Synopsis
Interaction with the immune system is one of the most well-established non-classical effects of vitamin D. For many years this was considered to be a manifestation of granulomatous diseases such sarcoidosis, where synthesis of active 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) is known to be dysregulated. However, recent reports have supported a role for 1,25(OH)2D3 in mediating normal function of both the innate and adaptive immune systems. Crucially, these effects appear to be mediated via localized autocrine or paracrine synthesis of 1,25(OH)2D3 from precursor 25-hydroxyvitamin D3 (25OHD3), the main circulating metabolite of vitamin D. As such, the ability of vitamin D to influence normal human immunity will be highly dependent on the vitamin D status of individuals, and may lead to aberrant response to infection or autoimmunity in those who are vitamin D-insufficient. The potential health significance of this has been underlined by increasing awareness of impaired vitamin D status in populations across the globe. The following review article will describe in more detail some of the recent developments with respect to vitamin D and the immune system, together with possible clinical implications.
Keywords: vitamin D, CYP27b1, toll-like receptor, macrophage, cathelicidin, regulatory T-cells
Introduction
Historical perspective
Non-classical actions of vitamin D were first recognized thirty years ago when receptors for active 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) were detected in various neoplastic cells lines 23,59. Other studies immediately following this showed that binding of 1,25(OH)2D3 to the vitamin D receptor (VDR) promoted antiproliferative and prodifferentiation responses in cancer cells 1,18, highlighting an entirely new facet of vitamin D action. The spectrum of non-classical responses to vitamin D was then extended to include actions on cells from the immune system 2,13. This interaction was further endorsed by the observation that some patients with the granulomatous disease sarcoidosis present with elevated circulating levels of 1,25(OH)2D3 and associated hypercalcemia 11,72. In these patients the high serum 1,25(OH)2D3 is due to increased activity of the enzyme 25-hydroxyvitamin D-1α-hydrooxylase (1α-hydroxylase). However, in contrast to normal subjects where 1α-hydroxylase is classically localized in the kidney, the increased synthesis of 1,25(OH)2D3 in patients with sarcoidosis involves 1α-hydroxylase activity in disease-associated macrophages 4,6,9. Thus, it was concluded that the immune system had the potential to both synthesize 1,25(OH)2D3 and elicit autocrine or paracrine responses from immune cells expressing the vitamin D receptor 38.
Despite these early advances, the precise nature of the interaction between vitamin D and the immune system remained unresolved for many years. Some pieces of the puzzle were easier to complete than others. For example, it became evident that dysregulation of 1,25(OH)2D3 was not restricted to sarcoidosis but was a common feature of many granulomatous disorders and some forms of cancer 39. Likewise, at least in vitro, it was possible to potently regulate a range of immune cell functions using 1,25(OH)2D3 or its synthetic analogs 35,97. However, the key remaining question concerned whether or not vitamin D could act as a physiological regulator of normal immune responses. Answers to this question began to appear about five years ago and new information on the fundamental nature of vitamin D sufficiency/insufficiency has provided a fresh perspective on non-classical actions of vitamin D. As a consequence, there is now a much broader acceptance that vitamin D plays an active role in regulating specific facets of human immunity. This will be detailed in the following review together with discussion on the possible impact of vitamin D insufficiency and vitamin D supplementation on normal immune function and human disease.
Vitamin D and innate immunity
Macrophages, vitamin D and cathelicidin
Consistent with the earlier seminal observations of extra-renal 1α-hydroxylase activity in patients with sarcoidosis, the effects of vitamin D on macrophage function have been central to many of the new observations implicating vitamin D in the regulation of immune responses. In common with natural killer cells (NK) and cytotoxic T-lymphocytes (cytotoxic T-cells), macrophages and their monocyte precursors play a central role in initial non-specific immune responses to pathogenic organisms or tissue damage – so called cell-mediated immunity. Their role is to phagocytose pathogens or cell debris and then eliminate or assimilate the resulting waste material. In addition, macrophages can interface with the adaptive immune system by utilizing phagocytic material for antigen presentation to T-lymphocytes (T-cells).
For many years, the key action of vitamin D on macrophages was thought to be its ability to stimulate differentiation of precursor monocytes to more mature phagocytic macrophages 1,2,45,93. This concept was supported by observations showing differential expression of VDR and 1α-hydroxylase during the differentiation of human monocytes macrophages 49. The latter report also emphasized early studies showing that normal human macrophages were able to synthesize 1,25(OH)2D3 when stimulated with interferon gamma (IFNγ) 46. Localized activation of vitamin D, coupled with expression of endogenous VDR was strongly suggestive of an autocrine or intracrine system for vitamin D action in normal monocytes/macrophages.
However, confirmation of such a mechanism was only obtained in 2006 when Robert Modlin and colleagues carried out DNA array analyses to define innate immunity genes that were specifically modulated in monocytes by Mycobacterium tuberculosis (M. tb). In a seminal investigation both the VDR and the gene for 1α-hydroxylase (CYP27B1) were shown to be induced following activation of the principal pathogen recognition receptor for M. tb, toll-like receptor 2/1 (TLR2/1) 56. Subsequent experiments confirmed that precursor 25OHD3 was able to induce intracrine VDR responses in monocytes that had been treated with a TLR2/1 activator. In particular, the TLR2/1–25OHD3 combination stimulated expression of the antibacterial protein cathelicidin, so that vitamin D was able to promote monocyte killing of M. tb 56. Notably, the ability to promote expression of the antibacterial protein following a TLR2/1 challenge was directly influenced by the 25OHD3 status of the donor serum used for monocyte culture 56. More recently, we have shown that vitamin D supplementation in vivo can also enhance TLR2/1-induced cathelicidin expression 5. Cathelicidin was identified several years ago as a target for transcriptional regulation by 1,25(OH)2D3-liganded VDR, in that its gene promoter contains a functional vitamin D response element (VDRE) 30,100. Interestingly, this VDRE occurs within a small interchangeable nuclear element (SINE) sequence which only appears to be present in the cathelicidin gene promoter of higher primates, suggesting that vitamin D regulation of this facet of innate immunity is a relatively recent evolutionary development 30.
Recent reports have underlined the importance of cathelicidin as a target for vitamin D but also suggest that this mechanism may be more complex than initially thought. As yet, the precise signal system by which TLR activation induces expression of VDR and 1α-hydroxylase remains unclear. Promoter-reporter analysis of the events involved in transcriptional regulation of CYP27B1 suggest that TLR4-mediated induction of the enzyme involves JAK-STAT, MAP kinase and nuclear factor kappB (NF-κB) pathways, and that these synergize with IFNγ-mediated induction of CYP27B192. However, other studies have proposed that TLR2/1 induction of 1α-hydroxylase occurs indirectly as a consequence of TLR2/1 induced interleukin-15 (IL-15) which is a potent inducer of CYP27B1 and 1α-hydroxylase activity 50. In a similar fashion, interleukin 17A (IL-17A) has been shown to enhance 1,25(OH)2D3-mediated induction of cathelicidin, although this response does not appear to involve transcriptional regulation of 1α-hydroxylase or increased VDR sensitivity 77. One pathway that has been poorly studied in this regard concerns the enzyme 24-hydroxylase, which is conventionally considered to function by inactivating 1,25(OH)2D3. The gene for 24-hydroxylase (CYP24) is potently induced by 25OHD3 following TLR2/1 activation of monocytes 56 but, as yet, it is unclear whether this involves the non-metabolic splice variant form of CYP24 known to be expressed by macrophages 82.
Regulation of the antibacterial protein by 1,25(OH)2D3 has been described for a wide variety of cell types other than macrophages, including keratinocytes 84,85,100, lung epithelial cells 104, myeloid cell lines 30,85,100 and placental trophoblasts 54. In some cases 54,84, this appears to involve an intracrine response similar to that reported for monocytes. However, the mechanisms controlling local synthesis of 1,25(OH)2D3 in these cells vary considerably. In keratinocytes, low baseline expression of 1α-hydroxylase is enhanced following epidermal wounding by transforming growth factor beta (TGFβ) 84. The resulting rise in 1,25(OH)2D3 concentrations upregulates expression of TLR2 and TLR4 by keratinocytes, thereby priming these cells for further innate immune responses to pathogens or tissue damage 84. By contrast, in trophoblasts, induction of cathelicidin and subsequent bacterial killing by 25OHD3 appears to be due to constitutive 1α-hydroxylase activity, which is not further enhanced by TLR activation 54. The latter may be due to the rapid non-immune induction of 1α-hydroxylase and VDR which occurs within the placenta during early gestation 24.
Although most of the studies of vitamin D-mediated innate immunity have focused on the role of 1,25(OH)2D3-bound VDR as a pivotal transcriptional regulator of cathelicidin, it is also important to recognize that other ligands may interact with the VDR 58. For example, recent studies of bilary epithelial cells have shown that cathelicidin expression can be induced in a VDR-dependent fashion by bile salts 19. This provides a mechanism for maintaining bilary sterility, although additive effects of 1,25(OH)2D3 also highlight a novel therapeutic application for vitamin D in the treatment of primary bilary cirrhosis. Conversely, other compounds may act to disrupt normal 1,25(OH)2D3-VDR-mediated immunity. The polycyclic aromatic hydrocarbon benzo(A)pyrene, a prominent product of cigarette smoking, has been shown to attenuate vitamin D-mediated induction of macrophage cathelcidin in a VDR-dependent fashion by stimulating expression of 24-hydroxylase, and vitamin D catabolism 64. The precise mechanism by which this occurs has yet to be determined but these data suggest that some toxic compounds are actively detrimental to vitamin D-mediated immunity.
The observations detailed above show clearly that vitamin D is a potent stimulator of mechanisms associated with pathogen elimination. In subsequent sections the clinical importance of this with respect to vitamin D insufficiency and immune-related diseases is discussed in more detail. However, one key question that immediately arises from the current observations is why there is a need to involve the vitamin D system in the TLR-induction of innate immunity. As previously described, VDR-mediated transcriptional regulation of cathelicidin is a relatively recent evolutionary change and was presumably advantageous when primates (including early Homo sapiens) were exposed to abundant sunlight, thereby priming high serum levels of vitamin D. Other benefits of incorporating vitamin D into innate immune regulation include the fact that it is associated with key feedback control pathways. As already mentioned, vitamin D has its own catabolic enzyme in the form of 24-hydroxylase which sensitively attenuates responses to 1,25(OH)2D3 and, in the case of the CYP24 splice variant, may also attenuate synthesis of this vitamin D metabolite 82. However, vitamin D may also provide feedback regulation of immune activation pathways in that 1,25(OH)2D3 has been shown to potently downregulate expression of monocyte TLR2 and TLR4, thereby suppressing inflammatory responses that are normally activated by these receptors 83. Thus, by utilizing both CYP24 and TLR regulatory mechanisms, vitamin D may help to promote appropriate innate immune responses whilst preventing an over-elaboration of innate immune responses and the tissue damage frequently associated with this.
Dendritic cells and antigen presentation
In addition to the phagocytic acquisition and elimination of pathogens and cell debris, innate immunity also involves the presentation of resultant antigen to cells involved in the adaptive arm of the immune system (see Figure 1). Although several cells are able to do this, the most well-recognized group of professional antigen presenting cells (APCs) are dendritic cells (DCs). Expression of VDR by purified tissue DCs was first reported in 198715. Subsequent studies using populations of DCs isolated from skin (Langerhans cells) provided evidence that 1,25(OH)2D3 could act to attenuate antigen presentation 20. However, it was not until the later advent of in vitro monocyte-derived DC models that the effects of vitamin D metabolites on antigen presentation were fully elucidated. In 2000 parallel studies by the Adorini and Kumar groups showed that 1,25(OH)2D376 and its synthetic analogs 34 inhibited the maturation of monocyte-derived DCs, thereby suppressing their capacity to present antigen to T-cells. Based on these observations, it was proposed that vitamin D could act to promote tolerance and this was endorsed by studies of pancreatic islet transplantation in which lower rejection rates were observed in 1,25(OH)2D3-treated mice 32. Crucially this response to 1,25(OH)2D3 appeared to be due to decreased DC maturation and concomitant enhancement of suppressor or regulatory T-cells (Treg) 32. Further studies have underlined the importance of Treg generation 68 as part of the interaction between vitamin D and the immune system and this is discussed in greater detail in later sections of this review.
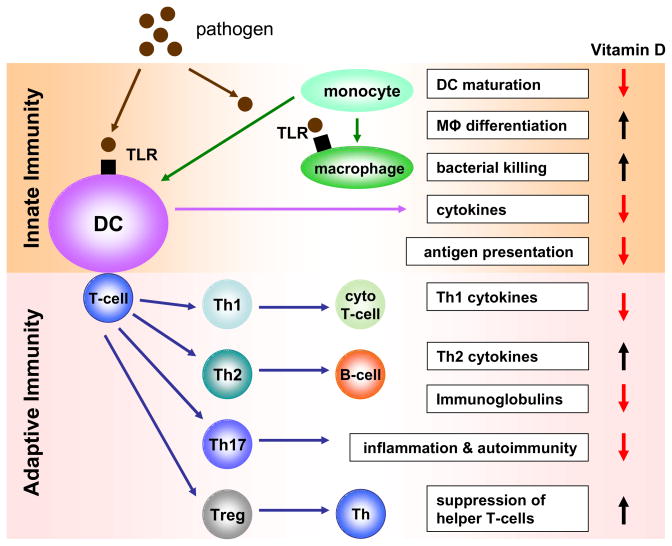
Figure 1. Effects of vitamin D on innate and adaptive immunity.
Schematic representation of the principal innate and adaptive immune responses to a pathogenic challenge, and the positive or negative regulation of these responses by vitamin D. TLR, toll like receptor; DC, dendritic cell, Mφ, macrophage; T-cell, T-lymphocyte; cyto T-cell, cytotoxic T-cell; B-cell, B-lymphocyte; Treg, regulatory T-cell.
Although regulation of DC maturation represents at potential target for 1,25(OH)2D3 and its synthetic analogs as treatment for autoimmune disease and host-graft rejection, another perspective was provided by the observation that DCs express 1α-hydroxylase in a similar fashion to macrophages 25,40. Data from monocyte-derived DCs showed that 1α-hydroxylase expression and activity increases as DCs differentiate towards an a mature phenotype 40. Functional analyses showed that treatment with 25OHD3 suppresses DC maturation and inhibits T-cell proliferation, confirming the existence of an intracrine pathway for vitamin D similar to that observed for macrophages 40. Interestingly, mature DCs showed lower levels of VDR than immature DCs or monocytes 40. This reciprocal organization of 1α-hydroxylase and VDR expression may be advantageous in that mature antigen-presenting DCs may be relatively insensitive to 1,25(OH)2D3, thereby allowing induction of an initial T-cell response. However, the high levels of 1,25(OH)2D3 being synthesized by these cells will be able to act on VDR-expressing immature DCs and thus prevent their further development 41. In this way, paracrine action of locally produced 1,25(OH)2D3 will allow initial presentation of antigen to T-cells whilst preventing continued maturation of DCs and over-stimulation of T-cells.
Although DCs are heterogeneous in terms of their location, phenotype and function, they are broadly divided into two groups based on their origin. Myeloid (mDCs) and plasmacytoid (pDCs) express different types of cytokines and chemokines and appear to exert complementary effects on T-cell responses, with mDCs being the most effective APCs 57 and pDCs being more closely associated with immune tolerance 91. It is therefore interesting to note that 1,25(OH)2D3 preferentially regulates mDCs, suggesting that the key effect of vitamin D in this instance is to suppress activation of naïve T-cells. Although in this study pDCs showed no apparent immune response to 1,25(OH)2D3, this does not preclude a role for vitamin D in the regulation of tolerogenic responses. One possibility is that local, intracrine, synthesis of 1,25(OH)2D3 will be more effective in achieving these responses. Alternatively, 1,25(OH)2D3 synthesized by pDCs may regulate tolerance through paracrine effects on VDR-expressing T-cells. This is discussed in further detail in the following section.
Vitamin D and adaptive immunity
Vitamin D and T-cell function
Resting T-cells express almost undetectable levels of VDR, but levels of the receptor increase as T-cells proliferate following antigenic activation 44,66,78. As a consequence, initial studies of the effects of vitamin D on T-cells focused on the ability of 1,25(OH)2D3 to suppress T-cell proliferation 44,66,78. However, the recognition that CD4+ effector T-cells were capable of considerable phenotypic plasticity, suggested that vitamin D might also influence the phenotype of T-cells. Lemire and colleagues first reported that 1,25(OH)2D3 preferentially inhibited T-helper 1 (Th1) cells which are a subset of CD4+ effector T-cells closely associated with cellular, rather than humoral, immune responses 52. Subsequent studies confirmed this observation and demonstrated that the cytokine profile of 1,25(OH)2D3-treated human T-cells was consistent with Th2 cells, a subset of CD4+ T-cells associated with humoral (antibody)-mediated immunity 14,70. The conclusion from these observations was that vitamin D promotes a T-cell shift from Th1 to Th2 and thus might help to limit the potential tissue damage associated with Th1 cellular immune responses. However, the validity of this generalization was called into question by studies using mouse T-cells in which 1,25(OH)2D3 was shown to inhibit cytokines associated with both Th1 (IFNγ) and Th2 (interleukin-4, IL-4). Subsequent analysis of immune cells from the VDR gene knockout mouse added further confusion by showing that these animals had reduced (rather than the predicted elevated) levels of Th1 cells 69. Thus, whilst in vitro vitamin D appears to broadly support a shift from Th1 to Th2 in CD4+ cells, it seems likely that in vivo its effects on T-cells are more complex.
The T-cell repertoire has continued to expand with the characterization of another effector T-cell lineage distinct from Th1 or Th2 cells, termed Th17 cells because of their capacity to synthesize interleukin-17 (IL-17) 36,101. Th17 cells play an essential role in combating certain pathogens but they have also been linked to tissue damage and inflammation 12,48. The precise role of vitamin D as a regulator of Th17 cells has yet to be fully elucidated but it is interesting to note that studies of animal models of the gastrointestinal inflammatory disease colitis have shown that treatment with 1,25(OH)2D3 reduces expression of IL-1721, whilst loss of 1,25(OH)2D3 as a result of CYP27b1 gene ablation leads to elevated levels of this cytokine 55. Thus, it possible that vitamin D exerts some of its effects on inflammation and autoimmune disease through the regulation of Th17 cells.
A fourth group of CD4+ T-cells, exert suppressor rather than effector functions and are known as regulatory T-cells or Tregs. In view of its early recognition as a suppressor of T-cell proliferation, it was anticipated that vitamin D would have effects on Tregs, and indeed in 2002 O’Garra and colleagues demonstrated that 1,25(OH)2D3, in conjunction with glucocorticoids, potently stimulated the generation of interleukin-10 (IL-10)-producing CD4+/CD25+ Tregs 10. Subsequent reports indicated that 1,25(OH)2D3 alone can induce Tregs 31, and it appears that preferential differentiation of Tregs is a pivotal mechanism linking vitamin D and adaptive immunity, with potential beneficial effects for autoimmune disease and host-graft rejection 33,62,89. This immunosuppressive mechanism is likely to be mediated by the induction of tolerogenic DCs as described in the previous section of the review 7,22,32, but direct effects on T-cells may also be important 95. In this latter study, it was notable that 1,25(OH)2D3 increased both IL-10-secretion and TLR9 expression by Tregs, suggesting a novel link between innate and adaptive immune responses 95.
Relative to the wealth of literature on CD4+ effector cells, our understanding of the effects of vitamin D on CD8+ suppressor T-cells remains somewhat limited. In contrast to CD4+ cells, CD8+ show poor antiproliferative response to 1,25(OH)2D378,98,99. However, VDR expression appears to be abundant in CD8+ cells suggesting that they are still potential targets for 1,25(OH)2D3. Indeed subsequent reports have shown that 1,25(OH)2D3 actively regulates cytokine production by CD8+ cells 103, and can also regulate the proliferation of CD8+ cells following specific immune stimuli 43. Despite this, 1,25(OH)2D3 does not appear to have a significant impact on animal disease models such as experimental autoimmune encephalomyelitis where CD8+ cells have been implicated 65.
Although many of the studies linking 1,25(OH)2D3 with adaptive immunity have focused on changes in T-cell proliferation and phenotype, it is important to recognize that other facets of T-cell function may also be affected by the hormone. In particular recent studies have shown that vitamin D can exert powerful effects on the homing of T-cells to specific tissues. Initial studies suggested that 1,25(OH)2D3 acts to inhibit migration of T-cells to lymph nodes 94. However, more recent reports have demonstrated an active role for vitamin D in promoting homing of T-cells to the skin via upregulation of chemokine receptor 10 (CCR10), the ligand for which, CCL27, is expressed by epidermal keratinocytes 87. Notably this T-cell homing response was induced by 25OHD3 as well as 1,25(OH)2D3 and the author suggested that both DCs and T-cells were possible sources of the local 1α-hydroxylase activity 87. In contrast to its positive effect on epidermal T-cell homing, vitamin D appears to exert a negative effect on chemokines and chemokine receptors associated with the GI tract 87. However, it seems likely that this is will be highly T-cell selective as newer studies using the VDR gene knockout mouse have demonstrated aberrant GI migration of a subset of CD8+ cells, and this effects appears to be closely linked to the increased risk of colitis in VDR knockout mice 105.
Vitamin D and B-cell function
Like T-cells, active but not inactive B-cells express the VDR 79. Consequently, initial studies indicated that 1,25(OH)2D3 could directly regulate B-cell proliferation 86 and immunoglobulin (Ig) production 79. Subsequent work contradicted this, suggesting instead that the ability of 1,25(OH)2D3 to suppress proliferation and immunoglobulin (Ig) production was due to indirect effects mediated via helper T-cells 51. However, more recent reports have demonstrated that 1,25(OH)2D3 does indeed exert direct effects on B cell homeostasis 17. In addition to confirming direct VDR-mediated effects on B cell proliferation and Ig production, this study also highlighted the ability of 1,25(OH)2D3 to inhibit the differentiation of plasma cells and class switched memory cells, suggesting a potential role for vitamin D in B cell-related disorders such as systemic lupus erythamtosus. Notably, expression of CYP27b1 was also detected in B-cells, indicating that B-cells may be capable of autocrine/intracrine responses to vitamin D 17. Indeed, this may be common to lymphocytes in general as CYP27b1 expression has also been detected in T-cells 87.
Vitamin D, the immune system and human health
For many years vitamin D status was defined simply by whether or not the patient in question exhibited symptoms of the bone disease rickets (osteomalacia in adults). However, an entirely new perspective on vitamin D status has arisen from the observation that serum levels of the main circulating form of vitamin D (25OHD3) as high as 75 nM correlate inversely with parathyroid hormone 16. This, has prompted the introduction of a new term - vitamin D ‘insufficiency’ - defined by serum levels of 25OHD3 that are sub-optimal (< 75 nM) but not necessarily rachitic (< 20 nM) 42. Unlike serum concentrations of 1,25(OH)2D3, which are primarily defined by the endocrine regulators of the vitamin D-activating enzyme, 1α-hydroxylase, circulating levels of 25OHD3 are a direct reflection of vitamin D status, which for any given individual will depend on access to vitamin D either through exposure to sunlight or through dietary intake. The net effect of this is that vitamin D status can vary significantly in populations depending on geographical, social or economic factors. As a result of these new parameters for vitamin D status, a consensus statement from the 13th Workshop on Vitamin D concluded that vitamin D insufficiency was a worldwide epidemic. Moreover, recent studies have shown that in the last ten years alone, serum vitamin D levels have on average fallen by 20% 28. The key question now being considered is what is the physiological and clinical impact of global vitamin D insufficiency beyond classical bone diseases such as rickets? Epidemiological studies have highlighted possible links between vitamin D insufficiency and a wide range of human diseases 42. The final section of the review will describe four of the key clinical problems which have been linked to the immunomodulatory properties of vitamin D.
Vitamin D and tuberculosis
The observation that vitamin D acts to promote innate immune responses to TLR-activation by M. tb 56, has provided a new perspective on observations made many decades ago concerning the beneficial effects of UV light exposure on the disease TB. As a consequence this has become the most well studied facet of the interaction between vitamin D and innate immunity 60. Initial studies to assess the effects of 25OHD status on ex vivo macrophage function have shown that supplementation with a single oral dose of 2.5 mg vitamin D enhances the ability of recipient macrophages to combat BCG infection in vitro 61. The potential benefits of vitamin D as treatment for tuberculosis (TB) have been further endorsed by a study which showed that adjunct vitamin D supplementation (0.25 mg vitamin D/day) of TB patients receiving conventional therapy for the disease reduced the time for sputum smear conversion from acid fast bacteria (AFB) positive to AFB-negative status 67. A recent double-blind randomized placebo-controlled trial showed that vitamin D supplementation had no effect on clinical outcomes or mortality amongst TB patients, although it should be emphasized that none of the supplemented patients in this study showed a significant rise in serum vitamin D levels 102.
Vitamin D and multiple sclerosis
Several epidemiology studies have reported association between vitamin D insufficiency and the incidence and/or severity of the autoimmune disease multiple sclerosis (MS) (reviewed in 80. These observations have been supported by analysis of animal models such as the experimental autoimmune encephalomyelitis (EAE) mouse, which shows increased disease severity under dietary vitamin D restriction 88. Conversely administration of 1,25(OH)2D3 to EAE mice confers disease protection through effects on cytokine synthesis and apoptosis of inflammatory cells 75,90. Some effects of 1,25(OH)2D3 on EAE appear to be dependent on IL-10 activity 89.
Vitamin D and type 1 diabetes
In common with MS, published reports suggest that there is a link between vitamin D deficiency and another autoimmune disease, type 1 diabetes (reviewed in 63). Low circulating levels of 25OHD3 have been reported in adolescents at the time of diagnosis of type 1 diabetes 53, and other data have documented the beneficial effects of vitamin D supplementation in protecting against type 1 diabetes 37. Another strand of evidence linking vitamin D with type 1 diabetes stems from the extensive genetic analyses that have explored the physiological impact of inherited variations in the genes for various components of the vitamin D metabolic and signaling system. Previous studies have indicated that some VDR gene haplotypes confer protection against diabetes 81 and more recently this has been expanded to show that genetic variants of the CYP27b1 gene also affect susceptibility to type 1 diabetes 8. Finally, in a similar fashion to animal model studies for MS, in vivo use of the non-obese diabetic (NOD) mouse as a model for type 1 diabetes has shown increased disease severity under conditions of dietary vitamin D restriction 29.
Vitamin D and Crohn’s disease
Several strands of evidence have linked vitamin D to the dysregulated immune responses observed with inflammatory bowel diseases such as Crohn’s disease. Firstly, epidemiology suggests that patients with Crohn’s disease have decreased serum levels of 25OHD373,74,96. Secondly, studies in vivo using various animal models indicate that 1,25(OH)2D3 plays a crucial role in the pathophysiology of experimentally-induced forms of inflammatory bowel disease 26,27,47,55. Finally, expression of 1α-hydroxylase has been detected in the human colon 106, with the vitamin D-activating enzyme being upregulated in disease-affected tissue from patients with Crohn’s disease 3. In the case of the latter, dysregulated colonic expression of 1α-hydroxylase was associated with increased circulating levels of 1,25(OH)2D3 indicating that, as with sarcoidosis, localized synthesis of this vitamin D metabolite can spill-over into the general circulation under conditions of persistent disease 3. Intriguingly, current studies have implicated aberrant innate immune handling of enteric microbiota as an initiator of the adaptive immune damage associated with Crohn’s disease 71. It is thus tempting to speculate that effects of vitamin D on this disease may involve both the activation of innate immunity, together with the suppression of adaptive immunity and associated inflammation.
Conclusions
It is almost thirty years since an interaction between vitamin D and the immune system was first documented. Although this was initially proposed as a non-classical effect of vitamin D associated with granulomatous diseases, our current view is now considerably changed. Recent studies have demonstrated a potential physiological role for vitamin D in regulating normal innate and adaptive immunity. Future studies will now need to focus on the clinical implications of vitamin D-mediated immunity and, in particular, the possible beneficial effects of supplementary vitamin D with respect to infectious and autoimmune diseases.
Acknowledgments
This work was supported by NIH grant RO1AR050626 to M.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe E, Miyaura C, Sakagami H, et al. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981;78:4990. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe E, Miyaura C, Tanaka H, et al. 1 alpha,25-dihydroxyvitamin D3 promotes fusion of mouse alveolar macrophages both by a direct mechanism and by a spleen cell-mediated indirect mechanism. Proc Natl Acad Sci U S A. 1983;80:5583. doi: 10.1073/pnas.80.18.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abreu MT, Kantorovich V, Vasiliauskas EA, et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53:1129. doi: 10.1136/gut.2003.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams JS, Gacad MA. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med. 1985;161:755. doi: 10.1084/jem.161.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams JS, Ren S, Liu PT, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adams JS, Sharma OP, Gacad MA, et al. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adorini L, Penna G, Giarratana N, et al. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J Steroid Biochem Mol Biol. 2004;89–90:437. doi: 10.1016/j.jsbmb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 8.Bailey R, Cooper JD, Zeitels L, et al. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes. 2007 doi: 10.2337/db07-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbour GL, Coburn JW, Slatopolsky E, et al. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med. 1981;305:440. doi: 10.1056/NEJM198108203050807. [DOI] [PubMed] [Google Scholar]
- 10.Barrat FJ, Cua DJ, Boonstra A, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell NH, Stern PH, Pantzer E, et al. Evidence that increased circulating 1 alpha, 25-dihydroxyvitamin D is the probable cause for abnormal calcium metabolism in sarcoidosis. J Clin Invest. 1979;64:218. doi: 10.1172/JCI109442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhalla AK, Amento EP, Serog B, et al. 1,25-Dihydroxyvitamin D3 inhibits antigen-induced T cell activation. J Immunol. 1984;133:1748. [PubMed] [Google Scholar]
- 14.Boonstra A, Barrat FJ, Crain C, et al. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 15.Brennan A, Katz DR, Nunn JD, et al. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61:457. [PMC free article] [PubMed] [Google Scholar]
- 16.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Sims GP, Chen XX, et al. Modulatory effects of 1,25-dihydroxyvitamin d3 on human B cell differentiation. J Immunol. 2007;179:1634. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 18.Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- 19.D’Aldebert E, Biyeyeme Bi Mve MJ, Mergey M, et al. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology. 2009;136:1435. doi: 10.1053/j.gastro.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Dam TN, Moller B, Hindkjaer J, et al. The vitamin D3 analog calcipotriol suppresses the number and antigen-presenting function of Langerhans cells in normal human skin. J Investig Dermatol Symp Proc. 1996;1:72. [PubMed] [Google Scholar]
- 21.Daniel C, Sartory NA, Zahn N, et al. Immune modulatory treatment of TNBS colitis with calcitriol is associated with a change of a Th1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2007 doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 22.Dong X, Bachman LA, Kumar R, et al. Generation of antigen-specific, interleukin-10-producing T-cells using dendritic cell stimulation and steroid hormone conditioning. Transpl Immunol. 2003;11:323. doi: 10.1016/S0966-3274(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 23.Eisman JA, Martin TJ, MacIntyre I, et al. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet. 1979;2:1335. doi: 10.1016/s0140-6736(79)92816-2. [DOI] [PubMed] [Google Scholar]
- 24.Evans KN, Bulmer JN, Kilby MD, et al. Vitamin D and placental-decidual function. J Soc Gynecol Investig. 2004;11:263. doi: 10.1016/j.jsgi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Fritsche J, Mondal K, Ehrnsperger A, et al. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102:3314. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 26.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Froicu M, Weaver V, Wynn TA, et al. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 28.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169:626. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giulietti A, Gysemans C, Stoffels K, et al. Vitamin D deficiency in early life accelerates Type 1 diabetes in non-obese diabetic mice. Diabetologia. 2004;47:451. doi: 10.1007/s00125-004-1329-3. [DOI] [PubMed] [Google Scholar]
- 30.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. Faseb J. 2005;19:1067. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 31.Gorman S, Kuritzky LA, Judge MA, et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 32.Gregori S, Casorati M, Amuchastegui S, et al. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 33.Gregori S, Giarratana N, Smiroldo S, et al. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 34.Griffin MD, Lutz WH, Phan VA, et al. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem Biophys Res Commun. 2000;270:701. doi: 10.1006/bbrc.2000.2490. [DOI] [PubMed] [Google Scholar]
- 35.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 36.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr Opin Immunol. 2006;18:349. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Harris SS. Vitamin D in type 1 diabetes prevention. J Nutr. 2005;135:323. doi: 10.1093/jn/135.2.323. [DOI] [PubMed] [Google Scholar]
- 38.Hewison M. Vitamin D and the immune system. J Endocrinol. 1992;132:173. doi: 10.1677/joe.0.1320173. [DOI] [PubMed] [Google Scholar]
- 39.Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 40.Hewison M, Freeman L, Hughes SV, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 41.Hewison M, Zehnder D, Chakraverty R, et al. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 2004;215:31. doi: 10.1016/j.mce.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 42.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 43.Iho S, Iwamoto K, Kura F, et al. Mechanism in 1,25(OH)2D3-induced suppression of helper/suppressor function of CD4/CD8 cells to immunoglobulin production in B cells. Cell Immunol. 1990;127:12. doi: 10.1016/0008-8749(90)90110-d. [DOI] [PubMed] [Google Scholar]
- 44.Karmali R, Hewison M, Rayment N, et al. 1,25(OH)2D3 regulates c-myc mRNA levels in tonsillar T lymphocytes. Immunology. 1991;74:589. [PMC free article] [PubMed] [Google Scholar]
- 45.Koeffler HP, Amatruda T, Ikekawa N, et al. Induction of macrophage differentiation of human normal and leukemic myeloid stem cells by 1,25-dihydroxyvitamin D3 and its fluorinated analogues. Cancer Res. 1984;44:5624. [PubMed] [Google Scholar]
- 46.Koeffler HP, Reichel H, Bishop JE, et al. gamma-Interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun. 1985;127:596. doi: 10.1016/s0006-291x(85)80202-3. [DOI] [PubMed] [Google Scholar]
- 47.Kong J, Zhang Z, Musch MW, et al. Novel Role of the Vitamin D Receptor in Maintaining the Integrity of the Intestinal Mucosal Barrier. Am J Physiol Gastrointest Liver Physiol. 2007 doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 48.Korn T, Oukka M, Kuchroo V, et al. Th17 cells: effector T cells with inflammatory properties. Semin Immunol. 2007;19:362. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreutz M, Andreesen R, Krause SW, et al. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood. 1993;82:1300. [PubMed] [Google Scholar]
- 50.Krutzik SR, Hewison M, Liu PT, et al. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lemire JM, Adams JS, Sakai R, et al. 1 alpha,25-dihydroxyvitamin D3 suppresses proliferation and immunoglobulin production by normal human peripheral blood mononuclear cells. J Clin Invest. 1984;74:657. doi: 10.1172/JCI111465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemire JM, Archer DC, Beck L, et al. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125:1704S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 53.Littorin B, Blom P, Scholin A, et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS) Diabetologia. 2006;49:2847. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 54.Liu N, Kaplan AT, Low J, et al. Vitamin D Induces Innate Antibacterial Responses in Human Trophoblasts via an Intracrine Pathway. Biol Reprod. 2009;80:398. doi: 10.1095/biolreprod.108.073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu N, Nguyen L, Chun RF, et al. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008;149:4799. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 57.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 58.Makishima M, Lu TT, Xie W, et al. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 59.Manolagas SC, Haussler MR, Deftos LJ. 1,25-dihydroxyvitamin D3 receptors in cancer. Lancet. 1980;1:828. doi: 10.1016/s0140-6736(80)91332-x. [DOI] [PubMed] [Google Scholar]
- 60.Martineau AR, Honecker FU, Wilkinson RJ, et al. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103:793. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 61.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin d enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 62.Mathieu C, Badenhoop K. Vitamin D and type 1 diabetes mellitus: state of the art. Trends Endocrinol Metab. 2005;16:261. doi: 10.1016/j.tem.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Mathieu C, Gysemans C, Giulietti A, et al. Vitamin D and diabetes. Diabetologia. 2005;48:1247. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 64.Matsunawa M, Amano Y, Endo K, et al. The Aryl Hydrocarbon Receptor Activator Benzo[a]pyrene Enhances Vitamin D3 Catabolism in Macrophages. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp044. [DOI] [PubMed] [Google Scholar]
- 65.Meehan TF, DeLuca HF. CD8(+) T cells are not necessary for 1 alpha,25-dihydroxyvitamin D(3) to suppress experimental autoimmune encephalomyelitis in mice. Proc Natl Acad Sci U S A. 2002;99:5557. doi: 10.1073/pnas.082100699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nunn JD, Katz DR, Barker S, et al. Regulation of human tonsillar T-cell proliferation by the active metabolite of vitamin D3. Immunology. 1986;59:479. [PMC free article] [PubMed] [Google Scholar]
- 67.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3. [PubMed] [Google Scholar]
- 68.O’Garra A, Barrat FJ. In vitro generation of IL-10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by Th1- and Th2-inducing cytokines. Immunol Lett. 2003;85:135. doi: 10.1016/s0165-2478(02)00239-0. [DOI] [PubMed] [Google Scholar]
- 69.O’Kelly J, Hisatake J, Hisatake Y, et al. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest. 2002;109:1091. doi: 10.1172/JCI12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Overbergh L, Decallonne B, Waer M, et al. 1alpha,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524–543) Diabetes. 2000;49:1301. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 71.Packey CD, Sartor RB. Commensal bacteria, traditional and opportunistic pathogens, dysbiosis and bacterial killing in inflammatory bowel diseases. Curr Opin Infect Dis. 2009;22:292. doi: 10.1097/QCO.0b013e32832a8a5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Papapoulos SE, Clemens TL, Fraher LJ, et al. 1, 25-dihydroxycholecalciferol in the pathogenesis of the hypercalcaemia of sarcoidosis. Lancet. 1979;1:627. doi: 10.1016/s0140-6736(79)91076-6. [DOI] [PubMed] [Google Scholar]
- 73.Pappa HM, Gordon CM, Saslowsky TM, et al. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118:1950. doi: 10.1542/peds.2006-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pappa HM, Grand RJ, Gordon CM. Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis. 2006;12:1162. doi: 10.1097/01.mib.0000236929.74040.b0. [DOI] [PubMed] [Google Scholar]
- 75.Pedersen LB, Nashold FE, Spach KM, et al. 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by inhibiting chemokine synthesis and monocyte trafficking. J Neurosci Res. 2007;85:2480. doi: 10.1002/jnr.21382. [DOI] [PubMed] [Google Scholar]
- 76.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 77.Peric M, Koglin S, Kim SM, et al. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008;181:8504. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Provvedini DM, Manolagas SC. 1 Alpha,25-dihydroxyvitamin D3 receptor distribution and effects in subpopulations of normal human T lymphocytes. J Clin Endocrinol Metab. 1989;68:774. doi: 10.1210/jcem-68-4-774. [DOI] [PubMed] [Google Scholar]
- 79.Provvedini DM, Tsoukas CD, Deftos LJ, et al. 1 alpha,25-Dihydroxyvitamin D3-binding macromolecules in human B lymphocytes: effects on immunoglobulin production. J Immunol. 1986;136:2734. [PubMed] [Google Scholar]
- 80.Raghuwanshi A, Joshi SS, Christakos S. Vitamin D and multiple sclerosis. J Cell Biochem. 2008;105:338. doi: 10.1002/jcb.21858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramos-Lopez E, Jansen T, Ivaskevicius V, et al. Protection from type 1 diabetes by vitamin D receptor haplotypes. Ann N Y Acad Sci. 2006;1079:327. doi: 10.1196/annals.1375.050. [DOI] [PubMed] [Google Scholar]
- 82.Ren S, Nguyen L, Wu S, et al. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J Biol Chem. 2005;280:20604. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- 83.Sadeghi K, Wessner B, Laggner U, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 84.Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schauber J, Dorschner RA, Yamasaki K, et al. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shiozawa K, Shiozawa S, Shimizu S, et al. 1 alpha,25-dihydroxyvitamin D3 inhibits pokeweed mitogen-stimulated human B-cell activation: an analysis using serum-free culture conditions. Immunology. 1985;56:161. [PMC free article] [PubMed] [Google Scholar]
- 87.Sigmundsdottir H, Pan J, Debes GF, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 88.Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005;175:4119. doi: 10.4049/jimmunol.175.6.4119. [DOI] [PubMed] [Google Scholar]
- 89.Spach KM, Nashold FE, Dittel BN, et al. IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:6030. doi: 10.4049/jimmunol.177.9.6030. [DOI] [PubMed] [Google Scholar]
- 90.Spach KM, Pedersen LB, Nashold FE, et al. Gene expression analysis suggests that 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by stimulating inflammatory cell apoptosis. Physiol Genomics. 2004;18:141. doi: 10.1152/physiolgenomics.00003.2004. [DOI] [PubMed] [Google Scholar]
- 91.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 92.Stoffels K, Overbergh L, Giulietti A, et al. Immune regulation of 25-hydroxyvitamin-d(3)-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 93.Tanaka H, Abe E, Miyaura C, et al. 1 alpha,25-dihydroxyvitamin D3 induces differentiation of human promyelocytic leukemia cells (HL-60) into monocyte-macrophages, but not into granulocytes. Biochem Biophys Res Commun. 1983;117:86. doi: 10.1016/0006-291x(83)91544-9. [DOI] [PubMed] [Google Scholar]
- 94.Topilski I, Flaishon L, Naveh Y, et al. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cells in vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur J Immunol. 2004;34:1068. doi: 10.1002/eji.200324532. [DOI] [PubMed] [Google Scholar]
- 95.Urry Z, Xystrakis E, Richards DF, et al. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J Clin Invest. 2009;119:387. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311. doi: 10.1177/0148607107031004311. [DOI] [PubMed] [Google Scholar]
- 97.Van Etten E, Decallonne B, Verlinden L, et al. Analogs of 1alpha,25-dihydroxyvitamin D3 as pluripotent immunomodulators. J Cell Biochem. 2003;88:223. doi: 10.1002/jcb.10329. [DOI] [PubMed] [Google Scholar]
- 98.Vanham G, Ceuppens JL, Bouillon R. T lymphocytes and their CD4 subset are direct targets for the inhibitory effect of calcitriol. Cell Immunol. 1989;124:320. doi: 10.1016/0008-8749(89)90134-2. [DOI] [PubMed] [Google Scholar]
- 99.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 100.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 101.Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 102.Wejse C, Gomes VF, Rabna P, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179:843. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 103.Willheim M, Thien R, Schrattbauer K, et al. Regulatory effects of 1alpha,25-dihydroxyvitamin D3 on the cytokine production of human peripheral blood lymphocytes. J Clin Endocrinol Metab. 1999;84:3739. doi: 10.1210/jcem.84.10.6054. [DOI] [PubMed] [Google Scholar]
- 104.Yim S, Dhawan P, Ragunath C, et al. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3) J Cyst Fibros. 2007;6:403. doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu S, Bruce D, Froicu M, et al. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci U S A. 2008;105:20834. doi: 10.1073/pnas.0808700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zehnder D, Bland R, Williams MC, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86:888. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]