Abstract
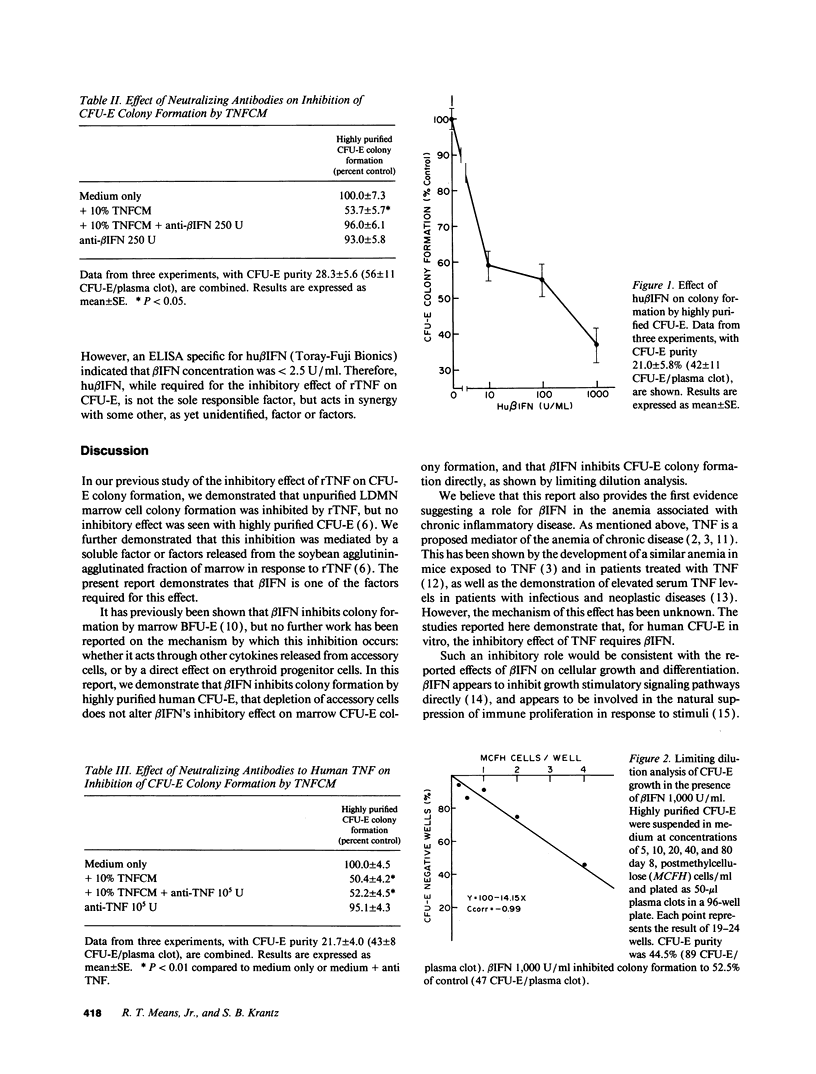
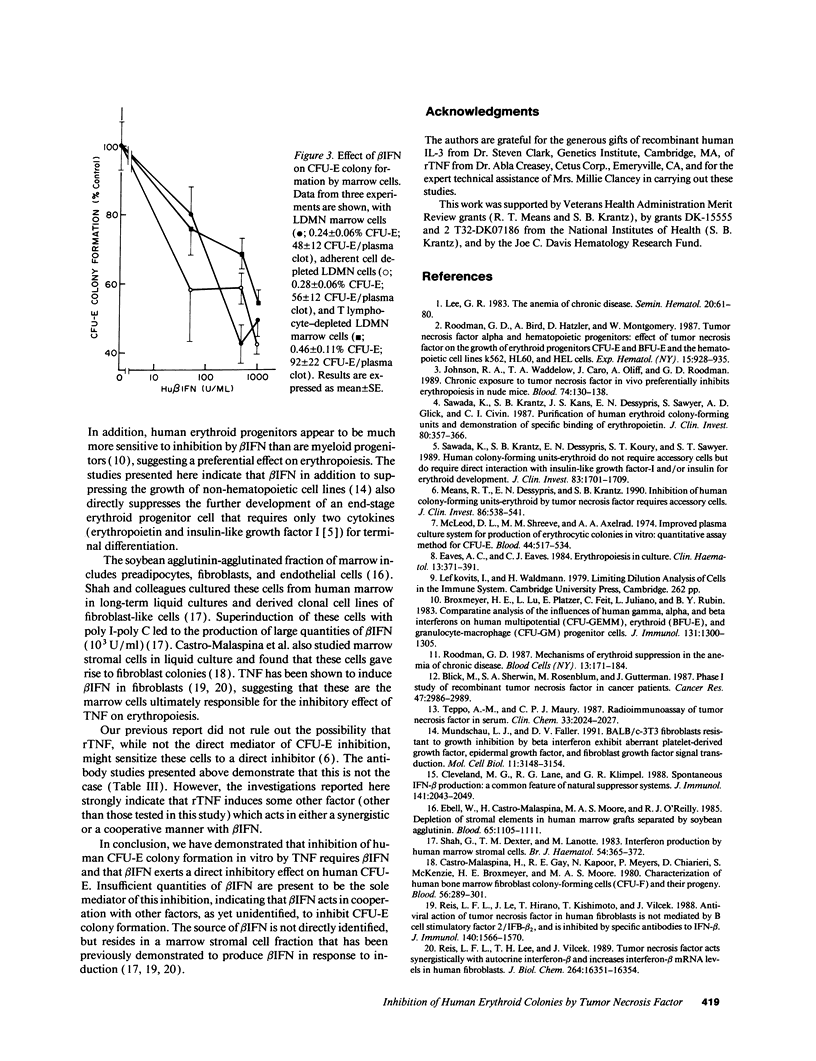
We have previously reported that inhibition of human CFU-erythroid (E) colony formation by tumor necrosis factor (TNF) is an indirect effect mediated by a soluble factor released from a fraction of marrow accessory cells which are predominantly stromal elements (Means, R. T., Jr., E. N. Dessypris, and S. B. Krantz. 1990. J. Clin. Invest. 86:538-541). Further studies reported here identify a mediator of this effect. The inhibitory effect of recombinant TNF on marrow CFU-E is ablated by neutralizing antibodies to human beta IFN, but not by antibodies to gamma IFN or IL-1. Anti-beta IFN also neutralizes the inhibitory effect of conditioned medium prepared from marrow cells exposed to TNF. Human beta IFN inhibits colony formation by unpurified marrow CFU-E as well as highly purified CFU-E generated from peripheral blood progenitors, and limiting dilution analysis shows that this is a direct inhibitory effect. TNF has been implicated in the pathogenesis of the anemia of chronic diseases since blood TNF levels are elevated in many patients with this syndrome, and since exposure to TNF produces a similar anemia in either humans or mice. The present study demonstrates that beta IFN is a required mediator of this inhibitory effect on erythropoiesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blick M., Sherwin S. A., Rosenblum M., Gutterman J. Phase I study of recombinant tumor necrosis factor in cancer patients. Cancer Res. 1987 Jun 1;47(11):2986–2989. [PubMed] [Google Scholar]
- Broxmeyer H. E., Lu L., Platzer E., Feit C., Juliano L., Rubin B. Y. Comparative analysis of the influences of human gamma, alpha and beta interferons on human multipotential (CFU-GEMM), erythroid (BFU-E) and granulocyte-macrophage (CFU-GM) progenitor cells. J Immunol. 1983 Sep;131(3):1300–1305. [PubMed] [Google Scholar]
- Castro-Malaspina H., Gay R. E., Resnick G., Kapoor N., Meyers P., Chiarieri D., McKenzie S., Broxmeyer H. E., Moore M. A. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980 Aug;56(2):289–301. [PubMed] [Google Scholar]
- Cleveland M. G., Lane R. G., Klimpel G. R. Spontaneous IFN-beta production. A common feature of natural suppressor systems. J Immunol. 1988 Sep 15;141(6):2043–2049. [PubMed] [Google Scholar]
- Eaves A. C., Eaves C. J. Erythropoiesis in culture. Clin Haematol. 1984 Jun;13(2):371–391. [PubMed] [Google Scholar]
- Ebell W., Castro-Malaspina H., Moore M. A., O'Reilly R. J. Depletion of stromal cell elements in human marrow grafts separated by soybean agglutinin. Blood. 1985 May;65(5):1105–1111. [PubMed] [Google Scholar]
- Johnson R. A., Waddelow T. A., Caro J., Oliff A., Roodman G. D. Chronic exposure to tumor necrosis factor in vivo preferentially inhibits erythropoiesis in nude mice. Blood. 1989 Jul;74(1):130–138. [PubMed] [Google Scholar]
- Lee G. R. The anemia of chronic disease. Semin Hematol. 1983 Apr;20(2):61–80. [PubMed] [Google Scholar]
- McLeod D. L., Shreeve M. M., Axelrad A. A. Improved plasma culture system for production of erythrocytic colonies in vitro: quantitative assay method for CFU-E. Blood. 1974 Oct;44(4):517–534. [PubMed] [Google Scholar]
- Means R. T., Jr, Dessypris E. N., Krantz S. B. Inhibition of human colony-forming-unit erythroid by tumor necrosis factor requires accessory cells. J Clin Invest. 1990 Aug;86(2):538–541. doi: 10.1172/JCI114741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundschau L. J., Faller D. V. BALB/c-3T3 fibroblasts resistant to growth inhibition by beta interferon exhibit aberrant platelet-derived growth factor, epidermal growth factor, and fibroblast growth factor signal transduction. Mol Cell Biol. 1991 Jun;11(6):3148–3154. doi: 10.1128/mcb.11.6.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis L. F., Ho Lee T., Vilcek J. Tumor necrosis factor acts synergistically with autocrine interferon-beta and increases interferon-beta mRNA levels in human fibroblasts. J Biol Chem. 1989 Oct 5;264(28):16351–16354. [PubMed] [Google Scholar]
- Reis L. F., Le J. M., Hirano T., Kishimoto T., Vilcek J. Antiviral action of tumor necrosis factor in human fibroblasts is not mediated by B cell stimulatory factor 2/IFN-beta 2, and is inhibited by specific antibodies to IFN-beta. J Immunol. 1988 Mar 1;140(5):1566–1570. [PubMed] [Google Scholar]
- Roodman G. D., Bird A., Hutzler D., Montgomery W. Tumor necrosis factor-alpha and hematopoietic progenitors: effects of tumor necrosis factor on the growth of erythroid progenitors CFU-E and BFU-E and the hematopoietic cell lines K562, HL60, and HEL cells. Exp Hematol. 1987 Oct;15(9):928–935. [PubMed] [Google Scholar]
- Roodman G. D. Mechanisms of erythroid suppression in the anemia of chronic disease. Blood Cells. 1987;13(1-2):171–184. [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Dessypris E. N., Koury S. T., Sawyer S. T. Human colony-forming units-erythroid do not require accessory cells, but do require direct interaction with insulin-like growth factor I and/or insulin for erythroid development. J Clin Invest. 1989 May;83(5):1701–1709. doi: 10.1172/JCI114070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K., Krantz S. B., Kans J. S., Dessypris E. N., Sawyer S., Glick A. D., Civin C. I. Purification of human erythroid colony-forming units and demonstration of specific binding of erythropoietin. J Clin Invest. 1987 Aug;80(2):357–366. doi: 10.1172/JCI113080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah G., Dexter T. M., Lanotte M. Interferon production by human marrow stromal cells. Br J Haematol. 1983 Jul;54(3):365–372. doi: 10.1111/j.1365-2141.1983.tb02111.x. [DOI] [PubMed] [Google Scholar]
- Teppo A. M., Maury C. P. Radioimmunoassay of tumor necrosis factor in serum. Clin Chem. 1987 Nov;33(11):2024–2027. [PubMed] [Google Scholar]



