Summary
Apoptosis is a highly organized cellular process that is critical for maintaining glandular homeostasis. We have used primary rat salivary acinar cells from the parotid and submandibular glands to investigate the critical regulatory events involved in apoptosis. Caspase-3 activity, cleavage of caspase substrates, and deoxyribonucleic acid (DNA) fragmentation were assayed in cells treated with etoposide, a DNA-damaging agent, or brefeldin A (BFA), a Golgi toxin. Dose–response studies showed that the sensitivity of both cell types to etoposide and BFA was similar, with 150 μM etoposide or 1.5 μM BFA inducing maximal caspase activation. However, BFA induced a more robust activation of caspase and DNA fragmentation in both cell types. Similar results were observed when the caspase cleavage of poly(adenosine 5′-diphosphate ribose) polymerase and protein kinase C delta were analyzed by Western blot. Analysis of the kinetics of apoptosis showed that caspase-3 activation was maximal at 8 h of etoposide or BFA treatment in the parotid cells and at 8–18 h in the submandibular cells. A similar time course was observed when DNA fragmentation was assayed, although maximal DNA fragmentation in BFA-treated cells was two- to threefold higher than that observed in etoposide-treated cells. Despite slight kinetic differences, it would appear that the apoptotic cascade is very similar in both primary parotid and submandibular acinar cells. Although limited in their long-term stability in culture, the use of primary, nonimmortalized salivary acinar cultures will also permit the use of specific transgenic animals to further characterize the molecular events involved in the regulation of salivary gland acinar cell apoptosis.
Keywords: apoptosis, primary culture, salivary gland, etoposide, brefeldin A
Introduction
Saliva is the first digestive fluid secreted into the alimentary canal. The functions of saliva are quite diverse and include moistening and facilitating bolus formation and swallowing (Mandel, 1987a; Fox, 1989), providing the necessary pH buffering capacity in oral cavity (Ek and Antonsson, 1993), ecological maintenance of antimicrobial and antifungal activities in the oral cavity (Mandel, 1989), homeostasis of calcium phosphate levels (Mandel, 1987b), and protection of the oral mucosal tissue (Tabak and Bowen, 1989). The absence of an adequate amount of saliva has a pronounced effect on oral health and quality of life for several million people in the United States. For example, increased respiratory infections, loss of adequate nutrition, severe oral bacterial and fungal infections, increased rate of dental caries, and risk of periodontal disease commonly occur in the absence of saliva (Mandel, 1987a; Tabak and Bowen, 1989).
Salivary gland hypofunction, leading to dry mouth or xerostomia, can occur as a result of chronic prescription drug treatment, particularly in the elderly, as a result of therapeutic X-ray irradiation of the head and neck regions, and during cancer chemotherapy (Fox, 1989). Certain systemic diseases (Atkinson et al., 1990; Nakamura et al., 1997) can also lead to salivary gland hypofunction such as the autoimmune disease, Sjögren’s syndrome. Aberrant salivary gland apoptosis may be one of the underlying pathogenic causes for the loss of salivary gland function (Polihronis et al., 1998) under each of these conditions. Apoptosis or programmed cell death is an important genetically controlled biological process involved in glandular development and homeostasis (Hofmann, 1999; Hengartner, 2000; Rich et al., 2000; Green and Evan, 2002). Two main molecular pathways have been described for the induction and regulation of cellular apoptosis: the extrinsic pathway or receptor-mediated pathway and the intrinsic or mitochondrial pathway (Zimmermann and Green, 2001). The extrinsic pathway is initiated through specific death receptors that activate intracellular signaling pathways that eventually lead to programmed cell death (Green, 2000). The intrinsic pathway is activated by various cellular stresses that activate signaling pathways, leading to the onset of apoptosis. Many of the intracellular events responsible for regulation of apoptosis appear to be similar in most animal cells, but there are specific cell-type and stimulus differences in its regulation (Allen et al., 1998; Salvesen, 1999). Both the intrinsic and extrinsic pathways lead to proteolytic cleavage and activation of procaspase-3, which plays a central role in the final execution of apoptosis (Cohen, 1997; Allen et al., 1998; Thornberry, 1998; Thornberry and Lazabnik, 1998) and subsequent cell death (Hengartner, 2000; Zimmermann and Green, 2001). Activated caspases cleave cellular proteins, including poly(adenosine 5′-diphosphate ribose) polymerase (PARP), an enzyme used for deoxyribonucleic acid (DNA) repair (Thornberry, 1998; Decker and Muller, 2002), protein kinase C delta (PKCδ) (Reyland et al., 1999), c-Cbl (Widmann et al., 1998), and Akt (Widmann et al., 1998).
Recently, we have initiated a series of investigations, using a rat parotid C5 acinar cell line (Anderson et al., 1999; Reyland et al., 1999, 2000; Matassa et al., 2001; DeVries et al., 2002; Limesand et al., 2003), to understand the important signaling pathways and critical intracellular events involved in salivary gland acinar apoptosis. This immortalized cell line, parotid C5, exhibits a high degree of cellular differentiation and near-normal acinar cell function (Redman et al., 1988, 1994) and has proved to be very beneficial in characterizing the apoptotic response of parotid cells. Yet, given the potential dysfunction of the cell cycle or cell death signal pathways during the immortalization process (Green and Evan, 2002), it is also important to access these signaling pathways using non-transformed cells. Although mature salivary acinar cells are difficult to maintain in primary culture, we have developed the necessary conditions for the transient growth and maintenance of moderate to well-differentiated rat salivary acinar cells in primary culture (Quissell et al., 1986; Redman et al., 1988). The current studies were conducted using slightly modified culture conditions (Limesand et al., 2003). We provide information about the regulatory events involved in salivary acinar apoptosis in a nontransformed cell system and compare the results obtained using primary cultures with our previously published data obtained from the parotid C5 cells.
Materials and Methods
Cells and cell culture
Primary rat parotid and submandibular acinar cells were prepared from 150-g rats purchased from Harlan (Indianapolis, IN). Animals were maintained in accordance with University of Colorado Health Sciences Center Laboratory Animal Care guidelines and protocols. Rats were anesthetized with sodium pentobarbital (60 mg/kg) and primary parotid, and submandibular acinar cells were prepared under sterile conditions as described previously (Redman et al., 1988, 1994). A 1% v/v cell suspension was seeded onto collagen-coated dishes (Falcon/Becton Dickinson, Fairlawn, NJ) in media similar to that used previously, with the exception that 10% fetal bovine serum was used (Anderson et al., 1999; Reyland et al., 1999). Cells reached approximately 80% confluency after 5 d in culture and were used at that time without further passage. To induce apoptosis, primary rat parotid or submandibular cells were treated for 24 h with varying doses of etoposide (50–200 μM) or brefeldin A (BFA) (0.3–6 μM). In kinetic studies, cells were treated with etoposide (150 μM) or BFA (2 μM) and harvested at the time points indicated. Etoposide and BFA were purchased from Sigma Chemical Co. (St. Louis, MO).
Caspase activation
Activation of caspase-3 was quantified using a BioMol QuantiZyme Colormetric Assay Kit (BioMol, Plymouth Meeting, PA). The adherent and floating cells were collected from a 100-mm2 dish and lysed in caspase lysis buffer supplemented with 0.1% Triton X-100, aprotinin (4 μg/ml), Prefebloc (0.5 mg/ml), and leupeptin (2 μg/ml), according to the manufacturer’s instructions and previously published reports (Anderson et al., 1999; Reyland et al., 1999). Caspase-3 activity in 15 μg of cellular lysate was measured by the cleavage of Ac-DEVD-pNA substrate, and absorbance at A405 was quantitated in a microtiter plate reader (Molecular Devices, Sunnyvale, CA) at 10-min intervals for 7 h. Enzyme-specific activity (pmol/min/μg protein) was measured in quadruplicate, and statistical analysis was performed using Microsoft Excel.
Deoxyribonucleic acid fragmentation
Deoxyribonucleic acid fragmentation was quantitated using a cell death detection enzyme-linked immunosorbent assay (Roche, Indianapolis, IN) as described previously (Reyland et al., 2000). Primary parotid and submandibular cells were seeded onto a 96-well Biocoat collagen plate (Falcon/Becton Dickinson) and treated similar to the 100-mm2 plates.
Immunoblotting
Cells were lysed in c-Jun N-terminal kinase lysis buffer (25 mM N-2-hydroxyethylpiperazine-N′-2-ethane-sulfonic acid, 20 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 0.1% Triton X-100, 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM ethylenediaminetetraacetic acid, and 0.5 mM dithiothreitol) supplemented with aprotinin (4 μg/ml), Prefebloc (0.5 mg/ml), and leupeptin (2 μg/ml), and the lysates were clarified by centrifugation at 4° C for 5 min at 13,000 rpm in a refrigerated Savant microcentrifuge. Protein concentrations were determined using the BCA Protein Assay Kit (Pierce Chemical Company, Rockford, IL). For immunoblotting, 100 μg of clarified whole cellular proteins were resolved on an 8% polyacrylamide gel, transferred to Immunobilon membrane (Millipore Corporation, Bedford, MA), and immunoblotted. Enhanced chemiluminescence lighting (Amersham Pharmacia Biotech, Arlington Heights, IL) was used according to the manufacturer’s instructions to detect immunoblotted proteins. Anti-PARP antibody was from Upstate Biotechnology (Lake Placid, NY), and anti-PKCδ antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Membranes were then stripped as described previously (Anderson et al., 1999), reblocked in Tris-buffered saline & Tween 20 (TBST) with 5% nonfat dry milk (Carnation), and detected with a second antibody.
Results
Etoposide and BFA induce apoptosis in primary rat parotid and submandibular acinar cells in a dose-dependent manner
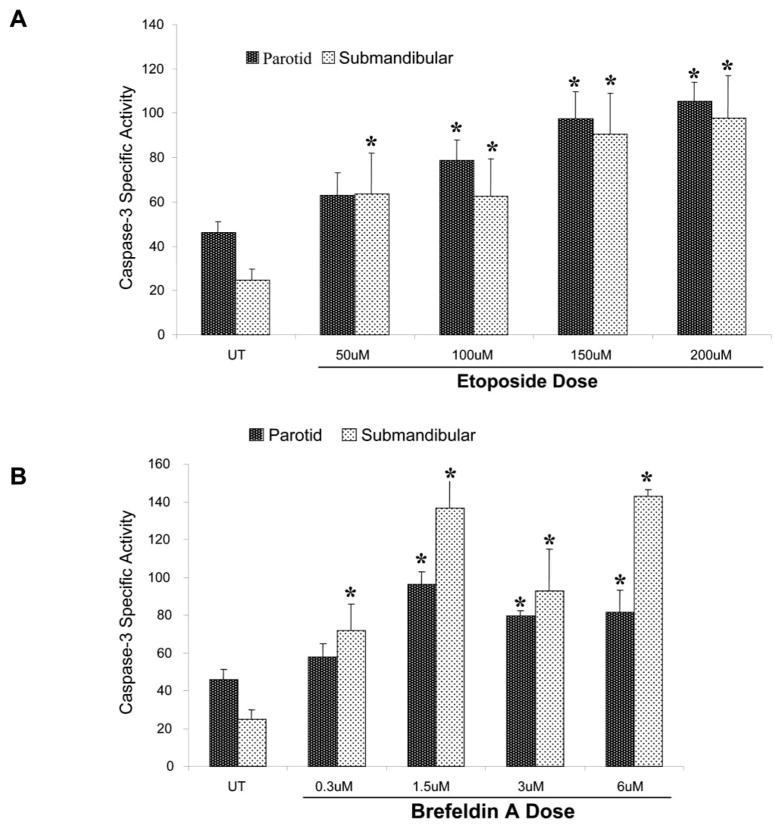
Programmed cell death or apoptosis is a genetically programmed process that occurs via a carefully controlled sequence of events. Using the parotid C5 cells, we have demonstrated previously that several types of apoptotic agents are able to induce caspase-3 activation and subsequent acinar cell apoptosis (Anderson et al., 1999; Reyland et al., 1999, 2000; Matassa et al., 2001). Etoposide is a chemotherapeutic drug that acts as a genotoxin by inhibiting topoisomerase II activity, leading to double-strained DNA breaks (Van Maanen et al., 1988). In contrast, BFA disrupts the Golgi and trans-Golgi network, resulting in an intracellular stress response (Guo et al., 1998; Matassa et al., 2001). To determine the efficacy of these two agents to induce apoptosis in primary parotid and submandibular acinar cells, subconfluent primary cultures of rat parotid and submandibular acinar cells were treated for 24 h with increasing concentrations of etoposide or BFA. This time point was chosen based on our previous studies on the C5 cell line (Anderson et al., 1999; Reyland et al., 1999, 2000; Matassa et al., 2001). Caspase-3 activation was assayed to assess the optimal dose for each of these apoptotic agents. As shown in Fig. 1A and B, 150 μM etoposide or 1.5 μM BFA treatment resulted in maximal caspase-3 activation. The magnitude of the response and the sensitivity to different concentrations of etoposide were similar in both the primary parotid and the submandibular cells. The magnitude of the response to BFA was greater than that observed with etoposide based on the fold increase in caspase-3 activity.
Fig. 1.
Induction of apoptosis in primary cultures of rat parotid and submandibular acinar cells by etoposide and brefeldin A (BFA). Subconfluent primary cultures of parotid and submandibular acinar cells were stimulated with increasing concentrations of etoposide (A) or BFA (B) for 24 h. The concentrations of etoposide and BFA are indicated under each column. All adherent and nonadherent cells were collected and lysed in caspase lysis buffer (BioMol QuantiZyme Colormetric Assay kit). Fifteen micrograms of cell lysate was used to analyze the level of enzyme-specific activity (pmol/min/μg protein) for each sample, and samples were measured in quadruplicate as described in the Materials and Methods. The results obtained with the primary parotid cells are indicated in black columns, whereas the results obtained with the primary submandibular cells are indicated in the stippled columns. Error bars represent standard error of the mean from four independent experiments. Student’s t-test P values were calculated using Microsoft Excel, and asterisks designate statistical significance (P ≤ 0.1) from the respective untreated control.
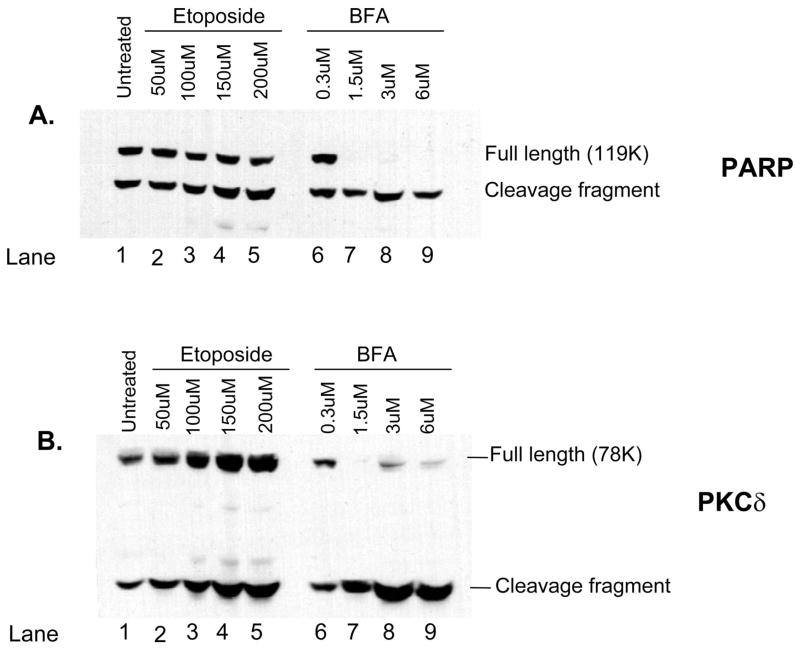
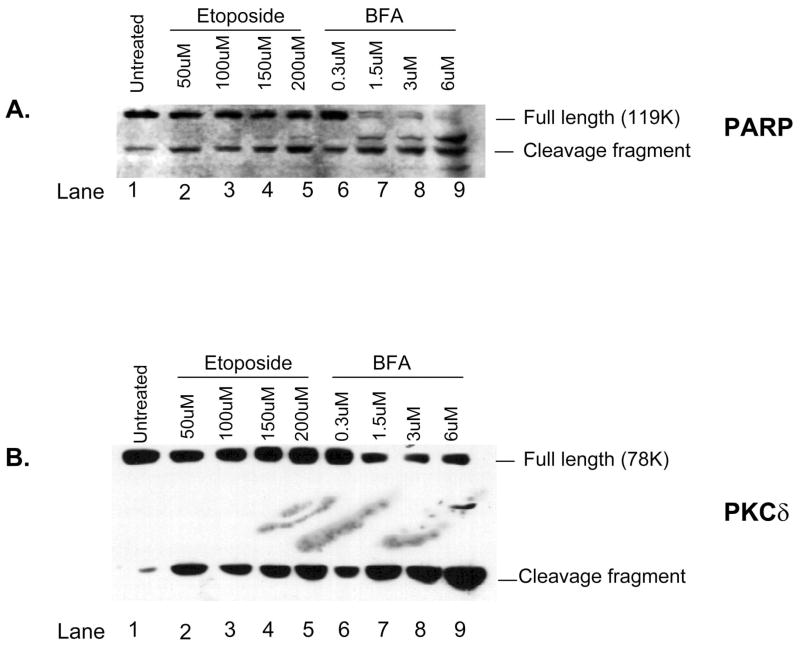
Activation of caspase-3 requires the cleavage of the procaspase-3 molecule (Cohen, 1997; Nicholson and Thornberry, 1997; Thornberry, 1998) and results in the subsequent cleavage of caspase-3 substrates such as PARP and PKCδ (Reyland et al., 1999, 2000). Treatment of primary parotid (Fig. 2A and B) and submandibular (Fig. 3A and B) cells with either etoposide or BFA resulted in a dose-dependent cleavage of both caspase-3 substrates at 24 h, a time point chosen based on our previous studies (Anderson et al., 1999; Reyland et al., 1999, 2000; Matassa et al., 2001) and preliminary studies on primary cells (data not shown). With regard to PARP, we were able to detect a small increase in its cleavage after etoposide treatment in both the primary parotid and the submandibular cells (Figs. 2A and 3A); however, statistically significant increase in the cleavage fragment was not obtained (P ≤ 0.2). A small increase in the PARP cleavage product was also detected after treatment of either the parotid or the submandibular cells with BFA; however, the decrease in the amount of full-length 119K PARP was clearly evident (lanes 6–9 of Figs. 2A and 3A). We conclude that cleavage of PARP may not be a good marker for etoposide-induced apoptosis of primary parotid and submandibular cells because of the extensive cleavage present in untreated cells. In contrast to the results obtained with PARP, we were readily able to detect a dose-dependent increase in the cleavage of PKCδ in both the primary parotid and the submandibular cells (Figs. 2B and 3B). Interestingly, in both cell types, substrate cleavage is much more robust after treatment with BFA than with etoposide. It is possible that there is an increase in the total amount of PKCδ after etoposide treatment as we have observed previously (DeVries et al., 2002). It is not clear whether this is due to increased transcription of PKCδ or increased stabilization of the cleavage fragment. It is known that other effector caspases, in addition to caspase-3, can contribute to the cleavage of both PARP and PKCδ. For instance, caspases 3 and 7 are known to cleave PARP (Decker and Muller, 2002), whereas PKCδ can be cleaved by caspase-3 and possibly other caspases (Denning et al., 1998). Thus, the difference in cleavage of these substrates in etoposide and BFA-treated cells suggests that these stimuli activate different subsets of effector caspases.
Fig. 2.
Cleavage of caspase-3 substrates poly(adenosine 5′-diphosphate ribose) polymerase (PARP) and PKCδ is induced by increasing doses of etoposide and brefeldin A (BFA) in primary cultures of rat parotid acinar cells. Subconfluent primary cultures of parotid acinar cells were treated for 24 h with increasing doses of etoposide (lanes 1–5) or BFA (lanes 6–9). Whole cell lysates were prepared as described in the Materials and Methods and immunoblotted with antibodies that detect PARP (panel A) or PKCδ (panel B). The position of the full-length and cleavage fragments of PARP and PKCδ are indicated on the right side of each panel. The concentration of etoposide and BFA used to treat each culture is shown on the top of each lane. Blots are representative of two independent experiments.
Fig. 3.
Cleavage of caspase-3 substrates poly(adenosine 5′-diphosphate ribose) polymerase (PARP) and PKCδ is induced by increasing doses of etoposide and brefeldin A (BFA) in primary cultures of rat submandibular acinar cells. Subconfluent primary cultures of submandibular acinar cells were treated for 24 h with increasing doses of etoposide (lanes 1–5) or BFA (lanes 6–9). Whole cell lysates were prepared as described in the Materials and Methods and immunoblotted with antibodies that detect PARP (panel A) or PKCδ (panel B). The positions of the full-length and cleavage fragments of PARP and PKCδ are indicated on the right side of each panel. The concentration of etoposide and BFA used to treat each culture is shown on the top of each lane. Blots are representative of two independent experiments.
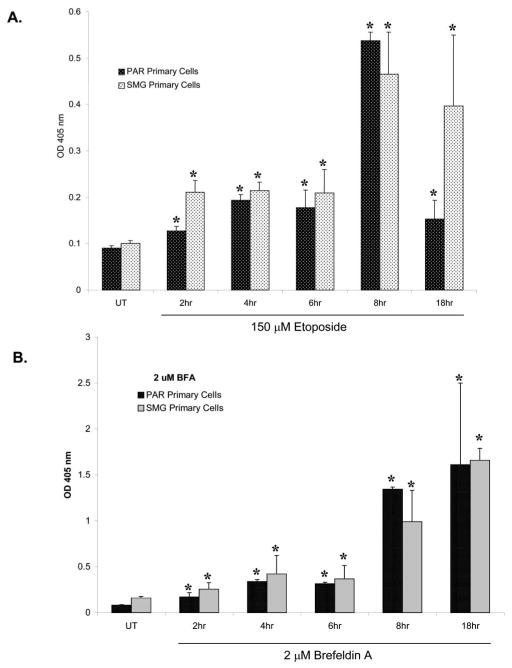
Temporal induction of apoptosis by etoposide and BFA in primary parotid and submandibular acinar cells
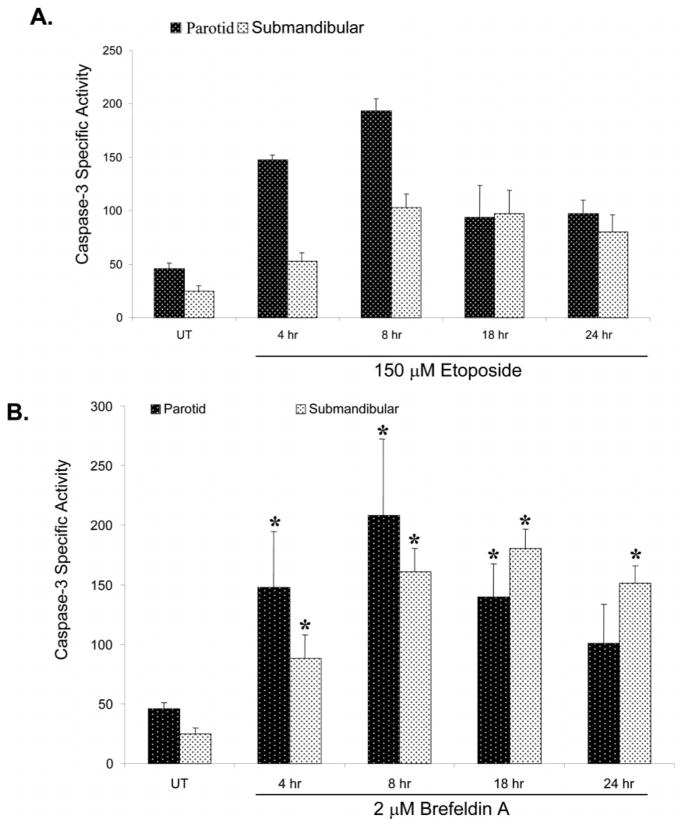
Programmed cell death is carried out by a specific series of events, including activation of initiator and effector caspases, and the breakdown of chromosomal DNA into 200 base-pair fragments. This latter process of DNA fragmentation requires caspase cleavage of the inhibitor of caspase-activated deoxyribonuclease, allowing activation of an apoptosis-specific DNA endonuclease (Enari et al., 1998; Sakahira et al., 1998). Thus, caspase cleavage and DNA fragmentation represent intermediate and late events, respectively, in the apoptotic pathway. To determine the kinetics of induction of apoptosis, subconfluent primary cultures of parotid and submandibular cells were treated with 150 μM etoposide or 2 μM BFA for various periods of time and caspase-3 activation, and DNA fragmentation was quantified. Within 4 h of treatment with either etoposide (Fig. 4A) or BFA (Fig. 4B), an increase in caspase-3 activity was observed in both the primary parotid and the submandibular acinar cells. Notably, the early response to etoposide (4 and 8 h) was of a higher magnitude (P ≤ 0.05) in the parotid cells than in the submandibular cells (Fig. 4A). Maximal activation of caspase-3 occurred within 8 h of treatment with etoposide in both the parotid and the the submandibular cells, after which it declined, presumably reflecting the loss of apoptotic cells. Deoxyribonucleic acid fragmentation increased (1.5- to 2.5-fold above basal) at 2, 4, and 6 h after addition of etoposide but increased dramatically (sixfold above basal) at 8 h of treatment in both cell types (Fig. 5A). A reduction in DNA fragmentation in the primary parotid cells was observed at 18 h of treatment with etoposide (Fig. 5A). It is possible that this decline in DNA fragmentation reflects the destruction of apoptotic cells; alternatively, there may be a small population of cells that rapidly undergo apoptosis, whereas the remainder of the cells remained viable. In BFA-treated submandibular cells, little or no increase in DNA fragmentation was observed until 8 h, whereas a further increase was observed at 18 h (Fig. 5B).
Fig. 4.
Kinetics of caspase-3 activation induced by etoposide and brefeldin A (BFA) in primary parotid acinar cells. Subconfluent primary cultures of parotid acinar cells were treated for various times with etoposide or BFA as indicated. Caspase-3 activation (pmol/min/μg protein) was quantified as described in the Materials and Methods. Error bars represent standard error from four caspase-independent experiments. Student’s t-test P values were calculated using Microsoft Excel, and asterisks designate statistical significance (P ≤ 0.1) from the respective untreated control.
Fig. 5.
Kinetics of deoxyribonucleic acid fragmentation induced by etoposide and brefeldin A (BFA) in primary submandibular acinar cells. Sub-confluent primary cultures of submandibular acinar cells were treated for various times with etoposide (A) or BFA (B). Deoxyribonucleic acid fragmentation was quantified as described in the Materials and Methods. Error bars represent standard error from two histone-independent experiments. Student’s t-test P values were calculated using Microsoft Excel, and asterisks designate statistical significance (P ≤ 0.05) from the respective untreated control.
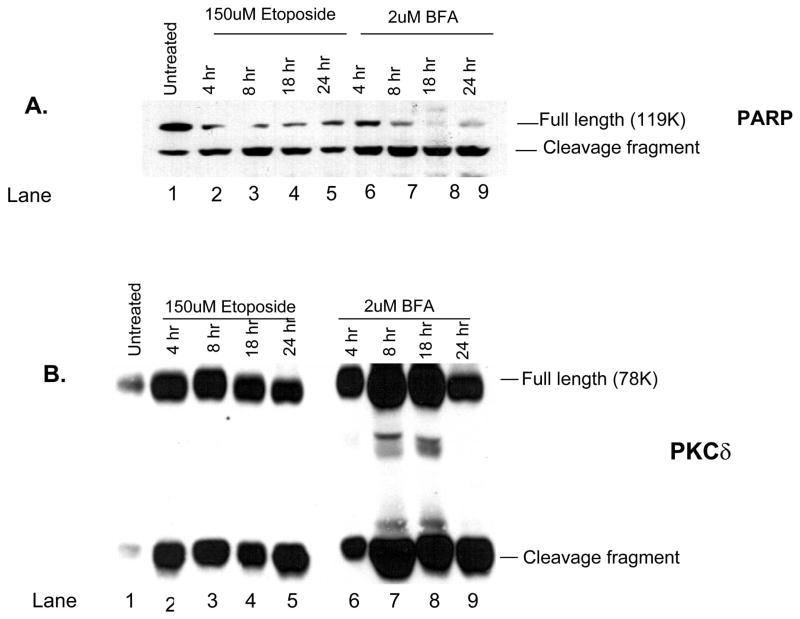
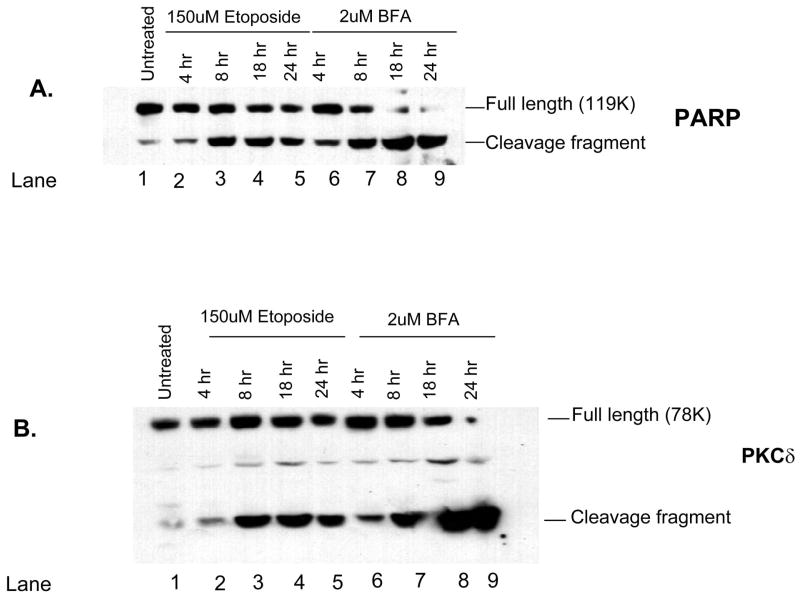
Immunoblot analysis was used to examine the cleavage of PARP and PKCδ over time in cells treated with etoposide or BFA. In primary parotid cells treated with either etoposide or BFA, PARP cleavage could be detected by 4 h, and the amount of cleaved PARP increased with time (Fig. 6A). This time point also corresponds to caspase activation and cleavage of substrates in the C5 cell line (Reyland et al., 1999, 2000; Matassa et al., 2001). In addition, caspase activation is required for cleavage of PKCδ because broad-spectrum caspase inhibitors such as ZVAD can block this effect (DeVries et al., 2002). Cleavage was more robust in BFA treatment cells, in agreement with the data shown in Figs. 3A and 4A. PKCδ cleavage was observed by 4 h in both cell types and was maximal by 8 h in most studies (Figs. 6B and 7B). Again, a more robust cleavage of PKCδ was observed in response to BFA in both cell types. As noted before, there may be an increase in the total amount of PKCδ protein in the study, as shown in Figs. 6 and 7. It is unclear whether this reflects increased transcription or altered stability of the PKCδ protein.
Fig. 6.
Cleavage of the caspase substrates poly(adenosine 5′-diphosphate ribose) polymerase (PARP) and PKCδ is induced by etoposide and brefeldin A (BFA) in primary rat parotid acinar cells. Subconfluent primary cultures of parotid acinar cells were left untreated (lane 1) and treated for the indicated times with 150 μM etoposide (lanes 2–5) or 2 μM BFA (lanes 6–9). Cell lysates were immunoblotted with anti-PARP (panel A) or anti-PKCδ (panel B). The positions of the full-length and cleavage fragments are indicated on the right side of each blot. The length of treatment (in h) with etoposide and BFA is indicated on the top of each lane. Blots are representative of two independent experiments.
Fig. 7.
Cleavage of the caspase substrates PARP and PKCδ is induced by etoposide and brefeldin A (BFA) in primary rat submandibular acinar cells. Subconfluent primary cultures of submandibular acinar cells were left untreated (lane 1) and treated for the indicated times with 150 μM etoposide (lanes 2–5) or 2 μM BFA (lanes 6–9). Cell lysates were immunoblotted with anti-PARP (panel A) or anti-PKCδ (panel B). The positions of the full-length and cleavage fragments are indicated on the right side of each blot. The length of the treatment (in h) with etoposide and BFA is indicated on the top of each lane. Blots are representative of two independent experiments.
Discussion
Established cell lines have been used quite extensively to understand the signaling pathways and key regulatory steps involved in apoptosis. Stable cell lines provide unique experimental opportunities and advantages for these types of investigations because they have a low basal level of apoptosis and can be manipulated in a variety of ways, including the use of DNA transfection protocols. The parotid C5 cell line has been very useful in studying the signaling pathways and key regulatory events involved in salivary gland acinar cell apoptosis (Anderson et al., 1999; Reyland et al., 1999, 2000; Matassa et al., 2001). One of the potential concerns, however, in using only immortalized cell lines is the possible modification of some of these critical signal pathways and regulatory steps during the process of cell transformation or immortalization (Green and Evan, 2002). Therefore, it is of value that studies use stable cell lines as well as primary nontransformed cells from the tissue of interest.
In these studies, we have used nontransformed rat primary salivary acinar cells from the parotid and submandibular glands to assess their response to specific apoptotic agents and to compare their responses with that observed in the parotid C5 cell line. Viable and actively growing primary salivary acinar cells can be maintained for about 3 wk in culture, but a moderate to high level of cell differentiation is maintained for only the first 12 to 15 d (Redman et al., 1988, 1994).
Our studies show that the primary parotid and submandibular acinar cells require a higher concentration of etoposide (150 μM) than that required in the parotid C5 cells (50 μM), to induce maximal activation of caspase-3 (Anderson et al., 1999). However, primary parotid and submandibular cells required lower levels of BFA (1.5 μM) than the C5 cells (3 μM) to induce maximal activation of caspase-3 (Matassa et al., 2001). This difference in sensitivity between the primary cells and the established cell line may relate to the mechanism of action of each of the apoptotic agents rather than to major differences in the apoptotic signal pathways present in these cells. Etoposide would be expected to have the greatest effects on cells that are rapidly synthesizing DNA. The C5 cells have a doubling time of 20 to 24 h (Quissell et al., 1998), whereas the rate of DNA synthesis in primary salivary acinar cells is significantly less (Redman et al., 1994). Therefore, it might be expected that the established cell line is more sensitive to etoposide.
Brefeldin A is a Golgi toxin (Hendricks et al., 1992; Tamaki and Yamashina, 2002). The level of differentiation of the primary acinar cells in culture is moderate to high, and the secretory pathway, including the Golgi apparatus, is fairly well maintained during the first 2 wk of culture (Redman et al., 1994). The primary acinar cells actively secrete proteins and glycoproteins because they lay down basement membrane and extracellular matrix during the first 2 wk in culture. In contrast, the Golgi system is less well maintained and less predominate in the parotid C5 cells (Quissell et al., 1998). This may explain the slightly enhanced sensitivity of the primary salivary acinar cells to BFA compared with that observed in the C5 cell line (1.5 and 3 μM, respectively). Kinetic studies revealed that there was a difference in the rate of caspase-3 activation in the primary cells when compared with the C5 cell line. Using a concentration of etoposide, which resulted in maximal apoptotic response for each culture system, the onset of apoptosis occurred at 8 h in the primary acinar cells (Fig. 4) instead of later (18 h) in the C5 cell line (Reyland et al., 1999; DeVries et al., 2002). Likewise, BFA induced maximal activation of caspase-3 by 8 h in the primary cells (Fig. 4), whereas it occurred later in the C5 cell line (Matassa et al., 2001).
Our studies suggest that the apoptotic cascade is very similar in both the C5 cells and the primary parotid and submandibular salivary acinar cells because it relates to agent specificity and timing. As we develop a further understanding of the molecular events involved in the regulation of salivary acinar apoptosis, additional comparative studies will need to be done to assess these observations in both cell systems. Although limited in their long-term stability in culture, the use of primary, nonimmortalized salivary acinar cultures will also permit the use of specific transgenic animals to further assess and characterize the molecular events involved in the regulation of salivary gland acinar cell apoptosis.
Acknowledgments
This work was supported in part by an NIH National Institutes of Dental and Craniofacial Research grant (PO1 DE 12798) to M. V. R., R. A. S., M. E. R., S. M. A., and D. O. Q. and a fellowship grant from the Sjogren’s Syndrome Foundation (PN0101-97) to K. H. L. K. H. L. is also a recipient of the NRSA fellowship grant DE 14315.
References
- Allen RT, Cluck MW, Agrawal DK. Mechanisms controlling cellular suicide: role of Bcl-2 and caspases. Cell Mol Life Sci. 1998;54:427–445. doi: 10.1007/s000180050171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Reyland ME, Hunter S, Deisher LM, Barzen KA, Quissell DO. Etoposide-induced activation of c-jun N-terminal kinase (JNK) correlates with drug-induced apoptosis in salivary gland acinar cells. Cell Death Differ. 1999;6:454–462. doi: 10.1038/sj.cdd.4400507. [DOI] [PubMed] [Google Scholar]
- Atkinson JC, Travis WD, Pillemer SR, Bermudez D, Wolff A, Fox PC. Major salivary gland function in primary Sjögren’s syndrome and its relationship to clinical features. J Rheumatol. 1990;17:318–322. [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker P, Muller S. Modulating poly (ADP-ribose) polymerase activity: potential for the prevention and therapy of pathogenic situations involving DNA damage and oxidative stress. Curr Pharm Biotechnol. 2002;3:275–283. doi: 10.2174/1389201023378265. [DOI] [PubMed] [Google Scholar]
- Denning MF, Wang YH, Nickoloff BJ, Wrone-Smith T. Protein kinase C delta is activated by caspase-dependent proteolysis during ultraviolet radiation-induced apoptosis in human keratinocytes. J Biol Chem. 1998;273:29995–30002. doi: 10.1074/jbc.273.45.29995. [DOI] [PubMed] [Google Scholar]
- DeVries TA, Neville MC, Reyland ME. Nuclear import of PKC delta is required for apoptosis: identification of a novel nuclear import sequence. EMBO J. 2002;21:6050–6060. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek B, Antonsson B-M. Studies of muscarinic receptor reserve linked to phosphoinositide hydrolysis in parotid gland and cerebral cortex. Acta Physiol Scand. 1993;147:289–295. doi: 10.1111/j.1748-1716.1993.tb09501.x. [DOI] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Fox PC. Saliva composition and its importance in dental health. Compend Contin Educ Dent Suppl. 1989;13:S457–S460. [PubMed] [Google Scholar]
- Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- Green DR, Evan GI. A matter of life and death. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- Guo H, Tittle TV, Allen H, Maziarz RT. Brefeldin A-mediated apoptosis requires the activation of caspases and is inhibited by Bcl-2. Exp Cell Res. 1998;245:57–68. doi: 10.1006/excr.1998.4235. [DOI] [PubMed] [Google Scholar]
- Hendricks LC, McClanahan SL, Palade GE, Farquhar MG. Brefeldin A affects early events but does not affect late events along the exocytic pathway in pancreatic acinar cells. Proc Natl Acad Sci USA. 1992;89:7242–7246. doi: 10.1073/pnas.89.15.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hofmann K. The modular nature of apoptotic signaling proteins. Cell Mol Life Sci. 1999;55:1113–1128. doi: 10.1007/s000180050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand KH, Barzen KA, Quissell DO, Anderson SA. Synergistic suppression of apoptosis in salivary acinar cells by IGF1 and EGF. Cell Death Differ. 2003;10:345–355. doi: 10.1038/sj.cdd.4401153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel ID. The functions of saliva. J Dent Res. 1987a;66:623–627. doi: 10.1177/00220345870660S203. [DOI] [PubMed] [Google Scholar]
- Mandel ID. Impact of saliva on dental caries. Compend Contin Educ Dent Suppl. 1987b;13:S476–S481. [PubMed] [Google Scholar]
- Mandel ID. The role of saliva in maintaining oral homeostasis. J Am Dent Assoc. 1989;119:298–304. doi: 10.14219/jada.archive.1989.0211. [DOI] [PubMed] [Google Scholar]
- Matassa AA, Carpenter L, Biden TJ, Humphries MJ, Reyland ME. PKC delta is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J Biol Chem. 2001;276:29719–29728. doi: 10.1074/jbc.M100273200. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Ikebe-Hiroki A, Shinohara M, Ohyama Y, Mouri T, Sasaki M, Shirasuna K, Nomoto K. An association between salivary gland disease and serological abnormalities in Sjogren’s syndrome. J Oral Pathol Med. 1997;26:426–430. doi: 10.1111/j.1600-0714.1997.tb00243.x. [DOI] [PubMed] [Google Scholar]
- Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Polihronis M, Tapinos NI, Theocharis SE, Economou A, Kittas C, Moutsopoulos HM. Modes of epithelial cell death and repair in Sjögren’s syndrome (SS) Clin Exp Immunol. 1998;114:485–490. doi: 10.1046/j.1365-2249.1998.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quissell DO, Barzen KA, Redman RS, Camden JM, Turner JT. Development and characterization of SV40 immortalized rat parotid acinar cell lines. In Vitro Cell Dev Biol. 1998;34:58–67. doi: 10.1007/s11626-998-0054-5. [DOI] [PubMed] [Google Scholar]
- Quissell DO, Redman RS, Mark MR. Short-term primary culture of acinar-intercalated duct complexes from rat submandibular glands. In Vitro Cell Dev Biol. 1986;22:469–480. doi: 10.1007/BF02623448. [DOI] [PubMed] [Google Scholar]
- Redman RS, Quissell DO, Barzen KA. Effects of dexamethasone, epidermal growth factor, and retinoic acid on rat submandibular acinar-intercalated duct complexes in primary culture. In Vitro Cell Dev Biol. 1988;24:734–742. doi: 10.1007/BF02623642. [DOI] [PubMed] [Google Scholar]
- Redman RS, Quissell DO, Barzen KA. Effects of oxygen, insulin, and glucagon concentrations on rat submandibular acini in serum-free primary culture. In Vitro Cell Dev Biol. 1994;30:833–842. doi: 10.1007/BF02639393. [DOI] [PubMed] [Google Scholar]
- Reyland ME, Anderson SM, Matassa AA, Barzen KA, Quissell DO. Protein kinase C delta is essential for etoposide-induced apoptosis in salivary gland acinar cells. J Biol Chem. 1999;274:19115–19123. doi: 10.1074/jbc.274.27.19115. [DOI] [PubMed] [Google Scholar]
- Reyland ME, Barzen KA, Anderson SM, Quissell DO, Matassa AA. Activation of PKC is sufficient to induce an apoptotic program in salivary gland acinar cells. Cell Death Differ. 2000;7:1200–1209. doi: 10.1038/sj.cdd.4400744. [DOI] [PubMed] [Google Scholar]
- Rich T, Allen RL, Wyllie AH. Defying death after DNA damage. Nature. 2000;407:777–783. doi: 10.1038/35037717. [DOI] [PubMed] [Google Scholar]
- Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- Salvesen GS. Programmed cell death and the caspases. APMIS. 1999;107:73–79. doi: 10.1111/j.1699-0463.1999.tb01528.x. [DOI] [PubMed] [Google Scholar]
- Tabak LA, Bowen WH. Roles of saliva (pellicle), diet, and nutrition of plaque formation. J Dent Res. 1989;68:1560–1566. [Google Scholar]
- Tamaki H, Yamashina S. Structural integrity of the Golgi stack is essential for normal secretory functions of rat parotid acinar cells: effects of brefeldin A and okadaic acid. J Histochem Cytochem. 2002;50:1611–1623. doi: 10.1177/002215540205001205. [DOI] [PubMed] [Google Scholar]
- Thornberry NA. Caspases: key mediators of apoptosis. Chem Biol. 1998;5:R97–R103. doi: 10.1016/s1074-5521(98)90615-9. [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazabnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- Van Maanen JM, Retel J, de Vries J, Pinedo HM. Mechanism of action of antitumor drug etoposide: a review. J Natl Cancer Inst. 1988;80:1526–1533. doi: 10.1093/jnci/80.19.1526. [DOI] [PubMed] [Google Scholar]
- Widmann C, Gibson S, Johnson GL. Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J Biol Chem. 1998;273:7141–7147. doi: 10.1074/jbc.273.12.7141. [DOI] [PubMed] [Google Scholar]
- Zimmermann KC, Green DR. How cells die: apoptosis pathways. J Allergy Clin Immunol. 2001;108:S99–S103. doi: 10.1067/mai.2001.117819. [DOI] [PubMed] [Google Scholar]