outcomes over the longer term. Fewer treatment options, limited access to and affordability of healthcare and the background burden of other important diseases, such as tuberculosis and viral hepatitis, may lead to different long-term mortality outcomes in low income settings.
In this study, we describe cause-specific mortality in high and low income settings where cART is available in the Asia Pacific HIV Observational Database. Our primary aim was to investigate rates and predictors of AIDS and non-AIDS mortality within a competing risks framework, and secondarily, to compare AIDS and non-AIDS mortality between high and low income settings in our study population. We hypothesised that markers of advanced HIV disease would best predict AIDS mortality, while age and possibly co-infection with hepatitis B/C virus (HBV/HCV) would be associated with non-AIDS mortality. We expected a similar risk of AIDS and non-AIDS mortality in high and low income settings after adjustment for important predictors of cause-specific mortality.
METHODS
Study Population
The Australian HIV Observational Database (AHOD) and the TREAT Asia HIV Observational Database (TAHOD) are observational clinical cohort studies of patients with HIV infection in Australia and countries in Asia and the Pacific region, respectively. As part of the International Epidemiologic Databases to Evaluate AIDS initiative, these databases are combined to form the Asia Pacific HIV Observational Database. TAHOD is based on the same methodology as AHOD and methods for both studies have been described elsewhere in detail [23, 24]. Briefly, data for AHOD are collected from 27 clinical sites throughout Australia, including hospitals, sexual health clinics and general medical practices. Prospective data collection commenced in 1999, with retrospective data provided where available. Written, informed consent is obtained from all patients recruited to AHOD at the time of enrolment. In TAHOD, data are collected from 17 clinical sites in Asia and the Pacific region. Prospective data collection for TAHOD commenced in 2003, with retrospective data provided where available. Written consent was not a requirement of sites in TAHOD unless required by the site's local ethics committee because data are collected in an anonymous form. Ethical approval for both AHOD and TAHOD was obtained from the University of New South Wales, Sydney, Australia, and all other relevant institutional review boards. All AHOD and TAHOD study procedures were developed in accordance with the revised 1975 Helsinki Declaration.
Data for both AHOD and TAHOD are transferred electronically to the National Centre in HIV Epidemiology and Clinical Research (NCHECR) every March and September and include the same set of core variables. All data are subject to standardised quality control procedures.
We included all patients recruited to AHOD and TAHOD by March 31, 2007, who commenced cART (three or more antiretroviral drugs in combination) after January 1, 1996, and had at least one follow-up visit. All data were anonymous and analysed centrally at the NCHECR.
Outcome
The primary endpoint was mortality from AIDS and non-AIDS causes. Initially, only date of death was reported in AHOD. From 2001, cause of death (COD) was collected by the AHOD study coordinator via direct contact with relevant study sites. A standardised COD form based on the Data collection on adverse events of anti-HIV Drugs (D:A:D) cohort study original COD form [21] was introduced to AHOD study sites in mid-2002 and TAHOD when the study commenced in 2003. In 2005, the more detailed CoDe case report form (CRF) for coding causes of death (www.cphiv.dk/CoDe) was introduced in both cohorts. Following a death, the CoDe CRF is completed by a clinician at the study site, with details of autopsy reports provided where relevant. After a CoDe CRF is received by the study coordinators, it is reviewed by an independent HIV specialist clinician at the NCHECR who determines the primary and secondary causes of death. In cases where inadequate information is provided to determine the COD, the study coordinator contacts personnel at the study site for further information. Patients are classified as having an unknown COD if no further information is obtained.
The primary underlying COD was used in this analysis. It was classified as AIDS or non-AIDS related using a modified version of the revised 1993 CDC case definition for AIDS [25], excluding CD4 counts <200 cells/μL. Deaths from non-AIDS causes were further categorised into the following: cardiovascular disease (CVD) related, liver related, non-AIDS cancers, (un)natural (including suicide, overdose and trauma), bacterial infection-related, and other/unknown (Table 1).
Table 1.
Non-AIDS causes of death in high and low income settings in the Australian HIV Observational Database (AHOD) and TREAT Asia HIV Observational Database (TAHOD) cohorts
| Classification of mortality from non-AIDS causes | Causes of death as recorded in the databases | All patients | AHOD – High income | TAHOD – High income | TAHOD – Low income | |
|---|---|---|---|---|---|---|
| N | % | N | N | N | ||
| TOTAL | 126 | |||||
| Cardiovascular disease - related | Myocardial infarction | 6 | 31.6 | 6 | 0 | 0 |
| Cardiac arrest/event/failure | 4 | 21.1 | 1 | 1 | 2 | |
| Intracerebral /cerebral haemorrhage | 2 | 10.5 | 2 | 0 | 0 | |
| Coronary death | 2 | 10.5 | 1 | 0 | 1 | |
| Cardio-pulmonary arrest | 1 | 5.3 | 1 | 0 | 0 | |
| Coronary artery atheroma | 1 | 5.3 | 1 | 0 | 0 | |
| Diabetes | 1 | 5.3 | 0 | 0 | 1 | |
| Stroke | 1 | 5.3 | 0 | 0 | 1 | |
| Vascular heart disease | 1 | 5.3 | 1 | 0 | 0 | |
| TOTAL | 19 | 100.0 | 13 | 1 | 5 | |
| Liver-related | Liver failure (+/- hepatitis history) | 6 | 37.5 | 6 | 0 | 0 |
| Chronic viral hepatitis/hepatitis C | 4 | 25.0 | 4 | 0 | 0 | |
| Liver cancer (hepatitis B/C history) | 3 | 18.8 | 1 | 2 | 0 | |
| Liver disease (alcoholic &/or hepatitis history) | 1 | 6.3 | 1 | 0 | 0 | |
| Cirrhosis | 1 | 6.3 | 0 | 0 | 1 | |
| Hepatorenal failure | 1 | 6.3 | 1 | 0 | 0 | |
| TOTAL | 16 | 100 | 13 | 2 | 1 | |
| Non-AIDS cancers | Lung cancer | 11 | 44.0 | 8 | 2 | 1 |
| Cancer | 3 | 12.0 | 3 | 0 | 0 | |
| Squamous cell carcinoma | 2 | 8.0 | 2 | 0 | 0 | |
| Oesophageal cancer | 2 | 8.0 | 1 | 1 | 0 | |
| Acute lymphoblastic leukaemia | 1 | 4.0 | 1 | 0 | 0 | |
| Brain tumour | 1 | 4.0 | 1 | 0 | 0 | |
| Colon cancer | 1 | 4.0 | 0 | 0 | 1 | |
| Gastrointestinal tumour | 1 | 4.0 | 1 | 0 | 0 | |
| Melanoma | 1 | 4.0 | 1 | 0 | 0 | |
| Mesothelioma | 1 | 4.0 | 1 | 0 | 0 | |
| Metastatic anal cancer | 1 | 4.0 | 1 | 0 | 0 | |
| TOTAL | 25 | 100 | 20 | 3 | 2 | |
| (un)natural | Drug overdose | 7 | 46.7 | 6 | 1 | 0 |
| Suicide | 5 | 33.3 | 3 | 1 | 1 | |
| Motor vehicle accident | 2 | 13.3 | 2 | 0 | 0 | |
| Head injury (possible suicide attempt) | 1 | 6.7 | 0 | 1 | 0 | |
| TOTAL | 15 | 100.0 | 11 | 3 | 1 | |
| Bacterial infection-related | Bacterial infection | 3 | 20.0 | 3 | 0 | 0 |
| Pneumonia (non-AIDS related)* | 4 | 26.7 | 1 | 1 | 2 | |
| Salmonella sepsis and/or organ failure | 2 | 13.3 | 0 | 2 | 0 | |
| Septicaemia, organ failure and/or peritonitis | 2 | 13.3 | 1 | 1 | 0 | |
| Appendiceal abscess* | 1 | 6.7 | 1 | 0 | 0 | |
| Leptospirosis | 1 | 6.7 | 0 | 0 | 1 | |
| Meningititis (bacterial, not tuberculosis-related) | 1 | 6.7 | 0 | 0 | 1 | |
| Pneumococcal septicaemia | 1 | 6.7 | 1 | 0 | 0 | |
| TOTAL | 15 | 100.0 | 7 | 4 | 4 | |
| Other/unknown | Epileptic seizure | 2 | 5.5 | 1 | 1 | 0 |
| Pancreatitis | 2 | 5.5 | 0 | 0 | 2 | |
| Kennedy's disease (inherited neuronopathy) | 1 | 2.8 | 1 | 0 | 0 | |
| Metabolic encephalitis | 1 | 2.8 | 1 | 0 | 0 | |
| Renal failure | 1 | 2.8 | 0 | 1 | 0 | |
| Unknown | 29 | 80.5 | 9 | 1 | 19 | |
| TOTAL | 36 | 100.0 | 12 | 3 | 21 | |
probably, but not conclusively, bacterial infection-related.
Statistical analysis
Follow-up was calculated from the start date of cART, or the date of cohort enrolment where cART initiation was identified retrospectively, until death or the most recent follow-up visit. Lost to follow-up was defined as no clinic visit in the 12 months prior to March 31, 2007 (cohort censoring date). Patients lost to follow-up were censored at their last clinic visit. We used an intent-to-continue treatment approach and ignored any changes to, interruptions or termination of, treatment after baseline.
The cumulative incidences of all categories of causes of death were calculated within a competing risks framework for each of the income-cohort groups [26].
We investigated predictors of AIDS and non-AIDS mortality using multivariate Cox proportional hazards models, taking into account competing risks [8, 27, 28]. First we created a stacked dataset with repeat observations for AIDS and non-AIDS mortality. An administrative censoring time (March 31, 2007) was then created for each patient who died of a COD that was not the COD of interest (the date the patient was otherwise presumed to have been alive at). Patients who died after the cohort censoring date were also censored at this date. Baseline covariates included in the univariate analysis were: age, sex, income-cohort group, HIV exposure, time since first positive HIV test, HBV co-infection, HCV co-infection, HIV viral load, prior AIDS, mono/dual therapy prior to cART and duration of prior cART exposure at cohort enrolment. Study sites were classified as low or high income if they were located in low to lower-middle income or upper-middle to high income countries, respectively, as per 2007 World Bank country income classifications [29]. HBV co-infection was defined as the detection of HBV surface antigen ever. HCV co-infection was defined as the detection of HCV antibody ever. Baseline CD4 count and HIV viral load were based on a single measure in the six months prior to baseline, closest to the baseline date. All viral load measures less than 400 copies/mL were replaced with the value 399 copies/mL because more sensitive assays were not uniformly available throughout the study period. CD4 count and calendar year were also included as time-dependent covariates. Covariates with a p-value of <0.10 were considered in the multivariate analysis. Two multivariate models were built to compare the effect of adjusting for CD4 count at baseline (model 1) and as a time-dependent covariate (model 2). The forward step-wise method was used, using the log-likelihood ratio statistic to assess contribution to the model. A two-sided p-value ≤0.05 was considered statistically significant. Missing data were included in all analyses as a separate category, except for tests for trend (ordinal categorical covariates) or tests for homogeneity (nominal categorical covariates) where they were excluded. We evaluated the effect of income-cohort setting on AIDS and non-AIDS mortality by adjusting the income-cohort variable for significant covariates in the above models.
Sensitivity analyses were also conducted where: unknown causes of death (n=29) were classified as AIDS deaths; sites with less than 80% follow-up were excluded; sites with more than 25% of deaths classified as unknown were excluded; patients with mono/dual therapy prior to cART were excluded; the earliest baseline date was set to January 1, 2003 (when cohort recruitment commenced in TAHOD); and every second time-dependent CD4 count was excluded in AHOD to approximate the frequency of measurements in TAHOD.
Data were analysed using Stata version 10 [30].
RESULTS
A total of 4252 patients were included in these analyses; 1594 from AHOD (high income), 1101 from TAHOD sites in high income countries and 1557 from TAHOD sites in low income countries. The median age in AHOD was 41 years (interquartile range (IQR), 35-49), 38 years (IQR, 33-46) in the TAHOD high income group and 36 years (IQR, 31-42) in the TAHOD low income group (Table 2). Ninety-four percent of patients in AHOD were male compared to 81% and 65% in TAHOD high and low income groups, respectively. Heterosexual contact was the primary HIV exposure at low income TAHOD sites (80%) and accounted for over half of infections at high income TAHOD sites. In contrast, homosexual contact (with or without injecting drug use) was the most common HIV exposure in AHOD (74%). A total of 215 of the 4252 patients died during follow-up; 89 died from AIDS, 97 from non-AIDS causes and 29 from unknown causes.
Table 2.
Patient characteristics at baseline in low and high income settings in the Australian HIV Observational Database (AHOD) and TREAT Asia HIV Observational Database (TAHOD) cohorts
| AHOD – high income | TAHOD - high income | TAHOD - low income | |||||
|---|---|---|---|---|---|---|---|
| n (%) | No. deaths (%) | n (%) | No. deaths (%) | n (%) | No. deaths (%) | ||
| Total | 1594 (100) | 123 (100) | 1101 (100) | 36 (100) | 1557 (100) | 56 (100) | |
| Age (years) | Median(IQR) | 41 (35,49) | 46 (38,53) | 38 (33,46) | 43 (34,63) | 36 (31,42) | 39 (32,46) |
| 15-29 | 110 (7) | 4 (3) | 176 (16) | 5 (14) | 292 (19) | 10 (18) | |
| 30-39 | 595 (37) | 39 (32) | 463 (42) | 10 (28) | 799 (51) | 20 (36) | |
| 40-49 | 543 (34) | 40 (32.5) | 282 (26) | 9 (25) | 340 (22) | 15 (27) | |
| 50+ | 346 (22) | 40 (32.5) | 180 (16) | 12 (33) | 126 (8) | 11 (20) | |
| Sex | Male | 1504 (94) | 117 (95) | 896 (81) | 25 (69) | 1016 (65) | 44 (79) |
| Female | 90 (6) | 6 (5) | 205 (19) | 11 (31) | 541 (35) | 12 (21) | |
| HIV exposure | Homosexual contact +/- IDU | 1181 (74) | 97 (79) | 408 (37) | 6 (17) | 87 (6) | 2 (4) |
| IDU +/- heterosexual contact | 32 (2) | 4 (3) | 35 (3) | 2 (6) | 76 (5) | 4 (7) | |
| Heterosexual contact | 134 (8) | 5 (4) | 620 (56) | 27 (75) | 1251 (80) | 41 (73) | |
| Other | 80 (5) | 13 (11) | 38 (3) | 1 (3) | 137 (9) | 8 (14) | |
| Missing | 167 (10) | 4 (3) | 0 | 0 | 6 (0) | 1 (2) | |
| Time since first positive HIV test (years) | No. with test | n = 1584 | n = 122 | n = 1101 | n = 36 | n = 1540 | n = 54 |
| Median (IQR) | 7 (4,12) | 9 (6,12) | 3 (2,5) | 2 (1,5) | 2 (1,4) | 1 (0,2) | |
| <5 | 539 (34) | 23 (19) | 793 (72) | 29 (81) | 1246 (80) | 47 (84) | |
| 5+ | 1045 (65) | 99 (80) | 308 (28) | 7 (19) | 294 (19) | 7 (12) | |
| Missing | 10 (1) | 1 (1) | 0 (0) | 0 (0) | 17 (1) | 2 (4) | |
| HBV co-infection | Negative | 788 (49) | 63 (51) | 737 (67) | 20 (56) | 605 (39) | 25 (45) |
| Positive | 50 (3) | 6 (5) | 83 (8) | 3 (8) | 65 (4) | 2 (4) | |
| Missing | 756 (47) | 54 (44) | 281 (26) | 13 (36) | 887 (57) | 29 (52) | |
| HCV co-infection | Negative | 697 (44) | 61 (50) | 755 (69) | 20 (56) | 455 (29) | 18 (32) |
| Positive | 113 (7) | 14 (11) | 47 (4) | 5 (14) | 75 (5) | 4 (7) | |
| Missing | 784 (49) | 48 (39) | 299 (27) | 11 (31) | 1027 (66) | 34 (61) | |
| Prior AIDS | No | 1273 (80) | 79 (64) | 498 (45) | 15 (42) | 478 (31) | 6 (11) |
| Yes | 321 (20) | 44 (36) | 603 (55) | 21 (58) | 1079 (69) | 50 (89) | |
| Mono/dual therapy prior to cART | No | 882 (55) | 46 (37) | 799 (73) | 28 (78) | 1420 (91) | 47 (84) |
| Yes | 712 (45) | 77 (63) | 302 (27) | 8 (22) | 137 (9) | 9 (16) | |
| Duration of prior cART exposure at cohort enrolment¥ (months) | No. starting cART prior to cohort enrolment | n = 1420 | n = 116 | n = 932 | n = 30 | n = 1244 | n = 42 |
| Median (IQR) | 33 (19,42) | 37 (27,43) | 24 (10,43) | 10 (4,39) | 12 (4,24) | 5 (3,14) | |
| 1-24 | 470 (33) | 26 (22) | 457 (49) | 20 (67) | 930 (75) | 36 (86) | |
| 25+ | 950 (67) | 90 (78) | 475 (51) | 10 (33) | 314 (25) | 6 (14) | |
| First cART regimen | NRTI based cART (+/-PI) | 999 (63) | 88 (71) | 487 (44) | 15 (42) | 191 (12) | 5 (9) |
| NNRTI based cART | 541 (34) | 29 (24) | 591 (54) | 20 (56) | 1363 (88) | 51 (91) | |
| NNRTI + PI cART | 54 (3) | 6 (5) | 23 (2) | 1 (3) | 3 (0) | 0 (0) | |
| Baseline regimen | Noneα | 143 (9) | 24 (19) | 29 (3) | 5 (14) | 27 (2) | 1 (2) |
| Mono/dual therapy | 140 (9) | 13 (11) | 68 (6) | 1 (3) | 51 (3) | 8 (14) | |
| NRTI based cART (+/- PI) | 590 (37) | 40 (33) | 371 (34) | 11 (31) | 129 (8) | 1 (2) | |
| NNRTI based cART | 616 (39) | 32 (26) | 604 (55) | 17 (47) | 1341 (86) | 46 (82) | |
| NNRTI + PI cART | 102 (6) | 14 (11) | 29 (3) | 2 (5) | 7 (0) | 0 (0) | |
| CD4 count (cells/μL)** | No. with CD4 count | n = 1541 | n = 114 | n = 1038 | n = 26 | n = 1283 | n = 36 |
| Median (IQR) | 450 (270,660) | 234 (49,442) | 290 (175,437) | 145 (60,253) | 223 (106,358) | 66 (23,218) | |
| >500 | 673 (42) | 23 (19) | 197 (18) | 1 (3) | 139 (9) | 1 (2) | |
| 351-500 | 318 (20) | 15 (12) | 205 (18) | 1 (3) | 194 (12) | 3 (5) | |
| 201-350 | 298 (19) | 24 (20) | 329 (30) | 6 (16) | 361 (23) | 6 (11) | |
| 101-200 | 153 (10) | 16 (13) | 186 (17) | 10 (28) | 279 (18) | 3 (5) | |
| ≤100 | 99 (6) | 36 (29) | 121 (11) | 8 (22) | 310 (20) | 23 (41) | |
| Missing | 53 (3) | 9 (7) | 63 (6) | 10 (28) | 274 (18) | 20 (36) | |
| HIV RNA (copies/mL)** | No. with HIV RNA | n = 1532 | n = 112 | n = 904 | n = 24 | n = 396 | n = 9 |
| Median (IQR) | 400 (400,4467) | 1650 (400,94599) | 400 (400,1100) | 11700 (400,98350) | 400 (400,10900) | 120000 (400,190000) | |
| ≤400 | 958 (60) | 47 (38) | 643 (58) | 11 (31) | 268 (17) | 3 (5) | |
| 401 - 10,000 | 258 (16) | 19 (16) | 82 (7) | 1 (3) | 28 (2) | 1 (2) | |
| 10,001 - 100,000 | 206 (13) | 22 (18) | 107 (10) | 8 (22) | 39 (2) | 0 (0) | |
| ≥100,001 | 110 (7) | 24 (19) | 72 (7) | 4 (11) | 61 (4) | 5 (9) | |
| Missing | 62 (4) | 11 (9) | 197 (18) | 12 (33) | 1161 (75) | 47 (84) | |
| Complete follow-up rate | 78% | 90% | 79% | ||||
| Median follow-up per patient (years) | 5.7 | 2.4 | 2.7 | 0.9 | 1.7 | 0.8 | |
None = not on antiretroviral treatment at the time of cohort enrolment, NRTI-based cART = NRTI +/- PI, no NNRTI, NNRTI-based cART = NRTI + NNRTI, no PI, NNRTI+PI cART = NNRTI + PI, +/- NRTI; Sum of all percentages for each variable may be > 100% due to rounding
the closest measure in the six months prior to baseline
among patients who started cART prior to cohort enrolment
only includes patients who started cART prior to cohort enrolment and were not on an ART regimen on the cohort enrolment date.
Cumulative incidences
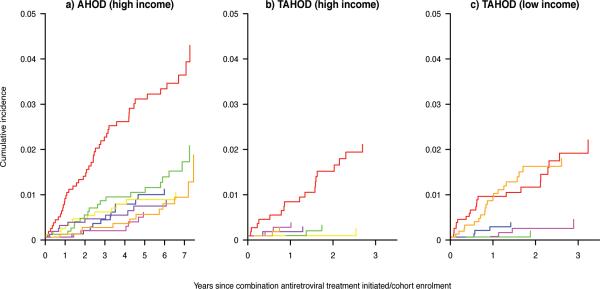
In all income-cohort groups, AIDS was the most common COD (Figure 1a, 1b, 1c). In the TAHOD low income group, the cumulative incidence for other/unknown causes was similar to AIDS (Figure 1c); 19 of these 21 deaths were of unknown cause (Table 1). In AHOD, non-AIDS cancers had the second highest cumulative incidence, followed by cardiovascular disease- and liver-related causes. The cumulative probability of dying from non-AIDS COD by three years follow-up was low in the TAHOD high and low income groups.
Figure 1.
Cumulative incidences of each specific causes of death in: (a) the Australian HIV Observational Database high income sites; (b) TREAT Asia HIV Observational Database (TAHOD) high income sites; and (c) TAHOD low income sites.
Red = AIDS; Green=Non-AIDS cancers; Blue=Cardiovascular disease-related; Yellow=Liver-related; Pink = Bacterial infection-related; Purple = (un)natural; Orange = unknown/other.
Predictors of AIDS and non-AIDS mortality
CD4 count (baseline and time-dependent) and HIV viral load were associated with both AIDS and non-AIDS mortality in the univariate analysis (Table 3). Age and HIV exposure were also associated with non-AIDS mortality, while prior AIDS, mono/dual therapy prior to cART and calendar year were associated with AIDS mortality; all were included in the multivariate analysis.
Table 3.
Unadjusted cause-specific hazard ratios for AIDS and non-AIDS mortality in the Australian HIV Observational Database (AHOD) and TREAT Asia HIV Observational Database (TAHOD) cohorts
| Univariate analysis | Non-AIDS mortality | AIDS mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | P* | HR | 95% CI | p | P* | ||
| Baseline (fixed) covariates | |||||||||
| Age (years) | 15-29 | 1.00 | <0.001 | 1.00 | 0.465 | ||||
| 30-39 | 1.04 | (0.50 - 2.16) | 0.921 | 0.87 | (0.43 - 1.76) | 0.699 | |||
| 40-49 | 1.44 | (0.69 - 2.99) | 0.333 | 1.12 | (0.54 - 2.31) | 0.760 | |||
| 50+ | 3.28 | (1.60 - 6.72) | 0.001 | 1.11 | (0.50 - 2.44) | 0.805 | |||
| Sex | Male | 1.00 | 1.00 | ||||||
| Female | 0.76 | (0.44 - 1.32) | 0.334 | 0.99 | (0.55 - 1.76) | 0.964 | |||
| Cohort - country income level | AHOD – high income | 1.00 | 0.110 | 1.00 | 0.932 | ||||
| TAHOD –high income | 0.56 | (0.32 - 0.99) | 0.044 | 0.99 | (0.56 - 1.74) | 0.968 | |||
| TAHOD – low income | 0.99 | (0.64 - 1.53) | 0.977 | 0.91 | (0.53 - 1.55) | 0.721 | |||
| HIV Exposure | Homosexual contact +/- IDU | 1.00 | 0.005 | 1.00 | 0.668 | ||||
| IDU +/- heterosexual contact | 1.58 | (0.68 - 3.66) | 0.284 | 1.46 | (0.52 - 4.09) | 0.475 | |||
| Heterosexual contact | 0.86 | (0.57 - 1.31) | 0.486 | 0.99 | (0.61 - 1.60) | 0.959 | |||
| Other | 2.36 | (1.33 - 4.17) | 0.003 | 1.50 | (0.67 - 3.35) | 0.319 | |||
| Missing | 0.43 | (0.13 - 1.36) | 0.151 | 0.43 | (0.10 - 1.77) | 0.243 | |||
| Time since first positive HIV test (years) | <5 | 1.00 | 0.214 | 1.00 | 0.373 | ||||
| 5+ | 1.26 | (0.88 - 1.80) | 0.205 | 1.26 | (0.83 - 1.92) | 0.274 | |||
| Missing | 4.29 | (1.00 - 18.45) | 0.050 | 2.66 | (0.38 - 18.52) | 0.322 | |||
| Hepatitis C virus co-infection | Negative | 1.00 | 0.386 | 1.00 | 0.886 | ||||
| Positive | 1.38 | (0.66 - 2.87) | 0.392 | 0.92 | (0.28 - 2.98) | 0.887 | |||
| Missing | 0.66 | (0.46 - 0.96) | 0.028 | 1.29 | (0.84 - 1.97) | 0.246 | |||
| Hepatitis C virus co-infection | Negative | 1.00 | 0.135 | 1.00 | 0.116 | ||||
| Positive | 1.57 | (0.88 - 2.79) | 0.128 | 1.83 | (0.88 - 3.84) | 0.107 | |||
| Missing | 0.60 | (0.41 - 0.87) | 0.007 | 0.99 | (0.63 - 1.54) | 0.959 | |||
| Prior AIDS | No | 1.00 | 1.00 | ||||||
| Yes | 1.17 | (0.81 - 1.68) | 0.408 | 3.08 | (1.98 - 4.78) | <0.001 | |||
| Mono/dual therapy prior to cART | No | 1.00 | 1.00 | ||||||
| Yes | 1.30 | (0.91 - 1.86) | 0.145 | 1.65 | (1.08 - 2.51) | 0.020 | |||
| Time since cART initiation (months) | 0 | 1.00 | 0.500 | 1.00 | 0.929 | ||||
| 1-24 | 0.74 | (0.42 - 1.29) | 0.285 | 0.94 | (0.46 - 1.91) | 0.858 | |||
| 25+ | 0.73 | (0.43 - 1.26) | 0.257 | 1.02 | (0.51 - 2.03) | 0.952 | |||
| CD4 count (cells/μL) | >200 | 1.00 | <0.001 | 1.00 | <0.001 | ||||
| 101-200 | 1.64 | (0.97 - 2.78) | 0.064 | 3.77 | (1.78 - 8.02) | 0.001 | |||
| ≤100 | 3.23 | (2.08 - 5.03) | <0.001 | 17.58 | (9.92 - 31.16) | <0.001 | |||
| Missing | 3.22 | (1.88 - 5.53) | <0.001 | 15.57 | (8.21 - 29.53) | <0.001 | |||
| HIV RNA (copies/mL) | ≤400 | 1.00 | 0.034 | 1.00 | <0.001 | ||||
| 401 - 10,000 | 1.29 | (0.71 - 2.36) | 0.406 | 2.73 | (1.07 - 6.93) | 0.035 | |||
| ≥10,001 | 1.69 | (1.04 - 2.75) | 0.035 | 11.53 | (6.01 - 22.11) | <0.001 | |||
| Missing | 1.96 | (1.28 - 3.01) | 0.002 | 5.81 | (2.87 - 11.75) | <0.001 | |||
| Time-dependent covariates | |||||||||
| CD4 count (cells/μL) | >200 | 1.00 | <0.001 | 1.00 | <0.001 | ||||
| 101-200 | 2.60 | (1.53 - 4.45) | <0.001 | 8.75 | (4.13 - 18.55) | <0.001 | |||
| ≤100 | 7.34 | (4.89 - 11.01) | <0.001 | 50.10 | (27.24 - 92.17) | <0.001 | |||
| Missing | 10.75 | (4.58 - 25.26) | <0.001 | 31.14 | (10.41 - 93.15) | <0.001 | |||
| Calendar year | 1999-2001 | 1.00 | 0.166 | 1.00 | 0.032 | ||||
| 2002-2004 | 1.04 | (0.64 - 1.67) | 0.884 | 1.04 | (0.59 - 1.82) | 0.887 | |||
| 2005-2007 | 0.70 | (0.41 - 1.18) | 0.178 | 0.52 | (0.28 - 0.98) | 0.042 | |||
Bold type indicates significant covariates on univariate analysis
P-overall for test for trend (ordinal categorical covariates) and test for homogeneity (nominal categorical covariates).
Multivariate analyses
Significant predictors of non-AIDS mortality in model 1, which excluded CD4 count as a time-dependent covariate, were older age (HR, 4.08 for >50 years compared to 15-29 years; 95% CI 1.95-8.54), HIV exposure and lower baseline CD4 count (Table 4). A lower CD4 count was also associated with a higher risk of AIDS mortality, as were prior AIDS (HR, 2.07; 95%CI, 1.26-3.39) and higher HIV viral load at baseline. The risk of AIDS mortality decreased by almost 70% in 2005-2007 compared to 1999-2001.
Table 4.
Adjusted cause-specific hazard ratios for AIDS and non-AIDS mortality in the Australian HIV Observational Database (AHOD) and TREAT Asia HIV Observational Database (TAHOD) cohorts
| Multivariate analysis | Non-AIDS mortality | AIDS mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | P* | HR | 95% CI | p | P* | ||
| Model 1 (including baseline CD4 count) | |||||||||
| Baseline (fixed) covariates | |||||||||
| Age (years) | 15-29 | 1.00 | <0.001 | ||||||
| 30-39 | 1.18 | (0.56 - 2.49) | 0.672 | ||||||
| 40-49 | 1.68 | (0.80 - 3.55) | 0.173 | ||||||
| 50+ | 4.08 | (1.95 - 8.54) | <0.001 | ||||||
| HIV Exposure | Homosexual contact +/- IDU | 1.00 | 0.028 | ||||||
| IDU +/- heterosexual contact | 1.46 | (0.60 - 3.55) | 0.405 | ||||||
| Heterosexual contact | 0.74 | (0.49 - 1.13) | 0.163 | ||||||
| Other | 1.77 | (0.97 - 3.20) | 0.061 | ||||||
| Missing | 0.39 | (0.12 - 1.23) | 0.108 | ||||||
| Prior AIDS | No | 1.00 | |||||||
| Yes | 2.07 | (1.26 - 3.39) | 0.004 | ||||||
| Mono/dual therapy prior to cART | No | 1.00 | |||||||
| Yes | 1.60 | (0.99 - 2.58) | 0.053 | ||||||
| HIV RNA (copies/mL) | ≤400 | 1.00 | 0.385 | 1.00 | <0.001 | ||||
| 401 - 10,000 | 1.25 | (0.67 - 2.31) | 0.481 | 2.64 | (1.01 - 6.90) | 0.047 | |||
| ≥10,001 | 1.28 | (0.77 - 2.14) | 0.345 | 5.38 | (2.61 - 11.08) | <0.001 | |||
| Missing | 1.64 | (0.96 - 2.79) | 0.068 | 1.52 | (0.65 - 3.52) | 0.333 | |||
| CD4 count (cells/μL) | >200 | 1.00 | <0.001 | 1.00 | <0.001 | ||||
| 101-200 | 1.61 | (0.96 - 2.69) | 0.070 | 2.66 | (1.14 - 6.19) | 0.023 | |||
| ≤100 | 3.71 | (2.36 - 5.83) | <0.001 | 8.70 | (4.35 - 17.41) | <0.001 | |||
| Missing | 3.50 | (2.04 - 6.03) | <0.001 | 16.21 | (6.94 - 37.84) | <0.001 | |||
| Time-dependent covariates | |||||||||
| Calendar year | 1999-2001 | 1.00 | 0.006 | ||||||
| 2002-2004 | 0.63 | (0.36 - 1.10) | 0.107 | ||||||
| 2005-2007 | 0.33 | (0.16 - 0.66) | 0.002 | ||||||
| Model 2 (including time-dependent CD4 count) | |||||||||
| Baseline (fixed) covariates | |||||||||
| Age group | 15-29 | 1.00 | <0.001 | ||||||
| 30-39 | 1.13 | (0.54 - 2.34) | 0.745 | ||||||
| 40-49 | 1.64 | (0.79 - 3.41) | 0.186 | ||||||
| 50+ | 4.29 | (2.10 - 8.79) | <0.001 | ||||||
| HIV Exposure | Homosexual contact +/- IDU | 1.00 | 0.063 | ||||||
| IDU +/- heterosexual contact | 1.29 | (0.52 - 3.20) | 0.583 | ||||||
| Heterosexual contact | 0.79 | (0.52 - 1.19) | 0.256 | ||||||
| Other | 1.75 | (0.98 - 3.12) | 0.061 | ||||||
| Missing | 0.42 | (0.13 - 1.31) | 0.135 | ||||||
| Prior AIDS | No | 1.00 | |||||||
| Yes | 1.60 | (0.99 - 2.57) | 0.054 | ||||||
| Mono/dual therapy prior to cART | No | 1.00 | |||||||
| Yes | 1.40 | (0.89 - 2.21) | 0.149 | ||||||
| HIV RNA (copies/mL) | ≤400 | 1.00 | 0.712 | 1.00 | <0.001 | ||||
| 401 - 10,000 | 1.16 | (0.62 - 2.18) | 0.637 | 1.95 | (0.75 - 5.07) | 0.171 | |||
| ≥10,001 | 1.08 | (0.63 - 1.87) | 0.775 | 4.21 | (2.07 - 8.55) | <0.001 | |||
| Missing | 1.30 | (0.81 - 2.10) | 0.272 | 1.95 | (0.86 - 4.42) | 0.108 | |||
| Time-dependent covariates | |||||||||
| CD4 count (cells/μL) | >200 | 1.00 | <0.001 | 1.00 | <0.001 | ||||
| 101-200 | 2.63 | (1.54 - 4.49) | <0.001 | 7.28 | (3.34 - 15.83) | <0.001 | |||
| ≤100 | 8.59 | (5.66 - 13.03) | <0.001 | 34.97 | (18.01 - 67.90) | <0.001 | |||
| Missing | 11.56 | (5.08 - 26.32) | <0.001 | 27.07 | (7.97 - 91.96) | <0.001 | |||
| Calendar year | 1999-2001 | 1.00 | 0.238 | ||||||
| 2002-2004 | 0.79 | (0.44 - 1.43) | 0.443 | ||||||
| 2005-2007 | 0.55 | (0.27 - 1.13) | 0.102 | ||||||
Bold type indicates significant covariates in multivariate model; normal type indicates covariates with p<0.1 on univariate analysis, adjusted for the final significant multivariate model
P-overall for test for trend (ordinal categorical covariates) and test for homogeneity (nominal categorical covariates).
In model 2 (incorporating time-dependent CD4 counts), older age remained a significant predictor of non-AIDS mortality (HR, 4.29 for >50 years compared to 15-29 years; 95% CI 2.10-8.79). Lower CD4 counts were associated with both outcomes, although the magnitude of risk was much greater for AIDS (HR for ≤100 vs. >200 cells/μL, 34.97; 95%CI 18.01-67.90) than non-AIDS mortality (HR for ≤100 vs. >200 cells/μL, 8.59; 95% CI 5.66-13.03). A baseline HIV viral load ≥10,001 copies/mL (vs. ≤400 copies/mL) was also associated with AIDS mortality (HR, 4.21; 95%CI, 2.07-8.55). Similar results were obtained in sensitivity analyses.
Association between income-cohort group and AIDS and non-AIDS mortality
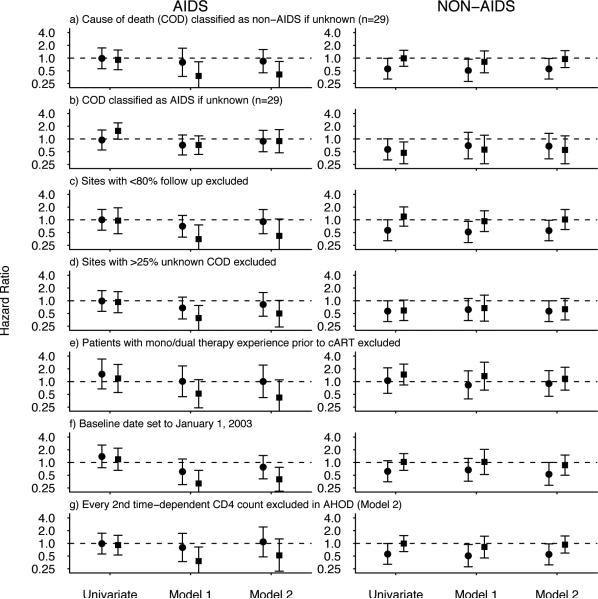
There was no significant difference in the risk of AIDS or non-AIDS mortality between the income-cohort groups on the univariate analysis, except for a marginally lower risk of non-AIDS mortality in the TAHOD high income group compared to AHOD (HR, 0.56; 95%CI, 0.32-0.99) (Figure 2a). When unknown COD were classified as AIDS deaths, both TAHOD high and low income groups had a lower risk of non-AIDS mortality than AHOD and the risk of AIDS mortality was higher in TAHOD low income sites (HR, 1.55; 95%CI, 0.99-2.41) (Figure 2b). Adjustment for significant covariates in model 1 and model 2 had a broadly similar effect across all sensitivity analyses; it reduced the risk of AIDS mortality in the TAHOD low income group, but had little effect on the risk of non-AIDS mortality (Figure 2). Recoding unknown COD as AIDS deaths resulted in little difference between AHOD and TAHOD in terms of AIDS mortality in the adjusted analyses (Figure 2b). Exclusion of every second time-dependent CD4 count in AHOD also slightly increased the hazard ratio for the TAHOD low income group in model 2 (Figure 2g).
Figure 2.
Unadjusted and adjusted hazard ratios for the risk of AIDS and non-AIDS mortality in the Australian HIV Observational Database (high income), TREAT Asia HIV Observational Database (TAHOD) high and low income sites, when: (a) unknown causes of death (COD) were classified as non-AIDS deaths; (b) unknown COD were classified as AIDS deaths; (c) sites with <80% follow-up were excluded; (d) sites with >25% unknown COD were excluded; (e) patients with mono/dual therapy experience prior to cART were excluded; (f) the baseline date was set to January 1, 2003; and (g) every 2nd time-dependent CD4 count was excluded in AHOD (model 2).
Closed circle represents TAHOD high income sites; closed square represents TAHOD low income sites; dashed line represents reference group (AHOD).
DISCUSSION
In patients who started cART in the Asia Pacific region, immunodeficiency (as indicated by CD4 count) was associated with non-AIDS mortality, although to a lesser extent than AIDS mortality. Older age was also associated with an increased risk of non-AIDS mortality. Other risk factors traditionally associated with AIDS remained important predictors of AIDS mortality in the cART era. While the risk of dying from AIDS decreased over the study period, this was largely due to differences in CD4 count over time. We found a lower risk of dying from non-AIDS causes in high income Asian countries compared to Australia, but no difference in AIDS mortality between the two settings. Less conclusive was the relative risk of dying from AIDS and non-AIDS causes in low income Asian countries compared to Australia because of the relatively high proportion of unknown COD at low income sites.
Our finding that immunodeficiency is related to non-AIDS mortality in a diverse clinical population in the Asia Pacific region is consistent with previous studies in high income settings [9, 13, 31-35]. It also parallels previous studies of morbidity in patients with HIV infection where immunodeficiency has been associated with non-AIDS cancers [36, 37]. In this study, sample size limited us from further investigating the relationship between immunodeficiency and more specific non-AIDS COD in the Asia Pacific region.
The association between older age and non-AIDS mortality in our study is also consistent with other studies of non-AIDS morbidity and mortality in high income settings [18, 21, 31, 35, 37]. This finding reflects the ageing nature of HIV affected populations in the cART era and possible drug-related toxicities. Despite this demographic transition and shift in the treated history of HIV disease, patients who present with risk factors traditionally associated with AIDS remain at higher risk of AIDS mortality in the cART era [33].
Consistent with previous reports of declining AIDS mortality in the cART era [9, 14], we observed an almost 70% reduction in the risk of dying from AIDS between 1999-2001 and 2005-2007 in our study. This was largely explained by differences in CD4 count over time. As AHOD and TAHOD are relatively closed cohorts, this study is not ideal to investigate the association between calendar year and mortality.
Contrary to our hypothesis, we did not find any significant associations between viral hepatitis co-infection and non-AIDS mortality in our study. This may, in part, be due to limited statistical power. It may also be a consequence of under ascertainment of HBV and HCV co-infection in TAHOD. Missing values for hepatitis test results in TAHOD may reflect low testing rates despite the relatively high prevalence of HBV and HCV in Asia [38, 39]. In contrast, patients without hepatitis test results recorded in AHOD were generally not tested because they had no known risk factors and were, therefore, presumed negative.
The overall follow-up rates in each of the income-cohort groups in our study were relatively high, although there were lower rates of follow-up at some sites. Similar to other HIV cohorts [22], lower rates of follow-up were observed at low income sites, which may have led to under ascertainment of deaths in this setting. To assess the effect of follow-up rates, we performed a sensitivity analysis to exclude sites with follow-up rates <80%. This analysis yielded similar results for both AIDS and non-AIDS mortality.
The most important limitation of our study was the relatively large proportion of unknown COD at TAHOD low income sites. To explore their effect on our results, we performed various sensitivity analyses. First we assumed that patients with AIDS were more likely to be under close surveillance prior to death and classified unknown COD as non-AIDS deaths. In this scenario, there was no difference in AIDS mortality between the different income-cohort groups and TAHOD high income sites had a lower risk of non-AIDS mortality than AHOD. Unexpectedly, adjustment for CD4 count (baseline or time-dependent), HIV viral load and prior AIDS led to a decreased risk of AIDS mortality in the TAHOD low income group compared to AHOD. This was only partly accounted for by differences in the frequency of CD4 count measurements in AHOD and TAHOD. In a second scenario, we grouped unknown COD as AIDS deaths on the basis that the average last CD4 count prior to death in patients with other/unknown COD at TAHOD low income sites was low and comparable to that in the AIDS death group. In this analysis, the TAHOD low income group had an increased risk of AIDS mortality compared to AHOD, which decreased after adjustment for significant covariates in the predictor models. This was more in line with the expected direction of any cause-specific mortality differences between the income-cohort groups. Further, TAHOD low income sites had a lower risk of non-AIDS mortality than AHOD, comparable to that at TAHOD high income sites. The same pattern was observed for non-AIDS mortality when sites with more than 25% of deaths classified as unknown were excluded.
Other limitations of this study include enrolment of patients into AHOD and TAHOD after they started cART and inclusion of patients with mono/dual therapy experience prior to starting cART in this analysis. To address these limitations, we included duration of cART exposure prior to cohort enrolment and mono/dual therapy prior to cART as covariates in the competing risks model; neither variable was significantly associated with mortality from AIDS or non-AIDS causes in the final model. We also conducted a sensitivity analysis excluding patients with mono/dual therapy experience prior to cART, of which the vast majority were from AHOD. In this analysis, the risk of non-AIDS mortality was similar in all income-cohort groups.
The introduction of a new, standardised COD form in AHOD in 2002 may have led to differential misclassification bias for specific COD between the periods 1999-2002 and 2003-2007. To investigate whether the change in data collection affected our results, we conducted a sensitivity analysis with the baseline date set to January 1, 2003. We found similar results, suggesting the new COD form had little effect on the classification of COD into the broader categories of AIDS and non-AIDS causes used in our analysis.
This study has several strengths. To our knowledge, this is the first study to report mortality outcomes beyond 12 months of cART in low and high income settings internationally. Further, we believe this is the first study to investigate mortality from AIDS versus non-AIDS causes in low and high income settings during the cART era. The use of competing risks methods in our analysis enabled simultaneous comparison of risk factors for AIDS and non-AIDS mortality and was less likely to overestimate mortality [33], both of which are advantages over standard survival methods. Finally, use of data from two observational cohort studies founded on the same methodology is a major strength of our study, reducing the likelihood that methodological differences can explain the findings.
In summary, improved immunological outcomes are likely to reduce mortality from both AIDS and non-AIDS causes in patients with HIV infection, although this needs to be evaluated in large clinical studies. The increased risk of non-AIDS mortality with age highlights the importance of managing risk factors for other chronic diseases in patients with HIV infection in the cART era. We found some indication of a lesser risk of non-AIDS mortality at sites in high, and possibly low, income countries in Asia compared to Australia. The association between country-income level and AIDS mortality warrants further investigation and requires improved COD ascertainment in low income settings. Finally, the results of our study can only be generalised to patients receiving cART in the Asia Pacific region. Notably, those receiving cART represent only a small fraction of the overall number of individuals in need of treatment in low-middle income countries in the Asia Pacific region at this time [40].
Supplementary Material
SUGGESTED ONLINE SUPPLEMENTARY DATA
Table A1. Crude mortality rates in high and low income settings in the Australian HIV Observational Database (AHOD) and TREAT Asia HIV Observational Database (TAHOD) cohorts
ACKNOWLEDGEMENTS
KF and ML developed the analysis concept and KF drafted the analysis plan. KF coordinated the study, managed the data, conducted all statistical analyses and drafted the manuscript. All authors discussed the analysis plan, contributed to interpretation of the analysis results and commented on drafts of the manuscript. All authors approved the final manuscript draft for journal submission.
The authors would like to thank Dr Preeyaporn Srasuebkul for statistical advice on this paper. The authors also thank participating sites and steering committee members (Appendix 1) and all patients who participated in this study.
The TREAT Asia HIV Observational Database and the Australian HIV Observational Database are part of the Asia Pacific HIV Observational Database and are initiatives of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the National Institute of Allergy and Infectious Diseases (NIAID) of the U.S. National Institutes of Health (NIH), as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA) (grant no. U01AI069907), and the Dutch Minisitry of Foreign Affairs, through a partnership with Stichting Aids Fonds. The National Centre in HIV Epidemiology and Clinical Research is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the institutions mentioned above.
Appendix 1 The Australian HIV Observational Database (AHOD) and the TREAT Asia HIV Observational Database (TAHOD)
The Australian HIV Observational Database
D Ellis, General Medical Practice, Coffs Harbour, NSW; J Chuah*, D Lester, W Fankhauser, B Dickson, Gold Coast Sexual Health Clinic, Miami, QLD; M Bloch, T Franic, S Agrawal, Holdsworth House General Practice, Darlinghurst, NSW; R Moore, P Cortissos, S Edwards, P Locke The Carlton Clinic, Carlton, VIC; D Nolan, S Mallal, C Forsdyke, S Bulgannawar, Department of Clinical Immunology, Royal Perth Hospital, Perth, WA; NJ Roth*, J Nicholson, Prahran Market Clinic, South Yarra, VIC; D Allen, Holden Street Clinic, Gosford, NSW; D Smith, C Mincham, C Gray Lismore Sexual Health & AIDS Services, Lismore, NSW; D Baker*, R Vale, East Sydney Doctors, Darlinghurst, NSW; D Russell, J Leamy, C Remington, P Brown Cairns Sexual Health Service, Cairns, QLD; C O'Connor; D Templeton, Royal Prince Alfred Hospital Sexual Health, Camperdown, NSW; D Sowden, A Walker*, Clinic 87, Sunshine Coast & Cooloola HIV Sexual Health Service, Nambour, QLD; D Orth; D Youds, Gladstone Road Medical Centre, Highgate Hill, QLD; E Jackson, D Hunter, K McCallum, Blue Mountains Sexual Health and HIV Clinic, Katoomba, NSW; T Read, J Silvers, Melbourne Sexual Health Centre, Melbourne, VIC; A Kulatunga, P Knibbs, Communicable Disease Centre, Royal Darwin Hospital, Darwin, NT; A Mijch, J Hoy, K Watson*, M Bryant, The Alfred Hospital, Melbourne, VIC; M Gotowski, S Taylor, L Stuart-Hill, Bligh Street Clinic, Tamworth, NSW; D Cooper, A Carr, M Lacey, K Hesse, G Keogh, R Norris, St Vincent's Hospital, Darlinghurst, NSW; R Finlayson, I Prone, Taylor Square Private Clinic, Darlinghurst, NSW; MT Liang, Nepean Sexual Health and HIV Clinic, Penrith, NSW; M Kelly, P Negus, PL Am,brose, H Magon, AIDS Medical Unit, Brisbane, QLD; K Brown, N Skobalj, Illawarra Sexual Health Clinic, Warrawong, NSW; L Wray, H Lu, Sydney Sexual Health Centre, Sydney, NSW; W Donohue, A Lohmeyer, The Care and Prevention Programme, Adelaide University, Adelaide, SA; I Woolley, M Giles, Monash Medical Centre, Clayton, VIC; Dubbo Sexual Health Centre, Dubbo, NSW; P Canavan*, National Association of People Living with HIV/AIDS; C Lawrence*, National Aboriginal Community Controlled Health Organisation; I Zablotska*, National Centre in HIV Social Research, University of NSW, Sydney; B Mulhall*, Department of Public Health and Community Medicine, University of Sydney, Sydney, NSW; M Law*, K Petoumenos*, K Falster*, S Marashi Pour*, C Bendall* National Centre in HIV Epidemiology and Clinical Research, University of NSW, Sydney; NSW. *Steering Committee member during 2007-2008.
The TREAT Asia HIV Observational Database
CV Mean*, V Saphonn* and K Vohith, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia; FJ Zhang*†, HX Zhao and N Han, Beijing Ditan Hospital, Beijing, China; PCK Li*‡ and MP Lee, Queen Elizabeth Hospital, Hong Kong, China; N Kumarasamy* and S Saghayam, YRG Centre for AIDS Research and Education, Chennai, India; S Pujari* and K Joshi, Institute of Infectious Diseases, Pune, India; TP Merati* and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia; S Oka*‡ and M Honda, International Medical Centre of Japan, Tokyo, Japan; JY Choi* and SH Han, Division of Infectious Diseases, Dept. of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea; C KC Lee* and R David, Hospital Sungai Buloh, Kuala Lumpur, Malaysia; A Kamarulzaman* and A Kajindran, University of Malaya, Kuala Lumpur, Malaysia; G Tau*, Port Moresby General Hospital, Port Moresby, Papua New Guinea; R Ditangco* and R Capistrano, Research Institute for Tropical Medicine, Manila, Philippines; YMA Chen*, WW Wong and YW Yang, Taipei Veterans General Hospital and AIDS Prevention and Research Centre, National Yang-Ming University, Taipei, Taiwan; PL Lim*, CC Lee and E Foo, Tan Tock Seng Hospital, Singapore; P Phanuphak*, and M Khongphattanayothing, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand; S Sungkanuparph* and B Piyavong, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; T Sirisanthana* and W Kotarathititum, Research Institute for Health Sciences, Chiang Mai, Thailand; J Chuah*, Gold Coast Sexual Health Clinic, Miami, Queensland, Australia; K Frost*, J Smith* and B Nakornsri, The Foundation for AIDS Research, New York, USA; DA Cooper*, MG Law*, K Petoumenos, R Oyomopito and J Zhou*, National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, Sydney, Australia. * TAHOD Steering Committee member; † Current Steering Committee chair; ‡ co-chair.
Footnotes
CONFLICTS OF INTEREST
MG Law has received research grants, consultancy and/or travel grants from Abbott; Boehringer Ingelheim; Bristol-Myers Squibb; Gilead; GlaxoSmithKline; Janssen-Cilag; Johnson & Johnson; Merck Sharp & Dohme; Pfizer; Roche and CSL Ltd. The other writing committee members have no conflicts of interest to declare.
REFERENCES
- 1.Egger M, Hirschel B, Francioli P, Sudre P, Wirz M, Flepp M, et al. Impact of new antiretroviral combination therapies in HIV infected patients in Switzerland: prospective multicentre study. BMJ. 1997;315:1194–1199. doi: 10.1136/bmj.315.7117.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ewings FM, Bhaskaran K, McLean K, Hawkins D, Fisher M, Fidler S, et al. Survival following HIV infection of a cohort followed up from seroconversion in the UK. AIDS. 2008;22:89–95. doi: 10.1097/QAD.0b013e3282f3915e. [DOI] [PubMed] [Google Scholar]
- 3.Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, Monforte AdA, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 4.Mocroft A, Vella S, Benfield TL, Chiesi A, Miller V, Gargalianos P, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. Lancet. 1998;352:1725–1730. doi: 10.1016/s0140-6736(98)03201-2. [DOI] [PubMed] [Google Scholar]
- 5.Nash D, Katyal M, Shah S. Trends in predictors of death due to HIV-related causes among persons living with AIDS in New York City: 1993-2001. J Urban Health. 2005;82:584–600. doi: 10.1093/jurban/jti123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ormaasen V, Sandvik L, Dudman SG, Bruun JN. HIV related and non-HIV related mortality before and after the introduction of highly active antiretroviral therapy (HAART) in Norway compared to the general population. Scand J Infect Dis. 2007;39:51–57. doi: 10.1080/00365540600904779. [DOI] [PubMed] [Google Scholar]
- 7.Palella FJ, Jr., Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 8.Babiker A, Darbyshire J, Pezzotti P, Porter K, Rezza G, Walker SA, et al. Changes over calendar time in the risk of specific first AIDS-defining events following HIV seroconversion, adjusting for competing risks. Int J Epidemiol. 2002;31:951–958. doi: 10.1093/ije/31.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smit C, Geskus R, Walker S, Sabin C, Coutinho R, Porter K, Prins M. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. AIDS. 2006;20:741–749. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 10.Antiretroviral Therapy Cohort Collaboration Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhaskaran K, Hamouda O, Sannes M, Boufassa F, Johnson AM, Lambert PC, Porter K. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA. 2008;300:51–59. doi: 10.1001/jama.300.1.51. [DOI] [PubMed] [Google Scholar]
- 12.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995-2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 13.Petoumenos K, Law MG. Risk factors and causes of death in the Australian HIV Observational Database. Sex Health. 2006;3:103–112. doi: 10.1071/sh05045. [DOI] [PubMed] [Google Scholar]
- 14.Mocroft A, Brettle R, Kirk O, Blaxhult A, Parkin JM, Antunes F, et al. Changes in the cause of death among HIV positive subjects across Europe: results from the EuroSIDA study. AIDS. 2002;16:1663–1671. doi: 10.1097/00002030-200208160-00012. [DOI] [PubMed] [Google Scholar]
- 15.Pacheco AG, Tuboi SH, Faulhaber JC, Harrison LH, Schechter M. Increase in Non-AIDS Related Conditions as Causes of Death among HIV-Infected Individuals in the HAART Era in Brazil. PLoS ONE. 2008;3:e1531. doi: 10.1371/journal.pone.0001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palella FJ, Jr., Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43:27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 17.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 18.d'Arminio A, Sabin CA, Phillips AN, Reiss P, Weber R, Kirk O, et al. Cardio- and cerebrovascular events in HIV-infected persons. AIDS. 2004;18:1811–1817. doi: 10.1097/00002030-200409030-00010. [DOI] [PubMed] [Google Scholar]
- 19.Fontas E, van Leth F, Sabin CA, Friis-Moller N, Rickenbach M, d'Arminio Monforte A, et al. Lipid profiles in HIV-infected patients receiving combination antiretroviral therapy: are different antiretroviral drugs associated with different lipid profiles? J Infect Dis. 2004;189:1056–1074. doi: 10.1086/381783. [DOI] [PubMed] [Google Scholar]
- 20.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 21.Friis-Moller N, Sabin CA, Weber R, d'Arminio Monforte A, El-Sadr WM, Reiss P, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 22.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 23.Australian HIV Observational Database Rates of combination antiretroviral treatment change in Australia, 1997-2000. HIV Med. 2002;3:28–36. doi: 10.1046/j.1464-2662.2001.00094.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Kumarasamy N, Ditangco R, Kamarulzaman A, Lee CK, Li PC, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38:174–179. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 26.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;446:496–509. [Google Scholar]
- 28.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 29.World Bank . World Bank list of economies (July 2007) 2007. [Google Scholar]
- 30.StataCorp . Stata statistical software: Release 10.0. Stata Corporation; College Station, TX: 2007. [Google Scholar]
- 31.Baker JV, Peng G, Rapkin J, Abrams DI, Silverberg MJ, MacArthur RD, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emery S, Neuhaus JA, Phillips AN, Babiker A, Cohen CJ, Gatell JM, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. J Infect Dis. 2008;197:1133–1144. doi: 10.1086/586713. [DOI] [PubMed] [Google Scholar]
- 33.Lau B, Gange SJ, Moore RD. Risk of non-AIDS-related mortality may exceed risk of AIDS-related mortality among individuals enrolling into care with CD4+ counts greater than 200 cells/mm3. J Acquir Immune Defic Syndr. 2007;44:179–187. doi: 10.1097/01.qai.0000247229.68246.c5. [DOI] [PubMed] [Google Scholar]
- 34.Smith DK, Gardner LI, Phelps R, Hamburger ME, Carpenter C, Klein RS, et al. Mortality rates and causes of death in a cohort of HIV-infected and uninfected women, 1993-1999. J Urban Health. 2003;80:676–688. doi: 10.1093/jurban/jtg074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 36.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 37.Monforte A, Abrams D, Pradier C, Weber R, Reiss P, Bonnet F, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS. 2008;22:2143–2153. doi: 10.1097/QAD.0b013e3283112b77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 39.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 40.WHO. UNICEF. UNAIDS . Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector: Progress report. 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUGGESTED ONLINE SUPPLEMENTARY DATA
Table A1. Crude mortality rates in high and low income settings in the Australian HIV Observational Database (AHOD) and TREAT Asia HIV Observational Database (TAHOD) cohorts