Abstract
Background
White matter hyperintensities (WMH) have an effect on cognition and are increased in severity among individuals with amnestic mild cognitive impairment (aMCI). The influence of WMH on progression of aMCI to Alzheimer’s disease (AD) is less clear.
Methods
Data were drawn from a three-year prospective, double blind, placebo controlled clinical trial that examined the effect of donepezil or vitamin E on progression from aMCI to AD. WMH from multiple brain regions were scored on MR images obtained at entry into the trial from a subset of 152 study participants using a standardized visual rating scale. Cox proportional hazards models adjusting for age, education and treatment arm were used to investigate the role of WMH on time to progression.
Results
55 of the 152 (36.2 %) aMCI subjects progressed to AD. Only periventricular hyperintensities (PVH) were related to an increased risk of AD within three years (HR = 1.59, 95 % CI = 1.24–2.05, p-value < 0.001). Correcting for medial temporal lobe atrophy or the presence of lacunes did not change statistical significance.
Conclusion
PVH are associated with an increased risk of progression from aMCI to AD. This suggests that PVH, an MRI finding thought to represent cerebrovascular damage, contributes to AD onset in vulnerable individuals independent of Alzheimer pathology.
Keywords: Alzheimer’s disease, MCI (mild cognitive impairment), MRI, cerebrovascular disease
Introduction
Recent data suggest that older individuals who have considerable, but circumscribed cognitive impairment may be in a transition phase between normal aging and dementia that is often denoted as mild cognitive impairment (MCI) [1–3]. MCI may be divided into multiple subtypes [4]. Individuals with the amnestic subtype of MCI (aMCI) have memory impairment as the primary cognitive deficit and a subsequently high likelihood of progressing to clinically probable Alzheimer’s disease (AD) [5–8]. While it has been suggested that most individuals with aMCI are in the earliest stages of AD, cerebrovascular disease (CVD) is also associated with this clinical syndrome [9–11].
Amongst individuals with extensive white matter hyperintensities (WMH), a radiological condition that is thought to reflect cerebrovascular disease, clinically relevant episodic memory impairment may result from dysfunction of working memory and of executive control processes [12, 13]. Additional studies support this notion by showing that WMH are associated with reduced frontal glucose metabolism [14, 15]. Although substantial work has been done to study the role of neurodegenerative processes (pathologically characterized by plaques and tangles) on progression from aMCI to dementia, studies examining the impact of CVD markers on progression to dementia are more limited and have resulted in opposing results [16–18]. We therefore examined the impact of WMH on a group of 152 individuals with aMCI. We hypothesized that increased WMH would be associated with an increased likelihood of risk for progressing to AD.
Methods
Subjects
Subjects were drawn from the prospective, double-blind placebo controlled study to test the efficacy of donepezil and vitamin E on the progression of aMCI to dementia [19]. The details of study rationale, design and subject characteristics for the parent study and the MRI sub-study have been previously described, including description of previous qualitative estimates of medial temporal atrophy measures that were used as part of this study [19–21]. In brief, 769 participants were recruited from 69 Alzheimer’s Disease Cooperative Study (ADCS) centers in the United States and Canada. Inclusion was based on criteria for amnestic MCI and modified to utilize the Logical Memory II subtest of the Wechsler Memory Scale-Revised adjusted for education [2, 22]. Additional requirements included a Clinical Dementia Rating (CDR) scale score of 0.5 and insufficient impairment to meet National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria for AD [23, 24]. The study was conducted according to Good Clinical Practice guidelines, the Declaration of Helsinki, and the U.S. Code of Federal Regulations title 21 Part 50 (Protection of Human Subjects) and title 21 Part 56 (Institutional Review Boards). Written informed consent was obtained from all participants and study partners who had knowledge of the participants’ functional activities. A data and safety monitoring board reviewed the blinded safety data every three months during the trial. Subjects were followed-up for three years and time to progression to dementia was recorded. AD was the clinically determined etiology for dementia in 99 % of the subjects. There was no significant treatment effect in the parent study.
A subset of 195 individuals received a research brain MRI examination at entry to the study as part of an ancillary study [25, 26]. These individuals were selected based solely on their willingness to undergo a research MRI and the availability of suitable MRI machinery at clinical sites that participated in the parent trial. No other criteria were used to select these subjects and subjects from 24 separate sites of the parent study were enrolled into this sub-study. Subjects of the MRI sub-study closely represented subjects of the parent study [25]: Percentage male participants was 56 % (53 % in the parent study), mean age 73 (same as parent study), Mini-Mental State Examination (MMSE) score 27.5 (versus 27.3 in the parent study), Clinical Demenita Rating (CDR) score 1.8 (same as in parent study).
MRI studies
The imaging protocol included a 3D T1-weighted gradient echo sequence, with 124 contiguous, 1.6 mm thick coronal slices and 2D proton density (PD) and T2-weighted spinecho sequences with 24 transverse slices, slice thickness 5 mm. MRI data were sent from the participating centers to a central location at the Mayo Clinic in Rochester, Minnesota for quality check, storage and analysis. For this study, images were stripped from identity data and transferred to the Imaging of Dementia and Aging (IDeA) laboratory at the University of California at Davis. Of the original 195 scans, WMH from 43 MRIs could not be read due to image artefact or incomplete image acquisition of the T2-weighted series, leaving 152 subjects with MRI for analysis.
MRI visual rating
One independent rater (EvS), who was blinded to all demographic, treatment related and outcome data, applied a semi-quantitative visual rating scale for the analysis of WMH [27]. Using this scale, deep WMH were assessed on a 0–6 scale in different brain regions, where score 0 reflects no WMH, and score 6 confluent lesions. The regions assessed were the frontal, parietal, occipital and temporal lobes, basal ganglia and infratentorial regions. A total deep WMH score (D-WMH) was composed by summing up the scores of the frontal, parietal, occipital and temporal regions (range 0–36). In addition, periventricular hyperintensities (PVH) were assessed on a scale ranging from 0–2 scale in three regions (frontal and occipital caps and bands). A periventricular lesion was operationally defined as adjacent to the ventricle and when exceeding 10 mm, a periventricular score of 2 was given and the excess of the lesion was scored in the region in which it was situated as deep WMH. A total periventricular score was composed of the scores of these three regions (range 0–6).
A total WMH score (T-WMH) was created by summing up the scores for D-WMH and PVH. Intra-observer variability was good with an intraclass correlation coefficient (ICC) of 0.92. The number of lacunes was assessed, where a lacune was defined as a T1-hypointense and T2-hyperintense CSF-like lesion surrounded by white matter or subcortical gray matter with a minimum diameter of 2 mm and was not located in areas with a high prevalence of widened perivascular spaces (vertex, anterior commissure). Medial temporal lobe atrophy (MTA) was assessed on this data set in an earlier study by one independent rater (PS) on coronal T1 images, using a qualitative visual rating scale [20, 28]. This scale ranges from 0–4 for both left and right medial temporal lobe region with higher scores indicative of increased atrophy. The scores for the left and right medial temporal lobe region were averaged and used as a general measure of medial temporal atrophy (Fig. 1).
Fig. 1.
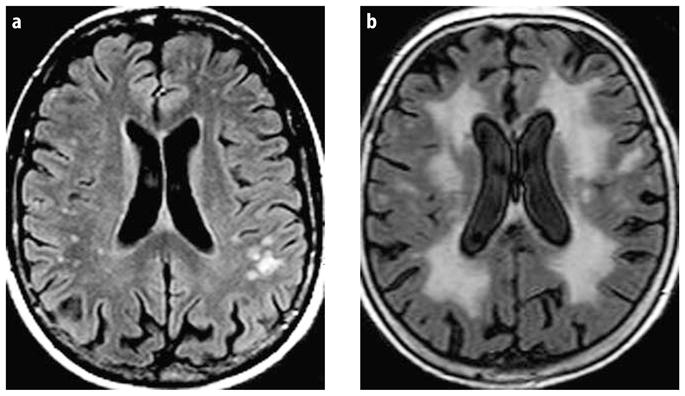
Examples of different severity of a D-WMH and b PVH are shown
Statistical analyses
The primary outcome of interest was time of progression to AD, according to the NINCDS-ARDA criteria. Those that had not converted were considered censored at their last assessment. Cox proportional hazards models were used to assess the association of qualitative white matter ratings with progression to AD. Models were adjusted for age and education (model 1). In a second step (model 2), we added MTA score, and treatment arm and in model 3 number of lacunes was added to correct for the presumed influence of the AD process, treatment effect of donepezil or vitamin E, and vascular subcortical changes other than WMH. Kaplan-Meier curves were generated to illustrate the findings, by comparing those in the highest 25th percentile of white matter ratings to the remainder of the subjects. Mean differences in T-WMH, D-WMH, and PVH scores between individuals who converted to AD and those who did not were assessed using Mann-Whitney tests. All assumptions of the models were checked both graphically and numerically and were met by the data.
Results
Demographics and WMH scores of the total group of 152 subjects, converters (subjects who progressed to dementia, n = 55) and non-converters (subjects who did not progress to dementia, n = 97) are presented in Table 1.
Table 1.
Demographic characteristics of the study group
| Total group (n = 152) | Converters (n = 55) | Non-converters (n = 97) | |
|---|---|---|---|
| Mean age in years, (SD) | 72.5 (6.6) | 73.4 (6.6) | 72.0 (6.7) |
| Mean years of education (SD) | 15 (3) | 15 (3) | 15 (3) |
| Gender (% male) | 54.2 % | 48.1 % | 57.6 % |
| MMSE (SD) | 27.9 (1.8) | 26.9 (1.9) | 27.9 (1.7)** |
| Mean T-WMH score (SD) | 13.4 (8.4) | 13.7 (9.7) | 13.1 (7.7) |
| Mean PVH score (SD) | 3.6 (1.2) | 3.9 (1.4) | 3.5 (1.1) |
| Mean D-WMH score (SD) | 7.3 (5.4) | 7.4 (6.2) | 7.2 (5.0) |
| Mean basal ganglia score (SD) | 1.5 (2.2) | 1.5 (2.2) | 1.6 (2.3) |
| Mean infratentorial score (SD) | 0.9 (1.7) | 0.9 (1.8) | 0.9 (1.7) |
| Number of subjects with lacunes | 13 | 7 | 6 |
| Mean MTA score (SD) | 1.2 (0.8) | 1.3 (0.9) | 1.1 (0.7)* |
D-WMH Deep White Matter Hyperintensities; PVH Periventricular Hyperintensities; T-WMH Total White Matter Hyperintensities
P < 0.05;
P < 0.001
The demographics of this MRI subgroup were similar to parent study. The mean age was 72.4 ± 6.6 years, the mean educational achievement was 15.0 ± 3.0 years and women made up 55.3 % of the sample. Mean baseline MMSE scores were nearly identical between the parent study (27.3 ± 1.8) and the current study (27.5 ± 1.8). Randomization by treatment arm was also well balanced in this study with 30 % randomized to donepezil, 32 % to vitamin E and 38 % to placebo.
A total of 55 subjects (36 %) converted to dementia over the 3-year study period. Subjects progressing to dementia were slightly older and included a higher percentage of women, but the differences were not significant. Converters, however, performed worse on baseline MMSE testing (26.8 ± 1.9) than did non-converters (27.9 ± 1.6), p < 0.001). Mean WMH scores amongst those who converted to dementia also tended to be higher. These differences were small and non-significant, although a trend was found for the PVH ratings (p = 0.057). Lacunes were seen in 13 subjects, of which seven converted. Mean (SD) scores were higher in subjects with lacunes than in subjects without lacunes for T-WMH (19.4 ml ± 12.2 vs. 12.8 ± 7.8), D-WMH (10.6 ± 7.6 vs. 7.0 ± 5.1) and PVH (4.5 ± 1.4 vs. 3.5 ± 1.2). Mean MTA ratings were significantly higher in converters than non-converters.
Table 2 shows the additional risk of progression to dementia with each one-point increase on the WMH rating scale. Only PVH was significantly associated with an increased risk of progression after correcting for age and education. A one-point increase in the rating was associated with a 59 % increased hazard of progression.
Table 2.
Hazard ratios (95 % confidence intervals) of increase of 1 point WMH
| HR (95 % CI) Model 1 | HR (95 % CI) Model 2 | HR (95 % CI) Model 3 | |
|---|---|---|---|
| PVH | 1.59 (1.24–2.05)** | 1.49 (1.15–1.93)* | 1.42 (1.08–1.87)* |
| D-WMH | 1.02 (0.97–1.08) | 0.99 (0.94–1.05) | 0.97 (0.92–1.03) |
| Basal ganglia hyperintensities | 1.06 (0.94–1.20) | 1.05 (0.92–1.20) | 1.01 (0.88–1.16) |
| Infratentorial hyperintensities | 1.08 (0.90–1.28) | 1.06 (0.89–1.27) | 1.01 (0.84–1.21) |
| T-WMH | 1.03 (0.99–1.06) | 1.01 (0.97–1.05) | 1.00 (0.96–1.04) |
p < 0.05;
p < 0.001
Model 1: Age and education included in the model
Model 2: Age, education, MTA, and treatment arm included in the model
Model 3: Age, education, MTA, treatment arm, and presence of lacunes included in the model
This is also illustrated by Fig. 2, which shows the relationship between the highest quartile of PVH (scores > 4) as compared to lower scores (scores ≤ 4) and progression to AD over time. Correcting for MTA and number of lacunes did not change the significance of this association, even though total PVH and MTA ratings were significantly correlated (r = 0.31).
Fig. 2.
The relationship between high total periventricular hyperintensities score (scores > 4; upper 25th percentile of scores) and low total periventricular hyperintensities score (scores < 4) and progression to dementia are shown
In order to compare the relative risk for progression to dementia related to PVH and MTA, which were rated on different scales, we also fitted the Model 2 after z-score transformation. One standard deviation increase in the total PVH rating was associated with a 64 % increased hazard (β = 0.49, SE = 0.17, p-value = 0.003, HR = 1.64, 95 % CI = 1.18–2.27), while one standard deviation increase in MTA rating was associated with a 42 % increased hazard (β = 0.35, SE = 0.15, p = 0.04, HR = 1.42, 95 % CI = 1.014–1.99).
Since ApoE genotype was a powerful predictor of progression from MCI to dementia in the parent study, we also assessed the relationship between WMH, MTA, and ApoE genotype. Mean PVH, D-WMH, T-WMH and MTA scores were significantly higher in ApoE4 allele carriers as compared to the non-carriers (p < 0.01 for the WMH scores and p = 0.046 for MTA).
Discussion
Our results indicate that PVH, and not deep subcortical WMH, increase the likelihood of progressing from amnestic MCI to AD. This is in line with previous cross-sectional studies [29]. Earlier studies showed the influence of PVH on decline in cognition in non-demented elderly and risk of dementia [30, 31]. Moreover, the effect of PVH on progression to dementia was unchanged after correction for atrophy of the medial temporal lobe suggesting that WMH lesions may have effects independent of a presumed AD process and may increase the likelihood of clinically evident AD through an additive mechanism. This adds to the growing body of evidence that vascular factors increase lifetime risk of AD [16, 32–34].
Quantitative measures of WMH and medial temporal lobe atrophy differ notably between AD subjects and healthy controls [35]. If we assume that most individuals with amnestic MCI have at least some AD pathology, WMH would be expected to increase the likelihood of progression to dementia as a second mechanism for brain injury similar to studies of stroke and AD [36–38]. Moreover, since WMH can lead to deficits in cognitive areas other than memory, such as executive function or attention, WMH related brain injury may result in additional cognitive deficits that would contribute to dementia diagnostic criteria [39, 40]. The above mentioned studies, however, did not differentiate between deep WMH and PVH, a notion that creates confusion and needs clarification for future studies [41].
In our study, deep WMH did not substantially add to the risk of progression to dementia. There are at least two possible explanations for this finding. First, it may be that qualitative estimates of PVH closely estimate total WMH volume [42]. Second, it is possible that the periventricular region includes functionally important (cholinergic) neural pathways [43, 44]. Of course, the combined effect of total volume and location may be important. Finally, the significance of the PVH finding may be a limitation to the qualitative scoring method as neuropathological research suggests that the substrate of larger deep and periventricular lesions (a likely co-occurrence when PVH scores are high) is similar [45]. Irrespective of potential etiology, qualitative estimates of PVH seem to reflect the cognitive effect of subcortical subtotal vascular disease better than the deep WMH and can therefore serve as a better surrogate marker for disease in this population of amnestic MCI subjects.
ApoE4 genotype has been found to increase the risk of developing AD, presumably by increasing amyloid beta protein (Aβ) deposition and enhancing the vulnerability of neurons for Aβ [46]. We found an association between ApoE4 and increased MTA and WMH scores. ApoE is also important to cholesterol metabolism and the ApoE4 genotype is associated with increased risk for cardiovascular and possibly cerebrovascular disease [47]. The association of ApoE4 with both MTA and WMH may, therefore, reflect a role in the development of vascular lesions as well as AD pathology and contribute to the apparent association between AD pathology, vascular factors and dementia.
One limitation of this study could be the use of the visual scale for the assessment of WMH. Visual scales are not always linear and the effect measured could be limited due to ceiling effects [48]. Our findings, however, showed a significant relationship of PVH with clinical data, indicating that the PVH assessment was sensitive enough in this population. An effect of WMH in general on progression from MCI to dementia, however, has not consistently been found. Small subject groups and limited number of individuals, who convert to dementia during the period of observation, may account for these discrepancies. In addition, we used the MTA scale, a qualitative rating scale. Visual MTA scores may not reflect hippocampal atrophy as precise as volumetric measurement of this structure on MRI, although differences between visual and volumetric scoring methods seem small with respect to clinical and cognitive characteristics [49].
Results of double-blind, placebo-controlled, clinical trials to test efficacy and safety of drugs proven to be efficacious in AD, such as cholinesterase inhibitors, and vitamin E in MCI populations are being carried out and the first results are becoming available [19, 50, 51]. In animal models, permanent oxidative stress is a major contributor to neurodegeneration, leading to the investigation of protective effect of vitamin E as anti-oxidative agent [52]. However, intervention studies have not been able to demonstrate an effect in humans [53]. Besides neurodegeneration, oxidative stress could lead to vascular disease, especially WMH when this stress is chronic and subtotal. Vascular disease could therefore interfere with the effect of anti-oxidative agents, complicating the assessment of treatment effects. Cholinesterase-inhibitors reduce cognitive deficits in AD and VaD, but no effect has been demonstrated in prevention of AD [54]. These agents do not alter the underlying disease but optimize cholinergic status, leading to a modest and temporary improvement of symptoms. Subjects with vascular lesions could benefit from cholinesterase inhibitors since the lesions might disrupt important cholinergic pathways [44]. Given the results of this study and others vascular lesions seem to interact with degenerative disease, and prevention of these lesions, such as PVH, through control of vascular risk factors may prove helpful in reducing the likelihood of dementia for at risk populations [18, 55, 56].
Acknowledgments
Research supported by grants from the Institute for the Study of Aging 990802 and 2002073, NIA P30 AG10129 and NIA U01-10483.
Contributor Information
Elisabeth C. W. van Straaten, Email: i.vanstraaten@vumc.nl, Dept. of Neurology and Alzheimer Center, VU Medical Center, De Boelelaan 1117, P.O. Box 7057, 1007 MB, Amsterdam, The Netherlands, Tel.: +31-20/444-0679, Fax: +31-20/444-0715
Danielle Harvey, Division of Biostatistics, Dept. of Public Health Sciences, University of California at Davis, USA.
Philip Scheltens, Dept. of Neurology and Alzheimer Center, VU Medical Center, De Boelelaan 1117, P.O. Box 7057, 1007 MB, Amsterdam, The Netherlands, Tel.: +31-20/444-0679, Fax: +31-20/444-0715.
Frederik Barkhof, Dept. of Radiology, VU University Medical Center, Amsterdam, The Netherlands.
Ronald C. Petersen, Dept. of Neurology, Mayo Clinic, Rochester, USA
Leon J. Thal, Dept. of Neurosciences, University of California, San Diego, USA
Clifford R. Jack, Jr., Dept. of Radiology, Mayo Clinic, Rochester, USA
Charles DeCarli, Dept. of Neurology and Imaging of Dementia and Aging (IDeA) Laboratory, Center for Neuroscience, University of California at Davis, USA.
References
- 1.Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Mol Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 3.Price JL, Morris JC. Tangles and plaques in nondemented aging and «preclinical» Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 6.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 7.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 9.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Carmelli D. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 2001;58:643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 10.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 11.Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 12.Englund E. Neuropathology of white matter lesions in vascular cognitive impairment. Cerebrovasc Dis. 2002;13(Suppl 2):11–15. doi: 10.1159/000049144. [DOI] [PubMed] [Google Scholar]
- 13.Nordahl CW, Ranganath C, Yonelinas AP, DeCarli C, Reed BR, Jagust WJ. Different mechanisms of episodic memory failure in mild cognitive impairment. Neuropsychologia. 2005;43:1688–1697. doi: 10.1016/j.neuropsychologia.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeCarli C, Murphy DG, Tranh M, et al. The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology. 1995;45:2077–2084. doi: 10.1212/wnl.45.11.2077. [DOI] [PubMed] [Google Scholar]
- 15.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeCarli C. The role of cerebrovascular disease in dementia. Neurologist. 2003;9:123–136. doi: 10.1097/00127893-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 17.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf H, Ecke GM, Bettin S, Dietrich J, Gertz HJ. Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? A longitudinal study. Int J Geriatr Psychiatry. 2000;15:803–812. doi: 10.1002/1099-1166(200009)15:9<803::aid-gps190>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 20.DeCarli C, Frisoni GB, Clark CM, et al. Qualitative estimates of medial temporal atrophy as a predictor of progression from mild cognitive impairment to dementia. Arch Neurol. 2007;64:108–115. doi: 10.1001/archneur.64.1.108. [DOI] [PubMed] [Google Scholar]
- 21.Grundman M, Petersen RC, Ferris SH, et al. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. WMS-R Wechsler Memory Scale – Revised Manual. New York: Harcourt Brace Jovanovich Inc; 1987. [Google Scholar]
- 23.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Grundman M, Sencakova D, Jack CR, Jr, et al. Brain MRI hippocampal volume and prediction of clinical status in a mild cognitive impairment trial. J Mol Neurosci. 2002;19:23–27. doi: 10.1007/s12031-002-0006-6. [DOI] [PubMed] [Google Scholar]
- 26.Grundman M, Jack CR, Jr, Petersen RC, et al. Hippocampal volume is associated with memory but not monmemory cognitive performance in patients with mild cognitive impairment. J Mol Neurosci. 2003;20:241–248. doi: 10.1385/jmn:20:3:241. [DOI] [PubMed] [Google Scholar]
- 27.Scheltens P, Barkhof F, Leys D, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- 28.Scheltens P, Leys D, Barkhof F, et al. Atrophy of medial temporal lobes on MRI in «probable» Alzheimer’s disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burns JM, Church JA, Johnson DK, et al. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Arch Neurol. 2005;62:1870–1876. doi: 10.1001/archneur.62.12.1870. [DOI] [PubMed] [Google Scholar]
- 30.de Groot JC, de Leeuw FE, Oudkerk M, et al. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002;52:335–341. doi: 10.1002/ana.10294. [DOI] [PubMed] [Google Scholar]
- 31.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol. 2004;61:1531–1534. doi: 10.1001/archneur.61.10.1531. [DOI] [PubMed] [Google Scholar]
- 32.de la Torre JC. Vascular basis of Alzheimer’s pathogenesis. Ann N Y Acad Sci. 2002;977:196–215. doi: 10.1111/j.1749-6632.2002.tb04817.x. [DOI] [PubMed] [Google Scholar]
- 33.Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 34.Pansari K, Gupta A, Thomas P. Alzheimer’s disease and vascular factors: facts and theories. Int J Clin Pract. 2002;56:197–203. [PubMed] [Google Scholar]
- 35.van der Flier WM, Middelkoop HA, Weverling-Rijnsburger AW, et al. Interaction of medial temporal lobe atrophy and white matter hyperintensities in AD. Neurology. 2004;62:1862–1864. doi: 10.1212/01.wnl.0000125337.65553.8a. [DOI] [PubMed] [Google Scholar]
- 36.Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. 2004;62:1148–1155. doi: 10.1212/01.wnl.0000118211.78503.f5. [DOI] [PubMed] [Google Scholar]
- 37.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA. 1997;277:813–817. [PubMed] [Google Scholar]
- 38.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 39.Cummings JL, Benson DF. Psychological dysfunction accompanying subcortical dementias. Annu Rev Med. 1988;39:53–61. doi: 10.1146/annurev.me.39.020188.000413. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatry Association. Diagnostic and statistical manual of mental disorders. 4. Washington: American Psychiatry Association; 1994. [Google Scholar]
- 41.Barkhof F, Scheltens P. Is the whole brain periventricular? J Neurol Neurosurg Psychiatry. 2006;77:143–144. doi: 10.1136/jnnp.2005.075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeCarli C, Fletcher E, Ramey V, Harvey D, Jagust WJ. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Desmond DW. The neuropsychology of vascular cognitive impairment: is there a specific cognitive deficit? J Neurol Sci. 2004;226:3–7. doi: 10.1016/j.jns.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Selden NR, Gitelman DR, Salamon-Murayama N, Parrish TB, Mesulam MM. Trajectories of cholinergic pathways within the cerebral hemispheres of the human brain. Brain. 1998;121(Pt 12):2249–2257. doi: 10.1093/brain/121.12.2249. [DOI] [PubMed] [Google Scholar]
- 45.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 46.Olichney JM, Hansen LA, Lee JH, Hofstetter CR, Katzman R, Thal LJ. Relationship between severe amyloid angiopathy, apolipoprotein E genotype, and vascular lesions in Alzheimer’s disease. Ann N Y Acad Sci. 2000;903:138–143. doi: 10.1111/j.1749-6632.2000.tb06360.x. [DOI] [PubMed] [Google Scholar]
- 47.DeCarli C, Reed T, Miller BL, Wolf PA, Swan GE, Carmelli D. Impact of apolipoprotein E epsilon4 and vascular disease on brain morphology in men from the NHLBI twin study. Stroke. 1999;30:1548–1553. doi: 10.1161/01.str.30.8.1548. [DOI] [PubMed] [Google Scholar]
- 48.van Straaten EC, Fazekas F, Rostrup E, et al. Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke. 2006;37:836–840. doi: 10.1161/01.STR.0000202585.26325.74. [DOI] [PubMed] [Google Scholar]
- 49.Visser PJ, Verhey FR, Hofman PA, Scheltens P, Jolles J. Medial temporal lobe atrophy predicts Alzheimer’s disease in patients with minor cognitive impairment. J Neurol Neurosurg Psychiatry. 2002;72:491–497. doi: 10.1136/jnnp.72.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology. 2004;63:651–657. doi: 10.1212/01.wnl.0000134664.80320.92. [DOI] [PubMed] [Google Scholar]
- 51.Thal LJ, Ferris SH, Kirby L, et al. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30:1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- 52.Ancelin ML, Christen Y, Ritchie K. Is antioxidant therapy a viable alternative for mild cognitive impairment? Examination of the evidence. Dement Geriatr Cogn Disord. 2007;24:1–19. doi: 10.1159/000102567. [DOI] [PubMed] [Google Scholar]
- 53.Hull M, Berger M, Heneka M. Disease-modifying therapies in Alzheimer’s disease: how far have we come? Drugs. 2006;66:2075–2093. doi: 10.2165/00003495-200666160-00004. [DOI] [PubMed] [Google Scholar]
- 54.Burns A, O’Brien J, Auriacombe S, et al. Clinical practice with anti-dementia drugs: a consensus statement from British Association for Psychopharmacology. J Psychopharmacol. 2006;20:732–755. doi: 10.1177/0269881106068299. [DOI] [PubMed] [Google Scholar]
- 55.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- 56.Hanon O, Forette F. Prevention of dementia: lessons from SYST-EUR and PROGRESS. J Neurol Sci. 2004;226:71–74. doi: 10.1016/j.jns.2004.09.015. [DOI] [PubMed] [Google Scholar]



