Abstract
Purpose
To investigate the effect of recombinant replication-defective adenovirus containing DN(dominant-negative)Ku70 fragment on the response of tumor cells to multiple small radiation doses. Ultimate goal is to demonstrate the feasibility of using this virus in gene-radiotherapy to enhance the radiation response of tumor cells.
Materials and Methods
Human colorectal HCT8 and HT29 carcinoma cells were plated in glass tubes, infected with virus (25 MOI) and irradiated with single doses or 0-5 doses of 3 Gy with 6 h intervals. Hypoxia was induced by flushing 100% N2. Cells were trypsinized 0 or 6 h after (final) irradiation, and cell survival determined by colony formation. Survival data were fitted to L-Q model or exponential line.
Results
Virus infection enhanced the radiation response of HCT8 and HT29 cells. Virus enhancement ratio (VER) for single dose irradiation at surviving fraction of 0.1 was ~1.3 for both oxic and hypoxic HCT8, and 1.4 and 1.1 for oxic and hypoxic HT29, respectively. Similar VER of 1.2–1.3 was observed for both oxic and hypoxic cells irradiated with multiple doses but these values were smaller than values found for DNKu70-transfected Rat-1 cells. This difference is discussed. The OERs for HCT8 and HT29 receiving fractionated doses were 1.2 and 2.0, respectively, and virus-infection slightly altered them.
Conclusion
Infection of recombinant replication-defective adenovirus containing DNKu70 fragment enhanced the response of human colorectal cancer cells to single and multiple doses.
Keywords: Ku70, dominant-negative Ku70, fractionation, DNA repair, PLD repair, gene-radiotherapy
INTRODUCTION
DNA double strand break (DSB) is a major lethal damage induced by ionizing radiation in mammalian cells. DNA non-homologous end-joining is a principal repair pathway for the DNA-DSB and essential for the survival of irradiated cells (1–3). A major participant in this pathway is the DNA dependent protein kinase (DNA-PK) complex, which consists of two components: a 450 kDa catalytic subunit, DNA-PKcs, and a heterodimeric protein, Ku. The latter consists of two tightly associated polypeptides of 70 and 80 kDa (Ku70 and Ku80, respectively), and has double-strand DNA end-binding activity, thereby targeting the complex to DNA ends (4, 5). As revealed by studies in past decades, an absence of or a defect in the DNA-PK subunit results in deficiency in DNA-DSB repair with enhanced radiation response (6–8). Of significance to radiation biology and oncology is that targeting compartment(s) of the DNA-PK complex could inhibit repair of the radiation-induced DNA-DSB, enhancing radiation response of tumor cells.
Our research group has been investigating the potential of inhibiting the function of DNA-PK complex to improve radiotherapy, and recently extending to gene-radiotherapy in preclinical research (9–11). Data obtained from structure and function analyses of Ku70/80 (1–3) have led to a hypothesis that a construct with a deletion of the N-terminal region of Ku70 might be a candidate for the use in gene-radiotherapy. This construct was shown to have a dominant-negative activity (designated as DNKu70), and when stably transduced in Rat-1 cells and U-87 human glioblastoma cells, resulted in increased radiation sensitivity under both well-oxygenated and oxygen-deficient conditions (9). Importantly, the radiation enhancement in Rat-1 fibroblasts transfected with DNKu70 fragment; DNKu70 cells, was greater at a small radiation dose such as 3 Gy than at a large dose (11). Survival curve analysis using linear-quadratic (L-Q) model demonstrated a larger α/β ratio with an increased α value for both oxic and hypoxic DNKu70 cells compared to parental cells, suggesting a decreased repair capability in DNku70 cells. Interestingly, enhancement ratios observed following multiple small doses was greater than those obtained for single doses.
Clinical gene-radiotherapy requires introduction of the effector cDNA into tumors with targeting vectors. We have constructed the recombinant replication-deficient adenovirus containing the DNKu70 fragment, infected human colorectal carcinoma cells with them, and demonstrated that adenovirus-mediated expression of the DNKu70 fragment enhanced the response of cells to single radiation dose (9, 10). This study further investigates the effect of this virus-infection on the response to fractionated small doses and of delayed trypsinization of human colorectal carcinoma cells.
MATERIALS AND METHODS
Cell Culture and DNKu70 containing recombinant adenovirus
Human colorectal HCT8 and HT29 adenocarcinoma cells (ATCC, Manassas, VA) were used. HCT8 cells with wild type p53 and HT29 cells with mutated p53 were maintained in RPMI medium 1640 and McCoy’s 5a medium (Mediatech, Herndon, VA), respectively, each supplemented with 10% fetal calf serum (Gemini Bio-Product, West Sacramento, CA) and antibiotics (1% penicillin-streptomycin; Mediatech). Doubling times of exponentially proliferating HCT8 and HT29 cells were 18.3 and 16.7 h, respectively.
Construction of recombinant adenovirus containing a DNKu70 fragment has been described (9). In this study, a virus dose of 25 MOI (multiplicity of infection) was added 24–48 hours after cell plating and treatment was initiated 48 h thereafter unless otherwise stated. In previous study and in a pilot experiment, 50 MOI was given to HCT8 cells, but caused division delay and prolonged cell cycle time, but 25 MOI did not cause such effect. A strong expression of DNKu70 proteins was observed on the Western blot 2 days after infecting 25 MOI into HCT8 cells.
Irradiation under oxic and hypoxic conditions
As previously described (11), cells were irradiated with a Cs-137 unit at a dose rate of ~2.0 Gy/min. For both oxic and hypoxic irradiations, cells were plated in glass tubes (tube-type flasks with plating surface of ~5 cm2). To make cells hypoxic, medium was replaced with the same medium containing HEPES (25 mM) ~30 min before irradiation. Tubes were tightly shielded with rubber stoppers, connected with vinyl tubing and 100% N2 gas was flushed for 15 min at a flow rate of 1.5 l/min, and irradiated ~10 min later. Either immediately or 6 h after irradiation, stoppers were removed and the cells prepared for survival assays.
Single dose irradiation and survival assay
To determine the cell survival following irradiations, 3 days colonies (3 days after cell plating) were irradiated, trypsinized and plated for colony formation assay as previously describes (11).
Exponentially proliferating cells were trypsinized and counted. Thirty thousands cells were plated in each tube, and incubated for 3 days. During this incubation cells formed colonies, the majority of which contained >30 cells; thus they were named 3 days colonies. Cells were irradiated under oxic or hypoxic conditions as described above. Following a single irradiation, cells were trypsinized either immediately or 6 h post-irradiation; then counted, plated in 60 mm diameter Petri dishes with 105 heavily irradiated cells and incubated in a humidified incubator with 5% CO2 gas flow for 9–14 days. Colonies were stained and counted. Surviving fractions (SF) were calculated by dividing the number of colonies counted by the number of cells plated [PE (test)] and then corrected by plating efficiency of control cells [PE (control)].
Multiple radiation doses
For the multiple irradiations, three days colonies were irradiated with 0 to 5 doses of 3 Gy each (total 0–15 Gy) with 6 h intervals. The number of cells at the first irradiation was ~5×105. Six h after the last fraction, cells were trypsinized, counted and plated for colony formation. Between the irradiations, cells were kept in a 37°C incubator. For the hypoxic irradiation, cells were kept hypoxic until trypsinization. Throughout the fractionation, medium pH had been maintained above 7.0.
In previous study on DNKu70 cells, we noticed changes in the population size that were due to cell proliferation or loss during 0–30 h of multiple doses. Thus, the SF was further corrected by this population change; i.e., the SF [PE (test)/PE (control)] was multiplied by the cell ratio [number of cells (test)/number of cells (control)] where the latter indicates the change in cell number. Notably, this ratio equals to “clonogen ratio” (11). This concept was also applied in this study.
Survival curve analysis, and calculation of virus enhancement ratio (VER) and oxygen enhancement ratio (OER)
Survival curves following single dose irradiation were fitted to L-Q model, and α and β values were calculated. The exponential regression analysis was used to fit the cell survival curve following multiple doses and D0 (the dose to reduce survival by a factor of 1/e) was calculated. These analyses were made using commercially available software.
VER and OER were obtained at various survival levels. Radiation doses to reduce survival to given levels were calculated, and VER and OER were obtained as a ratio of these doses.
RESULTS
Single Radiation Dose
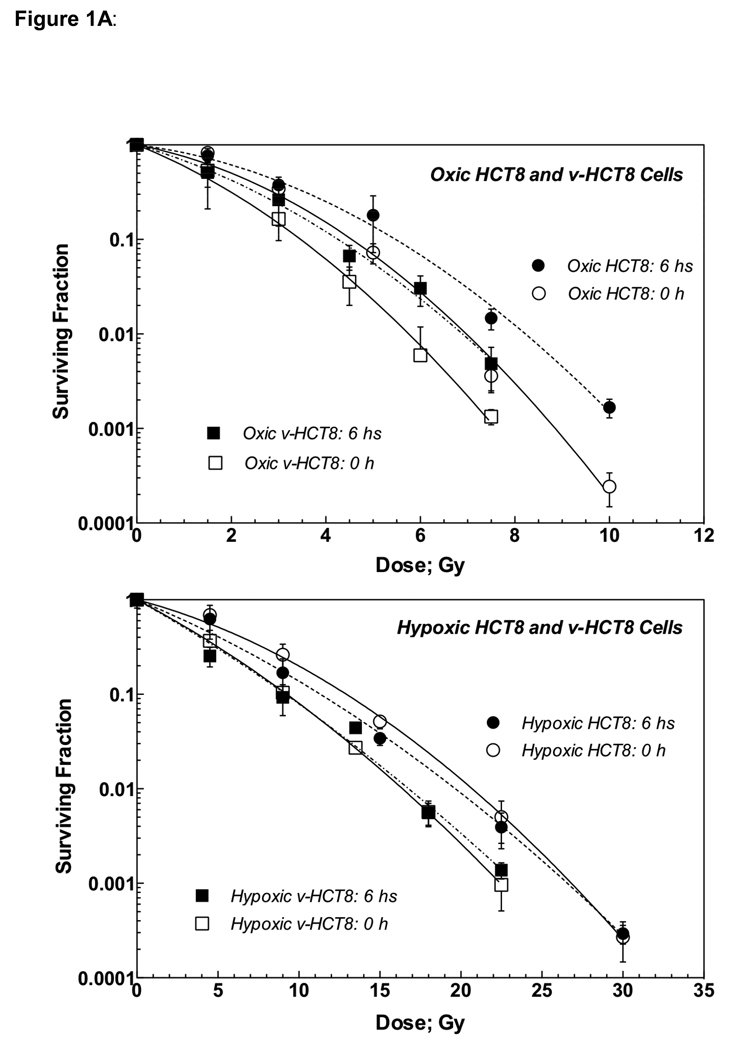
Figure 1A shows radiation survival curves for HCT8 (circles) and virus-infected HCT8 cells (v-HCT8; squares) irradiated under oxic (upper panel) and hypoxic (lower) conditions. Open and solid symbols indicate cells trypsinized 0 or 6 h post-irradiation. Survival curves for v-HCT8 were steeper than those for HCT8 regardless of irradiation condition and timing of trypsinization, indicating that virus-infection enhanced the radiation response of HCT8 cells. Both oxic HCT8 and v-HCT8 cells trypsinized 6 h post-irradiation were more resistant to radiation than cells trypsinized immediately after irradiation, suggestive of PLD (potentially lethal damage) repair. This was not observed in cells irradiated under hypoxic conditions.
Figure 1.
Figure 1A: Radiation dose-cell survival curves of parental HCT8 and v-HCT8 (DNKu70 virus-infected) cells irradiated under aerobic (upper) and hypoxic (lower) conditions in vitro. Open symbols with solid lines and solid symbols with dotted lines indicate cells trypsinized immediately and 6 h post-irradiation, respectively. Circles and squares show HCT8 and v-HCT8, respectively.
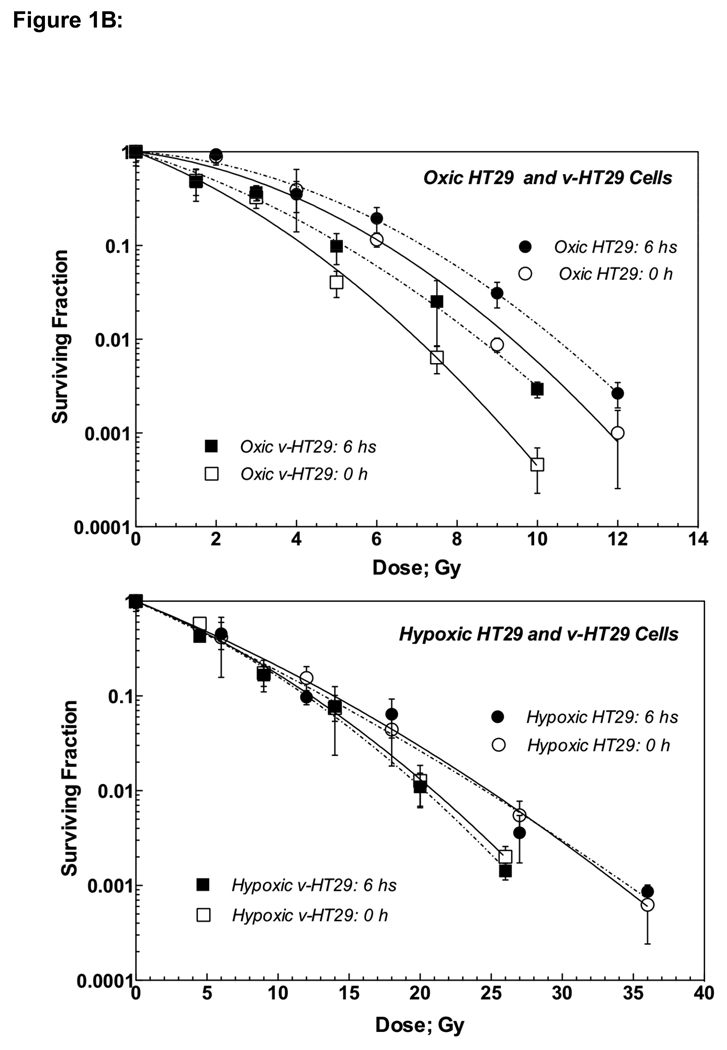
Figure 1B: Radiation dose-cell survival curves of parental HT29 and v-HT29 (DNKu70 virus-infected) cells irradiated under aerobic (upper) and hypoxic (lower) conditions in vitro. Open symbols with solid lines and solid symbols with dotted lines indicate cells trypsinized immediately and 6 h post-irradiation, respectively. Circles and squares show HT29 and v-HT29, respectively.
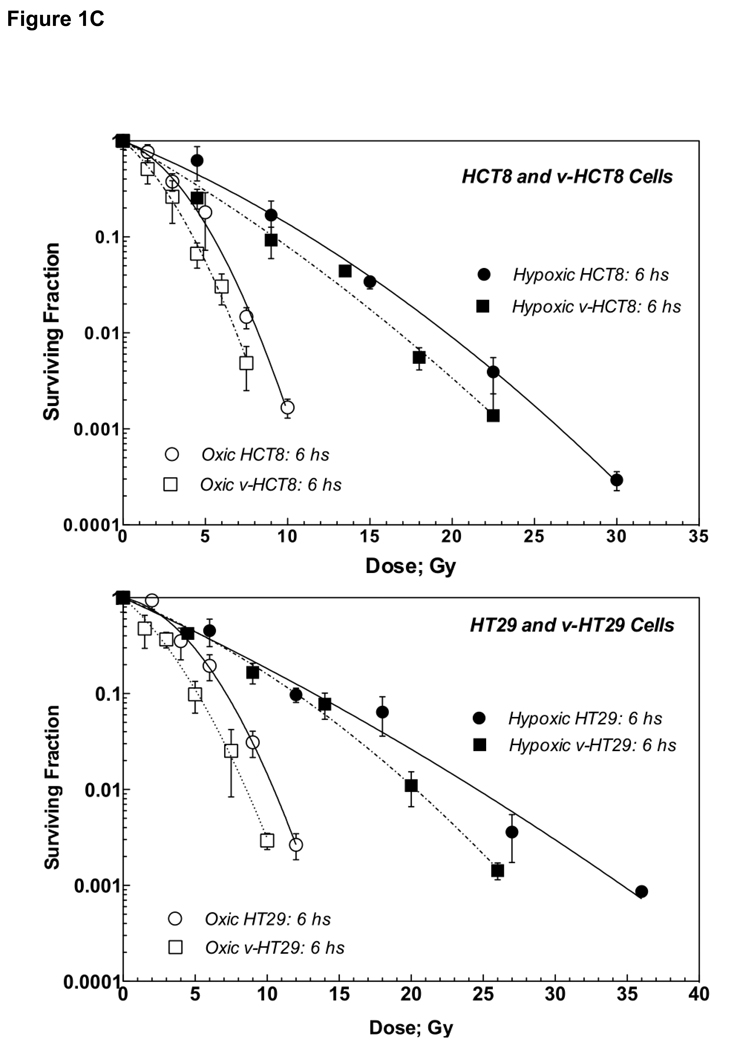
Figure 1C: Survival curves of cells irradiated under aerobic and hypoxic conditions in vitro shown together for comparison. Cells trypsinized 6 h after irradiation is shown. Upper panel shows HCT8 and v-HCT8, and lower panel HT29 and v-HT29 (DNKu70 virus-infected) cells. Open and solid symbols indicate cells irradiated under aerobic and hypoxic conditions, respectively. Circles and squares symbols indicate parental and virus-infected, respectively.
Alpha and beta values and α/β ratios are shown in Table 1A together with VER and OER at different survival levels. Notably, the α/β ratios were larger for v-HCT8 than for HCT8 due to an increase in the α value rather than a decrease in the β value, suggesting a decreased repair capability in v-HCT cells.
Table 1.
| Table 1A: α, β, α/β, OER (oxygen enhancement ratio) and VER (virus enhancement ratio) for HCT8 cells infected with recombinant adenovirus containing DNKu70 fragment. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| HCT8 Cells | α ± SE | β ± SE | α/β ± SE | VER at SF of | OER at SF of | ||||
| 0.5 | 0.1 | 0.01 | 0.5 | 0.1 | 0.01 | ||||
| Cont. (O)-0h | −0.216 ± 0.078 | −0.0637 ± 0.0092 | 3.39 ± 1.33 | ||||||
| Cont. (O)-6h | −0.142 ± 0.059 | −0.0508 ± 0.0069 | 2.79 ± 1.22 | ||||||
| Virus (O)-0h | −0.458 ± 0.067 | −0.0594 ± 0.0107 | 7.71 ± 1.80 | 1.55 | 1.31 | 1.21 | |||
| Virus (O)-6h | −0.333 ± 0.068 | −0.0486 ± 0.0107 | 6.86 ± 2.06 | 1.53 | 1.29 | 1.19 | |||
| Cont. (H)-0h | −0.104 ± 0.012 | −0.00572 ± 0.00047 | 18.23 ± 2.58 | 2.57 | 2.84 | 2.97 | |||
| Cont. (H)-6h | −0.161 ± 0.017 | −0.00372 ± 0.00065 | 43.33 ± 8.77 | 1.55 | 2.07 | 2.39 | |||
| Virus (H)-0h | −0.207 ± 0.003 | −0.00449 ± 0.00016 | 46.15 ± 1.76 | 1.65 | 1.40 | 1.26 | 2.42 | 2.67 | 2.85 |
| Virus (H)-6h | −0.222 ± 0.036 | −0.00310 ± 0.00189 | 71.72 ± 45.26 | 1.32 | 1.23 | 1.17 | 1.79 | 2.15 | 2.44 |
| Table 1B: α, β, α/β, OER (oxygen enhancement ratio) and VER (virus enhancement ratio) for HT29 cells infected with recombinant adenovirus containing DNKu70 fragment. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| HT29 Cells | α ± SE | β ± SE | α/β ± SE | VER at SF of | OER at SF of | ||||
| 0.5 | 0.1 | 0.01 | 0.5 | 0.1 | 0.01 | ||||
| Cont. (O)-0h | −0.125 ± 0.068 | −0.0391 ± 0.0067 | 3.19 ± 1.82 | ||||||
| Cont. (O)-6h | −0.067 ± 0.033 | −0.0356 ± 0.0033 | 1.89 ± 0.95 | ||||||
| Virus (O)-0h | −0.385 ± 0.069 | −0.0387 ± 0.0081 | 9.95 ± 2.74 | 1.87 | 1.48 | 1.34 | |||
| Virus (O)-6h | −0.302 ± 0.046 | −0.0275 ± 0.0054 | 10.99 ± 2.73 | 1.83 | 1.38 | 1.22 | |||
| Cont. (H)-0h | −0.139 ± 0.005 | −0.00186 ± 0.00015 | 75.09 ± 6.62 | 1.61 | 2.23 | 2.65 | |||
| Cont. (H)-6h | −0.158 ± 0.025 | −0.00119 ± 0.00114 | 133.31 ± 111.1 | 1.19 | 1.85 | 2.35 | |||
| Virus (H)-0h | −0.139 ± 0.014 | −0.00384 ± 0.00064 | 36.22 ± 7.05 | 1.06 | 1.13 | 1.19 | 2.85 | 2.93 | 2.99 |
| Virus (H)-6h | −0.142 ± 0.025 | −0.00418 ± 0.00115 | 33.86 ± 9.30 | 0.98 | 1.10 | 1.21 | 2.23 | 2.32 | 2.37 |
Figure 1B shows radiation survival curves for HT29 (circles) and v-HT29 (squares) irradiated under oxic (upper panel) and hypoxic (lower) conditions. Open and solid symbols indicate cells trypsinized 0 and 6 h post-irradiation, respectively. The survival curves of oxic v-HT29 cells were steeper than those of HT29 regardless timing of trypsinization, indicating virus-enhanced radiation response. On the other hand, hypoxic v-HT29 cells were more sensitive than HT29 only at high doses. Similar to HCT8, both HT29 and v-HT29 required oxygen to undertake PLD repair.
Table 1B lists α, β and α/β for HT29 and v-HT29 cells together with VER and OER at different survival levels. Similar to HCT8 and v-HCT8, the α and α/β of oxic v-HT29 were greater than those for oxic HCT8. However, α/β ratios were smaller for hypoxic v-HT29 than for hypoxic HT29 with an increase in β.
The VERs for both v-HCT8 and v-HT29 cells were generally greater at higher survival levels and decreased with a decreasing survival though this was no true for hypoxic v-HT29 cells. This suggests that a greater VER could be obtained following multiple small doses (except hypoxic v-HT29). Conversely, the OER was greater at lower survival levels, suggesting low OER following multiple small doses. Survival curves for all test cells trypsinized 6 h after irradiation are shown in Figure 1C for easy comparison.
Multiple Radiation Doses
To investigate the effect of multiple small doses, cells were left oxic or made hypoxic and received 0–5 doses of 3 Gy each with 6 h intervals. Six hours after the final dose (e.g., for cells received two 3 Gy doses, 6 h after the second 3 Gy dose), cells were trypsinized and survivals determined by colony formation.
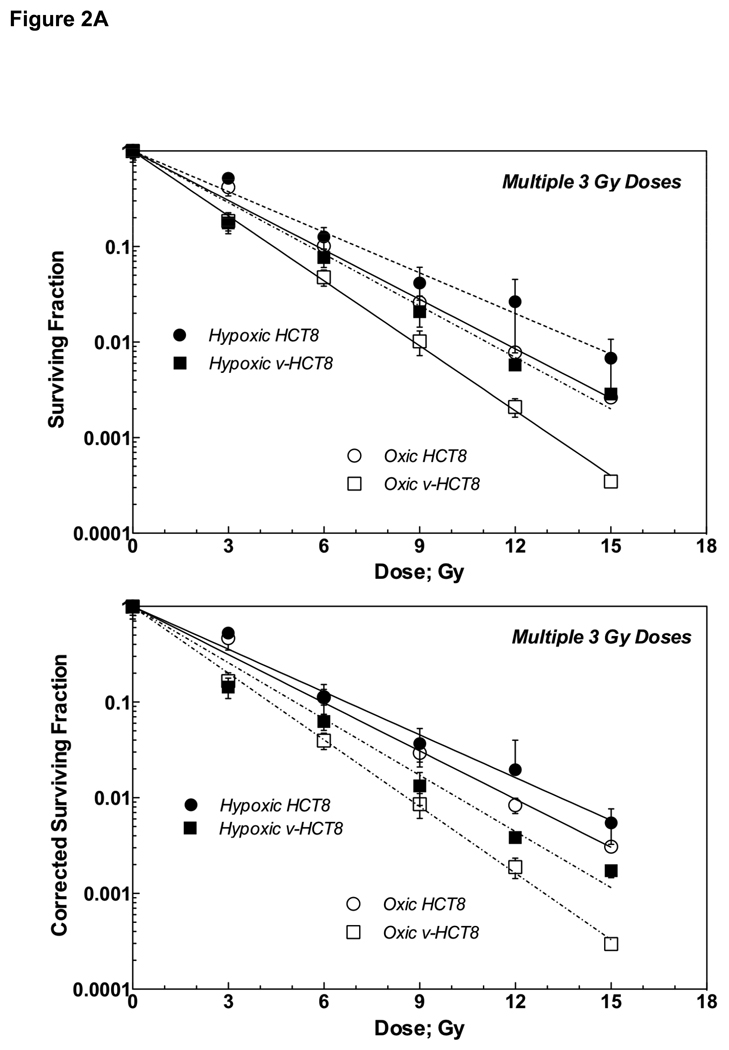
As shown in Figure 2A upper panel, exponential survival curves for both oxic and hypoxic v-HCT8 cells were steeper than the corresponding curves for HCT8 cells, indicative virus-enhanced radiation response. The slope of each survival curve was expressed by D0, and the VER and OER were calculated as D0 ratios. The VERs for oxic and hypoxic cells were 1.31 and 1.27, respectively. Of interest were the small OER values of 1.22 and 1.26 for HCT8 and v-HCT8 cells, respectively (Table 2A).
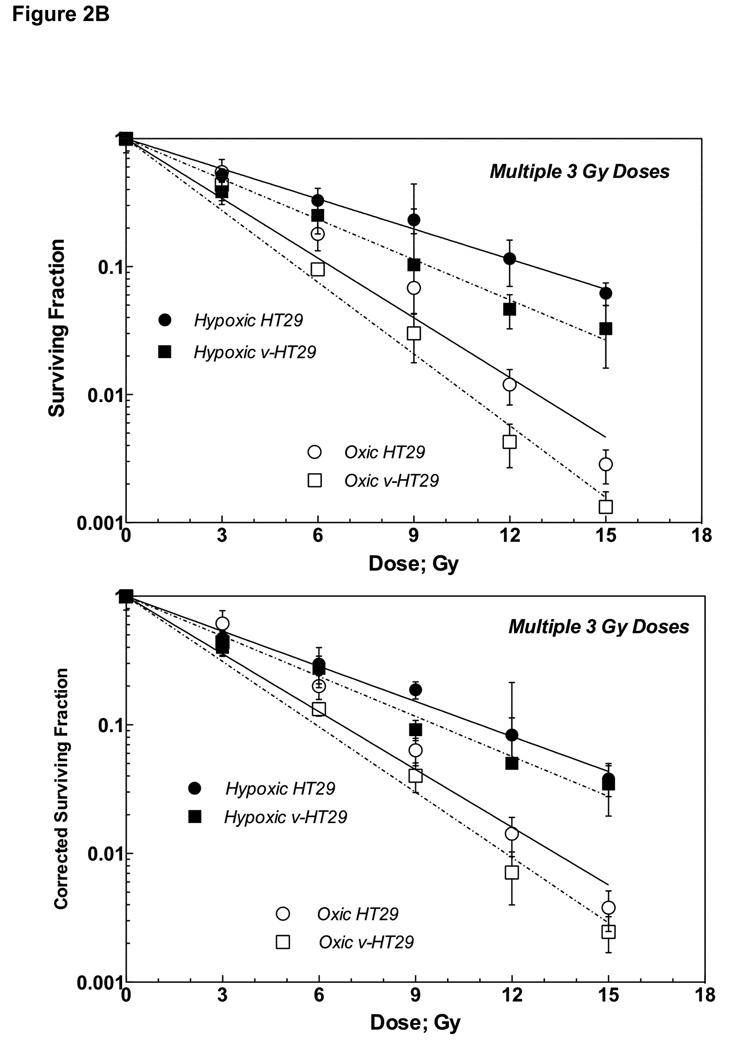
Figure 2.
Figure 2A: Survival curves (upper) and corrected survival curves (lower) of parental HCT8 and v-HCT8 (DNKu70 virus-infected) cells following 0–5 doses of 3 Gy each given under aerobic (open symbols) or hypoxic (closed symbols) conditions in vitro. Circles and squares symbols indicate parental HCT8 and v-HCT8 (DNKu70 virus-infected), respectively.
Figure 2B: Survival curves (upper) and corrected survival curves (lower) of parental HT29 and v-HT29 (DNKu70 virus-infected) cells following 0–5 doses of 3 Gy each given under aerobic (open symbols) or hypoxic (solid symbols) conditions in vitro. Circles and squares symbols indicate parental HT29 and v-HT29 (DNKu70 virus-infected), respectively.
Table 2.
| Table 2A: D0, VER (virus enhancement ratio) and OER (oxygen enhancement ratio) for HCT8 cells treated with fractionated radiation doses. | |||
|---|---|---|---|
| Non-corrected Survival Curve | |||
| Cells | D0 ± SE | OER ± SE | VER ± SE |
| Control (Oxic) | 2.52 ± 0.05 | ||
| Control (Hypoxic) | 3.07 ± 0.10 | 1.22 ± 0.05 | |
| Virus (Oxic) | 1.92 ± 0.02 | 1.31 ± 0.03 | |
| Virus (Hypoxic) | 2.41 ± 0.08 | 1.26 ± 0.04 | 1.27 ± 0.06 |
| Corrected Survival Curve | |||
| Cells | D0 ± SE | OER ± SE | VER ± SE |
| Control (Oxic) | 2.59 ± 0.06 | ||
| Control (Hypoxic) | 2.91 ± 0.09 | 1.13 ± 0.13 | |
| Virus (Oxic) | 1.87 ± 0.02 | 1.38 ± 0.16 | |
| Virus (Hypoxic) | 2.22 ± 0.08 | 1.19 ± 0.17 | 1.31 ± 0.19 |
| Table 2B: D0, VER (virus enhancement ratio) and OER (oxygen enhancement ratio) for HT29 cells treated with fractionated radiation doses. | |||
|---|---|---|---|
| Non-corrected Survival Curve | |||
| Cells | D0 ± SE | OER ± SE | VER ± SE |
| Control (Oxic) | 2.79 ± 0.16 | ||
| Control (Hypoxic) | 5.54 ± 0.14 | 1.98 ± 0.05 | |
| Virus (Oxic) | 2.32 ± 0.08 | 1.20 ± 0.08 | |
| Virus (Hypoxic) | 4.14 ± 0.13 | 1.78 ± 0.04 | 1.34 ± 0.05 |
| Corrected Survival Curve | |||
| Cells | D0 ± SE | OER ± SE | VER ± SE |
| Control (Oxic) | 2.90 ± 0.16 | ||
| Control (Hypoxic) | 4.78 ± 0.01 | 1.64 ± 0.13 | |
| Virus (Oxic) | 2.57 ± 0.01 | 1.13 ± 0.07 | |
| Virus (Hypoxic) | 4.19 ± 0.01 | 1.63 ± 0.01 | 1.14 ± 0.05 |
Figure 2A lower panel shows corrected survivals and Table 2A bottom half lists the values of corresponding parameters. They indicate no significant cell proliferation or loss during the total treatment length of 30 h.
Like HCT8 and v-HCT8, both oxic and hypoxic v-HT29 cells showed steeper survival curves compared to oxic and hypoxic HT29 cells, indicative of virus-enhanced radiation response (Figure 2B upper panel). The VER values for oxic and hypoxic cells were 1.20 and 1.34, and OER values for HT29 and v-HT29 were 1.98 and 1.78, respectively (Table 2B).
The lower panels in Figure 2B and Table 2B show the corrected survival curves and the corresponding parameter values, respectively. No significant changes in D0 values were observed except that of hypoxic HT29, but both VER and OER were slightly decreased, compared to the uncorrected data.
Virus Infection with Higher MOI
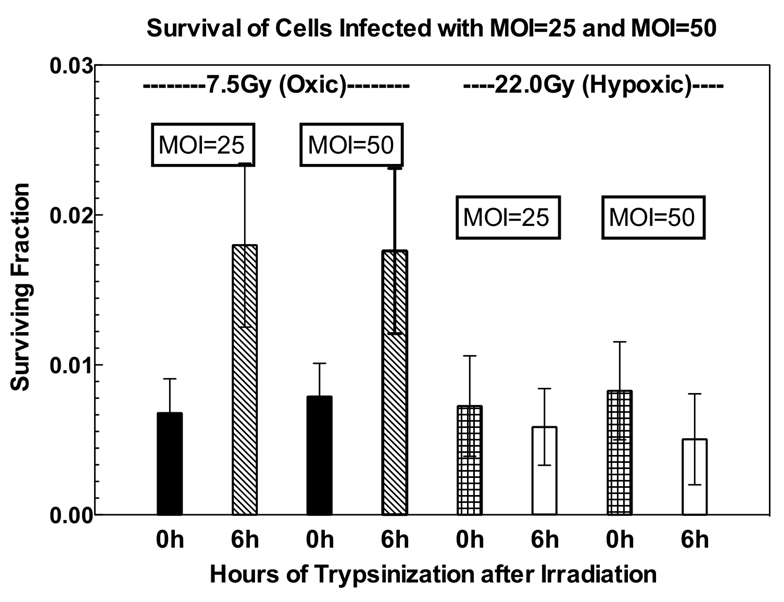
We had previously performed similar studies using DNKu70-transfected Rat-1 fibroblast; DNKu70 cells (11). Comparison of the present results with those of this previous study showed two distinct differences. First, virus infected cells were able to repair PLD, though this was completely absent in DNKu70-transfected cells. Second, in the previous study, the OER was lower for DNKu70-transfected cells in a fractionation regime, compared to the untransfected controls. Since it was possible that our protocol might only be partially inhibiting Ku70, we tested the effect of higher MOI on HT29. In addition, time interval between virus infection and irradiation was prolonged to three days to ensure complete expression of DNKu70 proteins. HT29 cells were infected with 50 MOI and irradiated with a 7.5 Gy or a 22 Gy under oxygenated or hypoxic conditions, respectively. Figure 3 demonstrates insignificant differences in SFs between cells received 25 and those received 50 MOI. Cells infected with 50 MOI equally exhibited PLD repair.
Figure 3.
Surviving fractions of v-HT29 cells irradiated 7.5 Gy or 22.0 Gy under aerobic or hypoxic conditions. HT29 ells were infected with 25 or 50 MOI and irradiated 3 days later. Cells were trypsinized immediately (0h) or 6 hours (6h) after irradiation for survival assay.
DISCUSSION
This study demonstrated that the infection of recombinant adenovirus containing a DNKu70 fragment into the human colorectal carcinoma HT29 cells enhanced the response to ionizing radiation, and also confirmed our previous finding of this virus-enhanced radiation response of HCT8 cells (9). Previous studies also showed that DNKu70-transfection enhanced the radiation response of human glioblastoma cells and rat fibroblasts (9, 11). L-Q model analysis suggested that this enhancement was due to an increase in the α value that led to a larger α/β ratio relative to that of the parental cells (11). This increased α value was also observed in this study in both oxic and hypoxic v-HCT8 and in oxic v-HT29 cells. It has been well-documented that late-responding normal tissue with a large repair capability is characterized by a small α/β ratio with a small α value, while early-responding normal tissue with a small repair capability by a large α/β ratio with a large α value (12). An increase in the α observed in our studies likely indicates an increase in the non-repairable damage in cells infected with DNKu70-carrying virus and in cells transfected with DNKu70. As a result, VER is greater at high survivals and decreases with decreasing survival (Table 1).
An exception from this observation was the survival curves of hypoxic v-HT29 cells that showed smaller α/β and larger β than hypoxic HT29 (Tables 1B). This led to an increased VER with decreasing survival. This cause is unknown but a possible explanation may be in the different status of p53. Adenovirus-mediated wild-type p53 was shown to sensitize tumor cells by inhibiting DNA repair (13). To check this possibility, radiation survival curves of U-87 MG (malignant glioblastoma) and HCT8 cells infected with 50 MOI DNKu70-virus in our previous study (9) were fitted to L-Q model (Table 3). The radiation response of U-87 with wild-type p53 was strongly enhanced by DNKu70-virus infection with a substantial increase in the α/β ratios.
Table 3.
The L-Q model analysis of U87 malignant glioma cells and HCT8 colorectal carcinoma cells based on previous report (13). V-U87 and v-HCT8 cells were treated with 50 MOI DNKu70-carrining virus 2 days before irradiation given under oxygenated (O) and 0.5% oxygen hypoxic conditions.
| Cell Lines (% Oxygen) |
Alpha | Beta | Alpha/Beta Ratio |
DNKu70-ER at SF of | ||
|---|---|---|---|---|---|---|
| 0.5 | 0.1 | 0.01 | ||||
| U87 MG (O) | −0.121 | −0.0146 | 8.32 | |||
| v-U87 MG (O) | −0.426 | 0 | Infinite | 2.40 | 1.68 | 1.31 |
| U87 MG (0.5%) | −0.0347 | −0.0012 | 28.92 | |||
| v-U87 MG (0.5%) | −0.151 | −0.00026 | 581.06 | 2.98 | 2.13 | 1.69 |
| HCT8 (O) | −0.0795 | −0.0409 | 1.94 | |||
| v-HCT8 (O) | −0.396 | −0.0354 | 11.17 | 2.12 | 1.56 | 1.36 |
| HCT8 (0.5%) | −0.0701 | −0.0061 | 11.43 | |||
| v-HCT8 (0.5%) | −0.0992 | −0.0154 | 6.43 | 1.51 | 1.54 | 1.55 |
Our observation that VER was greater at high survivals led to a hypothesis that multiple small doses could be effectively combined with gene-therapy, and a previous study using the DNKu70-transfected cells provided data consistent with this hypothesis (11). For both HCT8 and HT29, the VERs obtained in the fractionation experiment were broadly comparable to the VERs obtained at SF 0.1 in the single-dose protocol. The value of 1.20 for oxic v-HT29 was smaller than 1.38, the value at SF 0.1 in the single dose survival curve, and that of 1.34 for hypoxic v-HT29 was larger than 1.10, the value at SF 0.1 in the single dose survival curve. Notably, hypoxic DNKu70 cells used in previous study showed greater ERs than both hypoxic v-HCT8 and v-HT29 cells.
Another benefit of using small doses might be small OERs because, as is well known, the OER is lower at low doses. This is likely due to inhibited repair of both sublethal and potentially lethal damages under hypoxia (14, 15). As a result, all OERs for fractionation were between 1.13 and 1.98 (Table 2) while the difference in the OERs between parental and virus-infected cells were insignificant.
We have observed some significant differences in the effect of DNKu70 between DNKu70-virus infection and DNKu70 transfection. Both oxic v-HCT8 and v-HT29 showed the PLD repair, while oxic DNKu70 cells did not. Inhibition of PLD repair in DNKu70 cells resulted in large DNKu70 ERs at high survival levels, leading to large ERs following multiple small doses. However, unlike DNKu70 transfection, virus-infection did not inhibit this repair, resulting in smaller ERs following multiple doses compared to DNKu70 cells. We predicted that this difference might be due to a small virus dose rather than the disparity in DNKu70 insertion methods; transfection and infection. A previous study using a virus dose of 50 MOI; i.e., twice a dose used in this study, demonstrated a VER at SF 0.1 of 1.56 for oxic v-HCT8 (Table 4) that is slightly larger than the VER of 1.31 obtained in present study (Table 1). However, at low survivals such as 0.01, the VERs for 50 and 25 MOI were 1.36 and 1.21, respectively; i.e., a smaller difference than found at SF 0.1. In addition, 50 MOI to HT29 failed inhibiting the PLD repair. These data suggests that the effect of increasing virus dose may be smaller than predicted.
Table 4.
Ratio of radiation sensitivity of parental or wild type cells over that of radiosensitive mutants or cells with reduced Ku70/80 activity. Radiation dose to reduce surviving fraction to 0.1 [D(0.1)] was obtained by authors for each cell type from published figures. Ratio is the ratio of D(0.1) of parental or mutant cells to D(0.1) of cells with reduced activity.
| Cell Types | Ratio | Reference |
|---|---|---|
| Radiosensitive Mutant-1 | Wild/Mutant | |
| DSB Repair-deficient V79 (sxi-1 – 4) | 2.2 – 4.2 | Lee SE, 1995 (17) |
| DSB Repair-deficient CHO (xrs-6) | 7.6 | Ross GM, 1995 (21) |
| Ku80-deficient CHO (xrs-6) | 8.6 | Singleton BK, 1997 (23) |
| Ku80-deficient ES | 3.7 | Nussenzweig A, 1997 (19) |
| Ku80-deficient CHO (xrs-6) | 5.0 | Marangoni, E 2000 (18) |
| Ku80-deficient CHO (xrs-6) | 6.6 | Okayasu R, 2006 (20) |
| Ku80-deficient MEF | 3.9 | Wang H, 2008 (24) |
| Ku80-deficient MEF | 5.9 | Schulte-Uentrop L, 2008(22) |
| Radiosensitive Mutant-2 | Transfectant/Mutant | |
| NHEJ-deficient V79 (XR-V15B) | 2.3 (Ku86*) | Smider V, 1994 (26) |
| NHEJ-deficient V79 (XR-V15B) | 1.0 (Ku70*) | Smider V, 1994 (26) |
| NHEJ-deficient V79 (XR-V15B) | 3.0 (Ku70 & 86*) | Smider V, 1994 (26) |
| Ku80-deficient CHO (xrs-6) | 3.4 (Ku80*) | Taccioli GE, 1994 (27) |
| DSB Repair-deficient CHO (xrs-6) | 6.6 (Ku80*) | Ross GM, 1995 (21) |
| NHEJ-deficient V79 (XR-V15B) | 1.6 - >3.0 (Ku86*) | Errami A, 1996 (25) |
| Knock out | Parent/Knock out | |
| Ku70−/− ES | 2.3, 3.2 | Gu Y, 1997 (29) |
| Ku70−/− (Ku70−/−/RAD54−/−) DT40 | 0.8 (6.0) | Utsumi H, 2001 (30) |
| Ku70−/− DT40 | 1.5 | Adachi N, 2001 (28) |
| Ku70−/− MEF | 5.2 | He F, 2003 (9) |
| Ku antisence | Parent/Antisense | |
| MRC5V1 Human fibroblasts | 1.8 (Ku86*) | Marangoni E, 2000 (32) |
| M059K Malignant glioma | 1.8 (Ku86*) | Belenkov AI, 2002 (31) |
| RERFV1 Human lung sq.c.ca | 1.5 – 1.7 (Ku70*) | Omori S, 2002 (33) |
| FSa-II Mouse fibrosarcoma | 1.5 (Ku70-virus*) | Li GC, 2003 (10) |
| siRNA | Parent/Transfectant | |
| HeLa Human cervical ca. | 1.4 (Ku70*) | Ayen, IS, 2005 (34) |
| A549 Human Lung ca. | 1.5∞ (Ku80*) | Nimura Y, 2007 (35) |
| H1299 Human Lung ca. | 1.4∞ (Ku80*) | Nimura Y, 2007 (35) |
| RT112 Human Bladder ca. | 1.2 (Ku80*) | Negroni A, 2008 (36) |
| DNKu70/80 | Parent/Transfectant | |
| CHO cells | 2.0 (DNKu80*) | Marangoni, E, 2000 (18) |
| Rat-1 fibroblasts | 1.5 – 1.9 (DNKu70*) | He, F, 2007 (9) |
| U-87 Human Glioblastoma | 1.8 (DNKu70*) | He, F, 2007(9) |
| Rat-1 fibroblasts | 1.3 – 1.8 (DNKu70*) | Urano, M, 2008 (11) |
| DNKu70-virus | Parent/Virus-infected | |
| U-87 Human Glioblastoma | 1.8 (50 MOI**) | He, F, 2007 (9) |
| HCT8 Human Colorectal ca. | 1.5 (50 MOI**) | He, F, 2007 (9) |
| HCT8 Human Colorectal ca. | 1.3 (25 MOI**) | Present |
| HT29 Human Colorectal ca. | 1.5 (25 MOI**) | Present |
[Note]
Abbreviations: V79 & CHO=Chinese hamster lung & ovary cells; MEF=mouse embryo fibroblasts; ES=embryonic stem cells; DT40=Chicken B-lymphocyte;
Transfected cDNA, siRNA or fragment.
Virus dose.
Values obtained by extrapolating to SF of 0.1.
Another difference was in the response of hypoxic cells to multiple small doses. The DNKu70-ERs calculated as a ratio of D0 for hypoxic DNKu70-7 and DNKu70-11 cells; 2.23 and 4.61, respectively, were much greater than the VER for hypoxic v-HCT8 and v-HT29; 1.27 and 1.34, respectively. Substantial differences in the OER values were also noted. The OERs of 2.13 for DNKu70-7 and 1.04 for DNKu70-11 were smaller than that of 3.51 for parental Rat-1, while the OERs of 1.26 for v-HCT8 and 1.78 for v-HT29 cells were nearly identical to that of 1.22 and 1.78 for parental HCT8 and HT29 cells, respectively. As previously discussed (11), the large DNKu70-ER and small OER for DNKu70 cells were in part due to the increased death of DNKu70 cells and unpredicted proliferation of Rat-1 cells under hypoxic conditions. Namely, OER values in fractionated treatments were dynamically influenced by cell proliferation and death during the treatment period.
We have observed throughout the present and on-going experiments (16) that hypoxia kills cells mostly exponentially with the duration of hypoxia. The SFs following 30 h at 0% oxygen of HCT8, v-HCT8, HT29 and v-HT29 cells were 0.16, 0.11, 0.55 and 0.52, respectively, indicating that hypoxic survival shown in figure 2 resulted from the combined effects of radiation and hypoxia. This hypoxia-induced lethal damage contributed to the small OERs shown in Table 2. Using these data, we calculated true OERs, resulting in an increase in the OER for HCT8, v-HCT8, HT29 and v-HT29 to 1.9, 2.1, 2.5 and 2.3, respectively. Notably, the difference in the hypoxia-induced cell death between parental and virus-infected cells was small in both cell lines. During the treatment period, changes in the number of cells per tube were minor; namely, the number of cells increased ~15–20% under oxic conditions but decreased ~10–20% under hypoxia in both parental and virus-infected cells. These changes in the cell number were too small to significantly modify the corrected survival. Another factor taken into account may be a change in the radiation sensitivity of cells kept under prolonged hypoxia. However, our pilot study showed that the changes were insignificant at 3 Gy although sensitivity increased at higher doses (data not shown).
The role of Ku70/80 in the repair process of DNA-DSBs that follow radiation or chemical insults has been extensively investigated. Most studies have reported a large difference in radiation sensitivity between radiosensitive mutant or repair-deficient cells and wild type (17–24) or between Ku70/80 cDNA transfected cells and parental cells (21, 25–27). It is not easy tabulating these published data to compare radiation sensitivity of various types of cells, because of the lack of consensus in the way comparing radiation sensitivity. To simplify these comparisons, we obtained the radiation doses to reduce survival to 0.1 [(D(0.1)] from each reported survival curve and compared these doses as we did for calculating the VER. All calculated values are shown as ratios in Table 4, indicating that repair-deficient mutants and Ku80 cDNA transfectants are 1.5 to 7.6 times more sensitive and 3.0 to 6.6 times more resistant, respectively, than corresponding wild-type cells. Another method for this type of studies is to knock out Ku70/80 cDNA. Ku70/80 knocked-out cells are 1.3 to 5.2 times more sensitive than the parental cells (9, 27–30).
Molecular methods have been developed to enhance radiation response by reducing Ku70/80 activity, including transfection of Ku70/80 antisence, Ku70/80 siRNA, or DNKu70 cDNA, and infection of DNKu70- or antisence-virus. These methods enhanced the radiation response, as tabulated in Table 4, by a factor of 1.2 to 1.9 (9–11, 31–36) that is much smaller than factors obtained for mutant and knocked-out cells. These gene insertion methods have been established with clinical applications in mind for the safe and successful insertion of anti-Ku70/80 cDNA or fragment. However, the large ratios obtained between radiosensitive mutants and their wild type cells and between Ku70/80 knocked-out and parental cells may suggest that further improvement in the insertion method(s) could substantially increase the enhancement of radiation response.
ACKNOWLEDGMENTS
This study was supported by NIH-NCI grants: CA56909, CA109772, and P01 CA115675. We acknowledge James Russell, PhD for his assistance in editing this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reprint request: Muneyasu Urano.
Conflict of Interest Notification
None.
REFERENCES
- 1.Gullo C, Au M, Feng G, Teoh G. The biology of Ku and its potential oncogenic role in cancer - Review. Bioch Biophy Acta. 2006;1765:223–234. doi: 10.1016/j.bbcan.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Velerie K, Povirk LF. Regulation and mechanisms of mammalian double-strand break repair. Oncogene. 2003;22:5792–5812. doi: 10.1038/sj.onc.1206679. [DOI] [PubMed] [Google Scholar]
- 3.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- 4.Tuteja R, Tuteja N. Ku Autoantigen: A Multifunctional DNA-Binding Protein. Crit Rev Biochem Mol Biol. 2000;35:1–33. doi: 10.1080/10409230091169177. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Dong X, Myung K, et al. Identification of two domains of the p70 Ku protein mediating dimerization with p80 and DNA binding. J Biol Chem. 1998;273:842–848. doi: 10.1074/jbc.273.2.842. [DOI] [PubMed] [Google Scholar]
- 6.Kurimasa A, Ouyang H, Dong L-J, et al. Catalytic subunit of DNA-dependent protein kinase: Impact on lymphocyte development and tumorigenesis. Proc Natl Acad Sci USA. 1999;96:1403–1408. doi: 10.1073/pnas.96.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nussenzweig A, Chen C, Soares VdaC, et al. Requirement for Ku80 in growth and immunoglobulin V(D)J recombination. Nature. 1996;382:551–555. doi: 10.1038/382551a0. [DOI] [PubMed] [Google Scholar]
- 8.Ouyang H, Nussenzweig A, Kurimasa A, et al. Ku70 is required for DNA repair but not for T cell antigen receptor gene recombination in vivo. J Exp Med. 1997;186:921–929. doi: 10.1084/jem.186.6.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He F, Li L, Kim D, et al. Adenovirus-mediated expression of a dominant-negative Ku70 fragment radiosensitizes human tumor cells under aerobic and hypoxic conditions. Cancer Res. 2007;67:634–642. doi: 10.1158/0008-5472.CAN-06-1860. [DOI] [PubMed] [Google Scholar]
- 10.Li GC, He F, Shao X, et al. Adenovirus-mediated heat-activated antisense Ku70 expression radiosensitizes tumor cells in vitro and in vivo. Cancer Res. 2003;63:3268–3274. [PubMed] [Google Scholar]
- 11.Urano M, Huang Y, He F, et al. Response to multiple radiation doses of fibroblasts over-expressing dominant-negative KU70. Int J Radiat Oncol Biol Phys. 2008;71:533–541. doi: 10.1016/j.ijrobp.2007.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thames HD, Withers HR, Peters LJ, et al. Changes in early and late radiation responses with altered dose fractionation: Implications for dose-survival relationships. Int J Radiat Oncol Biol Phys. 1982;8:219–226. doi: 10.1016/0360-3016(82)90517-x. [DOI] [PubMed] [Google Scholar]
- 13.Sah NK, Munshi A, Nishikawa T, Mukhopadhyay T, Roth JA, Meyn RE. Adenovirus-mediated wild-type p53 radiosensitizes human tumor cells by suppressing DNA repair capacity. Mol Cancer Ther. 2003;2:1223–1231. [PubMed] [Google Scholar]
- 14.Spiro IJ, Kennedy KA, Stickler R, Ling CC. Cellular and molecular repair of x-ray-induced damage: Dependence on oxygen tension and nutritional status. Radiat Res. 1985;101:144–155. [PubMed] [Google Scholar]
- 15.Rao BS, Hopwood LE. Effect of hypoxia on recovery from damage induced by heat and radiation in plateau-phase CHO cells. Radiat Res. 1985;101:312–325. [PubMed] [Google Scholar]
- 16.Urano M, He F, Minami A, et al. Hypoxia-induced lethal damage is enhanced by inhibiting the repair of DNA damage but reparable when reoxygenated. 55th Ann Radiat Res Meeting Abstract. 2009:156. [Google Scholar]
- 17.Lee SE, Pulaski CR, He DM, et al. Isolation of mammalian cell mutants that are X-ray sensitive, impaired in DNA double-strand break repair and defective for V(D)J recombination. Mutat Res. 1995;336:279–291. doi: 10.1016/0921-8777(95)00002-2. [DOI] [PubMed] [Google Scholar]
- 18.Marangoni E, Foray N, O'Driscoll M, et al. A Ku80 fragment with dominant-negative activity imparts a radiosensitive phenotype to CHO-K1 cells. Nucleic Acids Res. 2000;28:4778–4782. doi: 10.1093/nar/28.23.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nussenzweig A, Sokol K, Burgman P, et al. Hypersensitivity of Ku80-deficient cell lines and mice to DNA damage. Proc Natl Acad Sci USA. 1997;94:13588–13593. doi: 10.1073/pnas.94.25.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okayasu R, Okada M, Okabe A, et al. Repair of DNA Damage induced by accelerated heavy ions in mammalian cells proficient and deficient in the non-homologous end-Joining pathway. Radiat Res. 2006;165:59–67. doi: 10.1667/rr3489.1. [DOI] [PubMed] [Google Scholar]
- 21.Ross GM, Eady JJ, Mithal NP, et al. DNA strand break rejoining defect in xrs-6 Is complemented by transfection with the human Ku80 gene1. Cancer Res. 1995;55:1235–1238. [PubMed] [Google Scholar]
- 22.Schulte-Uentrop L, El-Awady RA, Schliecker L, et al. Distinct roles of XRCC4 and Ku80 in non-homologous end-joining of endonuclease and ionizing radiation-induced DNA double-strand breaks. Nucl Acids Res. 2008;36:2561–2569. doi: 10.1093/nar/gkn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singleton BK, Priestley A, Steingrimsdottir H, et al. Molecular and biochemical characterization of xrs mutants defective in Ku80. Mol Cell Biol. 1997;17:1264–1273. doi: 10.1128/mcb.17.3.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Wang X, Zhang P, Wang Y. The Ku-dependent non-homologous end-joining but not other repair pathway is inhibited by high linear energy transfer ionizing radiation. DNA Repair. 2008;7:725–733. doi: 10.1016/j.dnarep.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Errami A, Smider V, Rathmell WK, et al. Ku86 defines the genetic defect and restores X-ray resistance and V(D)J recombination to complementation group 5 hamster cell mutants. Mol Cell Biol. 1996;16:1519–1526. doi: 10.1128/mcb.16.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smider V, Rathmell WK, Lieber MR, Chu G. Restoration of X-ray resistance and V(D)J recombination in mutant cells by Ku cDNA. Science. 1994;266:288–291. doi: 10.1126/science.7939667. [DOI] [PubMed] [Google Scholar]
- 27.Taccioli GE, Gottlieb TM, Blunt T, et al. Ku80: product of the XRCC5 and its role in DNA repair and V(D)J recombination. Science. 1994;265:1442–1445. doi: 10.1126/science.8073286. [DOI] [PubMed] [Google Scholar]
- 28.Adachi N, Ishino T, Ishii Y, et al. DNA ligase IV-deficient cells are more resistant to ionizing radiation in the absence of Ku70. Proc Natl Acad Sci USA. 2001;98:12109–12113. doi: 10.1073/pnas.201271098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu Y, Jin S, Gao Y, et al. Ku70-deficient embryonic stem cells have increased ionizing radiosensitivity, defective DNA end-binding activity, and inability to support V(D)J recombination. Proc Natl Acad Sci USA. 1997;94:8076–8081. doi: 10.1073/pnas.94.15.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utsumi H, Tano K, Takata M, et al. Requirement for repair of DNA double-strand breaks by homologous recombination in split-dose recovery. Radiat Res. 2001;155:680–686. doi: 10.1667/0033-7587(2001)155[0680:rfrodd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Belenkov AL, Paiement J-P, Panasci LC, et al. An antisense oligonucleotide targeted to human Ku86 messenger RNA sensitizes M059K malignant glioma cells to ionizing radiation, bleomycin, and etoposide but not DNA cross-linking agents. Cancer Res. 2002;62:5888–5896. [PubMed] [Google Scholar]
- 32.Marangoni E, LeRomancer M, Foray N, et al. Transfer of Ku86 antisense decreases the radioresistance of human fibroblasts. Cancer Gene Therapy. 2000;7:339–346. doi: 10.1038/sj.cgt.7700111. [DOI] [PubMed] [Google Scholar]
- 33.Omori S, Takiguchi U, Suda A, et al. Suppression of a DNA double-strand break repair gene, Ku70, increases radio- and chemo-sensitivity in a human lung carcinoma cell line. DNA Repair. 2002;1:299–310. doi: 10.1016/s1568-7864(02)00006-x. [DOI] [PubMed] [Google Scholar]
- 34.Ayene IS, Ford LP, Koch CJ. Ku protein targeting by Ku70 small interfering RNA enhances human cancer cell response to topoiomerase II inhibitor and γ radiation. Mol Cancer Ther. 2005;4:529–536. doi: 10.1158/1535-7163.MCT-04-0130. [DOI] [PubMed] [Google Scholar]
- 35.Nimura Y, Kawata T, Uzawa K, et al. Silencing Ku80 using small interfering RNA enhanced radiation sensitivity in vitro and in vivo. Int J Oncol. 2007;30:1477–1484. [PubMed] [Google Scholar]
- 36.Negroni A, Strolnati L, Grollino MG, et al. Radioresistance in a tumour cell line correlates with radiation inducible Ku70/80 end-binding activity. Int J Radiat Biol. 2008;84:265–276. doi: 10.1080/09553000801953318. [DOI] [PubMed] [Google Scholar]