The secreted morphogen Wingless promotes Drosophila wing growth by fueling a wave front of Fat-Dachsous signaling that recruits new cells into the wing primordium.
Abstract
During development, the Drosophila wing primordium undergoes a dramatic increase in cell number and mass under the control of the long-range morphogens Wingless (Wg, a Wnt) and Decapentaplegic (Dpp, a BMP). This process depends in part on the capacity of wing cells to recruit neighboring, non-wing cells into the wing primordium. Wing cells are defined by activity of the selector gene vestigial (vg) and recruitment entails the production of a vg-dependent “feed-forward signal” that acts together with morphogen to induce vg expression in neighboring non-wing cells. Here, we identify the protocadherins Fat (Ft) and Dachsous (Ds), the Warts-Hippo tumor suppressor pathway, and the transcriptional co-activator Yorkie (Yki, a YES associated protein, or YAP) as components of the feed-forward signaling mechanism, and we show how this mechanism promotes wing growth in response to Wg. We find that vg generates the feed-forward signal by creating a steep differential in Ft-Ds signaling between wing and non-wing cells. This differential down-regulates Warts-Hippo pathway activity in non-wing cells, leading to a burst of Yki activity and the induction of vg in response to Wg. We posit that Wg propels wing growth at least in part by fueling a wave front of Ft-Ds signaling that propagates vg expression from one cell to the next.
Author Summary
Under normal conditions, animals and their various body parts grow until they achieve a genetically predetermined size and shape—a process governed by secreted organizer proteins called morphogens. How morphogens control growth remains unknown. In Drosophila, wings develop at the larval stage from wing primordia. Recently, we discovered that the morphogen Wingless promotes growth of the Drosophila wing by inducing the recruitment of neighboring cells into the wing primordium. Wing cells are defined by the expression of the “selector” gene vestigial. Recruitment depends on the capacity of wing cells to send a short-range, feed-forward signal that allows Wingless to activate vestigial in adjacent non-wing cells. Here, we identify the molecular components and circuitry of the recruitment process. We define the protocadherins Fat and Dachsous as a bidirectional ligand-receptor system that is controlled by vestigial to generate the feed-forward signal. Further, we show that the signal is transduced by the conserved Warts-Hippo tumor suppressor pathway via activation of its transcriptional effector Yorkie. Finally, we propose that Wingless propels wing growth by fueling a wave front of Fat-Dachsous signaling and Yorkie activity that propagates vestigial expression from one cell to the next.
Introduction
Growth is a fundamental property of animal development. Under normal conditions, animals of a given species, as well as their various body parts, achieve a characteristic size, shape, and pattern under tight genetic control. However, the basis of this control is poorly understood.
Morphogens, such as secreted factors of the Wingless/Int (Wnt), Bone Morphogenetic Protein (BMP), and Hedgehog (Hh) families, control growth. For example, in the classic paradigm of the Drosophila wing, the morphogens Wingless (Wg, a Wnt) and Decapentaplegic (Dpp, a BMP) drive a rapid ∼200-fold increase in cell number and mass that occurs during larval life [1],[2],[3],[4],[5]. Removal of either morphogen results in truncated wings [4],[5],[6],[7]. Conversely, their ectopic expression induces supernumerary wings [1],[2],[4],[5],[8].
Another system involved in growth is the evolutionarily conserved Warts-Hippo tumor suppressor pathway [9],[10],[11],[12]. This pathway includes the Warts (Wts) and Hippo (Hpo) kinases, the FERM domain proteins Expanded (Ex) and Merlin (Mer), and the accessory proteins Salvador (Sav) and Mob-as-tumor-suppressor (Mats). All of these proteins limit growth by mediating the phosphorylation and cytosolic retention of the transcriptional co-activator Yorkie (Yki)/YES Associated Protein (YAP) [9],[11], preventing Yki from up-regulating genes that promote growth [9],[13],[14].
In Drosophila, two protocadherins, Dachsous (Ds) and Fat (Ft), have been implicated as a ligand-receptor pair that acts, via the atypical myosin Dachs (D), to regulate Wts kinase activity [11],[15],[16],[17],[18],[19]. Previous studies have shown that morphogens such as Wg, Dpp, and Hh direct the formation of opposing, tissue-wide gradients of Ds and Ft activity [20],[21],[22],[23],[24]. Further, it has been proposed that the differential (i.e., slope) of Ds-Ft signaling across each cell sets the level of Wts activity and thereby governs the rate of growth and division on a cell-by-cell basis [24],[25] (see also [26]). In support, experiments that create sharp disparities in morphogen receptor activity or Ds-Ft signaling down-regulate Wts-Hpo activity and induce abnormal growth [24],[25],[27]. Conversely, experiments that flatten Ds-Ft signaling (e.g. uniform over-expression of Ds) suppress growth [22],[24],[25],[28].
Ft and Ds are also important for planar cell polarity (PCP), in which cells within epithelial sheets adopt a common orientation, e.g. as manifest by their secreting hairs that point in the same direction [20],[21],[29],[30],[31]. In this case, the ligand-receptor relationship between the two proteins appears more complex [23],[32]. Cells that express only Ds or only Ft can polarize their neighbors, whereas cells that lack either Ds or Ft cannot respond to their neighbors. Hence, in PCP, Ds and Ft each have intrinsic signaling activities, and both are required to receive and transduce each signal [23],[32].
Recently, we defined a new mechanism for the control of Drosophila wing growth by morphogen [33],[34]. Focusing on Wg, we showed that morphogen propels growth at least in part by fueling a reiterative process of recruitment of non-wing cells into the wing primordium. Recruitment depends on a special, auto-regulatory property of vestigial (vg), the selector gene that defines the wing state [35]. This is the capacity of vg expressing cells to send a feed-forward (FF) signal that induces neighboring cells to activate vg in response to Wg [33],[34]. Early in larval life, specialized “border” cells along the boundary between the dorsal (D) and ventral (V) compartments are induced to express Vg and secrete Wg. These cells initiate the FF recruitment process, which then reiterates, propagating vg expression from cell to cell in response to Wg spreading from the border cells.
In our initial analysis of the recruitment process, we speculated that Ft and Ds might be involved in the FF mechanism [33]. Here, we confirm this speculation and show that Ft is required for cells both to send and, together with Ds, to receive the FF signal, concordant with the dual ligand and receptor activities of both proteins in PCP. Further, we show that Ft and Ds transduce the FF signal via D, the Wts-Hpo pathway, and Yki to activate vg expression and initiate a new cycle of FF signaling. Based on these findings, we posit that Wg (and likely Dpp) promote wing growth by fueling the propagation of a wave front of Ft-Ds signaling that transiently suppresses the Wts-Hpo pathway and elevates Yki activity to recruit new cells into the wing primordium.
Results
The vg FF Signal
The main phase of wing growth begins early in larval life with the segregation of the prospective wing primordium into D and V compartments [36],[37],[38]. Short-range Notch signaling across the D-V boundary activates the vg Boundary Enhancer (BE) to generate a stripe of vg expressing “border cells” [35],[39]. It also induces border cells to secrete Wg [40],[41],[42], which activates and sustains vg expression in surrounding cells via the vg Quadrant Enhancer (QE) (Figure 1A, 1B) [4],[5],[33],[34],[35], driving the rapid increase of the wing primordium from a population of ∼25–50 cells to one of ∼5,000–10,000 cells.
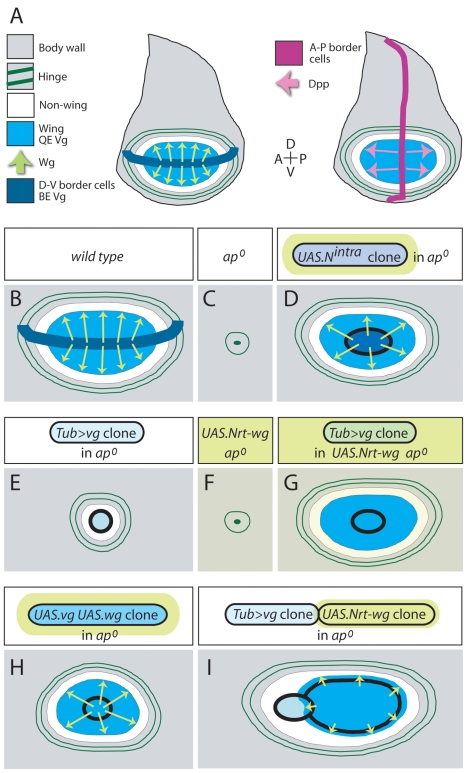
Figure 1. Feed-forward signaling: context and criteria.
(A) Context. Two diagrams of the mature wing imaginal disc are shown, depicting control of wing growth by Wg (left) and Dpp (right) and keys for the relevant primordia, signals, and gene expression domains. Early in larval life, the wing disc is subdivided into distal (prospective wing; turquoise/white) and proximal (prospective hinge and body wall; grey) domains. Feed-forward (FF) signaling operates only in the distal domain, to induce non-wing cells (white) to enter the wing primordium (turquoise). Both domains are further subdivided into D and V compartments by activity of the selector gene ap in the D compartment (not depicted). DSL-Notch signaling across the D-V compartment boundary defines a population of specialized border cells (dark blue) that express wg and vg, the latter mediated by the vg Boundary enhancer (BE). The wing disc is also divided into anterior (A) and posterior (P) compartments, with A cells just anterior to the A-P boundary secreting Dpp (for simplicity only shown in A). Following the D-V segregation, vg expressing wing cells send a short-range feed-forward (FF) signal (not depicted) that acts together with Wg and Dpp to activate Quadrant enhancer (QE) dependent vg expression (turquoise) in abutting non-wing cells; newly recruited wing cells serve as a source for new FF signal, propagating recruitment of neighboring non-wing cells into the wing primordium in response to Wg and Dpp (see Figure 8A). Wg and Dpp are also required (i) to maintain QE-dependent vg expression in cells once they are recruited into the wing primordium, (ii) to sustain the survival and growth of wing cells, so defined, and (iii) to act indirectly, through the action of Vg, to produce an additional signal that induces proliferation of surrounding non-wing cells for recruitment into the growing wing primordium [33],[34]. The hinge primordium, which encircles the prospective wing, contains two concentric rings of wg expressing cells (dark green) that serve as landmarks as well as potential sources for cryptic Wg signal in apo discs. (B–I) Criteria. FF signaling is monitored by assaying QE-dependent gene expression. (B) wild type. Here, as in the remaining panels, the genotype is indicated above and the QE response below for each of several experimental paradigms used to define the FF signal [33],[34]. Wg signal is depicted by Chartreuse arrows or wash. QE activity and formation of wing tissue (turquoise) indicates a positive response. (C) The apo condition serves as the ground state for assaying FF signaling. In the absence of ap, no D-V segregation occurs, no D-V border cells are specified and the nascent wing primordium ceases to express vg, yielding a population of “non-wing” cells that either die or sort out during subsequent development, unless they are induced to activate QE-dependent vg expression in response to Wg and the FF signal generated by an experimental manipulation (Dpp is provided, independently, by A-P border cells). As diagrammed, mature apo discs lack wing (turquoise) and non-wing (white) territories, as well as the distal portion of the hinge primordium, reducing the inner ring of Wg expression to a small patch, encircled by a rudimentary outer ring. (D) Cells that express constitutively active forms of Notch in apo discs (e.g., UAS.Nintra clones) behave like ectopic D-V border cells. They express wg and vg, induce neighboring non-wing cells to activate QE-dependent vg expression, and recruit surrounding cells to join a rapidly expanding wing primordium. (E,F) Providing only Vg expressing cells [e.g., Tub>vg clones; (E)] or only ectopic Wg signal [uniform expression of Neurotactin-Wg (UAS.Nrt-Wg), a membrane tethered form of Wg] fails to induce QE activity, except within Tub>vg expressing cells, where the combination of cryptic Wg input and exogenous Vg activity weakly activates the QE cell-autonomously (E, light turquoise wash). (G–I) Generating Vg expressing cells in the presence of Wg signal, whether in the form of ubiquitous Nrt-Wg expression (G), co-expression of ectopic Wg (H), or abutting clones of Nrt-Wg expressing cells (I), induces long-range propagation of QE-dependent vg expression and rescue of wing tissue. Note that in the last condition (I), FF signaling can propagate throughout the Nrt-Wg clone and extend to abutting wild type cells (which receive the Nrt-Wg signal) but does not go further owing to inadequate Wg signal in the surround.
D-V compartmentalization depends on the heritable activation of the selector gene apterous (ap) in D, but not V, cells [36],[43]. In ap null discs (henceforth apo discs), the D-V segregation fails, vg and wg expressing border cells are not specified, and the nascent wing primordium is subsequently lost (Figures 1C, 2B). However, it is possible to rescue wing development in apo discs by experimental protocols that provide both Wg and a population of ectopic Vg expressing cells (Figure 1D–I; Figure 2G,H) [33],[34]. Under these conditions, the ectopic Vg expressing cells induce neighboring cells that receive Wg to activate QE-dependent vg expression (turquoise shading in Figure 1), and these newly recruited vg expressing cells can similarly induce their non-expressing neighbors, the process reiterating to increase the size of the wing primordium [33],[34].
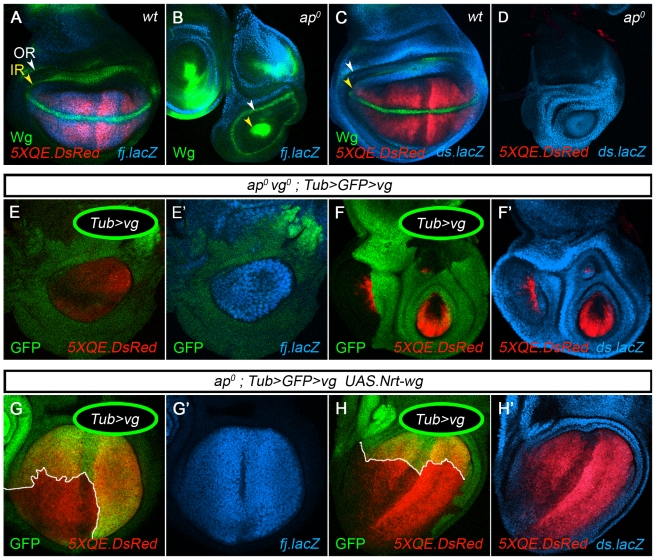
Figure 2. Vestigial activates four-jointed and represses dachsous.
(A–D) fj-lacZ, ds-lacZ, and 5XQE.DsRed reporter expression in mature wild type and apo discs counter-stained for Wg (A–C, only); note that 5XQE.DsRed expression is reduced in the vicinity of the A-P compartment boundary, as also apparent in (G,H). In wild type discs (A,C), fj-lacZ and 5XQE.DsRed are co-expressed in the wing pouch in a domain complementary to that of ds-lacZ (the inner (IR) and outer (OR) rings of Wg in the hinge primordium are indicated by yellow and white arrow heads). In apo discs (B,D), the wing pouch is absent, as indicated by the collapse of the IR to a small circular patch surrounded by the OR: ds-lacZ is expressed uniformly in place of fj-lacZ and 5XQE.DsRed in the territory encircled by the OR. (E,F). fj-lacZ, ds-lacZ, and 5XQE.DsRed reporter expression in apo vgo discs that contain clones of Tub>vg cells (marked by the absence of GFP). Clones located in the prospective wing domain develop cell-autonomously as wing tissue and express fj-lacZ and 5XQE.DsRed instead of ds-lacZ. (G,H) Clones of Tub>vg cells in UAS.Nrt-wg expressing apo discs (as in Figure 1G). Clones (outlined in white, marked by the absence of GFP) induce the long-range propagation of QE-dependent vg expression, as visualized by the domain of 5XQE.DsRed expression. Recruitment into wing tissue correlates with the up-regulation of fj-lacZ expression and the down-regulation of ds-lacZ expression. Here, and in subsequent figures, genotypes, clone markers, and antibody stains are indicated on the panels, coded by color (clones marked by the absence of GFP are shown as open circles with green borders; those marked positively are shown as filled circles), or in boxes above the panels (in all cases in which a UAS transgene is indicated in a box, its expression is driven by a Gal4 driver that is uniformly active in the prospective wing territory; white/turquoise domain as in Figure 1A, 1B; see Materials and Methods for exact genotypes).
These results establish that Vg expressing cells send a short-range, inductive signal that is required, together with Wg, to activate QE-dependent vg expression in neighboring cells. We term this Vg-dependent, Vg-inducing signal the FF signal [33],[34].
In the experiments below, we exploit the same experimental protocols (Figure 1C–I) to identify gene functions that are required to send and/or to receive the FF signal. We monitor the results of these manipulations by assaying QE activity as visualized by the expression of 1XQE.lacZ and 5XQE.DsRed reporters, as well as endogenous Vg [33],[35]; all three responses behave similarly, and we use them interchangeably.
FF Signaling Correlates with Steep, Vg-Dependent Differentials in Opposing Ft and Ds Signals
During normal development, vg activity drives production of the FF signal, and transduction of the signal occurs at the periphery of the wing primordium, where recruitment occurs. Strikingly, two genes involved in Ft-Ds signaling, four-jointed (fj) and ds, itself, are expressed at peak levels in complementary domains that abut at the wing periphery, fj in the vg ON domain (Figure 2A) and ds in the vg OFF surround (Figure 2C). fj encodes a Golgi resident ecto-kinase that functions in PCP to potentiate signaling by Ft and inhibit signaling by Ds [20],[21],[23],[44],[45],[46]. Hence, vg may generate the FF signal by activating fj transcription and repressing ds transcription to create steep and opposing differentials in Ft and Ds signaling between wing and non-wing cells.
One prediction of this hypothesis is that Vg should be both necessary and sufficient to activate fj and repress ds in prospective wing cells. To test this, we used fj-lacZ and ds-lacZ reporters to monitor the consequences of ectopically expressing Vg in apo discs.
Mature apo discs lack the wing primordium as well as adjacent portions of the hinge primordium (Figures 1C, 2B); the remaining cells (which correspond to the rest of the prospective hinge and body wall) express high levels of ds-lacZ (Figure 2D) but not fj-lacZ (Figure 2B). To determine if Vg is sufficient to activate fj-lacZ and repress ds-lacZ, we generated clones of Tub>vg cells in apo discs that are also vgo (to eliminate any contribution from endogenous Vg activity). Such clones express moderate levels of exogenous Vg, a few fold lower than the peak endogenous level observed in wild type discs, and rescue wing development cell-autonomously [33]. They also express fj-lacZ and repress ds-lacZ (Figure 2E, 2F). Thus, ectopic Vg acts cell-autonomously to up-regulate fj and down-regulate ds in apo vgo discs.
A second prediction of the hypothesis that vg generates the FF signal by activating fj and repressing ds is that FF propagation should correlate with the up-regulation of fj transcription at the expense of ds transcription. To test this we analyzed the effects of Tub>vg clones on fj-lacZ and ds-lacZ expression in apo discs supplemented with exogenous Wg, a context in which they induce long-range propagation of QE-dependent vg expression and wing growth (as in Figure 1G; [33]).
As previously shown, Tub>vg clones generated in such discs cell-autonomously activate peak levels of QE-dependent vg expression and induce the long-range propagation of QE-dependent vg expression in surrounding tissue (Figure 2G, 2H; [33]). They also induce the long-range propagation of fj-lacZ expression at the expense of ds-lacZ expression (Figure 2G, 2H), establishing a correlation between FF propagation and the control of fj and ds transcription by vg.
Two additional properties of Tub>vg clones are important to note. First, Tub>vg clones activate fj-lacZ and repress ds-lacZ only in the prospective wing (white/turquoise territory depicted in Figure 1A, 1B) and not in the prospective hinge and body wall (grey territory in Figure 1A, 1B), as is also the case for activation of the QE (Figure 2E, 2F). This is expected, as the FF recruitment process operates only in the prospective wing, where the selector gene teashirt is off, and not in the more proximal domains where it is on [33],[34].
Second, Tub>vg clones activate QE-dependent vg expression, albeit weakly, in apo discs, even in the absence of exogenous Wg, despite the fact that these discs are devoid of D-V border cells, the normal source of Wg required for QE activity. As previously shown [33],[34], this response depends on low levels of cryptic Wg, possibly emanating from the surrounding hinge primordium, which allows the QE to be activated cell-autonomously by the exogenous Vg produced by the Tub>vg transgene.
Both the presence of cryptic Wg signal in apo discs as well as the restriction of FF propagation to the prospective wing territory are relevant preconditions for the experiments presented below.
Ft and Ds Suppress QE-Dependent vg Expression in the Absence of FF Signal
Given that fto and dso discs show extra wing growth, we previously speculated that Ft and Ds normally suppress QE activity in non-wing cells and that the FF signal acts as an antagonist to alleviate this suppression, allowing the QE to respond to Wg [33]. Accordingly, the removal of either protein should mimic receipt of the FF signal and alleviate the block to Wg-dependent activation of the QE. We tested this prediction by assaying QE activity in fto apo and dso apo discs, either in the presence or absence of exogenous Wg.
As described above, apo discs do not activate QE-dependent vg expression and fail to sustain a wing primordium (Figures 1C and 2B) [33],[34]. In contrast, fto apo discs show at least partial rescue of the wing primordium, and cells within the primordium express both 5XQE.DsRed and Vg, albeit at barely detectable levels (Figure 3B and unpublished data; the rescue observed is due to this low level Vg activity, as it does not occur in fto apo vgo discs). Hence, prospective wing cells in these discs behave as if they have constitutively activated the FF signal transduction pathway but can mount only a weak QE response owing to the low levels of cryptic Wg available [34].
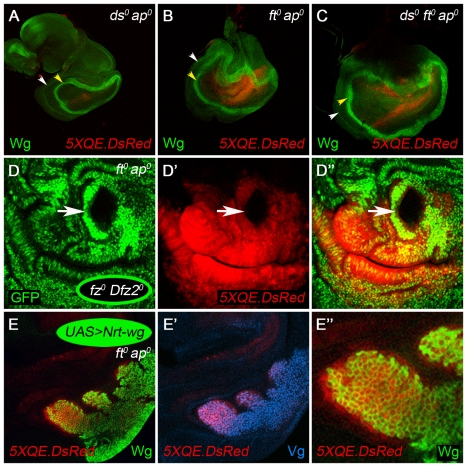
Figure 3. Fat and Dachsous are required to block Quadrant enhancer activity in the absence of feed-forward signal.
(A–C) Removal of either, or both, Ft and Ds causes constitutive, low-level QE activity (monitored by 5XQE.DsRed expression) in apo discs. apo discs that are dso, fto, or dso fto form wing pouches that express the 5XQE.DsRed reporter and are encircled by the Wg IR and OR, in contrast to single mutant apo discs (Figure 2B). Note that the level of 5XQE.DsRed expression is very low, especially in the dso apo disc, consistent with the presence of only cryptic levels of Wg; note also that some DsRed expression within the rescued pouch appears outside of the Wg IR because it is in a fold, underneath. (D) The 5XQE.DsRed response observed in fto apo discs depends on Wg input. 5XQE.DsRed expression is lost in clones of fzo Dfz2o cells in the wing pouch of fto apo discs (a single fzo Dfz2o clone is indicated by an arrow). (E) Clones of UAS.Nrt-wg cells induce normal, peak expression of both the 5XQE.DsRed reporter and endogenous Vg within the clone and in adjacent cells (the low levels of 5XQE.DsRed and Vg expression in surrounding cells can only be detected, as in A–D, using more intense laser illumination).
This interpretation is supported by two experiments that show that QE activity in fto apo discs is Wg dependent. First, the QE response is abolished in clones of fzo Dfz2o cells, which are unable to transduce Wg (Figure 3D) [47]. Second, clones of cells that express a membrane tethered form of Wg (Nrt-Wg; [4],[5]) under Gal4/UAS control (henceforth, UAS.Nrt-wg clones) drive peak levels of Vg and 5XQE.DsRed expression in fto apo discs, both within the clones and in abutting cells (Figure 3E; unpublished data). By contrast, Nrt-Wg fails to rescue Vg expression or wing development in apo discs that are wild type for ft (Figure 1F) [33], confirming that it is the absence of Ft activity in fto apo discs that allows them to activate the QE in response to Wg.
dso apo discs behave similarly to fto apo discs, except that they express even lower levels of 5XQE.DsRed and Vg, and the rescued wing primordium is smaller (Figure 3A; unpublished data). Nevertheless, as in fto apo discs, both responses are activated to peak levels by UAS.Nrt-wg clones (Figure S1). The effect of removing ds appears to be additive to that of removing ft: the rescued wing primordium in triply mutant, dso fto apo discs tend to be larger, on average, than those in fto apo discs (Figure 3B, 3C). The distinct and additive effects of removing Ft and Ds suggest that neither condition corresponds to normal, peak activation of the FF transduction pathway. Instead, as we describe below, each appears to lock the FF transduction pathway into a state of weak, constitutive activity, rendering the level of QE activity refractory to the presence or absence of incoming FF signal.
We conclude that Ft and Ds are normally required in non-wing cells to block QE activity and that receipt of the FF signal alleviates this suppression, allowing the QE to be activated by Wg. Below, we present evidence that Ft, itself, corresponds to the FF signal sent by wing cells and that Ft and Ds function in non-wing cells to receive and transduce this signal.
Ft Is Required for Sending the FF Signal
If, as we posit above, vg generates the FF signal by up-regulating Ft signaling at the expense of Ds signaling, wing cells should require ft, but not ds, to induce QE-dependent vg expression in neighboring non-wing cells. To test this, we generated dso and fto clones in apo discs. Given that the loss of either Ds or Ft mimics reception of the FF signal, such clones should cell-autonomously activate QE-dependent vg expression and survive as wing tissue in apo discs. Accordingly, they should serve as ectopic sources of FF signal, allowing us to determine if their capacity to send FF signal depends on either Ds or Ft activity.
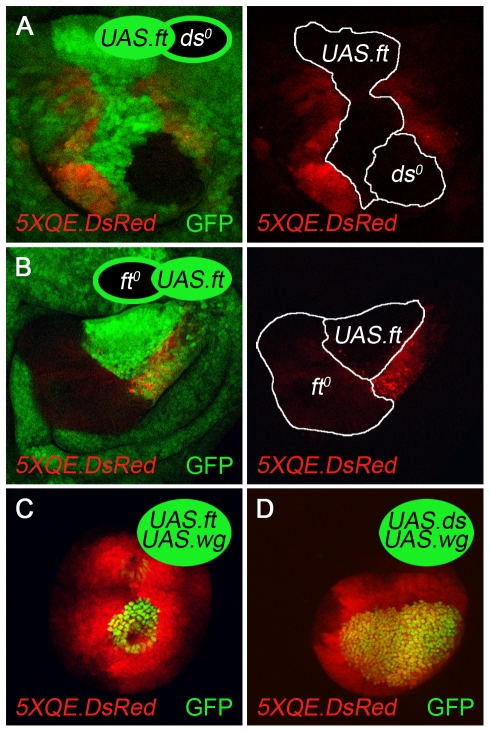
As expected from the behavior of entirely mutant dso apo and fto apo discs (Figure 3A, 3B), both dso and fto clones survive and develop as wing tissue in apo discs (Figure 4A, 4B). However, they express only cryptic, low levels of 5XQE.DsRed and Vg (Figure 4B; unpublished data; see also Figure 4D, 4E), like cells within the wing primordia of dso apo and fto apo mutant discs (Figure 3A, 3B). Strikingly, dso clones also act non-autonomously to induce higher levels of QE activity in neighboring cells (Figure 4A). In contrast, fto clones do not (Figure 4B). Thus, it appears that Ft, but not Ds, is required to send the FF signal.
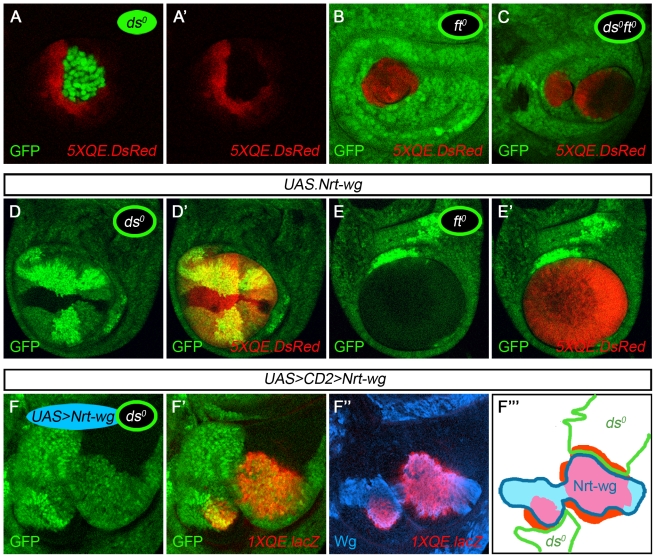
Figure 4. Fat is required in vestigial expressing cells to send feed-forward signal.
(A–C) Clones of dso, fto, and dso fto cells in apo discs. The dso clone (A) is marked positively by the expression of GFP to allow the non-autonomous induction of 5XQE.DsRed expression to be clearly distinguished from the clone. Conversely, fto and dso fto clones (B,C) are marked negatively, by the absence of GFP, to visualize the strictly cell-autonomous expression of the 5XQE.DsRed transgene. 5XQE.DsRed is expressed only at cryptic low levels within dso, fto, and dso fto clones (as in entirely dso apo, fto apo, and dso fto apo discs; Figure 3A–C) and is not detectable in (A) at the level of laser illumination used to generate this image. However, dso clones induce surrounding, wild type cells to express much higher levels of 5XQE.DsRed expression, in contrast to fto and dso fto clones, indicating that they generate ectopic FF signal. We infer that the absence of Ds activity in the dso cells constitutively activates the FF signal transduction pathway but only at a low level relative to the peak response of surrounding, wild type cells to ectopic FF signal sent by the clone. (D,E) Clones of dso and fto cells in apo discs that express UAS.Nrt.wg uniformly under Gal4 control (“UAS.Nrt-wg” discs in all subsequent panels; the non-autonomous induction of 5XQE.DsRed expression appears as yellow in D'). dso clones activate 5XQE.DsRed expression cell-autonomously and serve as a potent source of FF signal, inducing surrounding cells to express the 5XQE.DsRed reporter and join a growing wing primordium. Conversely, most fto clones show only a strictly cell-autonomous response (exceptions appear to be associated with ectopic FF signal generated by sibling ft+/ft+ clones, as documented in Figure S2). (F) An apo disc containing abutting dso and UAS.Nrt-wg clones marked, respectively, by the absence of GFP and the expression of Nrt-Wg (F''' depicts the experiment in cartoon form). The dso clones behave like Tub>vg clones (Figure 1I): they induce high levels of QE-dependent vg expression (monitored by both 1XQE.lacZ and endogenous Vg expression) in the abutting Nrt-Wg cells within the prospective wing domain. Moreover, QE activation propagates over many cell diameters within the Nrt-Wg clone and extends to adjacent cells across the clone border. Finally, the QE response is also up-regulated in dso cells that abut the Nrt-Wg clone, in response to the tethered Wg signal.
To determine if the non-autonomous induction of QE activity by dso clones is due specifically to Ft activity in the mutant cells, we generated dso fto clones. Such clones behave like fto clones in showing strictly cell-autonomous QE activity (Figure 4C). Hence, dso cells require Ft to generate ectopic FF signal.
Assaying FF signaling is limited in apo discs by the dependence of QE activity on cryptic Wg input (Figure 3D, 3E; [34]). We therefore repeated the dso and fto clone experiments, this time supplementing this cryptic Wg signal with uniformly expressed Nrt-Wg (as in Figure 1G).
In the presence of Nrt-Wg, dso clones expressed peak levels of Vg and 5XQE.DsRed cell-autonomously and induced the long-range propagation of both responses in surrounding cells (Figure 4D; unpublished data). Similar results were obtained when we supplied exogenous Wg by generating dso clones that express a UAS.wg transgene (using the MARCM technique [48]; unpublished data) and by generating UAS.Nrt-wg expressing clones next to dso clones in the same disc (Figure 4F). In the latter case, the dso clones behave indistinguishably from Tub>vg clones in the original experimental paradigm used to define the FF signal (Figure 1I; [33]): they induce the long-range propagation of peak levels of Vg and 5XQE.DsRed expression in abutting UAS.Nrt-wg clones (an effect that can extend to the immediate, wild type neighbors of the UAS.Nrt-wg clone). These results confirm that dso clones serve as ectopic sources of FF signal, capable of inducing QE-dependent vg expression in neighboring cells, provided that the responding cells also receive Wg.
In contrast, and with only limited exceptions (Figure S2), fto clones elicited a strictly cell autonomous response, both in Nrt-Wg expressing apo discs (Figure 4E) and when exogenous Wg was supplied using the MARCM technique (unpublished data). Such fto clones form ectopic wing primordia composed solely of mutant cells, excluding even cells of their wild type sibling clones from contributing to the rescued wing tissue (Figure 4E; the sibling clone is marked by elevated GFP staining; compare with the inclusion of the corresponding sibling cells in the case of dso clones, Figure 4D). The cell autonomous response of these fto clones is especially significant because all cells within such clones express peak levels of Vg and fj-lacZ (unpublished data) and hence should be potent sources of FF signal; nevertheless they behave as if devoid of the capacity to signal. Note that this failure cannot be attributed to a generic inability of fto cells to send intercellular signals. First, fto clones repolarize their neighbors, whereas dso fto clones do not, indicating that they have the capacity to send the Ds PCP signal [21],[23],[30],[44]. Second, we have verified by experiment that fto clones in the wing primordium can also send DSL-Notch, Wg, and Dpp signals (Figure S3).
Thus, we conclude that Ft is normally required in vg expressing cells to send the FF signal.
Ft and Ds Are Required for Receiving the FF Signal
Ft and Ds have a complex ligand-receptor relationship in PCP: both proteins have intrinsic signaling activity, and both are required, together, to receive and transduce each of the signals [23]. Hence, as in PCP, Ft may be required both to generate the FF signal in wing cells and, together with Ds, to receive the FF signal in non-wing cells. To test this, we generated abutting, sibling clones (“twin spots”) in which one clone is UAS.ft and the other is either dso or fto and assayed for the capacity of the UAS.ft clones to induce QE activity in neighboring wild type, dso, or fto cells (Figure 5A, 5B).
Figure 5. Generation and transduction of feed-forward signal by Fat and Dachsous in apo discs.
(A) Abutting, sibling clones of UAS.ft and dso cells marked, respectively, by high (2×) or no (0×) GFP expression in a background of moderate (1×) GFP expressing cells, and outlined in white. The UAS.ft clone has induced 5XQE.DsRed expression in neighboring wild type cells but not in the abutting dso cells. As noted in the legend to Figure 4A, the loss of ds is associated with the cell-autonomous activity of the 5XQE-DsRed reporter but only at cryptic, low level relative to the response induced in wild type cells by receipt of FF signal (and hence not detected at the level of laser illumination used in this image). (B) Abutting, sibling clones of UAS.ft and fto cells (marked as in A). The result is the same: like dso cells, the fto cells are refractory to induction of the 5XQE-DsRed transgene by abutting UAS.ft cells, in contrast to neighboring wild type cells. Similarly, as in the case of dso clones, 5XQE.DsRed transgene is expressed constitutively, but only at cryptic low level, in fto clones (as in Figure 4B) and is not readily detectable in this image. (C,D) UAS.ft UAS.wg (C) and UAS.ds UAS.wg (D) clones: The 5XQE.DsRed transgene is strongly expressed both within, and in a halo around, each clone.
UAS.ft clones express levels of Ft that are several fold higher than endogenous Ft (unpublished data) and generate ectopic FF signal in apo discs, as monitored by the induction of 5XQE.DsRed expression in adjacent wild type cells (Figure 5A, 5B; unpublished data). However, adjacent clones of fto cells appear unresponsive to this FF signal, even when they abut the UAS.ft clones over an interface of many cell diameters (Figure 5B). Instead, they express 5XQE.DsRed uniformly and at cryptic, low levels (as in Figure 3B), indicating that the FF transduction pathway is only weakly, albeit constitutively, active in fto cells. Similarly, although clones of dso cells can induce 5XQE.DsRed expression in abutting wild type cells (as in Figure 4A), they too appear to be incapable of responding to adjacent UAS.ft clones (Figure 5A).
Thus, clonal over-expression of Ft is sufficient to generate an ectopic FF signal, but abutting dso and fto cells are refractory to this signal. Notably, we detect either no, or very little, expression of Vg or the 5XQE.DsRed reporter in the Ft over-expressing cells, themselves. Hence, it appears that Ft itself, and not some other molecule under the control of Vg, is responsible for the FF signal sent by these cells.
Taken together with our preceding results, these findings indicate (i) that wing cells require Ft to generate FF signal and (ii) that non-wing cells require both Ft and Ds to receive the signal.
Complementary Roles for Ft and Ds in FF Signaling
Although wing cells require Ft, but not Ds, to send the FF signal, cells undergoing recruitment are also in position to receive an opposing Ds signal coming from non-wing cells on the other side, raising the possibility that this Ds input may also contribute to activating the QE and recruiting cells into the wing primordium.
To assess this, we generated Ds over-expressing clones in apo discs and asked if the resulting disparity in Ds signaling across the clone border is sufficient to induce the QE response in surrounding cells.
Clones of UAS.ds cells in apo discs generate levels of Ds that are several fold higher than endogenous Ds (which is expressed at peak levels in these discs, owing to the absence of vg activity). In the absence of exogenous Wg, such UAS.ds clones had little effect on surrounding cells, only occasionally inducing 5XQE.DsRed expression just outside the clone (unpublished data). However, when supplemented with exogenous Wg (using co-expression of a UAS.wg transgene), most UAS.ds clones induced 5XQE.DsRed expression both within the clone and in surrounding cells (Figure 5D), as is also the case for UAS.ft UAS.wg clones (Figure 5C).
Thus, Ds over-expressing clones, like Ft over-expressing clones, can induce neighboring cells to activate QE-dependent vg expression in apo discs, consistent with the possibility that recruitment of cells into the wing primordium normally depends on opposing Ft and Ds signals (Ft presented by wing cells and Ds presented by non-wing cells; see Discussion).
Transduction of the FF Signal by the Wts-Hpo Pathway and Yki
The Wts-Hpo pathway is known to function downstream of Ft and Ds, as well as the atypical myosin D, in the generic control of growth by the transcriptional co-activator Yki [11],[15],[16],[17],[18],[19]. Hence, it may similarly link reception of the FF signal by Ft and Ds to the induction of QE-dependent vg expression. D activity normally promotes Yki activity by inhibiting the Wts kinase (which would otherwise phosphorylate Yki and prevent it from gaining access to the nucleus). Hence, if the FF signal is transduced by the Wts-Hpo pathway, manipulations that promote Yki action (e.g., removal of Ex or Wts, or over-expression of D or Yki [9],[11]) should activate QE-dependent Vg expression cell-autonomously, subject to Wg input. Moreover, such QE-Vg expressing cells should, themselves, act as sources of ectopic FF signal and induce surrounding cells to activate the QE. We tested these predictions by manipulating D, Ex, Wts, and Yki function in apo discs, either with or without exogenous Wg.
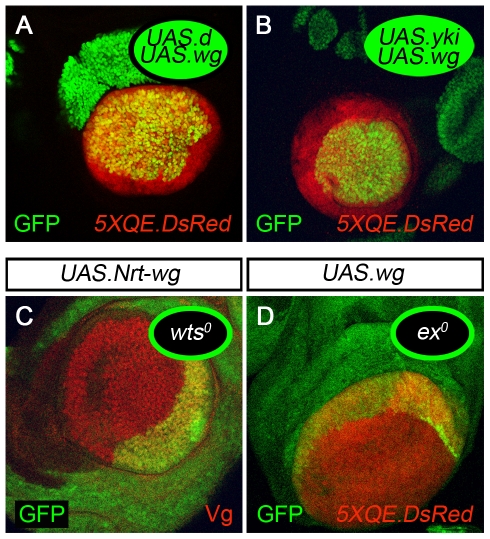
apo discs that uniformly over-express Yki, or which contain large clones of wtso cells, appear similar to fto apo discs (Figure 3B), forming wing primordia that express 5XQE.DsRed and Vg, albeit at barely detectable levels (Figure S1B, S1C; unpublished data). However, as in the case of fto apo and dso apo discs (Figure 3E; Figure S1A), clones of UAS.Nrt-wg cells in these apo wtso and apo UAS.yki discs induce peak levels of both Vg and 5XQE.DsRed expression within the clone and in adjacent cells (Figure S1B, S1C), indicating that both the removal of Wts as well as the over-expression of Yki constitutively activate the FF signal transduction pathway.
Corroborating these results, clones of UAS.d and UAS.yki cells that co-express UAS.wg in apo discs activate peak levels of 5XQE.DsRed expression, cell-autonomously, and can also induce 5XQE.DsRed expression in surrounding cells (Figure 6A, 6B). Likewise, clones of exo or wtso cells generated in UAS.Nrt-wg apo discs express peak levels of Vg and 5XQE.DsRed cell-autonomously and can induce both responses in the surround (Figure 6C, 6D).
Figure 6. Reducing or bypassing Warts-Hippo activity ectopically activates Quadrant enhancer-dependent vestigial expression.
(A,B) Clones of UAS.d (A) and UAS.yki (B) cells that co-express UAS.wg in apo discs. Both clones activate QE dependent gene expression cell-autonomously as monitored by 5XQE.DsRed expression. Both have also induced 5XQE.DsRed expression in neighboring cells encircling the clone. (C,D) Clones of wtso (C) and exo (D) cells in UAS.Nrt-wg apo discs. Both clones activate QE-dependent gene expression cell-autonomously and have also induced QE activity in neighboring cells (monitored by Vg in C, and 5XQE.DsRed expression in D).
These results link reception of the FF signal by Ft and Ds, via D, the Wts-Hpo pathway, and Yki, to activation of the QE.
D Is Required to Transduce the FF Signal
Of the various cytosolic components that function downstream of Ft and Ds, D is distinct in that it functions to promote, rather than to prevent, nuclear action of Yki and that it acts by repressing, rather than facilitating, Wts kinase activity [18],[19],[24],[49]. Hence, in the absence of D, Wts is constitutively active and Yki is excluded from the nucleus, irrespective of Ft-Ds signaling. Accordingly, removal of D should block transduction of the FF signal, preventing the recruitment of non-wing cells into the wing primordium. To test this, we performed the following four experiments.
First, we examined the consequences of generating dso apo, fto apo, and dso fto apo discs that are also null for d. Discs of all three genotypes appear indistinguishable from apo discs (unpublished data), as expected if D is not available to block Wts activity in the absence of Ds and/or Ft.
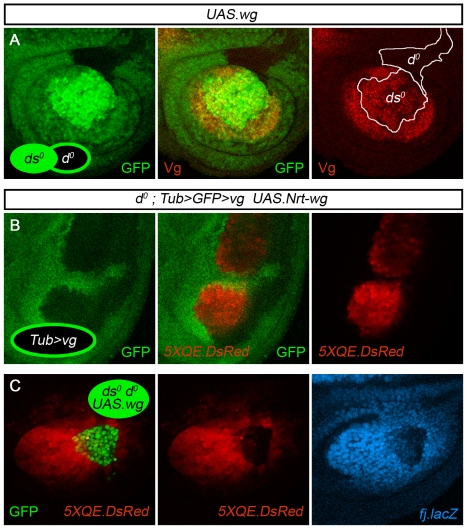
Second, we generated twin spots of sibling dso and do clones in UAS.wg apo discs. Under these conditions, the dso clones both expressed Vg and induced Vg expression in neighboring wild type cells but failed to induce detectable expression in abutting cells belonging to the do clone, resulting in their exclusion from the rescued wing pouch (Figure 7A).
Figure 7. Dachs is required to receive, but not to send, feed-forward signal.
(A) Sibling clones of dso and do cells in an UAS.wg apo disc (clones marked by 2× and 0× GFP expression, respectively, as in Figure 5A, and outlined in white). The dso clone expresses Vg and has induced Vg expression in abutting wild type cells but not in abutting do cells. As a consequence, the latter are unable to contribute to the rescued wing primordium. This result contrasts with the behavior of wild type clones that are generated as siblings of dso clones: as shown in Figure 4D, cells within such wild type clones can respond by activating the QE and joining the wing primordium. (B) Two clones of Tub>vg cells in an UAS.Nrt-wg do apo disc. Both clones express the 5XQE-DsRed reporter cell-autonomously and have induced a few adjacent cells to do the same, in marked contrast to the long-range propagation of QE-dependent Vg expression associated with Tub>vg clones generated in UAS.Nrt-wg apo discs that retain wild type d function (Figures 1G, 2G). (C) A UAS.wg dso do clone in an apo disc. The clone has induced the long-range propagation of 5XQE-DsRed and fj-lacZ expression in surrounding cells, but cells within the clone have failed to respond, or express only low levels of both reporters, indicating that they can send, but not receive, the FF signal.
Third, we generated clones of Tub>vg cells in both apo and do apo discs supplemented with uniform Nrt-Wg (as in Figure 1G). Such clones express peak levels of Vg and induce a long-range propagation of Vg and 5XQE.DsRed expression in apo discs (Figure 2G; [33]) but only a poorly penetrant and local induction of 5XQE.DsRed expression in abutting cells in do apo discs (Figure 7B).
Finally, we tested if the requirement for D in activating the QE is specific to transduction of the FF signal in “receiving” cells as opposed to production of the FF signal in “sending” cells by generating clones of dso do double mutant clones that co-express UAS.wg in apo discs. Such clones behave like corresponding clones of dso single mutant cells (Figure 4D) in that they induce 5XQE.DsRed expression in surrounding cells (Figure 7C). However, cells within the clone show either no or only low levels of 5XQE.DsRed expression.
We conclude that the loss of D activity severely and selectively compromises the capacity of non-wing cells to transduce the FF signal, blocking activation of the QE and recruitment into the wing primordium.
Discussion
During larval life, the Drosophila wing primordium undergoes a dramatic ∼200-fold increase in cell number and mass driven by the morphogens Wg and Dpp. Focusing on Wg, we previously established that this increase depends at least in part on a reiterative process of recruitment in which wing cells send a FF signal that induces neighboring cells to join the primordium in response to morphogen [33],[34]. As summarized in Figure 8, our present results identify Ft-Ds signaling, the Wts-Hpo tumor suppressor pathway, and the transcriptional co-activator Yki as essential components of the FF process and define the circuitry by which it propagates from one cell to the next. We consider, in turn, the nature of the circuit, the parallels between FF signaling and PCP, and the implications for the control of organ growth by morphogen.
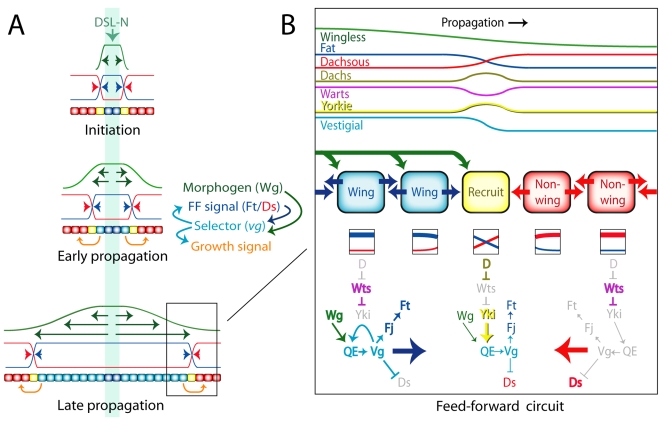
Figure 8. The vestigial feed-forward circuit, and the control of wing growth by morphogen.
(A) A model for the control of wing growth by Wg. Initiation (top): the main phase of wing growth begins with segregation of the wing disc into D-V compartments and the induction of specialized border cells (dark blue) by DSL-Notch signaling (mint green): Notch activity drives expression of both the morphogen Wg (dark green) as well as Boundary enhancer (BE) dependent expression of the wing selector gene vg. As detailed in (B), Vg activity up-regulates Ft signaling (blue) at the expense of Ds signaling (red) to generate the feed-forward (FF) signal. The FF signal then acts together with Wg to induce Quadrant enhancer (QE) expression of vg in non-wing cells (red), initiating a stable circuit of Wg-dependent vg expression that recruits the responding non-wing cell (yellow) into the wing primordium. Early propagation (middle): Vg activity in newly recruited wing cells (turquoise) generates new FF signal, which acts together with Wg secreted by border cells to induce QE-dependent vg expression in neighboring non-wing cells. It also leads to the production of an additional “growth” signal (orange arrows) that promotes proliferation of the surrounding population of non-wing cells from which new wing cells will be recruited. As shown to the right, Vg activity and FF signaling comprise an auto-regulatory cycle driven by Wg. Each turn of the cycle corresponds to the recruitment of a non-wing cell into the wing primordium and generates a new, non-wing cell for subsequent recruitment. Late propagation (bottom): The wing primordium increases in size, propelled by propagation of the FF recruitment cycle and proliferation of cells within and around the primordium, both fueled by Wg as it spreads from D-V border cells. FF forward propagation also depends on Dpp spreading from border cells along the A-P compartment boundary, which acts together with Wg to promote the outward growth of the wing primordium from the intersection between the D-V and A-P compartment boundaries (not depicted; Figure 1A). (B) The feed-forward circuit. Top: the signaling activities of Wg, Ft, and Ds as well as the transducing activities of D, Wts, and Yki are shown relative to vg transcription, and the recruitment of non-wing cells into the wing primordium (recruitment propagates from left to right, coloring as in A). Away from the recruitment interface, Ft and Ds signaling activities are weakly graded or flat, D and Yki activities are low, and Wts activity is high. At the recruitment interface, Ft and Ds signaling activities are steeply graded and opposite, generating a transient pulse in D activity, a dip in Wts activity, and a burst of Yki activity. Middle: Wg, Ft, and Ds signals are shown as green, blue, and red arrows. Only the cell undergoing recruitment (yellow) receives both Wg as well as steep and opposing Ft and Ds signals. Bottom: the regulatory circuits underlying the wing (vg ON; left) and non-wing (vg OFF; right) states as well as the transition that occurs during recruitment (vg OFF to vg ON; middle) are diagrammed relative to the landscapes of Wg, Ft, and Ds signaling upon which they depend. In wing cells, Wg input acts together with Vg to drive a positive auto-regulatory circuit of vg expression mediated by the Quadrant Enhancer (QE), and Vg up-regulates the expression of Fj while repressing that of Ds to enhance Ft signaling at the expense of Ds signaling (blue arrow). In non-wing cells, both the absence of Vg as well as the low level of nuclear Yki lead, by default, to low levels of Fj and high levels of Ds, enhancing Ds signaling at the expense of Ft signaling (red arrow). The box underneath each cell depicts the level and asymmetry of Ft and Ds inputs received from abutting cells on either side. Relatively uniform inputs (depicted by parallel lines in the boxes under wing and non-wing cells) cause modest, or no, polarization of the transducing activities of both proteins within each cell, suppressing the capacity of D to inhibit Wts activity and elevate nuclear import of Yki. Conversely, steep and opposite inputs (depicted by crossing lines in the box beneath the cell undergoing recruitment) cause a strong polarization, allowing D to inhibit Wts activity and induce a burst of Yki nuclear activity. Both Yki and Vg activate vg transcription via the QE by functioning as transcriptional co-activators for the same DNA binding protein, Sd (not depicted). Hence, as the level of Vg rises in cells undergoing recruitment, Vg can substitute for Yki to generate a stable circuit of Wg-dependent Vg auto-regulation that no longer requires Yki or FF input. Note that the depictions of vg expression, as well as of Ft and Ds signaling, as uniform away from the recruitment interface are simplifications. Instead, vg expression is weakly graded within the wing primordium in response to graded Wg signal, and the complementary patterns of fj and ds, upon which the signaling activities of Ft and Ds depend, are similarly graded. The resulting shallow differentials of Ft and Ds signaling may suffice to polarize cells in the plane of the epithelium (PCP). Nevertheless, the expression profiles of all three genes show a dramatic increase in steepness at the periphery of the wing primordium, and it is the resulting steepness in opposing Ds and Ft signals that we posit is essential to induce the burst of Yki nuclear activity upon which recruitment depends.
The vg FF Circuit
Sending the FF signal
We present several lines of evidence that expression of the wing selector gene vg drives production of the FF signal by promoting a non-autonomous signaling activity of Ft. First, we show that vg acts both to up-regulate fj and down-regulate ds, two outputs known to elevate an outgoing, signaling activity of Ft in PCP [20],[21],[23]. Second, we demonstrate that experimental manipulations that elevate Ft signaling—specifically, over-expression of Ft or removal of Ds—generate ectopic FF signal. Third, and most incisively, we show that ft is normally essential in wing cells to send FF signal.
Receiving the FF signal
We show that Ft and Ds are both required in non-wing cells to receive the FF signal, functioning in this capacity to prevent the activation of vg unless countermanded by FF input. Notably, the removal of either Ft or Ds from non-wing cells constitutively activates the FF signal transduction pathway, mimicking receipt of the FF signal. However, the pathway is only weakly activated in this condition and the cells are refractory to any further elevation in pathway activity.
Transducing the FF signal
Previous studies have defined a transduction pathway that links Ft-Ds signaling via the atypical myosin D to suppression of the Wts kinase and enhanced nuclear import of Yki [9],[10],[11],[12],[18],[19],[49]. Likewise, we find that Ft and Ds operate through the same pathway to transduce the FF signal. Specifically, we show that manipulations of the pathway that increase nuclear activity of Yki (over-expression of D or Yki, or loss of Wts or Ex) cause non-wing cells to adopt the wing state. Conversely, removal of D, an intervention that precludes down-regulation of Wts by Ft-Ds signaling, prevents non-wing cells from being recruited into the wing primordium.
Recruitment
To induce non-wing cells to become wing cells, transduction of the FF signal has to activate vg transcription. Activation is mediated by the vg QE [33],[34],[35] and depends on binding sites for Scalloped (Sd), a member of the TEAD/TEF family of DNA binding proteins that can combine with either Yki or Vg to form a transcriptional activator [50],[51],[52],[53],[54],[55],[56],[57]. Hence, we posit that Yki transduces the FF signal by entering the nucleus and combining with Sd to activate vg. In addition, we posit that once sufficient Vg produced under Yki-Sd control accumulates, it can substitute for Yki to generate a stable auto-regulatory loop in which Vg, operating in complex with Sd, sustains its own expression. Accordingly, we view recruitment as a ratchet mechanism. Once the auto-regulatory loop is established, neither FF signaling nor the resulting elevation in Yki activity would be required to sustain vg expression and maintain the wing state (Figure 8).
Morphogen as fuel for FF propagation
Both the activation of the QE by Yki as well as the maintenance of its activity by Vg depend on Wg and Dpp input [33],[34],[35],[50] and hence define distinct circuits of vg auto-regulation fueled by morphogen. For activation, the circuit is inter-cellular, depending on Ft-Ds signaling for vg activity to propagate from one cell to the next. For maintenance, the circuit is intra-cellular, depending on Vg to sustain its own expression. Accordingly, we posit that growth of the wing primordium is propelled by the progressive expansion in the range of morphogen, which acts both to recruit and to retain cells in the primordium (as diagrammed for Wg in Figure 8).
Ft-Ds Signaling: Parallels between FF Propagation and PCP
To date, Ft-Ds signaling has been studied in two contexts: the control of Yki target genes in tissue growth and the orientation of cell structures in PCP. Most work on tissue growth has focused on Yki target genes that control basic cell parameters, such as survival, mass increase, and proliferation (e.g., diap, bantam, and cyclinE). In this context, Ds and Ft are thought to function as a ligand-receptor pair, with tissue-wide gradients of Ds signal serving to activate Ft to appropriate levels within each cell [11],[18],[19],[24],[25]. In contrast, Ft and Ds behave as dual ligands and receptors in PCP, each protein having intrinsic and opposite signaling activity and both proteins being required to receive and orient cells in response to each signal [23],[32].
Here, we have analyzed a different, Yki-dependent aspect of growth, namely the control of organ size by the regulation of a selector gene, vg. In this case, Ft appears to correspond to a ligand, the FF signal, and Ds to a receptor required to receive the ligand—the opposite of the Ds-Ft ligand-receptor relationship inferred to regulate other Yki target genes. Moreover, as in PCP, we also find evidence that Ft and Ds operate as bidirectional ligands and receptors: like Ds, Ft is also required for receipt of the FF signal, possibly in response to an opposing signal conferred by Ds (Figure 8).
Studies of Ft-Ds interactions, both in vivo and in cell culture, have established that Ft and Ds interact in trans to form hetero-dimeric bridges between neighboring cells, the ratio of Ft to Ds presented on the surface of any given cell influencing the engagement of Ds and Ft on the abutting surfaces of its neighbors [28],[30],[44],[58]. These interactions are thought, in turn, to polarize the sub-cellular accumulation and activity of D [19],[24]. Accordingly, we posit that vg activity generates the FF signal by driving steep and opposing differentials of Ft and Ds signaling activity between wing (vg ON) and non-wing (vg OFF) cells. Further, we posit that these differentials are transduced in cells undergoing recruitment (yellow cells in Figure 8) by the resulting polarization of D activity, acting through the Wts-Hpo pathway and Yki to activate vg.
Thus, we propose that FF propagation and PCP depend on a common mechanism in which opposing Ft and Ds signals polarize D activity, both proteins acting as dual ligands and receptors for each other. However, the two processes differ in the downstream consequences of D polarization. For FF propagation, the degree of polarization governs a transcriptional response, via regulation of the Wts-Hpo pathway and Yki. For PCP, the direction of polarization controls an asymmetry in cell behavior, through a presently unknown molecular pathway.
FF propagation and PCP may also differ in their threshold responses to D polarization. We note that Figure 8 portrays vg expression and Ft-Ds signaling in an overly simplified form, in which the landscape is flat within frank wing and non-wing territories and steeply graded at the wing periphery, where recruitment occurs. In reality, vg expression is also graded, albeit weakly, within the wing primordium, due to the response of the QE to graded Wg and Dpp inputs [4],[50]. Hence, a shallow differential of Ft-Ds signaling reflecting that of Vg may be sufficient to orient cells in most of the prospective wing territories, but only cells in the vicinity of the recruitment interface may experience a steep enough differential to induce Yki to enter the nucleus and activate vg.
Finally, FF propagation and PCP differ in at least one other respect, namely, that they exhibit different dependent relationships between Ft and Ds signaling. In PCP, clonal removal of either Ft or Ds generates ectopic polarizing activity, apparently by creating an abrupt disparity in the balance of Ft-to-Ds signaling activity presented by mutant cells relative to that of their wild type neighbors [23]. By contrast, in FF propagation, only the removal of Ds, and not that of Ft, generates ectopic FF signal (Figure 4A–D). We attribute this difference to the underlying dependence of Ft and Ds signaling activity on vg. In dso cells, Ft signaling activity is promoted both by the absence of Ds and by the Vg-dependent up-regulation of fj. However, in fto cells, Ft is absent and Vg down-regulates ds, rendering the cells equivalent to dso fto cells (which are devoid of signaling activity in PCP [23]). Thus, for FF propagation, the underlying circuitry creates a context in which only the loss of Ds, but not that of Ft, generates a strong, ectopic signal. For PCP, no such circuit bias applies.
FF Signaling, the Steepness Hypothesis, and the Control of Growth by Morphogen
Morphogens organize gene expression and cell pattern by dictating distinct transcriptional responses at different threshold concentrations, a process that is understood conceptually, if not in molecular detail. At the same time, they also govern the rate at which developing tissues gain mass and proliferate, a process that continues to defy explanation.
One long-standing proposal, the “steepness hypothesis,” is that the slope of a morphogen gradient can be perceived locally as a difference in morphogen concentration across the diameter of each cell, providing a scalar value that dictates the rate of growth [26],[59],[60]. Indeed, in the context of the Drosophila wing, it has been proposed that the Dpp gradient directs opposing, tissue-wide gradients of fj and ds transcription, with the local differential of Ft-Ds signaling across every cell acting via D, the Wts-Hpo pathway, and Yki to control the rate of cell growth and proliferation [24],[25],[26]. The steepness hypothesis has been challenged, however, by experiments in which uniform distributions of morphogen, or uniform activation of their receptor systems, appear to cause extra, rather than reduced, organ growth [61],[62].
Our results provide an alternative interpretation. As discussed above and illustrated in Figure 8, we posit that “steepness,” as conferred by the local differential of Ft-Ds signaling across each cell, is not a direct reflection of morphogen slope but rather an indirect response governed by vg activity. Moreover, we propose that it promotes wing growth not by functioning as a relatively constant parameter to set a given level of Wts-Hpo pathway activity in all cells but rather by acting as a local, inductive cue to suppress Wts-Hpo pathway activity and recruit non-wing cells into the wing primordium.
How important is such local Ft-Ds signaling and FF propagation to the control of wing growth by morphogen? In the absence of D, cells are severely compromised for the capacity to transduce the FF signal (Figure 7), and the wing primordium gives rise to an adult appendage that is around a third the normal size, albeit normally patterned and proportioned [19]. A similar reduction in size is also observed when QE-dependent vg expression is obviated by other means [34]. Both findings indicate that FF signaling makes a significant contribution to the expansion of the wing primordium driven by Wg and Dpp. Nevertheless, wings formed in the absence of D are still larger than wings formed when either Wg or Dpp signaling is compromised [4],[5],[6],[7]. Hence, both morphogens must operate through additional mechanisms to promote wing growth.
Previously, we identified at least three other outputs of signaling by Wg (and likely Dpp) that work in conjunction with FF propagation [33],[34]. First, as discussed above, Wg is required to maintain vg expression in wing cells once they are recruited by FF signaling, and hence to retain them within the wing primordium. Second, it functions to provide a tonic signal necessary for wing cells to survive, gain mass, and proliferate at a characteristic rate (see also [62]). And third, it acts indirectly, via the capacity of wing cells, to stimulate the growth and proliferation of neighboring non-wing cells, the source population from which new wing cells will be recruited. All of these outputs, as well as FF propagation, depend on, and are fueled by, the outward spread of Wg and Dpp from D-V and A-P border cells. Accordingly, as we argue above, we think that wing growth is governed by the progressive expansion in the range of Wg and Dpp signaling.
Cell Fate Specification, Wts-Hpo Pathway Activity, and the Control of Organ Size
Our identification of Ft-Ds signaling, the Wts-Hpo pathway, and Yki as key components of the FF recruitment process provides a striking parallel with the recently discovered involvement of the Wts-Hpo pathway and Yki/YAP in regulating primordial cell populations in vertebrates, notably the segregation of trophectoderm and inner cell mass in early mammalian embryos [63] and that of neural and endodermal progenitor cells into spinal cord neurons and gut [57],[64]. As in the Drosophila wing, Wts-Hpo activity and YAP appear to function in these contexts in a manner that is distinct from their generic roles in the regulation of cell survival, growth, and proliferation, namely as part of an intercellular signaling mechanism that specifies cell type. We suggest that this novel employment of the pathway constitutes a new, and potentially general, mechanism for regulating tissue and organ size.
Materials and Methods
Generation and Analysis of Mutant Clones
(i) Flp/FRT mediated mitotic recombination [65],[66], (ii) “flp-out cassette” excision [67],[68],[69], and (iii) Mosaic analysis with a repressible cell marker (MARCM [48]) techniques were used, in conjunction with the Gal4/UAS method [70], to manipulate gene function in genetically marked clones of cells in developing wing imaginal discs (e.g., as in [33],[34]).
Animals were cultured at 25°C, and clones were induced during the first larval instar (24–48 h after egg laying) by heat shock induced expression of an Hsp70.flp transgene (usually 36°C for 20 min). Wing discs from mature third instar larvae were dissected, fixed, and processed for immuno-fluorescence by standard methods, using anti-Vg, anti-Wg, anti-HA, and anti-βGal antisera (as in Zecca and Struhl, 2007a,b [33],[34]).
vg QE activity was monitored by expression of 1XQE.lacZ and 5XQE.DSRed reporter transgenes as well as by the expression of Vg protein in the absence of DSL-Notch signaling [33],[34],[35]. In some experiments, expression of the fj-lacZ enhancer trap allele fjP1 [71], which is strongly up-regulated under Vg control, was also used in the absence of DSL-Notch input as a proxy for QE-dependent vg expression. All four assays gave similar results, with the 5XQE.DSRed and fj-lacZ reporters showing the greatest sensitivity.
The following amorphic mutant alleles and transgenes were employed (http://flybase.bio.indiana.edu/) [9],[19],[22],[24],[28],[33],[34]:
Mutant alleles: ap56f, dGC13, Df(2L)Exel6006, dsUA071, ds2D60b, exE1, fjP1, ft15, fzP21, Dfz2 C1, vg83b27R, and wtsX1.
Transgenes: UAS.Nintra, UAS.Nrt-wg, UAS.wg, Tubα1>GFP,y+>vg, C765.Gal4, nub.Gal4, Tubα1>Gal80>Gal4, UAS.dsGS, UAS.ft, UAS.d, UAS.yki, Hsp70.GFP.
Exact genotypes, by Figure panel:
(2A) y w 5XQE.DsRed/y w Hsp70.flp; FRT39 ap56f fjP1/+.
(2B) y w 5XQE.DsRed/y w Hsp70.flp; FRT39 ap56f fjP1/FRT39 ap56f.
(2C) y w 5XQE.DsRed/y w Hsp70.flp; ds2D60b FRT39 ap56f vg83b27R/+.
(2D) y w 5XQE.DsRed/y w Hsp70.flp; ds2D60b FRT39 ap56f vg83b27R/FRT39 ap56f.
(2E) y w Hsp70.flp/y w Hsp70.flp; ap56f vg83b27R 5XQE.DsRed/FRT39 ap56f vg83b27R fjP1; Tubα1>flu-GFP,y+>vg/+.
(2F) y w Hsp70.flp/y w Hsp70.flp; ap56f vg83b27R 5XQE.DsRed/ds2D60b FRT39 ap56f vg83b27R; Tubα1>flu-GFP,y+>vg/+.
(2G) y w 5XQE.DsRed/y w Hsp70.flp; FRT39 ap56f/Hsp70.flu-GFP FRT39 ap56f fjP1; Tubα1>flu-GFP,y+>vg UAS.Nrt-flu-wg/C765.Gal4.
(2H) y w 5XQE.DsRed/y w Hsp70.flp; FRT39 ap56f/ds2D60b FRT39 ap56f vg83b27R; Tubα1>flu-GFP,y+>vg UAS.Nrt-flu-wg/C765.Gal4.
(3A) y w 5XQE.DsRed/y w Hsp70.flp; dsUA071 FRT39 ap56f/dsUA071 FRT39 ap56f; UAS.wg/+.
(3B) y w 5XQE.DsRed/y w 5XQE.DsRed; ft15 FRT39 ap56f/dsUA071 ft15 FRT39 ap56f fjP1.
(3C) y w 5XQE.DsRed/y w 5XQE.DsRed; dsUA071 ft15 FRT39 ap56f/dsUA071 ft15 FRT39 ap56f fjP1; Tubα1>CD2,y+>Gal4/+.
(3D) y w 5XQE.DsRed/y w Hsp70.flp; ft15 FRT39 ap56f/ft15 FRT39 ap56f; fzP21 Dfz2C1 FRT2A/Hsp70.CD2 Hsp70.flu-GFP FRT2A.
(3E) y w 5XQE.DsRed/y w Hsp70.flp; ft15 FRT39 ap56f/ft15 FRT39 ap56f; UAS>CD2,y+>Nrt-flu-wg C765.Gal4/+.
(4A) y w 5XQE.DsRed/y w Hsp70.flp Tuba1.Gal4 UAS.GFPnls; dsUA071 FRT39 ap56f/Hsp70.flu-GFP Tubα1.Gal80 FRT39 ap56f fjP1.
(4B) y w 5XQE.DsRed/y w Hsp70.flp; ft15 FRT39 ap56f/Hsp70.flu-GFP Tubα1.Gal80 FRT39 ap56f fjP1; UAS.wg/+.
(4C) y w 5XQE.DsRed/y w Hsp70.flp; dsUA071 ft15 FRT39 ap56f fjP1/Hsp70.flu-GFP Tubα1.Gal80 FRT39 ap56f; C765.Gal4/+.
(4D) y w 5XQE.DsRed/y w Hsp70.flp; dsUA071 FRT39 ap56f/Hsp70.flu-GFP FRT39 ap56f; UAS.Nrt-flu-wg/C765.Gal4.
(4E) y w 5XQE.DsRed/y w Hsp70.flp; ft15 FRT39 ap56f/Hsp70.flu-GFP FRT39 ap56f; UAS.Nrt-flu-wg/C765.Gal4.
(4F) y w Hsp70.flp/y w Hsp70.flp; dsUA071 FRT39 ap56f/Hsp70.flu-GFP FRT39 ap56f; UAS>CD2,y+>Nrt-flu-wg C765.Gal4/1XQE.lacZ.
(5A) y w 5XQE.DsRed/y w Hsp70.flp; dsUA071 Tubα1.Gal80 FRT39 ap56f vg83b27R/Hsp70.flu-GFP FRT39 ap56f fjP1; UAS.ft/Tuba1.Gal4.
(5B) y w 5XQE.DsRed/y w Hsp70.flp; ft15 Tubα1.Gal80 FRT39 ap56f/Hsp70.flu-GFP FRT39 ap56f fjP1; UAS.ft/Tubα1.Gal4.
(5C) y w 5XQE.DsRed/y w Hsp70.flp UAS.GFPnls; FRT39 ap56f fjP1/FRT39 ap56f UAS.flu-wg; UAS.ft/Tubα1>Gal80,y+>Gal4.
(5D) y w 5XQE.DsRed/y w Hsp70.flp UAS.GFPnls; ap56f 1XQE.lacZ/dsUA071 FRT39 ap56f; UAS.ds/Tubα1>Gal80,y+>Gal4 UAS.wg.
(6A) y w 5XQE.DsRed/y w Hsp70.flp Tuba1.Gal4 UAS.GFPnls; FRT39 ap56f/Hsp70.flu-GFP Tubα1.Gal80 FRT39 ap56f fjP1; UAS.d/UAS.wg.
(6B) y w 5XQE.DsRed/y w Hsp70.flp UAS.GFPnls; FRT39 ap56f UAS.flu-wg/FRT39 ap56f fjP1; Tubα1>Gal80,y+>Gal4 UAS.yki/+.
(6C) y w Hsp70.flp/y w Hsp70.flp; nub-Gal4 FRT39 ap56f/ap56f UAS.flu-Nrt-wg; FRT82 wtsx1/FRT82 Hsp70.flu-GFP.
(6D) y w 5XQE.DsRed/y w Hsp70.flp; exe1 FRT39 ap56f/Hsp70.flu-GFP FRT39 ap56f fjP1; UAS.wg/C765.Gal4.
(7A) y w 5XQE.DsRed/y w Hsp70.flp; dsUA071 Hsp70.flu-GFP FRT39 ap56f/dGC13 FRT39 ap56f fjP1; UAS.wg/C765.Gal4.
(7B) y w 5XQE.DsRed/y w Hsp70.flp; dGC13 FRT39 ap56f fjP1/dGC13 FRT39 ap56f; Tubα1>flu-GFP,y+>vg UAS.Nrt-flu-wg/C765.Gal4.
(7C) y w 5XQE.DsRed/y w Hsp70.flp Tuba1.Gal4 UAS.GFPnls; dsUA071 dGC13 FRT39 ap56f/Hsp70.flu-GFP Tuba1.Gal80 FRT39 ap56f fjP1; UAS.wg/+.
(S1A) y w 5XQE-DsRed/y w Hsp70.flp; dsUA071 FRT39 ap56f/dsUA071 FRT39 ap56f; UAS.Nrt-flu-wg/Tubα1>Gal80,y+>Gal4.
(S1B) y w Hsp70.flp/y w Hsp70.flp; ap56f UAS>CD2,y+>Nrt-flu-wg/nub-Gal4 FRT39 ap56f; FRT82 wtsx1/FRT82 Hsp70.flu-GFP.
(S1C) y w Hsp70.flp/y w Hsp70.flp; ap56f 1XQE.lacZ/FRT39 ap56f; UAS>CD2,y+>Nrt-flu-wg C765.Gal4/UAS.yki.
(S2A) as (4E).
(S2B) y w 5XQE-DsRed/y w Hsp70.flp; ft15 FRT39 ap56f/Df(2L)Exel6006 Hsp70.flu-GFP FRT39 ap56f; UAS.Nrt-flu-wg/C765.Gal4.
(S2C) y w Hsp70.flp/y w Hsp70.flp; ft15 Tuba1.Gal80 FRT39 ap56f/Hsp70.flu-GFP FRT39 ap56f; UAS>CD2,y+>Nrt-flu-wg C765.Gal4/1XQE.lacZ.
(S3A) y w Hsp70.flp Tubα1.Gal4 UAS-GFPnls/y w Hsp70.flp; wgcx4 FRT39 ap56f/Hsp70.flu-GFP Tubα1.Gal80 FRT39 ap56f; UAS.Nintra/1XQE.lacZ.
(S3B) y w 5XQE-DsRed/y w Hsp70.flp Tubα1.Gal4 UAS-GFPnls; ft15 wgcx4 FRT39 ap56f/Hsp70.flu-GFP Tubα1.Gal80 FRT39 ap56f; lqf1227 Hsp70-CD2 FRT2A UAS.Nintra/+.
(S3C) y w Hsp70.flp Tubα1.Gal4 UAS-GFPnls/y w Hsp70.flp; ft15 FRT39 ap56f/Tubα1.Gal80 FRT39; UAS.wg/+.
(S3D) y w omb-lacZ/y w Hsp70.flp Tubα1.Gal4 UAS-GFPnls; ft15 FRT39 ap56f/Tubα1.Gal80 FRT39; UAS.dpp/+.
Supporting Information
Quadrant enhancer activity is Wingless dependent in apo discs that lack either Dachsous or Warts or that over-express Yorkie. (A) A UAS.Nrt-wg clone in a dso apo disc. Both Vg and 5XQE.DsRed are expressed at peak levels in the clone and in surrounding cells that abut the clone, as observed for UAS.Nrt-wg clones in fto apo discs (Figure 3E). (B) UAS.Nrt-wg clones generated in an apo disc largely composed of wtso clonal tissue; as in (A), Vg is strongly up-regulated in the UAS.Nrt-wg clones and abutting cells. (C) UAS.Nrt-wg clones generated in an UAS.yki apo disc; same outcome as in (A), except a 1XQE.lacZ transgene was used instead of the 5XQE.DsRed transgene.
(3.99 MB TIF)
Exceptional cases of local non-autonomous Quadrant enhancer activity associated with fto clones can be attributed to induction by their sibling 2× ft+ clones. (A) A fto clone (marked by the absence of GFP) associated with local, non-autonomous activity of the 5XQE.DsRed transgene (appears yellow in A') in an apo UAS.Nrt-wg disc. Note that this non-autonomous expression is associated with a sibling 2×ft+ clone (white arrow, marked by 2× GFP expression in a 1× GFP 1×ft+ background). In this experiment, 26/43 fto clones were associated with strictly cell-autonomous QE activity (as in Figure 4E): of these, 12/26 had an associated 2×ft+ twin (Figure 4E), and the remaining 14/26 clones had either no detectable twin (7/14) or a very small twin (<8 cells; 7/14). The remaining 17/43 fto clones showed local QE activity in neighboring cells: in 7/17 cases, this non-autonomous activity was associated with a 2×ft+ twin clone (as shown in this panel), and in the remaining 10/17 cases, 9/10 had no detectable twin, and 1/10 had a twin clone located elsewhere. Thus, the majority of fto clones analyzed in this experiment showed a strictly cell-autonomous response, and in 7/8 cases in which local, non-autonomous 5XQE.DsRed expression was observed and a 2×ft+ twin survived, the twin spot was associated with the 5XQE.DsRed expression. Based on these results, we attribute the exceptional cases of non-autonomous 5XQE.DsRed expression associated with fto clones to signaling by their 2×ft+ sibling clones, a conclusion further supported by experiments in panels (B) and (C). (B) A fto clone generated and marked as in (A), except under conditions in which its sibling 2×ft+ clone died, owing to homozygosity for Df(2L)Exel6006. Note the strictly cell-autonomous expression of the 5XQE.DsRed transgene. 39/45 clones generated in this experiment behaved in this way; 6/45 showed local non-autonomy. We have not determined how quickly the sibling 2×ft+ Df(2L)Exel6006 clones die after being generated in this experiment; it is possible that rare 2×ft+ Df(2L)Exel6006 clones survive long enough to induce self-sustaining vg and 5XQE.DsRed expression in neighboring cells prior to their loss. (C) A UAS.Nrt-wg 2×ft+ clone (marked by 2× GFP in a 1× GFP 1×ft+ background, and outlined in white in the right panel) and its fto sibling clone (marked by the absence of GFP) in an apo disc. Note the association of the 2×ft+ clone with ectopic Vg expression as well as the local induction of Vg expression by Nrt-Wg in neighboring cells (Nrt-Wg expression was also assayed, independently, in this clone; unpublished data). This result corroborates the evidence shown in (A) and (B), that 2×ft+ clones generated in 1×ft+ apo discs have the capacity to induce 5XQE-DsRed and vg expression.
(2.19 MB TIF)
fto clones can send Delta/Serrate/Lag2 (DSL), Wingless, and Decapentaplegic signals. (A) A UAS.Nintra wgo clone generated in an apo disc. Nintra encodes a constitutively active form of Notch; clones of UAS.Nintra wgo cells in apo discs up-regulate the expression of the Notch ligands Delta and Serrate and activate Notch in adjacent cells, as visualized by the induction of a ring of ectopic, Wg-expressing D-V border cells encircling the clone (no Wg is made within the clone, as it is wgo). These ectopic border cells suffice to initiate the long-range propagation of QE-dependent vg expression in surrounding cells, as indicated by the broad halo of 1XQE-lacZ expression. (B) A UAS.Nintra wgo fto clone generated an apo disc. Essentially the same experiment shown in (A), except that the clones are also fto. The result is the same (except that a 5XQE-DsRed reporter was used in place of the 1XQE-lacZ reporter), indicating that cells in the clone can send DSL signals to the surround, even though they are devoid of Ft. (C) A UAS.wg fto clone generated in a wild type disc. QE-dependent vg expression depends on the level of Wg input. As a consequence UAS.wg clones up-regulate Vg expression in surrounding cells within the wing pouch, as seen in this example, even though the clone is also fto. (D) A UAS.dpp fto clone generated in a wild type disc. Ectopic Dpp expressed by the clone has induced ectopic omb-lacZ expression in the surround, even though the clone is fto.
(3.03 MB TIF)
Acknowledgments
We thank Xiao-Jing Qiu for technical assistance; Seth Blair, Hitoshi Matakatsu, Mike Simon, and Ken Irvine for fly stocks; and José Casal, Peter Lawrence, Joseph Parker, and Andrew Tomlinson for advice and discussion.
Abbreviations
- ap
apterous
- BE
Boundary Enhancer
- BMP
Bone Morphogenetic Protein
- D
Dachs
- D
dorsal
- Dpp
Decapentaplegic
- Ds
Dachsous
- Ex
Expanded
- FF
feed-forward
- fj
four-jointed
- Ft
Fat
- Hh
Hedgehog
- Hpo
Hippo
- Nrt
Neuroactin
- PCP
planar cell polarity
- QE
Quadrant Enhancer
- Sav
Salvador
- Sd
Scalloped
- Tub
Tubulinα1
- V
ventral
- vg
vestigial
- Wnt
Wingless/Int
- Wts
Warts
- YAP
YES Associated Protein
- Yki
Yorkie
Footnotes
The authors have declared that no competing interests exist.
MZ is a research scientist and GS is an investigator of the Howard Hughes Medical Institute. This work was supported by the Howard Hughes Medical Institute (http://www.hhmi.org/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Zecca M, Basler K, Struhl G. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development. 1995;121:2265–2278. doi: 10.1242/dev.121.8.2265. [DOI] [PubMed] [Google Scholar]
- 2.Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a DPP morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 3.Lecuit T, Brook W. J, Ng M, Calleja M, Sun H, et al. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature. 1996;381:387–393. doi: 10.1038/381387a0. [DOI] [PubMed] [Google Scholar]
- 4.Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–844. doi: 10.1016/s0092-8674(00)81991-1. [DOI] [PubMed] [Google Scholar]
- 5.Neumann C. J, Cohen S. M. Long-range action of Wingless organizes the dorsal-ventral axis of the Drosophila wing. Development. 1997;124:871–880. doi: 10.1242/dev.124.4.871. [DOI] [PubMed] [Google Scholar]
- 6.Spencer F, Hoffmann F, Gelbart W. Decapentaplegic: a gene complex affecting morphogenesis in Drosophila melanogaster. Cell. 1982;28:451–461. doi: 10.1016/0092-8674(82)90199-4. [DOI] [PubMed] [Google Scholar]
- 7.Posakony L. G, Raftery L. A, Gelbart W. M. Wing formation in Drosophila melanogaster requires decapentaplegic gene function along the anterior-posterior compartment boundary. Mech Dev. 1990;33:69–82. doi: 10.1016/0925-4773(90)90136-a. [DOI] [PubMed] [Google Scholar]
- 8.Capdevila J, Guerrero I. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. The EMBO Journal. 1994;13:4459–4468. doi: 10.1002/j.1460-2075.1994.tb06768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Edgar B. A. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–273. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Reddy B. V, Irvine K. The Fat and Warts signaling pathways: new insights into their regulation, mechanism and conservation. Development. 2008;135:2827–2838. doi: 10.1242/dev.020974. [DOI] [PubMed] [Google Scholar]
- 12.Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat Rev Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- 13.Nolo R, Morrison C. M, Tao C, Zhang X, Halder G. The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr Biol. 2006;16:1895–1904. doi: 10.1016/j.cub.2006.08.057. [DOI] [PubMed] [Google Scholar]
- 14.Thompson B. J, Cohen S. M. The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell. 2006;126:767–774. doi: 10.1016/j.cell.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Silva E, Tsatskis Y, Gardano L, Tapon N, Mcneill H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen C. L, et al. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 17.Bennett F. C, Harvey K. F. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 18.Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, et al. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- 19.Mao Y, Rauskolb C, Cho E, Hu W. L, Hayter H, et al. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development. 2006;133:2539–2551. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- 20.Yang C. H, Axelrod J. D, Simon M. A. Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell. 2002;108:675–688. doi: 10.1016/s0092-8674(02)00658-x. [DOI] [PubMed] [Google Scholar]
- 21.Casal J, Struhl G, Lawrence P. A. Developmental compartments and planar polarity in Drosophila. Curr Biol. 2002;12:1189–1198. doi: 10.1016/s0960-9822(02)00974-0. [DOI] [PubMed] [Google Scholar]
- 22.Simon M. A. Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development. 2004;131:6175–6184. doi: 10.1242/dev.01550. [DOI] [PubMed] [Google Scholar]
- 23.Casal J, Lawrence P. A, Struhl G. Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development. 2006;133:4561–4572. doi: 10.1242/dev.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogulja D, Rauskolb C, Irvine K. D. Morphogen control of wing growth through the fat signaling pathway. Dev Cell. 2008;15:309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willecke M, Hamaratoglu F, Sansores-Garcia L, Tao C, Halder G. Boundaries of dachsous cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0805201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence P. A, Struhl G, Casal J. Do the protocadherins Fat and Dachsous link up to determine both planar cell polarity and the dimensions of organs? Nat Cell Biol. 2008;10:1379–1382. doi: 10.1038/ncb1208-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogulja D, Irvine K. Regulation of cell proliferation by a morphogen gradient. Cell. 2005;123:449–461. doi: 10.1016/j.cell.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 28.Matakatsu H, Blair S. S. Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development. 2004;131:3785–3794. doi: 10.1242/dev.01254. [DOI] [PubMed] [Google Scholar]
- 29.Adler P. N, Charlton J, Liu J. Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development. 1998;125:959–968. doi: 10.1242/dev.125.5.959. [DOI] [PubMed] [Google Scholar]
- 30.Strutt H, Strutt D. Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev Cell. 2002;3:851–863. doi: 10.1016/s1534-5807(02)00363-5. [DOI] [PubMed] [Google Scholar]
- 31.Rawls A, Guinto J, Wolff T. The cadherins fat and dachsous regulate dorsal/ventral signaling in the Drosophila eye. Curr Biol. 2002;12:1021–1026. doi: 10.1016/s0960-9822(02)00893-x. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence P. A, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zecca M, Struhl G. Recruitment of cells into the Drosophila wing primordium by a feed-forward circuit of vestigial autoregulation. Development. 2007;134:3001–3010. doi: 10.1242/dev.006411. [DOI] [PubMed] [Google Scholar]
- 34.Zecca M, Struhl G. Control of Drosophila wing growth by the vestigial quadrant enhancer. Development. 2007;134:3011–3020. doi: 10.1242/dev.006445. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Sebring A, Esch J. J, Kraus M. E, Vorwerk K, et al. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature. 1996;382:133–138. doi: 10.1038/382133a0. [DOI] [PubMed] [Google Scholar]
- 36.Diaz-Benjumea F. J, Cohen S. M. Interaction between dorsal and ventral cells in the imaginal disc directs wing development in Drosophila. Cell. 1993;75:741–752. doi: 10.1016/0092-8674(93)90494-b. [DOI] [PubMed] [Google Scholar]
- 37.Blair S. Compartments and appendage development in Drosophila. BioEssays. 1995;17:299–309. doi: 10.1002/bies.950170406. [DOI] [PubMed] [Google Scholar]
- 38.Irvine K. D, Vogt T. F. Dorsal-ventral signaling in limb development. Curr Opin Cell Biol. 1997;9:867–876. doi: 10.1016/s0955-0674(97)80090-7. [DOI] [PubMed] [Google Scholar]
- 39.Williams J. A, Paddock S. W, Vorwerk K, Carroll S. B. Organization of wing formation and induction of a wing-patterning gene at the dorsal/ventral compartment boundary. Nature. 1994;368:299–305. doi: 10.1038/368299a0. [DOI] [PubMed] [Google Scholar]
- 40.de Celis J. F, Garcia-Bellido A, Bray S. J. Activation and function of Notch at the dorsal-ventral boundary of the wing imaginal disc. Development. 1996;122:359–369. doi: 10.1242/dev.122.1.359. [DOI] [PubMed] [Google Scholar]
- 41.Diaz-Benjumea F. J, Cohen S. M. Serrate signals through Notch to establish a Wingless-dependent organizer at the dorsal/ventral compartment boundary of the Drosophila wing. Development. 1995;121:4215–4225. doi: 10.1242/dev.121.12.4215. [DOI] [PubMed] [Google Scholar]
- 42.Rulifson E. J, Micchelli C. A, Axelrod J. D, Perrimon N, Blair S. S. wingless refines its own expression domain on the Drosophila wing margin. Nature. 1996;384:72–74. doi: 10.1038/384072a0. [DOI] [PubMed] [Google Scholar]
- 43.Blair S. S, Brower D. L, Thomas J. B, Zavortink M. The role of apterous in the control of dorsoventral compartmentalization and PS integrin gene expression in the developing wing of Drosophila. Development. 1994;120:1805–1815. doi: 10.1242/dev.120.7.1805. [DOI] [PubMed] [Google Scholar]
- 44.Ma D, Yang C. H, McNeill H, Simon M. A, Axelrod J. D. Fidelity in planar cell polarity signalling. Nature. 2003;421:543–547. doi: 10.1038/nature01366. [DOI] [PubMed] [Google Scholar]
- 45.Strutt H, Mundy J, Hofstra K, Strutt D. Cleavage and secretion is not required for Four-jointed function in Drosophila patterning. Development. 2004;131:881–890. doi: 10.1242/dev.00996. [DOI] [PubMed] [Google Scholar]
- 46.Ishikawa H. O, Takeuchi H, Haltiwanger R. S, Irvine K. Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science. 2008;321:401–404. doi: 10.1126/science.1158159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen C. M, Struhl G. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development. 1999;126:5441–5452. doi: 10.1242/dev.126.23.5441. [DOI] [PubMed] [Google Scholar]
- 48.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 49.Cho E, Irvine K. Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development. 2004;131:4489–4500. doi: 10.1242/dev.01315. [DOI] [PubMed] [Google Scholar]
- 50.Kim J, Johnson K, Chen H. J, Carroll S, Laughon A. Drosophila Mad binds to DNA and directly mediates activation of vestigial by Decapentaplegic. Nature. 1997;388:304–308. doi: 10.1038/40906. [DOI] [PubMed] [Google Scholar]
- 51.Halder G, Polaczyk P, Kraus M. E, Hudson A, Kim J, et al. The Vestigial and Scalloped proteins act together to directly regulate wing-specific gene expression in Drosophila. Genes Dev. 1998;12:3900–3909. doi: 10.1101/gad.12.24.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simmonds A. J, Liu X, Soanes K. H, Krause H. M, Irvine K. D, et al. Molecular interactions between Vestigial and Scalloped promote wing formation in Drosophila. Genes Dev. 1998;12:3815–3820. doi: 10.1101/gad.12.24.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halder G, Carroll S. B. Binding of the Vestigial co-factor switches the DNA-target selectivity of the Scalloped selector protein. Development. 2001;128:3295–3305. doi: 10.1242/dev.128.17.3295. [DOI] [PubMed] [Google Scholar]
- 54.Goulev Y, Fauny J. D, Gonzalez-Marti B, Flagiello D, Silber J, et al. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol. 2008;18:435–441. doi: 10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 55.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Ren F, Zhang Q, Chen Y, Wang B, et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cao X, Pfaff S. L, Gage F. H. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22:3320–3334. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Matakatsu H, Blair S. S. Separating the adhesive and signaling functions of the Fat and Dachsous protocadherins. Development. 2006;133:2315–2324. doi: 10.1242/dev.02401. [DOI] [PubMed] [Google Scholar]
- 59.Lawrence P. A. The development of spatial patterns in the integument of insects. In: Counce S. J, Waddington C. H, editors. Developmental systems: insects. London, New York: Academic Press; 1973. pp. 157–209. [Google Scholar]
- 60.Day S. J, Lawrence P. A. Measuring dimensions: the regulation of size and shape. Development. 2000;127:2977–2987. doi: 10.1242/dev.127.14.2977. [DOI] [PubMed] [Google Scholar]
- 61.Schwank G, Restrepo S, Basler K. Growth regulation by Dpp: an essential role for Brinker and a non-essential role for graded signaling levels. Development. 2008;135:4003–4013. doi: 10.1242/dev.025635. [DOI] [PubMed] [Google Scholar]
- 62.Baena-Lopez L. A, Franch-Marro X, Vincent J-P. Wingless promotes proliferative growth in a gradient-independent manner. Science Signaling. 2009;2:ra60. doi: 10.1126/scisignal.2000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, et al. The hippo signaling pathway components lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Camargo F. D, Gokhale S, Johnnidis J. B, Fu D, Bell G, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 65.Golic K. G. Site-specific recombination between homologous chromosomes in Drosophila. Science. 1991;252:958–961. doi: 10.1126/science.2035025. [DOI] [PubMed] [Google Scholar]
- 66.Xu T, Harrison S. D. Mosaic analysis using FLP recombinase. Methods Cell Biol. 1994;44:655–681. doi: 10.1016/s0091-679x(08)60937-1. [DOI] [PubMed] [Google Scholar]
- 67.Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- 68.Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. [DOI] [PubMed] [Google Scholar]
- 69.Jiang J, Struhl G. Protein kinase A and hedgehog signaling in Drosophila limb development. Cell. 1995;80:563–572. doi: 10.1016/0092-8674(95)90510-3. [DOI] [PubMed] [Google Scholar]
- 70.Brand A. H, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 71.Villano J. L, Katz F. N. four-jointed is required for intermediate growth in the proximal-distal axis in Drosophila. Development. 1995;121:2767–2777. doi: 10.1242/dev.121.9.2767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quadrant enhancer activity is Wingless dependent in apo discs that lack either Dachsous or Warts or that over-express Yorkie. (A) A UAS.Nrt-wg clone in a dso apo disc. Both Vg and 5XQE.DsRed are expressed at peak levels in the clone and in surrounding cells that abut the clone, as observed for UAS.Nrt-wg clones in fto apo discs (Figure 3E). (B) UAS.Nrt-wg clones generated in an apo disc largely composed of wtso clonal tissue; as in (A), Vg is strongly up-regulated in the UAS.Nrt-wg clones and abutting cells. (C) UAS.Nrt-wg clones generated in an UAS.yki apo disc; same outcome as in (A), except a 1XQE.lacZ transgene was used instead of the 5XQE.DsRed transgene.
(3.99 MB TIF)
Exceptional cases of local non-autonomous Quadrant enhancer activity associated with fto clones can be attributed to induction by their sibling 2× ft+ clones. (A) A fto clone (marked by the absence of GFP) associated with local, non-autonomous activity of the 5XQE.DsRed transgene (appears yellow in A') in an apo UAS.Nrt-wg disc. Note that this non-autonomous expression is associated with a sibling 2×ft+ clone (white arrow, marked by 2× GFP expression in a 1× GFP 1×ft+ background). In this experiment, 26/43 fto clones were associated with strictly cell-autonomous QE activity (as in Figure 4E): of these, 12/26 had an associated 2×ft+ twin (Figure 4E), and the remaining 14/26 clones had either no detectable twin (7/14) or a very small twin (<8 cells; 7/14). The remaining 17/43 fto clones showed local QE activity in neighboring cells: in 7/17 cases, this non-autonomous activity was associated with a 2×ft+ twin clone (as shown in this panel), and in the remaining 10/17 cases, 9/10 had no detectable twin, and 1/10 had a twin clone located elsewhere. Thus, the majority of fto clones analyzed in this experiment showed a strictly cell-autonomous response, and in 7/8 cases in which local, non-autonomous 5XQE.DsRed expression was observed and a 2×ft+ twin survived, the twin spot was associated with the 5XQE.DsRed expression. Based on these results, we attribute the exceptional cases of non-autonomous 5XQE.DsRed expression associated with fto clones to signaling by their 2×ft+ sibling clones, a conclusion further supported by experiments in panels (B) and (C). (B) A fto clone generated and marked as in (A), except under conditions in which its sibling 2×ft+ clone died, owing to homozygosity for Df(2L)Exel6006. Note the strictly cell-autonomous expression of the 5XQE.DsRed transgene. 39/45 clones generated in this experiment behaved in this way; 6/45 showed local non-autonomy. We have not determined how quickly the sibling 2×ft+ Df(2L)Exel6006 clones die after being generated in this experiment; it is possible that rare 2×ft+ Df(2L)Exel6006 clones survive long enough to induce self-sustaining vg and 5XQE.DsRed expression in neighboring cells prior to their loss. (C) A UAS.Nrt-wg 2×ft+ clone (marked by 2× GFP in a 1× GFP 1×ft+ background, and outlined in white in the right panel) and its fto sibling clone (marked by the absence of GFP) in an apo disc. Note the association of the 2×ft+ clone with ectopic Vg expression as well as the local induction of Vg expression by Nrt-Wg in neighboring cells (Nrt-Wg expression was also assayed, independently, in this clone; unpublished data). This result corroborates the evidence shown in (A) and (B), that 2×ft+ clones generated in 1×ft+ apo discs have the capacity to induce 5XQE-DsRed and vg expression.
(2.19 MB TIF)
fto clones can send Delta/Serrate/Lag2 (DSL), Wingless, and Decapentaplegic signals. (A) A UAS.Nintra wgo clone generated in an apo disc. Nintra encodes a constitutively active form of Notch; clones of UAS.Nintra wgo cells in apo discs up-regulate the expression of the Notch ligands Delta and Serrate and activate Notch in adjacent cells, as visualized by the induction of a ring of ectopic, Wg-expressing D-V border cells encircling the clone (no Wg is made within the clone, as it is wgo). These ectopic border cells suffice to initiate the long-range propagation of QE-dependent vg expression in surrounding cells, as indicated by the broad halo of 1XQE-lacZ expression. (B) A UAS.Nintra wgo fto clone generated an apo disc. Essentially the same experiment shown in (A), except that the clones are also fto. The result is the same (except that a 5XQE-DsRed reporter was used in place of the 1XQE-lacZ reporter), indicating that cells in the clone can send DSL signals to the surround, even though they are devoid of Ft. (C) A UAS.wg fto clone generated in a wild type disc. QE-dependent vg expression depends on the level of Wg input. As a consequence UAS.wg clones up-regulate Vg expression in surrounding cells within the wing pouch, as seen in this example, even though the clone is also fto. (D) A UAS.dpp fto clone generated in a wild type disc. Ectopic Dpp expressed by the clone has induced ectopic omb-lacZ expression in the surround, even though the clone is fto.
(3.03 MB TIF)